Abstract
Kiosk-facilitated HIV self-testing has been shown to be accurate and well-accepted by emergency department (ED) patients. We investigated factors associated with patients who preferred self-testing over testing performed by health professionals in an ED-based HIV screening program. This opt-in program evaluation studied 332 patients in an inner-city academic ED from 2/2012–4/2012, when a kiosk-based HIV self-testing program was standard of care. The first kiosk in the 2-stage system registered patients and assessed their interest in screening, while the second kiosk gathered demographic and risk factor information and also provided self-testing instructions. Patients who declined to self-test were offered testing by staff. Broad eligibility included patients aged 18–64 who were not critically ill, English-speaking, able to provide informed consent, and registered during HIV program operational hours. Data analyzed using descriptive statistical analysis and chi-squared tests. 160 (48.2%) of 332 patients consenting to testing chose to use a kiosk to guide them performing self-testing. Patients aged 25–29 years and those whose primary ED diagnosis was not infectious disease-related were more likely to prefer HIV self-testing (OR=2.19, 95% CI: 1.17–4.10; OR=1.79, 95% CI: 1.03–3.12). HIV self-testing in the ED could serve as a complementary testing approach to the conventional modality.
Keywords: Emergency Department, HIV, Screening, Self-Testing
Introduction
The Centers for Disease Control and Prevention (CDC) has suggested that emergency departments (EDs) are critical venues for HIV screening.1 Since 2006, U.S. EDs have successfully identified thousands of previously undiagnosed cases of HIV.2 EDs can play a pivotal role towards improving the HIV Care Continuum in the United States.3–5
However, recent data demonstrated that HIV testing is only performed during 0.2% of U.S. ED visits for patients aged 13–64 years.6 Reasons for this gap are unclear but may be due to staff perception of opportunity, and work flow issues.7–12 Additionally, challenges exist in engaging patients in acute care for HIV testing because of their chief complaint issue or their concern on confidentiality issue.
Researchers have developed innovative strategies to adapt HIV testing into routine practice such as ED-based kiosk-facilitated HIV testing13. Our group developed a prototype computerized kiosk system as a means of offering HIV testing and other public health information to ED patients,14 after a pilot study using tablet-based kiosks for facilitating HIV testing in our ED.15 Following the success of our prototype system, we implemented a kiosk-facilitated HIV screening program to engage patients at ED registration. Our results demonstrated that a kiosk- driven program offering HIV testing is not only feasible but also engages more high-risk patients for testing.16
HIV self-testing in ED settings is another novel approach we have successfully explored to increase patient engagement without increasing burden on ED clinical staff.15 We also studied the feasibility of using a tablet kiosk to guide ED patients to perform HIV self-testing using a rapid oral fluid HIV test. This pilot study found that tablet-facilitated HIV self-testing was highly accurate and well-accepted by patients.15 Combining the successful outcomes from both the kiosk-facilitated HIV screening program and the tablet-based self-testing feasibility study, we implemented a pilot rapid oral fluid HIV self-testing program as an option for patients engaged in the kiosk-facilitated HIV screening program.
Our aim was to determine which factors were associated with patients who were more likely to accept kiosk-facilitated self-testing by exploring socio-demographic information, computer/kiosk technology experience, behavior patterns, and ED visit characteristics compared to those who chose conventional HIV testing by trained clinical staff.
Methods
Our program was implemented in an inner-city academic ED with approximately 65,000 annual patient visits in 2012 and an HIV seroprevalence rate of approximately 8%.5 The study took place on 20 weekdays between 3/20/2012 and 4/27/2012 when kiosk-based self-testing was standard of care. Eligibility included English-speaking patients aged 18–64 years able to provide informed consent, not critically ill, and registered as an ED patient during HIV program operational hours. Patients were directed by HIV testing staff to a touchscreen computerized kiosk near registration/triage area to respond to questions regarding general medical and public health information questions as well as their interest in HIV testing after the routine triage process. Then, eligible patients were directed to the backend kiosk where the HIV testing staff provided verbal opt-in consent for HIV testing. Consented patients then responded to a series of questions on the backend kiosk, including the option for either performing an HIV test for themselves or having trained testing staff to test them. The quality evaluation of HIV screening program was approved by the Johns Hopkins University Institutional Review Board.
The first kiosk in the 2-stage system registered patients and assessed their interest in screening, while the second kiosk served to gather demographic and risk factor information and provide self-testing instructions. This ‘‘Risk Assessment Module’’ included up to 26 screens, depending on user responses. Screens included: login; confirmation of desire to test; the option of selftesting; a series of screens regarding procedural instructions if self-testing was selected; sociodemographic data collection (including computer and kiosk experience); HIV risk assessment questions; and a survey on the patient’s preferences and ease of use regarding the kiosk program. The instructions guided patients in collecting and testing the oral fluid specimen (Figure 1). HIV test results were read by program staff and patients were asked to return to the booth 20 minutes later to receive their results. HIV testing staff were on hand to observe patients performing self-testing and to provide assistance as needed. Patients who declined self-testing were tested by trained testing staff.
Figure 1.

Selected Computerized Touch-Screen Kiosk Screens for HIV Self-Testing Instructions.
Demographic and visit data were obtained from ED administrative data while HIV testing/self- testing, computer/kiosk experience, risk behavior assessment were from the testing program. Data regarding ED diagnoses were coded according to the International Classification of Disease, Ninth Revision (ICD-9). We classified the primary ED diagnosis of each visit as infectious or non-infectious based on a previously described protocol (see eTable 1 in the Supplement).17 Data were first analyzed using descriptive analysis. Multiple imputation technique for missing values was performed for the questions when >20% of patients did not respond on the kiosk to minimize bias. Chi-square tests were performed for bivariate analysis and logistic regression was performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) followed by multiple imputation. Variables with a p-value <0.2 in bivariate analysis and other variables a priori considered as potential important confounders were entered into a full multivariate logistic regression model for stepwise model selection approach for a final multivariate regression model.
Results
During the study period, 332 ED patients consented for HIV testing. The majority were female (55%), African American (70%), and 18–39 years (66%) (Table 1). The majority had a triage acuity level of 3, at which patients required urgent attention from ED providers (69%). The leading chief complaint was abdominal pain (8%), followed by headache (4%), back pain (4%), and sore throat (3%). 20% of the patients had an infectious disease-related primary ED diagnosis.
Table 1.
Characteristics of 332 Patients Who Accepted for an HIV Test in an Emergency Department-Based HIV Screening Program by HIV Self-Testing Status
| Characteristics | Categories | Number | Number (%) Patients Who Chose to Perform HIV Self-Testing |
|---|---|---|---|
| Overall | 332 | 160 (48.2) | |
| Age (Years) | 18 – 24 | 106 | 50 (47.8) |
| 25 – 29 | 60 | 37 (61.7) | |
| 30 – 39 | 52 | 22 (42.3) | |
| 40 – 49 | 62 | 26 (41.9) | |
| 50 – 64 | 52 | 25 (48.1) | |
| Gender | Female | 184 | 90 (48.9) |
| Male | 147 | 70 (47.6) | |
| Transgender | 1 | 0 (0.0) | |
| Race | African American | 231 | 107 (46.3) |
| White | 40 | 22 (55.0) | |
| Other | 61 | 31 (50.8) | |
| Ethnicity | Hispanic | 6 | 4 (66.7) |
| Non-Hispanic | 326 | 156 (47.8) | |
| Highest Education Level | High school diploma or less | 155 | 72 (46.5) |
| Some college or more | 110 | 64 (58.2) | |
| Missing | 67 | 24 (35.8) | |
| High Risk Behavior* | Yes | 228 | 119 (52.2) |
| No | 37 | 17 (46.0) | |
| Missing | 67 | 24 (35.8) | |
| Computer Use | < 30 minutes | 114 | 60 (52.6) |
| (per Day) | ≥ 30 minutes | 152 | 77 (50.7) |
| Missing | 66 | 23 (34.8) | |
| Computer Use | < 3 days | 115 | 55 (47.8) |
| (per Week) | ≥ 3 days | 151 | 82 (54.3) |
| Missing | 66 | 23 (34.8) | |
| Previous Kiosk | Yes | 149 | 73 (49.0) |
| Experience | No | 120 | 64 (53.3) |
| Missing | 63 | 23 (36.5) | |
| Triage Acuity Level | 3 – “Urgent” | 229 | 112 (48.9) |
| 4 – “Less Urgent” | 97 | 44 (45.4) | |
| 5 – “Non-Urgent” | 1 | 0 (0.0) | |
| Missing | 5 | 4 (80.0) | |
| Chief Complaint | Abdominal Pain | 27 | 14 (51.9) |
| Headache | 14 | 7 (50.0) | |
| Back Pain | 12 | 7 (58.3) | |
| Sore Throat | 11 | 5 (45.5) | |
| Other | 268 | 160 (48.2) | |
| Primary ED Diagnosis | Infectious Diseases | 67 | 25 (37.3) |
| Other | 265 | 135 (50.9) |
High risk behaviors are defined as: men who have sex with men (MSM), injection drug use, having sex with both genders, having >4 sexual partners in the past year, having sex with >1 person in the past 3 months, having a new sexual partner in the past 3 months, ever having had receptive anal sex, having anal sex in the past 3 months, having a new anal sex partner in the past 3 months, having >1 anal sex partner in the past 3 months, having anonymous sex, having sex with an HIV-positive person, having sex with an injection drug user, having sex with MSM, having sex with someone without knowing their HIV status, having sex with anyone who trades sex for drugs or money, having sex against your will, not always using condoms when having sex, or having a partner who has an STD.
Among 332 patients, 160 (48.2%) patients opted for self-testing (Figure 2). No patients performing HIV self-tests were reactive for HIV; one patient tested by clinical staff had a reactive test. In the bivariate analysis, we found that patients who were 25–29 years or who did not have an infectious disease-related primary ED diagnosis were more likely to test themselves than their counterpart (p=0.017 and p=0.048, respectively). Patients with higher education levels (some college or higher) were marginally more likely to test themselves than those with high school education or less (p=0.092). Gender, race, high risk behavior for HIV infection, computer or kiosk technology experience, chief complaint, and triage acuity were not associated with HIV self-testing. In the multivariate analysis, age of 25–29 years and absence of an infectious disease- related primary ED diagnosis were associated with HIV self-testing (OR=2.19, 95% CI: 1.17, 4.10; OR=1.79, 95% CI: 1.03, 3.12, respectively) (Table 2).
Figure 2.
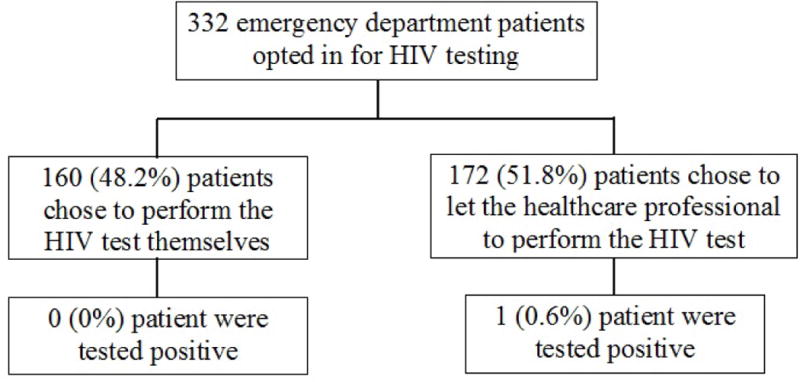
A Flow Diagram of Outcomes of 332 Emergency Department Patients Who Opted for HIV Self-Testing and Who Opted for HIV Testing Staff-Based Testing.
Table 2.
Multivariate Analysis of Factors Associated with Emergency Department Patients Who Opted to Choose Self-Testing for HIV.
| Characteristics | Categories† | Full Model
|
Final Model
|
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Age (years) | 25 – 29 | 1.85 (0.94, 3.64) | 2.19 (1.17, 4.10) |
| 18 – 24 or ≥ 30 | 1.00 | 1.00 | |
| Gender | Female | 1.02 (0.64, 1.60) | – |
| Male or Transgender | 1.00 | ||
| Race | African American | 1.13 (0.61, 2.09) | – |
| White | 0.97 (0.41, 2.25) | – | |
| Other Race | 1.00 | ||
| Highest Education | High School or Less | 1.49 (0.74, 2.98) | – |
| Level | Some College or Higher | 1.00 | |
| High Risk Behavior* | Yes | 0.68 (0.34, 1,37) | – |
| No | 1.00 | ||
| Computer Use | < 30 minutes | 0.70 (0.39, 1.25) | – |
| (per day) | ≥ 30 minutes | 1.00 | |
| Computer Use | < 3 days | 1.29 (0.63, 2.62) | – |
| (per week) | ≥ 3 days | 1.00 | |
| Previous Kiosk | Yes | 0.76 (0.46, 1.24) | – |
| Experience | No | 1.00 | |
| Triage Acuity Level | “Urgent” | 0.91 (0.55, 1.51) | – |
| “Less Urgent” or “Non-Urgent” | 1.00 | ||
| Primary ED Diagnosis | Non-Infectious Diseases | 1.71 (0.96, 3.03) | 1.79 (1.03, 3.12) |
| Infectious Diseases | 1.00 | 1.00 |
High risk behaviors are defined as: men who have sex with men (MSM), injection drug use, having sex with both genders, having >4 sexual partners in the past year, having sex with >1 person in the past 3 months, having a new sexual partner in the past 3 months, ever having had receptive anal sex, having anal sex in the past 3 months, having a new anal sex partner in the past 3 months, having >1 anal sex partner in the past 3 months, having anonymous sex, having sex with an HIV-positive person, having sex with an injection drug user, having sex with MSM, having sex with someone without knowing their HIV status, having sex with anyone who trades sex for drugs or money, having sex against your will, not always using condoms when having sex, or having a partner who has an STD.
Multiple imputation technique for missing values
Discussion
The results of this pilot HIV kiosk-self-testing program demonstrated that ED patients were accepting of HIV self-testing in the ED, as approximately half of the interested eligible patients chose to test themselves. The magnitude of preference and/or acceptability for self-testing was similar to the participation rate (49.5%) for our earlier ED HIV self-testing research study15 and was slightly lower than a hypothetical acceptance rate (56.2%) in a phone survey study in New York City.18 HIV self-testing in healthcare settings is an alternative, innovative approach to empower and engage patients in testing,19,20 and could minimize burden on clinical providers and eliminate the need for additional testing staff. Coupling self-testing with an electronic vending machine for dispensing the self-testing kit,21 may provide a cost-effective HIV testing program model with long-term sustainability in busy acute care settings.
Our study may be the first to investigate factors associated with preference of HIV self-testing in the clinical setting. We identified age of 25–29 years and absence of primary ED diagnosis of infectious diseases as two independent correlates with the preference of HIV self-testing in EDs. Younger age groups (18–24 years and 25–44 years versus 45–64 years) have been identified as key factors associated with perceived acceptability of HIV home testing, if the test were available based on a telephone survey study conducted in New York City prior to the Food and Drug Administration’s approval of the use of home self-testing HIV kits.18 It appears that HIV self-testing in EDs could engage more young adults for HIV testing, an important demographic group that is at higher risk for HIV infection. Interestingly, an acute infectious disease-related ailment was correlated with decreased willingness to self-test, even though patients expressed interest in HIV testing. One possible explanation is that this subset did not feel well enough due to acute symptoms of an acute infection) to follow the HIV self-testing kiosk instructions and chose conventional healthcare professional testing instead.
Some study aspects limit interpretation of our findings. First, a long-standing oral-fluid rapid HIV screening program was launched in 2005 and several research projects on HIV self-testing have been conducted in our ED. Our patients might be more familiar with the technology and procedures of the HIV test than those in other EDs without similar programs. Our acceptance rate of HIV self-testing might be higher and the factors associated with self-testing might be different from others. Additionally, approximately one-third of participants opted not to respond to socio-demographic, computer/kiosk experience, and risk behavior questions on the back-end kiosk. The data analysis on these variables might not truly reflect their influence on the acceptance of self-testing for HIV in the ED, even though an advanced multiple imputation technique for missing values was employed to mitigate the impact of missing data. Finally, we were not able to detect acute HIV infection using this third-generation oral-fluid point-of-care test.
In conclusion, approximately 50% of ED patients who accepted HIV testing chose to perform self-testing by following the instructions provided by a kiosk. Preference of self-testing was positively correlated with young age but negatively correlated with an ED primary diagnosis of infectious disease. With 50% of patients choosing conventional healthcare professional testing over self-testing in the ED, our findings indicate that ED patient self-testing for HIV is likely a complementary testing approach to the current, traditional format. Future research should be directed towards other novel strategies (e.g. electronic vending machine with a result reader) to couple with self-testing to increase patient engagement in HIV testing in acute care settings.
Acknowledgments
Source of Funding: HIV screening program for this study was support provided by Gilead Sciences, Inc.’s HIV FOCUS program and the Baltimore City Health Department. Hsieh, Rothman, and Gaydos are also supported by NIH U54EB007958. Dr Hsieh is also supported in part by a National Institutes of Health award, K01AI100681 from National Institute of Allergy and Infectious Diseases.
Footnotes
Part of this work was presented at the annual meeting of the Society for Academic Emergency Medicine, 14–18 May 2013, Atlanta, GA.
Conflicts of Interest: For all authors, none were declared.
References
- 1.Branson B, Handsfield H, Lampe M, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Results of the Expanded HIV Testing Initiative–25 jurisdictions, United States, 2007–2010. MMWR Morb Mortal Wkly Rep. 2011;60:805–10. [PubMed] [Google Scholar]
- 3.Gardner E, McLees M, Steiner J, Del Rio C, Burman W. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh Y, Kelen G, Laeyendecker O, Kraus C, Quinn T, Rothman R. HIV Care Continuum for HIV-Infected Emergency Department Patients in an Inner-City Academic Emergency Department. Ann Emerg Med. 2015;66:69–78. doi: 10.1016/j.annemergmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelen G, Hsieh Y-H, Rothman R, et al. Improvements in the continuum of HIV care in an inner-city emergency department. AIDS. 2016;30:113–20. doi: 10.1097/QAD.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoover J, Tao G, Heffelfinger J. Monitoring HIV testing at visits to emergency departments in the United States: very-low rate of HIV testing. J Acquir Immune Defic Syndr. 2013;62:90–4. doi: 10.1097/QAI.0b013e3182742933. [DOI] [PubMed] [Google Scholar]
- 7.Akhter S, Gorelick M, Beckmann K. Rapid human immunodeficiency virus testing in the pediatric emergency department: a national survey of attitudes among pediatric emergency practitioners. Pediatr Emerg Care. 2012;28:1257–62. doi: 10.1097/PEC.0b013e3182767add. [DOI] [PubMed] [Google Scholar]
- 8.Arbelaez C, Wright E, Losina E, et al. Emergency provider attitudes and barriers to universal HIV testing in the emergency department. J Emerg Med. 2012;42:7–14. doi: 10.1016/j.jemermed.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown J, Shesser R, Simon G. Establishing an ED HIV Screening Program: Lessons from the Front Lines. Acad Emerg Med. 2007;14:658–61. doi: 10.1197/j.aem.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Burke R, Sepkowitz K, Bernstein K, et al. Why don’t physicians test for HIV? A review of the US literature. AIDS. 2007;21:1617–24. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh Y-H, Jung J, Shahan J, Moring-Parris D, Kelen G, Rothman R. Emergency medicine resident attitudes and perceptions of HIV testing before and after a focused training program and testing implementation. Acad Emerg Med. 2009;16:1165–73. doi: 10.1111/j.1553-2712.2009.00507.x. [DOI] [PubMed] [Google Scholar]
- 12.Signer D, Peterson S, Hsieh Y, et al. Scaling Up HIV Testing in an Academic Emergency Department: An Integrated Testing Model with Rapid Fourth-Generation and Point-of- Care Testing. Public Health Rep. 2016;131:82–9. doi: 10.1177/00333549161310S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haukoos J, Hopkins E, Bender B, et al. Use of kiosks and patient understanding of opt- out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med. 2012;19:287–93. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 14.Rothman R, Gauvey-Kern M, Woodfield A, et al. Streamlining HIV Testing in the Emergency Department - Leveraging Kiosks to Provide True Universal Screening: A Usability Study. Telemed J E Health. 2014;20:122–7. doi: 10.1089/tmj.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaydos C, Solis M, Hsieh Y, Jett-Goheen M, Nour S, Rothman R. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int J STD AIDS. 2013;21:716–21. doi: 10.1177/0956462413487321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh Y-H, Gauvey-Kern M, Peterson S, et al. An emergency department registration kiosk can increase HIV screening in high risk patients. J Telemed Telecare. 2014;20:454–9. doi: 10.1177/1357633X14555637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong G, Conn L, Pinner R. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Myers J, Bodach S, Cutler B, Shepard C, Philippou C, Branson B. Acceptability of home self-tests for HIV in New York City, 2006. Am J Public Health. 2014;104:e46–8. doi: 10.2105/AJPH.2014.302271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high-and low-risk populations: a systematic review. PLoS Med. 2013;10:e1001414. doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youngs J, Hooper C. Ethical implications of HIV self-testing. J Med Ethics. 2015;41:809–13. doi: 10.1136/medethics-2014-102599. [DOI] [PubMed] [Google Scholar]
- 21.Young S, Klausner J, Fynn R, Bolan R. Electronic vending machines for dispensing rapid HIV self-testing kits: a case study. AIDS Care. 2014;26:267–9. doi: 10.1080/09540121.2013.808732. [DOI] [PMC free article] [PubMed] [Google Scholar]


