Abstract
Background
Exposure to advertisements for tobacco products and tobacco warning labels evokes emotions. This study evaluated the association of discrete positive and negative emotions with interest in alternative tobacco products.
Methods
In 2013, 1,226 US adult non-smokers and current smokers viewed advertisements for moist snuff, snus, and electronic cigarettes with various warning labels and then indicated their emotional responses in terms of anger, anxiety, sadness, guilt, disgust, discouragement, hope, and contentment. Outcomes were openness to using moist snuff, snus, and e-cigarettes in the future and interest in a free sample of each product. Data were analyzed in 2016.
Results
Hope was positively associated with openness and interest across all alternative tobacco products as was contentment for moist snuff and snus. Anger was negatively associated with openness to moist snuff and e-cigarettes, disgust negatively – to moist snuff and snus, and anxiety negatively – to e-cigarettes. Being a current smoker, ever trying a corresponding product, being male and younger age were associated with greater openness to and interest in moist snuff and snus. For e-cigarettes, being a current smoker, ever trying e-cigarettes, and being female were associated with greater openness, and being a current smoker was associated with greater odds of selecting a free sample.
Conclusions
Positive emotions, particularly hope, were consistently positively associated with interest in alternative tobacco products. Hope is widely used by tobacco and e-cigarette companies to advertise their products. Anti-tobacco messages should aim to lower hope associated with tobacco products, but increase hope for cessation or life without tobacco.
Keywords: alternative tobacco products, emotions, tobacco advertisements, hope, tobacco products, tobacco, smokeless, electronic cigarettes
INTRODUCTION
As rates of cigarette smoking declined (United States Department of Health and Human Services, 2014), tobacco manufacturers forayed into alternative tobacco products to generate revenue. Consequently, noncombustible alternative tobacco products such as snus and electronic cigarettes (e-cigarettes) have been aggressively marketed with overt or implied claims promoting them as less harmful alternatives to cigarettes, as smoking cessation aids, and as a means to circumvent smoke-free environments such as workplaces or airplanes (Curry, Pederson, & Stryker, 2011; Grana & Ling, 2014; Timberlake, Pechmann, Tran, & Au, 2011). Adoption of these alternative tobacco products has been rising, particularly for e-cigarettes. Between 2010–2013, ever e-cigarette use increased from 3.3% to 8.5% (King, Patel, Nguyen, & Dube, 2015). In 2014, this estimate increased further, with 14.9% of U.S. adults reporting ever trying e-cigarettes and 4.9% reporting use in the past 30 days (Weaver et al., 2016). Despite lower past 30 day use (2.1%), smokeless tobacco had higher reported ever use (17.9%) (Weaver et al., 2016).
Smokeless tobacco may have less adverse effects on the respiratory system than combustible cigarettes (Foulds, Ramstrom, Burke, & Fagerström, 2003). Electronic cigarettes contain lower levels of toxins than combustible cigarettes (Goniewicz et al., 2014) and some research suggests intensive use of e-cigarettes may help with cessation of combustible cigarettes (Biener & Hargraves, 2015). Early reviews and meta-analyses show conflicting findings, with limited data from two randomized clinical trials indicating that nicotine e-cigarettes might help people quit (Hartmann-Boyce et al., 2016), although a meta-analysis of studies found that e-cigarette use in the population is associated with lower odds of quitting (Kalkhoran & Glantz, 2016). While arguably safer than combustible cigarettes, noncombustible alternative tobacco products, such as smokeless tobacco products and e-cigarettes, are not harmless. Smokeless tobacco use has been linked to increased risk of oral, esophageal, and pancreatic cancer (Boffetta, Hecht, Gray, Gupta, & Straif, 2008) and heart disease (Boffetta & Straif, 2009; Henley, Thun, Connell, & Calle, 2005). E-cigarettes contain addictive nicotine and toxic and carcinogenic compounds (World Health Organization, 2014). The use of these alternative tobacco products might serve as a gateway to cigarette smoking (Dutra & Glantz, 2014; Klesges, Sherrill-Mittleman, Ebbert, Talcott, & Debon, 2010; Leventhal et al., 2015), or may encourage cigarette smokers to become dual users of both cigarettes and smokeless tobacco or e-cigarettes rather than quitting smoking (Pearson, Richardson, Niaura, Vallone, & Abrams, 2012). The research on potential warning labels for e-cigarettes has just begun with the FDA proposing a text warning for e-cigarettes as part of its deeming rule (United States Department of Health and Human Services, 2016). Examining the effects of potential warning labels for alternative tobacco products can inform impending regulations.
Cigarette warning labels increase perceived harm of smoking (Hammond, 2011; Hammond, Fong, McNeill, Borland, & Cummings, 2006; Mutti, Hammond, Reid, & Thrasher, 2013). Studies investigating the effects of warning labels have typically examined individuals’ cognitive responses such as perceived severity or harm (Hammond et al., 2006). However, emotion is another important factor influencing individuals’ everyday behaviors (Ferrer, Klein, Lerner, Reyna, & Keltner, 2014; Lerner, Li, Valdesolo, & Kassam, 2015) including economic choices (Cryder, Lerner, Gross, & Dahl, 2008; Lerner, Small, & Loewenstein, 2004) and health behaviors (Kiviniemi, Voss-Humke, & Seifert, 2007). Despite its relevance, emotion has been relatively understudied in the context of tobacco warning labels.
Emotions are thought to influence behavior by activating motivational states that fuel one’s behavioral inclination. Specifically, functional emotion theories (Frijda, 1986; Izard, 1977) and functional and utilitarian accounts of emotions (Keltner & Gross, 1999; Tamir, Chiu, & Gross, 2007) suggest that each emotion accompanies a distinct behavioral tendency that addresses the unique goal activated by the emotion thus serving a useful function for human survival. For instance, when we feel fear, which is caused by a perception of an overwhelming threat (Lazarus, 1991), we are motivated to protect ourselves against the threat (So, Kuang, & Cho, 2016). Disgust, which is triggered by a perception of being too close to an indigestible object or an idea (Lazarus, 1991), motivates behavioral tendency to dispose of the object that caused disgust (Han, Lerner, & Zeckhauser, 2012).
Thus, it is important to not only examine emotional arousal, but also to examine different types of “discrete emotions” (Izard, 1977), since different emotions activate different behavioral goals. Research on emotions and tobacco warning labels, however, has largely focused on the valence of the emotional arousal (i.e., whether positive or negative emotions were aroused; (Nascimento et al., 2008). This approach offers a limited understanding of the effects of warning labels since within the category of negative or positive emotions, different emotions can evoke drastically different perceptions and behavioral tendencies. A good example is the difference between anger and fear (Lerner & Keltner, 2000, 2001). While both emotions are considered to be “negative emotions,” anger promotes an approach tendency and motivates retributive behaviors (e.g., attacking the source; (Nabi, 2003), whereas fear activates an avoidance tendency and motivates protective behaviors against a threat (e.g., quitting smoking, anti-tobacco attitudes; (Hammond, McDonald, Fong, Brown, & Cameron, 2004; Shen, 2011). Thus, it is important to examine the effects of discrete emotions. Studies addressing discrete emotions and tobacco warning labels have focused on only a few emotions, predominantly fear (Cameron, Pepper, & Brewer, 2015; Kees, Burton, Andrews, & Kozup, 2010), and sometimes disgust (Hammond, Fong, McDonald, Brown, & Cameron, 2004). Anger has been studied as part of reactance to warning labels (Erceg-Hurn & Steed, 2011; Noar et al., 2015), which is conceptualized as a motivational state triggered by a threat to freedom (Brehm & Brehm, 1981; Dillard & Shen, 2005). Even fewer studies have linked emotions with tobacco use outcomes, finding that fear induced by cigarette warning labels predicted less smoking behavior and increased likelihood to quit (Hammond, Fong, et al., 2004) and that anxiety and fear mediated the effect of cigarette warning labels on attitudes and intentions to quit smoking (Emery, Romer, Sheerin, Jamieson, & Peters, 2014; Kees et al., 2010).
Another important gap in the literature concerns the relatively understudied role of positive emotions. Historically, positive emotions have received relatively scant scholarly attention due to a variety of reasons, including their diffusive action tendencies (Fredrickson, 1998). The bias towards negative emotions has also been present in health communication research, including anti-tobacco messages (Dunlop, Wakefield, & Kashima, 2008). More recently, however, scholars began examining roles of positive emotions in human behaviors such as economic decision making (Ifcher & Zarghamee, 2011), stress management and coping (Fredrickson, Tugade, Waugh, & Larkin, 2003), and health behaviors (Catellier & Yang, 2013).
Thus, in addition to taking a discrete emotions approach, it is also important to give comparable scholarly attention to the effects of positive discrete emotions. Of the positive emotions, hope is of particular interest in this study. Hope is evoked when a desired outcome is uncertain but possible (MacInnis & De Mello, 2005; Rossiter & Percy, 1991). Hope-inducing tactics include portraying a product as an innovative solution or a way to resolve an approach-avoidance conflict (MacInnis & De Mello, 2005). Tobacco and e-cigarette companies advertise alternative tobacco products with positive messages that could evoke hope (Figure 1). Similarly, the alternative warning label that tobacco companies have petitioned the FDA to use can elicit hopeful emotions. R.J. Reynolds in 2011 and Swedish Match in 2014 petitioned to change one of the current mandatory smokeless tobacco warning labels - “WARNING: This product is not a safe alternative to cigarettes”- to “WARNING: No tobacco product is safe, but this product presents substantially lower risks to health than cigarettes” (R.J. Reynolds, 2011). While the FDA has denied the R.J. Reynolds’s petition (U.S. Food and Drug Administration, 2015a), the decision on the Swedish Match application is still pending.
Figure 1.
An advertisement for Eversmoke electronic cigarettes http://ecigexperts.co.uk/eversmoke), blu electronic cigarettes (https://trinketsandtrash.org/detail.php?artifactid=6991&page=1) and Camel snus (https://trinketsandtrash.org/detail.php?artifactid=6926&page=1) that seem to be capitalizing on hope.
Another attempt at reassuring customers is the use by tobacco manufacturers of “FDA-approved” label as a marketing strategy to promote new tobacco products as meeting the FDA requirements or being “ FDA Approved,” as some e-cigarette products allude to be (U.S. Food and Drug Administration, 2015b). In addition, one of the current US cigarette warning labels states: “SURGEON GENERAL’S WARNING: Quitting smoking now greatly reduces serious risks to your health,” and the proposed FDA graphic warning labels contained “1–800-QUIT NOW,” which could be interpreted as a message of hope; although whether those messages evoke hope has not been studied.
Taken together, we propose a research question: What are the roles of different discrete emotional reactions to warning labels in one’s interest in alternative tobacco products? This study extends the existing research in several ways. First, we examined discrete emotions including positive emotions like hope. Second, we examined the effects of warning labels on three different alternative tobacco products (moist snuff, snus, and e-cigarettes). Currently, smokeless tobacco advertisements have to carry a text-based warning label (e.g., “WARNING: This product can cause mouth cancer” (Public Law 111-31, 2009), while e-cigarette advertising is unregulated. Thus, by examining the effects of alternative tobacco warning labels, this study may provide useful evidence in making decisions on regulating advertising for alternative tobacco products. Third, we evaluated warning labels placed on print tobacco advertisements rather than on the packaging, which has been a common message context in warning label research. This choice stems from the fact that warning labels on packages are typically seen by the users of the product, while advertisements placed in general readership magazines, websites, and outdoors are seen by a much broader audience of both users and non-users. Finally, we evaluated to what extent the aroused discrete emotions are associated with openness to trying and interest in a free sample of alternative tobacco products.
METHODS
Design
This study was a part of a larger investigation evaluating effects of different warning labels on perceptions of harm, attitudes towards, and openness to trying alternative tobacco products (moist snuff, snus, and e-cigarettes). Participants were randomized to one of six groups: five groups saw advertisements with one of the following warnings: 1) current smokeless tobacco warning label, “Warning: This product is not a safe alternative to cigarettes,” 2) graphic warning label (comprising current smokeless tobacco warning label “Warning: This product can cause mouth cancer” and a picture of a mouth sore), 3) R. J. Reynolds’s proposed “lower risk” label, “Warning: No tobacco product is safe, but this product presents substantially lower risks to health than cigarettes,” 4) “FDA-approved” label, and 5) advertisement with no label. The sixth group saw advertisements for a non-tobacco consumer product (such as a cell phone or gum). Participants saw advertisements online. In each condition, participants saw ads for three products that were presented in random order to mitigate order effects: Snus, moist snuff, and e-cigarettes. For each product, the ad shown was randomly drawn from three ads for each type of the alternative tobacco product. Warning labels covered the bottom 20% of the total ad area as required by law for smokeless tobacco warnings (“15 U.S.C. §4402(b)(2) (2012).”)). At the end of the study all participants saw a debriefing page stating that the warning labels they might have seen were used for research only and are not currently in use, and have not been approved by the FDA. Median study time was 14 minutes. For more information on the main study rationale, procedures, and the effects of exposure by condition on changes from pretest to posttest in perceived harm, attitudes, and openness to trying alternative tobacco products, see (Popova & Ling, 2014). For the purpose of this article, advertisements for tobacco products in combination with warning labels served as the stimuli to induce emotions. Thus, the control group that did not see any tobacco-related advertisement was excluded. The primary goal of this article was to examine the relationship between emotions evoked by tobacco-related advertisements and behavioral outcomes. The secondary goal was to evaluate how different warning labels on advertisements for tobacco products evoke different discrete emotions.
Participants
In 2013, a national sample of participants was recruited by Toluna (www.toluna-group.com), a survey market research company, through a variety of online (e.g., web banners, website referrals, affiliate marketing, pay-per-click) and offline recruitment strategies. The sample was screened to include only adults aged 18+ who were non-smokers (have not smoked 100 cigarettes in their lifetime) or current smokers (smoked at least 100 cigarettes in their entire life and were currently smoking cigarettes every day or some days). Non-smokers were included because some non-smokers are trying and using alternative tobacco products (Schoenborn & Gindi, 2015) and because they are exposed to tobacco advertisements and warning labels on them. In making regulatory decisions, FDA has to consider impact on the population as a whole, including non-smokers.
From the larger study of 1,471 individuals, the current study used 1,226 individuals who were exposed to tobacco advertisements, not including the control group that was not exposed to a tobacco advertisement. All participants completed electronic informed consent and all protocols were approved by the IRB at the University of California San Francisco.
Measures
Dependent Variables
Main outcome variables were openness to trying alternative tobacco products and interest in a free sample of alternative tobacco products evaluated after exposure to each ad. Participants were asked how open they were to trying each of the alternative tobacco products in the future with answers ranging from 1 (Not at all open) to 9 (Extremely open). Separately for each of the products, participants were asked if they would like a free sample of an alternative tobacco product. They could also select “Not interested in a free sample” option. In the end of the study, the participants were informed that no samples would be mailed to them and that this study did not endorse or promote tobacco use, similar to prior studies (Hammond, Doxey, Daniel, & Bansal-Travers, 2011; Popova, Neilands, & Ling, 2014).
Independent variables
Emotional responses to the ads
After seeing each ad, participants were asked “Think about the ad you just viewed. How much did the ad make you feel...?” with answers ranging on a 1 (not at all) to 9 (extremely) scale. Eight emotions were assessed; four with a single item each: guilt, disgust, discouragement, and hope; and four with two items each: anger (angry and annoyed, average correlation (Pearson’s r) among the three products r=.82), anxiety (worried and uneasy, average r=.81), sadness (sad and depressed, average r=.81), and contentment (amused and happy, average r=.68). These items were used previously in the study commissioned by the FDA to assess proposed cigarette graphic warning labels (Nonnemaker, Farrelly, Kamyab, Busey, & Mann, 2010)
Demographic variables included sex, age, race, ethnicity, education, and income. We measured ever use of moist snuff, snus, and e-cigarettes.
Statistical Analysis
The primary purpose of this article was to examine the associations of discrete emotions (explanatory variables) with openness to trying alternative tobacco products and interest in a free sample of those products (outcomes). Because we investigated the effects of emotions related to viewing tobacco-related advertisements, the control group, which viewed images unrelated to tobacco, was excluded. Effects of experimental conditions (different warning labels and control) on the outcomes (openness and interest) are reported elsewhere (Popova & Ling, 2014).
For the primary analyses of this article, we used multivariable hierarchical linear regression analyses for openness to trying alternative tobacco products (continuous outcomes) and multivariable hierarchical logistical regression analyses for interest in a free sample of alternative tobacco products (binary outcomes). Stratifying by smokers and non-smokers resulted in largely identical substantive conclusions, so we present the overall models in the main paper. Stratified analyses are available as supplemental tables. The same variable entry strategy was used for both types of regression: hierarchical with two blocks with being a smoker (yes/no), ever trying the respective tobacco product (yes/no), gender (male/female), age (continuous), being White (yes/no), and warning label condition entered in block 1 and the eight emotions reported in response to this product’s ads were entered in the block 2. Multicollinearity was assessed using the Variance Inflated Factor (VIF) with a value of 10 signifying potentially problematic multicollinearity (Hair, Anderson, Tatham, & Black, 1995; Neter, Wasserman, & Kutner, 1989). No predictors exhibited signs of problematic multicollinearity (VIF≥10).
The secondary analyses evaluated the effects of the tobacco-related warning labels on the emotions evoked, via 5 (label condition: current warning label, graphic warning label, lower risk, “FDA Approved,” no label) × 2 (smoker status: smoker, non-smoker) analyses of variance (ANOVAs). These ANOVAs were performed separately for each of the eight emotions and for three products. As in other analyses, because the focus of this investigation was on tobacco-related advertisements, the control group that saw advertisements for non-tobacco products was excluded. Analyses were conducted in 2016 using IBM SPSS Statistics, version 23.
RESULTS
The sample was 52% female, age range between 18 and 83 years (mean age was 43), 39% were White, 29% Black, 21% Asian, and 15% Hispanic. Among all participants, 21% ever tried moist snuff, 18% snus, and 37% e-cigarettes (Table 1). Descriptive statistics for emotions and outcome variables are presented in Table 2.
Table 1.
Participant Characteristics for the Overall Sample and Comparisons between Non-smokers and Smokers
| Characteristic n (%) | Total | Non-smokers (n=403) | Smokers (n=823) | χ2 (df) | p |
|---|---|---|---|---|---|
| Gender | 3.02 (1) | .08 | |||
| Male | 588 (48) | 179 (44.4) | 409 (49.7) | ||
| Female | 638 (52) | 224 (55.6) | 414 (50.3) | ||
| Age | 37.61 (3) | <.001 | |||
| 18–29 | 298 (24.3) | 68 (16.9) | 230 (27.9) | ||
| 30–44 | 387 (31.6) | 113 (28) | 274 (33.3) | ||
| 45–59 | 296 (24.1) | 109 (27) | 187 (22.7) | ||
| 60+ | 245 (20) | 113 (28) | 132 (16) | ||
| Race | 74.42 (6) | <.001 | |||
| White | 473 (38.6) | 217 (53.8) | 256 (31.1) | ||
| Black or African American | 358 (29.2) | 79 (19.6) | 279 (33.9) | ||
| American Indian or Alaska Native | 49 (4) | 11 (2.7) | 38 (4.6) | ||
| Asian | 255 (20.8) | 74 (18.4) | 181 (22) | ||
| Native Hawaiian or Other Pacific Islander | 32 (2.6) | 4 (1) | 28 (3.4) | ||
| Multiple Races | 39 (3.2) | 17 (4.2) | 22 (2.7) | ||
| Unknown | 20 (1.6) | 1 (0.2) | 19 (2.3) | ||
| Ethnicity | 16.60 (1) | <.001 | |||
| Hispanic | 178 (14.5) | 35 (8.7) | 143 (17.5) | ||
| Non-Hispanic | 1,040 (84.8) | 366 (90.8) | 674 (81.9) | ||
| Education | 5.42 (2) | .07 | |||
| High school or less | 261 (21.3) | 76 (18.9) | 185 (22.5) | ||
| Some college | 308 (25.1) | 92 (22.8) | 216 (26.2) | ||
| Bachelor’s degree or higher | 657 (53.6) | 235 (58.3) | 422 (51.3) | ||
| Income ($1,000) | 0.79 (2) | .67 | |||
| <25 | 316 (25.8) | 101 (25.1) | 215 (26.1) | ||
| 25–59.9 | 505 (41.2) | 162 (40.2) | 343 (41.7) | ||
| >60 | 405 (33.0) | 140 (34.7) | 265 (32.2) | ||
| Ever tried | |||||
| Moist snuff | 261 (21.3) | 5 (1.2) | 256 (31.1) | 144.0 (1) | <.001 |
| Snus | 218 (17.8) | 6 (1.5) | 212 (25.8) | 109.0 (1) | <.001 |
| E-cigarettes | 447 (36.5) | 9 (2.2) | 438 (53.2) | 303.6 (1) | <.001 |
Note: The total numbers for demographic variables do not always add up to n=1,226 due to small amounts of missing data.
Table 2.
Means (Standard Deviations) of Discrete Emotions and Outcomes for Three Alternative Tobacco Products (n=1,226)
| Emotions and Outcomes | Moist snuff | Snus | E-cigarettes |
|---|---|---|---|
| Emotions | |||
| Anger | 3.88 (2.80) | 3.79 (2.76) | 3.67 (2.76) |
| Anxiety | 3.91 (2.74) | 3.87 (2.77) | 3.73 (2.73) |
| Sadness | 3.62 (2.70) | 3.57 (2.72) | 3.45 (2.68) |
| Guilt | 3.09 (2.72) | 3.12 (2.72) | 3.04 (2.65) |
| Disgust | 4.60 (3.06) | 4.29 (3.03) | 3.80 (2.96) |
| Discouragement | 3.84 (2.92) | 3.75 (2.89) | 3.63 (2.89) |
| Hope | 2.96 (2.55) | 3.24 (2.62) | 3.83 (2.73) |
| Contentment | 2.93 (2.33) | 3.11 (2.38) | 3.38 (2.37) |
| Outcomes | |||
| Openness to using in the future | 2.74 (2.51) | 2.91 (2.56) | 4.68 (3.17) |
| Percent interested in a free sample | 30.2 | 34.5 | 56.1 |
Note: All variables (except percent interested in a free sample) are on 1–9 scale.
Standard deviations are in parentheses.
We first report the results of the primary analyses, examining the associations of demographics and discrete emotions with openness to trying alternative tobacco products and interest in a free sample of those products. Then we present the results of the secondary analyses showing the effects of different labels on tobacco advertisements on discrete emotions.
Factors Associated with Openness to Trying Alternative Tobacco Products
Smokers were more open to trying moist snuff (unstandardized regression coefficient, B=1.01, 95% confidence interval, CI=0.74, 1.29), snus (1.38 [1.1, 1.66]), and e-cigarettes (3.71 [3.37, 4.05]) compared to non-smokers (in the text, only results of models with demographics and smoking status are shown; for models with emotions as explanatory variables, see Table 3). Likewise, those who have ever tried moist snuff (2.02 [1.7, 2.35]), snus (1.65 [1.31, 2.0]), and e-cigarettes (1.01 [0.68, 1.34]) were significantly more open to trying the corresponding product in the future than those who have not tried that product. Females were less open to trying moist snuff (−0.54 [−0.79, −0.3]) and snus (−0.69 [−0.94, −0.44]), but more open to e-cigarettes (0.29 [0.01, 0.56]) than males were. Non-White participants were less open to trying moist snuff (−0.4 [−0.67, −0.13]) and snus (−0.54 [−0.81, −0.26]) compared to White participants, but only in the models without emotions as predictors. Older participants were less open to trying moist snuff (−0.03 [−0.04, −0.02]) and snus (−0.03 [−0.04, −0.02]) compared to younger participants. Participants who saw a graphic warning label were less open to snus (−0.58 [−0.97, −0.2]) and e-cigarettes (−0.48 [−0.91, −9.06]) compared to participants who saw an advertisement without a warning label, but only in the models without emotions as predictors. Adding emotions to the regressions explained an additional 6%–14% of the variance. Greater anger was associated with less openness to moist snuff (−0.1 [−0.18, −0.02]) and e-cigarettes (−0.13 [−0.24, −0.02]), and greater disgust with less openness to snus (−0.09 [−0.15, −0.02]). Greater hope was associated with greater openness to moist snuff (0.15 [0.07, 0.22]), snus (0.26 [0.19, 0.33]) and e-cigarettes (0.26 [0.19, 0.33]). Greater contentment was related to greater openness to moist snuff (0.29 [0.21, 0.37]) and snus (0.21 [0.13, 0.29]).
Table 3.
Multivariable Associations between Demographics, Past Tobacco Use, Warning Label Condition, and Emotions and Openness to Trying Alternative Tobacco Products
| Independent variables | Moist snuff | Snus | E-cigarettes | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| Current smoker | 1.01 (0.74, 1.29) | 0.62 (0.35, 0.89) | 1.38 (1.1, 1.66) | 0.85 (0.57, 1.12) | 3.71 (3.37, 4.05) | 3.12 (2.77, 3.46) |
| Ever tried product | 2.02 (1.7, 2.35) | 1.62 (1.31, 1.92) | 1.65 (1.31, 2) | 1.08 (0.75, 1.4) | 1.01 (0.68, 1.34) | 0.84 (0.52, 1.16) |
| Gender (female) | −0.54 (−0.79, −0.3) | −0.34 (−0.57, −0.11) | −0.69 (−0.94, −0.44) | −0.44 (−0.67, −0.21) | 0.29 (0.01, 0.56) | 0.28 (0.02, 0.54) |
| Non-white | −0.4 (−0.67, −0.13) | −0.13 (−0.39, 0.12) | −0.54 (−0.81, −0.26) | −0.2 (−0.46, 0.05) | −0.25 (−0.55, 0.06) | −0.16 (−0.46, 0.13) |
| Age | −0.03 (−0.04, −0.02) | −0.02 (−0.03, −0.01) | −0.03 (−0.04, −0.02) | −0.02 (−0.03, −0.01) | −0.01 (−0.02, 0) | −0.01 (−0.02, 0) |
| Current WL | 0.09 (−0.29, 0.47) | 0.19 (−0.17, 0.54) | −0.26 (−0.64, 0.13) | −0.04 (−0.39, 0.31) | −0.34 (−0.77, 0.09) | −0.08 (−0.49, 0.33) |
| Lower risk WL | −0.06 (−0.44, 0.32) | −0.05 (−0.4, 0.3) | −0.22 (−0.61, 0.16) | −0.18 (−0.53, 0.17) | 0.2 (−0.23, 0.63) | 0.25 (−0.15, 0.66) |
| FDA-approved WL | 0.12 (−0.25, 0.5) | 0.18 (−0.17, 0.53) | −0.11 (−0.49, 0.28) | −0.01 (−0.36, 0.34) | −0.12 (−0.54, 0.31) | −0.08 (−0.49, 0.33) |
| Graphic WL | −0.26 (−0.63, 0.12) | 0.07 (−0.29, 0.44) | −0.58 (−0.97, −0.2) | 0.03 (−0.34, 0.4) | −0.48 (−0.91, −0.06) | 0.3 (−0.15, 0.74) |
| Anger | −0.1 (−0.18, −0.02) | −0.06 (−0.15, 0.03) | −0.13 (−0.24, −0.02) | |||
| Anxiety | 0.04 (−0.06, 0.14) | 0.01 (−0.09, 0.11) | −0.04 (−0.16, 0.08) | |||
| Sadness | 0.05 (−0.05, 0.15) | 0.03 (−0.07, 0.13) | 0.03 (−0.09, 0.14) | |||
| Guilt | −0.05 (−0.12, 0.01) | −0.03 (−0.1, 0.04) | −0.07 (−0.15, 0.01) | |||
| Disgust | −0.04 (−0.1, 0.02) | −0.09 (−0.15, −0.02) | −0.03 (−0.12, 0.06) | |||
| Discouragement | −0.03 (−0.1, 0.05) | −0.02 (−0.1, 0.06) | −0.02 (−0.11, 0.07) | |||
| Hope | 0.15 (0.07, 0.22) | 0.26 (0.19, 0.33) | 0.26 (0.19, 0.33) | |||
| Contentment | 0.29 (0.21, 0.37) | 0.21 (0.13, 0.29) | 0.02 (−0.07, 0.1) | |||
| R2adj | 0.31 | 0.41 | 0.3 | 0.44 | 0.44 | 0.5 |
| Δ R2 | 0.1 | 0.14 | 0.06 | |||
Note: Multivariable linear regressions, unstandardized regression coefficients (n=1226). Boldface indicates statistical significance (p < .05)
WL – warning labels. Warning labels conditions are in comparison to the condition with no warning.
Factors Associated with Interest in a Free Sample of Alternative Tobacco Product
Smokers had significantly higher odds of choosing a free sample of moist snuff (adjusted odds ratio 7.3, 95% confidence interval, CI=4.53, 11.76), snus (6.98 [4.62, 10.55]), and e-cigarettes (20.0 [13.66, 29.26]) compared to non-smokers (results of models with demographics and smoking status only; for models with emotions as predictors, see Table 4). Those who ever tried moist snuff (3.32 [2.34, 4.7]) and snus (3.65 [2.52, 5.3]) had significantly higher odds of choosing a free sample of moist snuff and snus correspondingly, but ever trying e-cigarettes was significantly associated with interest in a free sample of e-cigarettes only in the model without emotions (1.43 [1.01, 2.04]). Women had significantly lower odds of choosing a free sample of moist snuff (0.52 [0.38, 0.7]) or snus (0.57 [0.43, 0.77]) than men did. Non-White participants had significantly higher odds of choosing free samples of moist snuff (1.76 [1.24, 2.51]) and snus (1.62 [1.16, 2.25]) compared to White participants, but only in the models that did not include emotions. Older participants had lower odds of choosing a free sample of moist snuff (0.97 [0.95, 0.98]) and snus (0.98 [0.96, 0.99]) compared to younger participants. Participants who saw a graphic warning label had lower odds of choosing free samples of snus (0.58 [0.37, 0.92]) compared to participants who saw an advertisement without a warning label, but only in the models without emotions as predictors. Greater anger (0.85 [0.74, 0.98]) and anxiety (0.84 [0.72, 0.98]) were associated with lower odds of choosing a free sample of e-cigarettes. Greater disgust was associated with lower odds of choosing a free sample of moist snuff (0.88 [0.79, 0.98]). Greater hope was associated with higher odds of choosing a free sample of moist snuff (1.17 [1.06, 1.29]), snus (1.29 [1.17, 1.41]), and e-cigarettes (1.23 [1.12, 1.34]). Greater contentment was related to higher odds of choosing a free sample of moist snuff (1.28 [1.15, 1.43]) and snus (1.21 [1.09, 1.35]).
Table 4.
Multivariable Associations between Demographics, Past Tobacco Use, Warning Label Condition, and Emotions and Interest in a Free Sample of Alternative Tobacco Products
| Moist snuff | Snus | E-cigarettes | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Current smoker | 7.3 (4.53, 11.76) | 4.77 (2.89, 7.87) | 6.98 (4.62, 10.55) | 4.78 (3.07, 7.46) | 20 (13.66, 29.26) | 14.12 (9.43, 21.14) |
| Ever tried product | 3.32 (2.34, 4.7) | 2.58 (1.76, 3.77) | 3.65 (2.52, 5.3) | 2.65 (1.76, 3.99) | 1.43 (1.01, 2.04) | 1.27 (0.88, 1.83) |
| Gender (female) | 0.52 (0.38, 0.7) | 0.61 (0.44, 0.86) | 0.57 (0.43, 0.77) | 0.67 (0.49, 0.92) | 1.2 (0.88, 1.62) | 1.28 (0.93, 1.76) |
| Non-white | 1.76 (1.24, 2.51) | 1.3 (0.88, 1.92) | 1.62 (1.16, 2.25) | 1.17 (0.81, 1.69) | 1.1 (0.78, 1.53) | 0.95 (0.67, 1.36) |
| Age | 0.97 (0.95, 0.98) | 0.98 (0.96, 0.99) | 0.97 (0.96, 0.98) | 0.98 (0.97, 0.99) | 0.99 (0.98, 1) | 0.99 (0.98, 1) |
| Current WL | 0.78 (0.48, 1.26) | 0.79 (0.47, 1.33) | 0.78 (0.49, 1.22) | 0.9 (0.55, 1.47) | 0.83 (0.52, 1.33) | 1.06 (0.64, 1.73) |
| Lower risk WL | 1.03 (0.64, 1.66) | 0.94 (0.56, 1.57) | 1.02 (0.65, 1.6) | 1.04 (0.64, 1.68) | 1.05 (0.65, 1.7) | 1.08 (0.65, 1.78) |
| FDA-approved WL | 1.15 (0.72, 1.85) | 1.17 (0.71, 1.95) | 1.08 (0.69, 1.69) | 1.21 (0.75, 1.95) | 1.07 (0.66, 1.74) | 1.11 (0.67, 1.83) |
| Graphic WL | 0.76 (0.47, 1.22) | 1.03 (0.59, 1.8) | 0.58 (0.37, 0.92) | 1.02 (0.6, 1.73) | 0.78 (0.49, 1.25) | 1.57 (0.9, 2.75) |
| Anger | 0.87 (0.75, 1) | 0.95 (0.82, 1.09) | 0.85 (0.74, 0.98) | |||
| Anxiety | 0.99 (0.83, 1.17) | 0.96 (0.82, 1.13) | 0.84 (0.72, 0.98) | |||
| Sadness | 1.02 (0.85, 1.21) | 1.03 (0.87, 1.22) | 1.12 (0.97, 1.29) | |||
| Guilt | 1.1 (0.98, 1.24) | 1 (0.89, 1.13) | 1.07 (0.96, 1.18) | |||
| Disgust | 0.88 (0.79, 0.98) | 0.92 (0.83, 1.02) | 0.93 (0.83, 1.05) | |||
| Discouragement | 1.08 (0.95, 1.22) | 0.99 (0.87, 1.13) | 1.07 (0.95, 1.21) | |||
| Hope | 1.17 (1.06, 1.29) | 1.29 (1.17, 1.41) | 1.23 (1.12, 1.34) | |||
| Contentment | 1.28 (1.15, 1.43) | 1.21 (1.09, 1.35) | 1.01 (0.91, 1.12) | |||
| Nagerlkerke R2adj | 0.41 | 0.5 | 0.38 | 0.49 | 0.47 | 0.52 |
Note: Multivariable logistic regressions (n=1226). AOR – adjusted odds ratios. Boldface indicates statistical significance (p < .05)
WL – warning labels. Warning labels conditions are in comparison to the condition with no warning.
Emotional responses to tobacco product ads with different labels
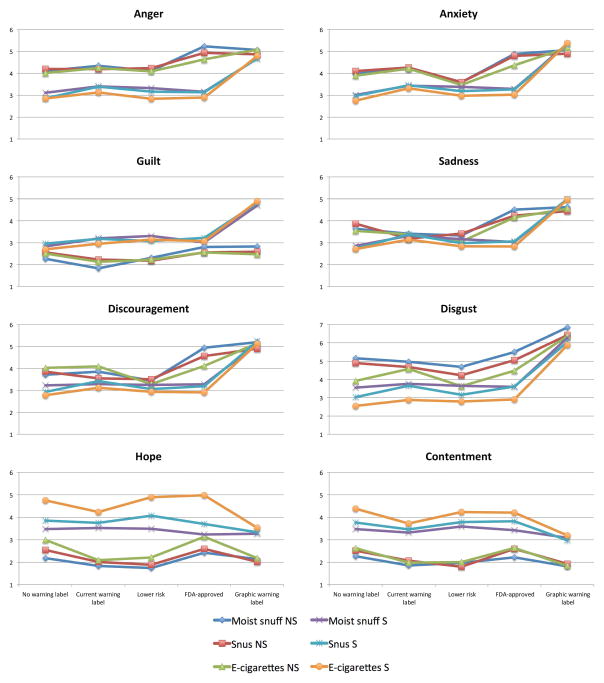
The main effect of condition was significant for almost all emotions in all products except for contentment for moist snuff and hope for moist snuff and snus (Supplemental Table 1). The graphic warning label condition evoked greater levels of negative emotions and lower levels of positive emotions than the condition with no warning. The main effect of being a smoker was significant across all emotions and products, such that smokers reported lower levels of negative and higher levels of positive emotions compared to non-smokers. The interaction effect between condition and being a smoker was significant for all products for anxiety, sadness, and guilt; for moist snuff and snus, but not for e-cigarettes for anger and discouragement, and was non-significant for contentment, hope and disgust. For example, a significant interaction for anxiety revealed that while non-smokers had greater levels of anxiety than smokers did in no warning, current warning, and “FDA approved” conditions, their level of anxiety dropped to the level of smokers in “Lower-risk” label, while smokers’ level of anxiety was raised to the level of anxiety in non-smokers in the graphic warning label condition (Figure 2, Supplemental Table 1).
Figure 2.
Levels of discrete emotions reported by non-smokers (NS) and smokers (S) in five different warning label conditions
DISCUSSION
When individuals see advertisements for alternative tobacco products with or without different warning labels they feel emotions – positive and negative. These emotions, in turn, are associated with interest in trying alternative tobacco products. Investigating how discrete emotions in response to different warning labels placed on advertisements for alternative tobacco products are associated with interest in these products, we found that hope and to a lesser extent contentment were consistently significantly and positively associated with interest in alternative tobacco products. Only select negative emotions were associated with interest in trying various alternative tobacco products. The more anger participants felt, the less open they were to trying moist snuff or e-cigarettes. Greater disgust was associated with less openness to moist snuff and snus. Participants who experienced greater levels of anxiety were less likely to select a free sample of e-cigarettes.
Emotional appeals have been widely used by tobacco companies to promote their products. Advertisements for alternative tobacco products frequently include positive imagery that may evoke hopeful emotions (Figure 1) with 95% of retail e-cigarette websites making health-related claims, 64% making smoking cessation–related claims, and 76% claiming that e-cigarettes do not produce secondhand smoke (Grana & Ling, 2014). While we do not have direct evidence that the companies tried to induce positive emotions, such as hope and contentment, through the ads used in this study, we know that tobacco companies have a long history of attempting to alleviate fears of health-concerned smokers by using positive appeals, as revealed in detailed analyses of previously secret internal tobacco industry documents (Anderson, Pollay, & Ling, 2006; Pollay, 2000). Tobacco companies promoted low-tar and low-nicotine brands as well as potential reduced-exposure products (PREPs) as an alternative to quitting smoking, reassuring the concerned smokers by explicitly presenting these products as a solution to the smoking problem and the guilt smokers feel, and by portraying smokers who used them as healthy, successful, and smart (Anderson et al., 2006; National Cancer Institute, 2008). The advertisements with health themes published in Time magazine in 1929–1984 were particularly frequent during or right before the major announcements about health effects of smoking (Warner, 1985). Today, advertisements for noncombustible alternative tobacco products portray e-cigarettes and snus as healthier alternatives to smoking and as potential smoking cessation aids (Grana & Ling, 2014; Trinkets & Trash Artifacts of the Tobacco Epidemic).
Tobacco companies have historically used contentment in their advertising. For example, a 1927 Camel national advertising campaign was called “The heights of contentment” (R. J. Reynolds Tobacco Company, 1927). A 1957 radio spot encouraged listeners to give fathers a gift of pipe tobacco for Father’s day: “It brings complete contentment – Mom will love the aroma, too” (underlined in the original) (The American Tobacco Company, 1957). Qualitative research commissioned by British American Tobacco (Carter, 2003), Philip Morris (Michael Scavone Inc., 1999), and RJ Reynolds (Pollay, 2000) showed that smokers associated advertisements for those brands with a sense of relaxation and contentment.
Given the history of use of positive emotions, such as contentment, in tobacco advertisements, it is not surprising that contentment and hope were positively associated with interest in alternative tobacco products. Positive emotions such as contentment and hope allow audiences to associate the alternative tobacco products with pleasant subjective feel and activate approach tendency towards the products (Fredrickson, 1998), which is manifested via interests in trying the products in this study.
We do not know exactly what “hope” meant to the respondents, but given the fact that the highest levels of hope were elicited by e-cigarette advertisements, especially among smokers, we can surmise that it might have been either hope of quitting smoking with the aid of e-cigarette or hope of continuing to use nicotine safely with a less harmful product. This is also consistent with findings from qualitative research with older (45+ years old) smokers, which revealed that e-cigarette advertisements evoked hopes that e-cigarettes might help them quit smoking (Cataldo, Petersen, Hunter, Wang, & Sheon, 2015). Future studies should investigate what “hope” means for smokers in the context of tobacco advertisements.
Research on the use of emotions by tobacco companies or by public health agencies to counteract these promotions has lacked examination of whether “emotional messages” actually evoke the intended emotions and the subsequent effect of discrete emotions on outcomes, such as intentions to use or quit these products. In evaluating emotional valence of the messages, past studies frequently employed panels of experts to determine whether “high emotion” messages elicited more emotions than “low emotion” messages, rather than evaluating the level of aroused emotions reported by participants (Biener, Ji, Gilpin, & Albers, 2004; Leshner, Bolls, & Thomas, 2009; Pechmann, Zhao, Goldberg, & Reibling, 2003). Using viewers’ interpretations of content has been shown to greatly increase the amount of explained variance (Potter & Tomasello, 2003). Our study improves over the past studies by evaluating the effects of different warning conditions on advertisements on viewers’ emotions. In this study, smokers felt greater levels of positive and less negative emotions than non-smokers did when they saw the advertisements, but seeing a graphic warning label increased the levels of negative emotions and eliminated the difference in negative emotions between smokers and non-smokers.
In this study, different emotions had differential effects, both in directionality and magnitude, on interest in trying alternative tobacco products. Therefore, future studies should take a discrete emotion approach to explain behavioral outcomes, with specific attention paid to positive emotions and especially hope, which was the emotion consistently associated with interest in alternative tobacco products. Future studies should examine effects of emotions on tobacco product use. This is particularly important given that the implementation of graphic warning labels on cigarettes in the US has been struck down in court partly because of the lack of evidence that emotional appeals precipitate tobacco cessation behavior (“R.J. Reynolds Tobacco Co. v. United States Food & Drug Admin., 845 F.Supp.2d 266 (D.C.C. 2012),”). Demonstrating that actual emotions as felt by those exposed to warning labels predict intentions and ultimately tobacco use behavior would provide the evidence for the implementation of graphic cigarette warning labels.
This study has implications for the development of anti-tobacco communications. In addition to using hard-hitting negative emotional appeals, such as the CDC’s Tips from Former Smokers campaign, researchers and public health agencies should explore more extensive use of positive emotions. For example, if the goal of a public health agency is to encourage people to be tobacco-free rather than continue using tobacco (as cigarettes and/or alternative tobacco products), messages should capitalize on positive emotions, particularly hope. Anti-tobacco messages could focus on giving smokers hope about quitting by using proven and regulated means, such as counseling, FDA-approved nicotine replacement therapy and by using messages that raise their self-efficacy. For example, California’s #TrulyFree Facebook campaign used uplifting messages with stories of real Californians who were “truly free” of all tobacco products (TobaccoFreeCA, 2015). Future studies should evaluate the role of hope in response to anti-tobacco ads.
Anti-tobacco campaigns should aim to reduce the feeling of contentment tobacco users might feel when exposed to tobacco advertisements. Based on our research, this could be achieved by placing graphic warning labels on tobacco advertisements. Furthermore, campaigns aimed at denormalizing tobacco use by showing the deceptive practices of the tobacco industry might raise consumers’ advertising or media literacy and might make them less susceptible to the positive emotions evoked by the advertisements (Pinkleton, Weintraub Austin, Cohen, Miller, & Fitzgerald, 2007).
Disgust was a deterrent for interest in smokeless tobacco. This conforms to previous qualitative studies reporting that smokers and women found snus disgusting and unappealing (Bahreinifar, Sheon, & Ling, 2013). However, the use of disgust (and possibly other negative emotions) may need to be tailored by tobacco use behavior: messages emphasizing lack of appeal of snus might be viewed by smokers as reinforcing smoking (Popova, Kostygina, Sheon, & Ling, 2014). Among Canadian smokers, disgust in response to cigarette warning labels was longitudinally associated with greater smoking quitting attempts and behavior (Hammond, Fong, et al., 2004).
Another emotion that was correlated with less interest in alternative tobacco products in this study, but has not been studied extensively in the tobacco literature, is anger. In general, emotion literature suggests that when an individual experiences anger, he/she is motivated to avoid or move against the source that made them angry (Roseman, 2011; Roseman, Wiest, & Swartz, 1994). Indeed, the findings suggest that angrier individuals were more motivated to avoid the use of alternative tobacco products. Given the findings, it would be fruitful to investigate where anger was directed (tobacco companies, regulatory agencies), which aspects of the advertisements, products, or tobacco warning labels are responsible for inducing anger, and if anger caused by different aspects of the labels (e.g., pictorial vs. text) results in different outcomes.
Anxiety was associated with less interest in free sample of e-cigarettes. Anxiety is closely related to fear (Lazarus, 1991), which is the emotion that has been studied the most in the context of tobacco warning labels (Cameron et al., 2015; Hammond, Fong, et al., 2004; Kees et al., 2010). A few studies have examined how fear evoked by the labels predict behavioral intentions, and found that fear evoked by graphic warning labels was a significant positive predictor of intentions to quit smoking (Kees et al., 2010) and quitting behavior at a follow-up (Hammond, Fong, et al., 2004). These few findings pertain to cigarettes. Similarly, we found that anxiety is also associated with less interest in e-cigarettes.
Different emotions were associated with interest in different products. Disgust was associated with reduced interest in moist snuff and snus only, while anxiety was related to lower interest in e-cigarettes, but not smokeless tobacco. This is perhaps not surprising as use of smokeless tobacco (and associated spitting, spitting jars, wads of chew) frequently are viewed as disgusting, especially among non-users (Sami et al., 2012). The same visceral response has not been seen in regards to electronic cigarettes, which are frequently viewed as modern and trendy (McDonald & Ling, 2015; Wagoner et al., 2016). In contrast, anxiety might be more pertinent to e-cigarettes where consumers are frequently uncertain about the health effects and unknown risks of these novel products (Majeed et al., in press).
This study is limited by the use of convenience sample; the findings might not be generalizable to the overall US population. However, the sample was demographically and geographically diverse. A single forced exposure to advertisements and a lack of behavioral outcome limit ecological validity. We measured emotions with self-report; however, it has been demonstrated that self-report of emotions correlates with physiological measurements and argued that self-report is a more reliable measure, especially for a specific emotional experience (Mewborn & Rogers, 1979; Robinson & Clore, 2002). The relationships studied between emotions and our outcomes are correlational in nature irrespective of the effects of experimental conditions on emotions.
This study examined emotional responses to advertisements for alternative tobacco products with various warning labels; future studies should evaluate how anti-smoking messages, for example, those produced by FDA and CDC, evoke discrete positive and negative emotions in both users and non-users of tobacco and how these emotional responses are related to subsequent cognitions, intentions, and behavior. Evaluations of the comparative effectiveness of anti-tobacco messages typically distinguished between messages with different themes, such as health effects of smoking, tobacco industry’s dishonesty, or short-term social consequences of smoking (Niederdeppe, Avery, Byrne, & Siam, 2014; Pechmann et al., 2003; Popova et al., 2015). Some attempts to explain the effects of these themes through emotional impact has been undertaken, for example, by analyzing if the message themes that changed behavioral intentions were more emotional in tone (Pechmann et al., 2003). Future research should explicitly evaluate the emotional responses of participants exposed to the anti-tobacco messages and analyze the relationship of these discrete emotions with outcomes.
In conclusion, this study adds to the emergent research on the correlates of use of alternative tobacco products (Richardson, Pearson, Xiao, Stalgaitis, & Vallone, 2014) by demonstrating that emotions evoked by advertisements with warning labels were associated with interest in alternative tobacco products. Among discrete emotions, hope was most consistently associated with interest in alternative tobacco products. Currently, hope is widely used in smokeless tobacco and e-cigarette advertisements. Public education campaigns should consider using messages that lower hope associated with tobacco products, but increase hope for cessation or life without tobacco.
Supplementary Material
Acknowledgments
We wish to dedicate this work in memoriam of Abby Prestin.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health (R01-CA141661, PI: Ling; R00CA187460, PI: Popova).
Footnotes
At the time of data collection, Dr. Popova was with the University of California, San Francisco. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Lucy Popova, School of Public Health, Georgia State University, Atlanta, GA.
Jiyeon So, Department of Communication Studies, University of Georgia, Athens, GA.
Angeline Sangalang, Annenberg School for Communication, University of Pennsylvania, Philadelphia, PA.
Torsten B. Neilands, Center for AIDS Prevention Studies, Department of Medicine, University of California, San Francisco, San Francisco, CA.
Pamela M. Ling, Division of General Internal Medicine, Department of Medicine, Center for Tobacco Control Research and Education, University of California, San Francisco, San Francisco, CA.
References
- 15 U.S.C. §4402(b)(2) (2012).
- Anderson SJ, Pollay RW, Ling PM. Taking ad-Vantage of lax advertising regulation in the USA and Canada: Reassuring and distracting health-concerned smokers. Social science & medicine. 2006;63(8):1973–1985. doi: 10.1016/j.socscimed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Bahreinifar S, Sheon NM, Ling PM. Is snus the same as dip? Smokers’ perceptions of new smokeless tobacco advertising. Tobacco Control. 2013;22:84–90. doi: 10.1136/tobaccocontrol-2011-050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Hargraves JL. A longitudinal study of electronic cigarette use in a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine & Tobacco Research. 2015;17(2):127–133. doi: 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Ji M, Gilpin EA, Albers AB. The impact of emotional tone, message, and broadcast parameters in youth anti-smoking advertisements. Journal of Health Communication. 2004;9(3):259–274. doi: 10.1080/10810730490447084. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ: British Medical Journal. 2009;339(b3060) doi: 10.1136/bmj.b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm SS, Brehm JW. Psychological reactance : a theory of freedom and control. New York: Academic Press; 1981. [Google Scholar]
- Cameron LD, Pepper JK, Brewer NT. Responses of young adults to graphic warning labels for cigarette packages. Tobacco Control. 2015;24(e1):e14–e22. doi: 10.1136/tobaccocontrol-2012-050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SM. The Australian cigarette brand as product, person, and symbol. Tob Control. 2003;12(Suppl 3):iii79–86. doi: 10.1136/tc.12.suppl_3.iii79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo JK, Petersen AB, Hunter M, Wang J, Sheon N. E-cigarette Marketing and Older Smokers: Road to Renormalization. American journal of health behavior. 2015;39(3):361–371. doi: 10.5993/AJHB.39.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catellier JRA, Yang ZJ. The role of affect in the decision to exercise: Does being happy lead to a more active lifestyle? Psychology of Sport and Exercise. 2013;14(2):275–282. [Google Scholar]
- Cryder CE, Lerner JS, Gross JJ, Dahl RE. Misery is not miserly sad and self-focused individuals spend more. Psychological Science. 2008;19(6):525–530. doi: 10.1111/j.1467-9280.2008.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry LE, Pederson LL, Stryker JE. The changing marketing of smokeless tobacco in magazine advertisements. Nicotine Tob Res. 2011;13(7):540–547. doi: 10.1093/ntr/ntr038. [DOI] [PubMed] [Google Scholar]
- Dillard JP, Shen L. On the nature of reactance and its role in persuasive health communication. Communication Monographs. 2005;72(2):144–168. [Google Scholar]
- Dunlop S, Wakefield M, Kashima Y. Can you feel it? Negative emotion, risk, and narrative in health communication. Media Psychology. 2008;11(1):52–75. [Google Scholar]
- Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among US adolescents: a cross-sectional study. JAMA pediatrics. 2014;168(7):610–617. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery LF, Romer D, Sheerin KM, Jamieson KH, Peters E. Affective and Cognitive Mediators of the Impact of Cigarette Warning labels. Nicotine & Tobacco Research. 2014;16(3):263–269. doi: 10.1093/ntr/ntt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg-Hurn DM, Steed LG. Does exposure to cigarette health warnings elicit psychological reactance in smokers? Journal of Applied Social Psychology. 2011;41(1):219–237. [Google Scholar]
- Ferrer R, Klein W, Lerner J, Reyna V, Keltner D. Emotions and health decision making: Extending the Appraisal Tendency Framework to improve health and health care. Cambridge, MA: Harvard University Press; 2014. pp. 1–25. [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerström K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12(4):349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Review of general psychology. 1998;2(3):300. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84(2):365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH. The emotions: studies in emotion and social interaction. New York: Cambridge University Press; 1986. [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, … Havel C. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Ling PM. “Smoking revolution”: A content analysis of electronic cigarette retail websites. American Journal of Preventive Medicine. 2014;46(4):395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JJ, Anderson R, Tatham R, Black W. Multivariate data analysis. 3. New York: Macmillan; 1995. [Google Scholar]
- Hammond D. Health warning messages on tobacco products: a review. Tobacco Control. 2011;20:327–337. doi: 10.1136/tc.2010.037630. [DOI] [PubMed] [Google Scholar]
- Hammond D, Doxey J, Daniel S, Bansal-Travers M. Impact of female-oriented cigarette packaging in the United States. Nicotine Tob Res. 2011;13(7):579–588. doi: 10.1093/ntr/ntr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Fong GT, McDonald PW, Brown KS, Cameron R. Graphic Canadian cigarette warning labels and adverse outcomes: evidence from Canadian smokers. American Journal of Public Health. 2004;94(8):1442–1445. doi: 10.2105/ajph.94.8.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Fong GT, McNeill A, Borland R, Cummings KM. Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii19–25. doi: 10.1136/tc.2005.012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, McDonald PW, Fong GT, Brown KS, Cameron R. The impact of cigarette warning labels and smoke-free bylaws on smoking cessation evidence from former smokers. Canadian Journal of Public Health. 2004;95(3):201–204. doi: 10.1007/BF03403649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lerner JS, Zeckhauser R. The disgust-promotes-disposal effect. Journal of risk and uncertainty. 2012;44(2):101–113. [Google Scholar]
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews. 2016;(9) doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16(4):347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Ifcher J, Zarghamee H. Happiness and Time Preference: The Effect of Positive Affect in a Random-Assignment Exper. The American Economic Review. 2011;101(7):3109–3129. [Google Scholar]
- Izard CE. Human emotions. Boom Koninklijke Uitgevers; 1977. [Google Scholar]
- Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. The Lancet Respiratory Medicine. 2016;4(2):116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees J, Burton S, Andrews JC, Kozup J. Understanding how graphic pictorial warnings work on cigarette packaging. Journal of Public Policy & Marketing. 2010;29(2):265–276. [Google Scholar]
- Keltner D, Gross JJ. Functional accounts of emotions. Cognition & Emotion. 1999;13(5):467–480. [Google Scholar]
- King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine & Tobacco Research. 2015;17(2):219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi MT, Voss-Humke AM, Seifert AL. How do I feel about the behavior? The interplay of affective associations with behaviors and cognitive beliefs as influences on physical activity behavior. Health Psychology. 2007;26(2):152–158. doi: 10.1037/0278-6133.26.2.152. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Sherrill-Mittleman D, Ebbert JO, Talcott GW, Debon M. Tobacco use harm reduction, elimination, and escalation in a large military cohort. Am J Public Health. 2010;100(12):2487–2492. doi: 10.2105/AJPH.2009.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS. Emotion and adaptation. New York: Oxford University Press; 1991. [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: Toward a model of emotion-specific influences on judgement and choice. Cognition & Emotion. 2000;14(4):473–493. [Google Scholar]
- Lerner JS, Keltner D. Fear, anger, and risk. Journal of Personality and Social Psychology. 2001;81(1):146. doi: 10.1037//0022-3514.81.1.146. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Li Y, Valdesolo P, Kassam KS. Emotion and decision making. Psychology. 2015;66:799–823. doi: 10.1146/annurev-psych-010213-115043. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Small DA, Loewenstein G. Heart strings and purse strings carryover effects of emotions on economic decisions. Psychological Science. 2004;15(5):337–341. doi: 10.1111/j.0956-7976.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Leshner G, Bolls P, Thomas E. Scare’em or disgust’em: The effects of graphic health promotion messages. Health Communication. 2009;24(5):447–458. doi: 10.1080/10410230903023493. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, … Audrain-McGovern J. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700–707. doi: 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis DJ, De Mello GE. The concept of hope and its relevance to product evaluation and choice. Journal of Marketing. 2005;69(1):1–14. [Google Scholar]
- Majeed BA, Weaver SR, Gregory KR, Whitney CF, Slovic P, Pechacek TF, Eriksen MP. Changing Perceptions of Harm of E-Cigarettes among U.S. Adults, 2012–2015. American Journal of Preventive Medicine. doi: 10.1016/j.amepre.2016.08.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald EA, Ling PM. One of several ‘toys’ for smoking: young adult experiences with electronic cigarettes in New York City. Tobacco Control. 2015;24(6):588–593. doi: 10.1136/tobaccocontrol-2014-051743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewborn CR, Rogers RW. Effects of threatening and reassuring components of fear appeals on physiological and verbal measures of emotion and attitudes. Journal of Experimental Social Psychology. 1979;15(3):242–253. [Google Scholar]
- Michael Scavone Inc. Qualitative research on an advertising exploratory for Cambridge. Philip Morris, USA by Michael Scavone, Inc; 1999. Apr 2, 1999 Retrieved from https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/-id=qznj0014. [Google Scholar]
- Mutti S, Hammond D, Reid JL, Thrasher JF. The Efficacy of Cigarette Warning Labels on Health Beliefs in the United States and Mexico. Journal of Health Communication. 2013;18(10):1180–1192. doi: 10.1080/10810730.2013.778368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi RL. Exploring the framing effects of emotion do discrete emotions differentially influence information accessibility, information seeking, and policy preference? Communication Research. 2003;30(2):224–247. [Google Scholar]
- Nascimento BE, Oliveira L, Vieira AS, Joffily M, Gleiser S, Pereira MG, … Volchan E. Avoidance of smoking: the impact of warning labels in Brazil. Tobacco Control. 2008;17(6):405–409. doi: 10.1136/tc.2008.025643. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Tobacco Control Monograph 19: The Role of the Media in Promoting and Reducing Tobacco Use. Bethesda, MD: 2008. [Accessed September 30, 2008]. http://cancercontrol.cancer.gov/tcrb/monographs/19/index.html. [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Applied linear regression models. Vol. 1127. Irwin Homewood, IL: 1989. [Google Scholar]
- Niederdeppe J, Avery R, Byrne S, Siam T. Variations in state use of antitobacco message themes predict youth smoking prevalence in the USA, 1999–2005. Tobacco Control, tobaccocontrol-2014–051836. 2014 doi: 10.1136/tobaccocontrol-2014-051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM, Hall MG, Francis DB, Ribisl KM, Pepper JK, Brewer NT. Pictorial cigarette pack warnings: a meta-analysis of experimental studies. Tobacco Control. 2015 doi: 10.1136/tobaccocontrol-2014-051978. tobaccocontrol-2014–051978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnemaker J, Farrelly MC, Kamyab K, Busey A, Mann N. Experimental Study of Graphic Cigarette Warning Labels: Final Results Report Prepared for Center for Tobacco Products, Food and Drug Administration. 2010 Retrieved from http://www.tobaccolabels.ca/research/experimental-study-of-graphic-cigarette-warning-labels-final-results-report-and-appendices-fda-usa-2010/
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. American Journal of Public Health. 2012;102(9):1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann C, Zhao G, Goldberg ME, Reibling ET. What to convey in antismoking advertisements for adolescents: The use of protection motivation theory to identify effective message themes. Journal of Marketing. 2003;67(2):1–18. [Google Scholar]
- Pinkleton BE, Weintraub Austin E, Cohen M, Miller A, Fitzgerald E. A statewide evaluation of the effectiveness of media literacy training to prevent tobacco use among adolescents. Health Communication. 2007;21(1):23–34. doi: 10.1080/10410230701283306. [DOI] [PubMed] [Google Scholar]
- Pollay RW. Targeting youth and concerned smokers: evidence from Canadian tobacco industry documents. Tobacco Control. 2000;9(2):136–147. doi: 10.1136/tc.9.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Kostygina G, Sheon NM, Ling PM. A qualitative study of smokers’ responses to messages discouraging dual tobacco product use. Health Education Research. 2014;29(2):206–221. doi: 10.1093/her/cyt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Linde BD, Bursac Z, Talcott GW, Modayil MV, Little MA, … Klesges RC. Testing antismoking messages for Air Force trainees. Tobacco Control. 2015 doi: 10.1136/tobaccocontrol-2015-052477. tobaccocontrol-2015–052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Ling PM. Nonsmokers’ responses to new warning labels on smokeless tobacco and electronic cigarettes: an experimental study. BMC public health. 2014;14(1):997. doi: 10.1186/1471-2458-14-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Neilands TB, Ling PM. Testing messages to reduce smokers’ openness to using novel smokeless tobacco products. Tob Control. 2014;23(4):313–321. doi: 10.1136/tobaccocontrol-2012-050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WJ, Tomasello TK. Building upon the experimental design in media violence research: The importance of including receiver interpretations. Journal of Communication. 2003;53(2):315–329. [Google Scholar]
- R. J. Reynolds Tobacco Company. 1927 On the heights of contentmentRetrieved from https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/lylw0099.
- Reynolds RJ. Citizen Petition. 2011 Retrieved from http://www.regulations.gov/-!documentDetail;D=FDA-2011-P-0573-0001.
- R.J. Reynolds Tobacco Co. v. United States Food & Drug Admin., 845 F.Supp.2d 266 (D.C.C. 2012) (
- Richardson A, Pearson J, Xiao H, Stalgaitis C, Vallone D. Prevalence, harm perceptions, and reasons for using noncombustible tobacco products among current and former smokers. American Journal of Public Health. 2014;104(8):1437–1444. doi: 10.2105/AJPH.2013.301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Episodic and semantic knowledge in emotional self-report: evidence for two judgment processes. Journal of Personality and Social Psychology. 2002;83(1):198. [PubMed] [Google Scholar]
- Roseman IJ. Emotional behaviors, emotivational goals, emotion strategies: Multiple levels of organization integrate variable and consistent responses. Emotion Review. 2011;3(4):434–443. [Google Scholar]
- Roseman IJ, Wiest C, Swartz TS. Phenomenology, behaviors, and goals differentiate discrete emotions. Journal of Personality and Social Psychology. 1994;67(2):206. [Google Scholar]
- Rossiter JR, Percy L. Emotions and Motivations in Advertising. Advances in consumer research. 1991;18(1) [Google Scholar]
- Sami M, Timberlake DS, Nelson R, Goettsch B, Ataian N, Libao P, Vassile E. Smokers’ perceptions of smokeless tobacco and harm reduction. Journal of public health policy. 2012;33(2):188–201. doi: 10.1057/jphp.2012.9. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS data brief. 2015;217:1–8. [PubMed] [Google Scholar]
- Shen L. The effectiveness of empathy-versus fear-arousing antismoking PSAs. Health Communication. 2011;26(5):404–415. doi: 10.1080/10410236.2011.552480. [DOI] [PubMed] [Google Scholar]
- So J, Kuang K, Cho H. Reexamining Fear Appeal Models from Cognitive Appraisal Theory and Functional Emotion Theory Perspectives. Communication Monographs. 2016;83(1):120–144. [Google Scholar]
- Tamir M, Chiu CY, Gross JJ. Business or pleasure? Utilitarian versus hedonic considerations in emotion regulation. Emotion. 2007;7(3):546. doi: 10.1037/1528-3542.7.3.546. [DOI] [PubMed] [Google Scholar]
- The American Tobacco Company. Half and Half Pipe Tobacco 20 second radio spot - Father’s Day. 1957 Retrieved from https://www.industrydocumentslibrary.ucsf.edu/docs/-id=nrbk0075.
- Timberlake DS, Pechmann C, Tran SY, Au V. A content analysis of Camel Snus advertisements in print media. Nicotine Tob Res. 2011;13(6):431–439. doi: 10.1093/ntr/ntr020. [DOI] [PubMed] [Google Scholar]
- TobaccoFreeCA. #TrulyFree. 2015 Retrieved from https://www.facebook.com/TobaccoFreeCA/
- Trinkets & Trash Artifacts of the Tobacco Epidemic. Marketing Smokeless Tobacco: Moist Snuff, Snus, Dissolvables. The online surveillance system and archive of tobacco products and tobacco industry marketing materials. Retrieved from http://www.trinketsandtrash.org/dissolvables/
- U.S. Food and Drug Administration. FDA Denies Citizen Petition to Change a Smokeless Tobacco Warning Statement. This Week in CTP - May 12, 2015. 2015a Retrieved from http://www.fda.gov/TobaccoProducts/NewsEvents/ucm446663.htm.
- U.S. Food and Drug Administration. Warning Letters to Vaperz Ltd, Knoxville Vapor, and Dr. K. 2015b Retrieved from http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm441314.htm, http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm441310.htm, http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm441302.htm.
- United States Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2014. [Google Scholar]
- United States Department of Health and Human Services. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final Rule (21 CFR Parts 1100, 1140 and 1143. 2016 Retrieved from http://federalregister.gov/a/2016-10685. [PubMed]
- Wagoner KG, Cornacchione J, Wiseman KD, Teal R, Moracco KE, Sutfin E. E-cigarettes, hookah pens and vapes: Adolescent and young adult perceptions of Electronic Nicotine Delivery Systems. Nicotine & Tobacco Research. 2016;18(10):2006–2012. doi: 10.1093/ntr/ntw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE. Tobacco industry response to public health concern: a content analysis of cigarette ads. Health Education & Behavior. 1985;12(1):115–127. doi: 10.1177/109019818501200111. [DOI] [PubMed] [Google Scholar]
- Weaver SR, Majeed BA, Pechacek TF, Nyman AL, Gregory KR, Eriksen MP. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. International journal of public health. 2016;61(2):177–188. doi: 10.1007/s00038-015-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Report on e-cigarettes to WHO Framework Convention on Tobacco Control. 2014 Retrieved from http://apps.who.int/gb/fctc/PDF/cop6/FCTC_COP6_10-en.pdf?ua=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.