Abstract
Gfi1b is a 37 kDa transcriptional repressor with six zinc-finger domains that is differentially expressed during hemato- and lymphopoiesis. We show here that transcription from the Gfi1b gene locus is silenced in the spleen but not in the bone marrow of transgenic mice that constitutively express Gfi1b under the control of the pan-hematopoietic vav promoter. Sequence analysis of the Gfi1b promoter showed the presence of potential Gfi1/Gfi1b-binding sites close to the mRNA start site. The expression of reporter gene constructs containing the Gfi1b core promoter appended to the luciferase gene were strongly repressed in the presence of exogenous Gfi1b. Moreover, analysis of combinatorial mutant mice that carry the vav-Gfi1b transgene and a green fluorescent protein-tagged Gfi1 gene locus demonstrated that the Gfi1 gene can be repressed by Gfi1b. Direct binding of Gfi1b and Gfi1 to the potential binding sites in the Gfi1b promoter could be demonstrated by gel-shift analyses in vitro. Chromatin-immunoprecipitation experiments showed that both the Gfi1b and the Gfi1 promoter are indeed occupied by Gfi1b in vivo. Hence, we conclude from our data that Gfi1b can auto-repress its own expression, but, in addition, is also able to cross-repress expression of the Gfi1 gene most likely in a cell type specific manner.
INTRODUCTION
Gfi1b and Gfi1 are small nuclear proteins with a theoretical molecular weight of 37 and 47 kDa, respectively [for review see (1,2)]. Both can repress RNA Pol II dependent transcription upon direct binding to cognate sites in target gene promoters. DNA binding to these sites which contain an AATC core sequence is mediated by six C2H2 type zinc fingers located at the C-terminus of both proteins. At the N-terminus, a 20 amino acid SNAG domain that is conserved between Gfi1 proteins of all mammalian species is indispensable for transcriptional repression (3,4). Within the SNAG domain and the zinc-finger motifs, amino acid sequences in Gfi1 and Gfi1b are almost identical. However, the area separating both the domains is not conserved and does not harbor any known domain homology. Hence, the function of this part of the proteins remains obscure but a role as a protein–protein interaction domain is conceivable.
Gfi1 and Gfi1b are both differentially expressed during lympho- and hematopoiesis (1,2). Gfi1 is most prominently present in the early stages of T-cell development, ceases in mature, resting CD4+ or CD8+ peripheral T-cells, but can be steeply re-induced upon antigenic stimulation (5–8). In contrast, Gfi1b appears to be largely absent from T-cells save for a reported expression in the earliest DN3 subset of the CD4− CD8− thymocyte population (9). Similarly, B-cell precursors in bone marrow and spleen show constitutive expression of Gfi1 but mature, resting B-cells lack Gfi1 and re-express it only after stimulation (8). In the myeloid lineage, granulocytes show high levels of Gfi1 expression whereas Gfi1b is completely absent and vice versa. Gfi1b is highest in erythroid precursors and megakaryocytes where Gfi1 can barely be detected (10,11). Outside the hematopoietic system, Gfi1 is expressed in precursors of sensory neurons, the retina, specific cells of the lung and in the CNS (12). Whether Gfi1b shows significant expression in these or other organs has not been thoroughly investigated.
Although both Gfi1 and Gfi1b are highly similar, it remains to be shown to what extent they are redundant or exert unique and specific functions. Gene knock-out studies have shown that loss of Gfi1 affects pre-T-cell differentiation, development of granulocytes, integrity of inner ear hair cells, and proliferation and self-renewal of hematopoietic stem cells (7,10,12–15). In contrast, the deletion of Gfi1b leaves the myeloid lineage and hematopoietic stem cells undisturbed, but has drastic effects on the development of erythroid cells and megakaryocytes (11). Since Gfi1b null mice die at mid gestation, the consequences of Gfi1b deletion on other cell types and organs could not be investigated.
The promoter of the Gfi1 gene contains a number of bona fide Gfi1-binding sites with the typical AATC core sequence. Two independent studies have shown that Gfi1 expression is regulated by a feedback loop through Gfi1 itself (8,16). Results from chromatin immunoprecipitation (ChIP) and reporter gene assays suggest that Gfi1 binds to sequences within its own promoter and can repress its own transcription (8,16). Interestingly, this auto-regulation appears to be confined to T-cells and is absent in myeloid cells (16), but the reason for this cell type specific auto-regulation is entirely unknown. Since the same DNA-binding motifs can be used by Gfi1 and Gfi1b, a cross-regulation of the Gfi1 gene by Gfi1b is possible, and evidence supporting such a regulatory mechanism has recently been published (16). By using a transgenic mouse model and several cell-based systems, we show that the Gfi1b gene, similar to the Gfi1 gene, underlies auto-regulation and that a direct transcriptional repression of the Gfi1b promoter by Gfi1b occurs in vivo. Furthermore, we confirm that Gfi1 expression can be repressed in vivo by Gfi1b using a Gfi1:GFP knock-in mouse mutant and animals that express Gfi1b as a transgene and, finally, we demonstrate that Gfi1 can repress transcription from the Gfi1b reporter. The data in this paper complete a picture in which, at least in lymphoid cells, both Gfi1 and Gfi1b are not only able to repress their own expression, i.e. under auto-regulatory control, but can also cross-regulate each other by mutually silencing their promoters.
MATERIALS AND METHODS
Generation of vav-Gfi1b transgenic mice
The transgene construct was generated by inserting the mGfi1b cDNA in a pIC19H-based plasmid containing the vav promoter and enhancer which was kindly provided by J. Adams (17). In order to measure the Gfi1b expression elicited by the transgene, a HindIII restriction site was removed from the inserted Gfi1b cDNAs by silent mutation. Transgenic mice were generated by the standard microinjection method. Mice were housed at the IFZ animal facility, University of Essen Medical School, in single ventilated cages under specific pathogen-free conditions according to German animal legislation. Mice that were used for analyses were healthy 4- to 6-week-old animals from a several generation backcross with C57BL/6 animals.
Mapping of DNase I hypersensitive sites
A single cell suspension from wt spleen was prepared, washed in ice cold phosphate-buffered saline and resuspended in 15 ml homogenization buffer (0.3 M sucrose, 60 mM KCl, 15 mM NaCl, 15 mM Tris, pH 7.5, 5 mM MgCl2, 0.5 mM EGTA and 0.2 mM AEBSF) per 107 cells. The nuclei were isolated by the addition of 15 ml homogenization buffer plus 1% NP40 per 107 cells, incubation for 5 min on ice and centrifugation at 1500 g. The nuclei were then washed 2-fold with homogenization buffer without NP40 and resuspended in 2 ml of DNase buffer (60 mM KCl, 15 mM NaCl, 15 mM Tris, pH 7.5, 5 mM MgCl2, 0.2 mM EGTA and 0.2 mM AEBSF). Three hundred microliter each of the nuclei suspension were incubated for 15 min on ice with 0, 80, 160, 240, 480 or 720 U of DNase I (Grade II, Roche). The reaction was stopped by adding 1.7 ml proteinase-K digestion buffer (60 mM Tris, pH 8.0, 25 mM EDTA, 1.2% SDS and 400 μg proteinase-K) to each vial and incubating at 37°C for 16 h. Nuclear DNA was prepared by phenol-extraction and ethanol-precipitation. The DNA was resolved in 400 μl TE (10 mM Tris, pH 7.5, 1 mM EDTA) and incubated with 20 μg RNase-A for 30 min at 37°C. The DNA was digested with 30 U of SmaI for 6 h at 37°C, ethanol-precipitated, redissolved in 25 μl TE, separated on a 0.8% agarose-gel, transferred to a solid support and hybridized using a PCR generated 296 bp probe directly downstream of the SmaI site. The result was visualized by autoradiography. Probe-primers: SmaUS, cctgggatccttcagtggcagag; and SmaLS, gtcctggaactccctctatagaac.
Primer extension analysis
This section deals with primer extension on bone marrow and spleen cells from wt and Gfi1b transgenic mice. Total RNA was prepared from each tissue (bone marrow BM, spleen SP) with Peq-Gold (PEQLAB) according to the manufacturer's protocol. Yeast t-RNA (Y) was used as a negative control. The primer for the primer extension analysis, 5′-gactgctctcggctcggtcaac-3′, was synthesized to hybridize the sense strand from nucleotides −34 to −13 in the first exon of the Gfi1b gene. The synthetic primer was labeled at its 5′ end with 32P-ATP using T4 polynucleotide kinase and hybridized to 1 μg total RNA in reverse transcriptase buffer at 58°C. After cooling to 43°C, the primer was extended with 200 U of SuperScript II RNase H–reverse transcriptase (Invitrogen) using 1 mM each of the four deoxynucleotides. After 1 h at 37°C, the reaction was neutralized and the DNA was collected. The reaction products were analyzed on a 7 M urea–6% polyacrylamide gel to determine the size of the extended product. The gel was exposed to an autoradiography film (Kodak BioMax).
Gel-shift analysis
The Myc-tagged Gfi1 and Gfi1b proteins were produced with the TNT(TM) Rabbit Reticulocyte Lysate System (Promega). Unprogramed lysate was used as control.
One micorliter of lysate was incubated for 20 min at room temperature with 7 μl of gel-shift buffer (40 mM KCl, 30 mM HEPES-KOH, pH 7.6, 1 mM MgCl2, 5% Glycerol, 15% Ficoll, 5 mM DTT, 0.25 μg/μl poly(dI·dC) with or without 1 pM (20 000 Cz-c.p.m.) 32P-labeled double-stranded oligonucleotides. When indicated for test of specificity, 50 pM unlabeled target or non-target oligo was added to compete for binding with the protein of interest. After preincubation for 20 min, anti-Myc antibody was added to the protein:DNA complex for 10 min to further retard its mobility (supershift) during electrophoresis. Protein:DNA complexes were separated using a native acrylamide gel (4% PAA, 0.5× TBE) and visualized by autoradiography. Oligos: Gfi1b-shift-US1, gatccacaaataatcagatgaaaacaggaggg; Gfi1b-shift-LS, gatcccctccgattttcaatctgattatttgtg; Ideal US, gatcaaaataaatcacagcat; Ideal LS, gatcatgctgtgatttatttt; CRE US, gatccttggctgacgtcagagagagct; CRE LS, ctctctgacgtcagccaag; SAT-A US, tatggcgaggaaaactgaaaaaggtggaaaatttagaaatgt; SAT-A LS, acatttctaaattttccacctttttcagttttcctcgccata; SAT-B US, aaactgaaaatcatggaaaatgagaaacat; SAT-B LS, atgtttctcattttccatgattttcagttt; IKAROS US, tgacagggaatacacattcccaaaagc; and IKAROS LS, gcttttgggaatgtgtattccctgtca.
ChIP
ChIP procedures were performed as described previously (8). Primers used for DNA amplification of the Gfi1b locus were: Gfi1b-ChIP-US1, tctaaagcaaggatgagggactgta; and Gfi1b-ChIP-LS1, gtgtcaaaatctggcggctgcagc. After 32–35 cycles of amplification, PCR products were run on 1.5% agarose gel and analyzed by ethidium bromide staining. Primers to amplify sequences of the Gfi1 locus were as described previously (8).
Transient transfection and reporter gene assay
To generate Gfi1b full-length and deletion construct expression plasmids, the Gfi1b cDNA was PCR amplified, cloned using the TOPO-PCR cloning kit (Invitrogen) and PCR-products were sequenced and subcloned into the pCDNA3.1 (Invitrogen) mammalian expression plasmid. Primers used for amplification were: Full1BUS, gaattccgcgatgccacggtcctttctagtg; dSnagUS, gaattccgcgatggcacagggtgatgagctg; Full1BLS, tcaggatcccttgagattgtgttgactctc; dZNF6LS, tcaggatcccttgaagcctgtgtgcttgcg; and dZNF5LS, tcaggatcccttctcacctgtgtggatgta. For the VP16 activation domain (AD) fusion proteins, first a HindIII, EcoRI VP16 AD fragment from the pVP16 yeast expression plasmid was cloned into pCDNA3. Then the polylinker was mutated by the insertion of an adapter into the BamHI/XbaI sites to generate in-frame cloning sites for Gfi1b, followed by EcoRI/BamHI cloning of the Gfi1b inserts from the pCDNA3-Gfi1b clones described above into the pCDNA-VP16 vector. Primers for the generation of the adapter were: VP16poly3US, gatctcgaattcagaagcttctcgagctggatcctaagtga; and VP16poly3LS, ctagtcacttaggatccagctcgagaagcttctgaattcga. Luciferase reporter plasmids were generated by the insertion of PCR-amplified mouse genomic sequences covering the regions from −798 to +31 (Gfi1b-long-promoter) or from −511 to +31 (Gfi1b-short-promoter) into the pGL3-basic vector (Promega). Primers for the amplification of the promoter sequences were: 1Bprom9050US, ctgcgctggcctgggtacccag; and 1Bprom9896LS, ggcagatctgactgctctcggctcggtcaacg. As a control, an oligo covering the Gfi1/Gfi1b-binding site in the Gfi1b promoter was inserted into the BglII site in the polylinker of the pGL3-promoter vector (Promega). Primers for the generation of the binding site were Gfi1b-shift-US1 and Gfi1b-shift-LS1 as described above. In 12 well plates, 5 × 104 NIH-3T3 cells per well were seeded and transfected 24 h later with 1 μg luciferase construct, and the indicated amount of a cytomegalovirus-driven Gfi1b Myc tagged expression plasmid using Rotifect according to the manufacturer's instructions. The amount of DNA was kept constant in each transfection by adding empty vector where necessary. All DNA constructs were confirmed by sequencing. After 48 h, cells were lysed at 4°C in 25 mM Tris (pH 7.8), 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, 1% Triton X-100, 2 mM DTT, 0.3 mM phenylmethlysulfonyl fluoride and 2 μg/ml aprotinin. The luciferase activity was measured by luminometry after diluting the lysate 1:3 or 1:5 in 25 mM glycyl glycine, 15 mM MgSO4, 15 mM K2HPO4, 4 mM EGTA, 40 μM ATP, 40 μM DTT and 0.3 μg/ml of luciferin. Expression of Gfi1b or Gfi1b mutants was confirmed by immunoblotting using an affinity purified Gfi1b-specific rabbit polyclonal antibody.
Retrovirus production and generation of transformed pre-B lines expressing p210 BCR-ABL
Plasmid constructs, pSRaMSCV-p210-tkneo and MSCV-GFP-IRESp210 (a gift from Dr O. N. Witte), were used to produce helper-free retroviral stocks by transient over expression in 293T cells as described previously (18). For estimation of the viral titers, viral stocks were serially diluted and used to infect 3T3 indicator cell lines. Viral titers were determined by counting green fluorescent protein (GFP) positive cells (MSCV-GFP-IRESp210) or by counting G418-resistant colonies after selection (pSRaMSCV-p210- tkneo). Routinely, we obtained titers of ≥5 × 105/ml. For generation of transformed pre-B lines, fresh bone marrow from tibias and femurs of 4- to 8-week-old C56Bl/6 mice (wild-type and Gfi1b transgene) was infected with retroviral stocks of different BCR-Abl constructs and plated at a density of 5 × 106 per 6 cm dish as described previously (18). All pre-B lines were fed twice a week and analyzed after they became stroma independent.
RESULTS
Auto-regulation of the Gfi1b gene in vivo
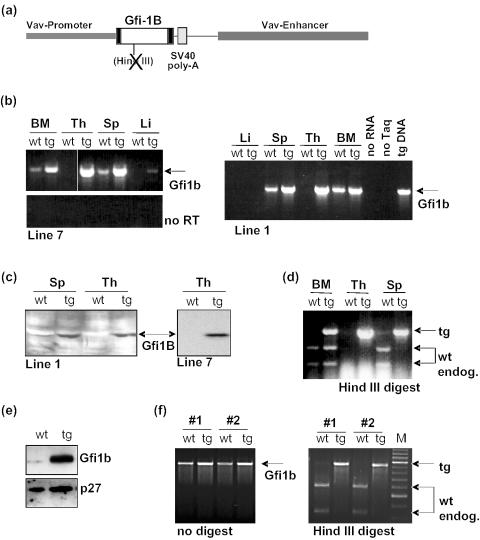
To gain more insight into the molecular function of Gfi1b, we have generated transgenic mice in which a constitutive expression of Gfi1b was targeted to all hematopoietic cells by the vav promoter enhancer element, which has been previously described to be effective in directing pan-hematopoietic expression [Figure 1a and (17)]. The animals harbor a Gfi1b cDNA with a disrupted HindIII restriction site to facilitate the distinction between endogenous (wt) and transgenic (tg) Gfi1b transcripts by RT–PCR (Figure 1a). High-level expression of the Gfi1b transgene both on RNA and protein level was reached in bone marrow, thymus and spleen (Figure 1b and c) of two different, independently generated lines of mice (Line 1 and 7). Save for a slightly higher number of B220+ cells in bone marrow, spleen and peripheral blood, flowcytometric analysis did not reveal major alterations of lymphoid or myeloid subsets in spleen and bone marrow or the pre-T-cell subsets in the thymus of 4- to 8-week-old vav-Gfi1b transgenic mice compared with age-matched littermates (data not shown). Surprisingly, however, endogenous (wt) Gfi1b transcripts were absent in spleen, but not in bone marrow of vav Gfi1b transgenic mice (Figure 1d). In thymus, endogenous expression of Gfi1b was not detected under the conditions employed here and the only measurable Gfi1b-specific transcripts originated from the Gfi1b transgene (Figure 1b–d).
Figure 1.
Generation and analysis of vav-Gfi1b transgenic mice. (a) Schematic representation of the vav-Gfi1b construct used to generate transgenic mice. The Gfi1b cDNA was placed under the control of the enhancer and promoter elements of the vav gene. To terminate transcription, a SV 40 polyadenylation signal was inserted at the end of the Gfi1b cDNA. (b) Expression of the vav-Gfi1b transgene was detected by RT–PCR in bone marrow (BM), spleen (Sp) and thymus (Th) of two mouse lines (1 and 7). In both the lines, higher levels of mRNA were apparent in all the three organs of vav-Gfi1b transgenic mice (tg) than in wild-type animals (wt). Endogenous Gfi1b mRNA was present in bone marrow and spleen of wt mice but was absent in thymus and liver (Li) from wt mice. The signal in the transgenic liver from line 7 is very probably due to the contamination with blood cells. (c) Significantly higher levels of Gfi1b protein were detected by immunoblot in extracts from spleen (Sp) and thymus (Th) from vav-Gfi1b transgenic mice (tg) than from wild-type animals (wt). (d) HindIII digestion of RT–PCR products distinguishes between endogenous (wt) and transgenic Gfi1b expression. Endogenous Gfi1b expression is lost in spleen but not in bone marrow of vav-Gfi1b transgenic mice. (e) Detection of Gfi1b protein expression levels by immunoblot in cell lines established after infection of bone marrow from wt or vav-Gfi1b transgenic mice (tg) with a pSRα vector driving the expression of the p210 BCR/Abl fusion protein. Levels of p27 were analyzed to ensure uniform loading of the gel. (f) Expression of the wt and transgene Gfi1b-alleles was detected by RT–PCR in two independent BCR/Abl transformed B-cell lines (1 and 2) before and after HindIII digestion. Endogenous Gfi1b mRNA can be detected in both cell lines derived from wt bone marrow but is absent in the cell lines established from bone marrow of vav-Gfi1b transgenic mice.
To test whether the absence of endogenous Gfi1b transcripts in spleen is due to a bona fide auto-regulation of the Gfi1b gene in B-cells or due to different expression levels in wt or vav-Gfi1b transgenic mice or due to different subpopulations of cells, we generated permanent B-cell lines from wt and vav-Gfi1b transgenic mice by infecting bone marrow cells with retroviral vectors (MSCV or pSRalpha type) expressing the BCR/Abl fusion protein. Four B220+ B-cell lines, two from transgenic bone marrow and two from wt bone marrow were generated with each retrovirus. The cell lines derived from vav-Gfi1b transgenic mice expressed a significantly higher amount of Gfi1b protein than cell lines from wt bone marrow (Figure 1e). When RNA from these cell lines was analyzed by RT–PCR, no endogenous Gfi1b transcript could be detected in cells derived from the vav-Gfi1b transgenic mice (Figure 1f), suggesting a direct and complete auto-regulation of the endogenous Gfi1b gene by Gfi1b itself in B-cells.
The sequence of the Gfi1b promoter is partially conserved and harbors several Gfi1-binding sites
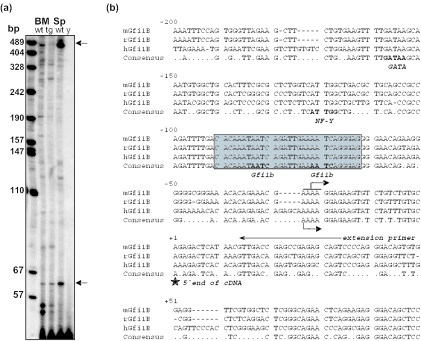
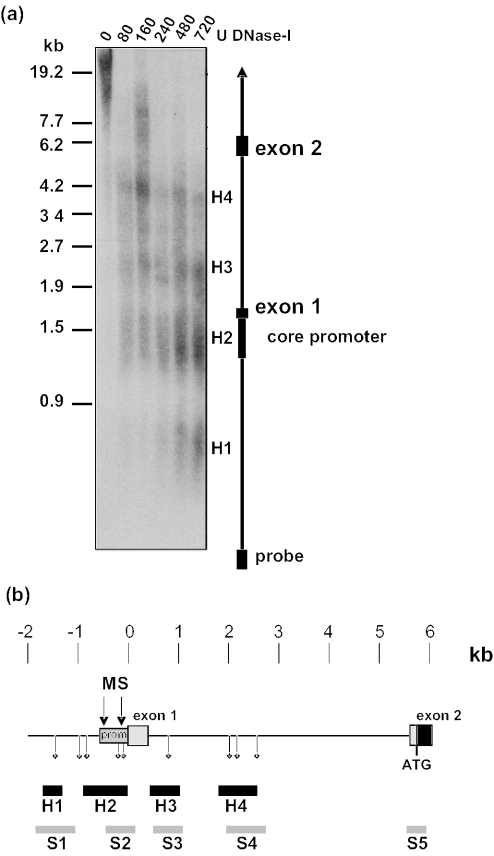
Primer extension experiments with RNA from wt spleen or bone marrow cells and a primer located within exon 1 of Gfi1b yielded two major fragments of 60 and ∼490 bp in size and a number of minor signals (Figure 2a), suggesting the existence of two major and several minor mRNA start sites in the Gfi1b promoter. A closer inspection of the murine Gfi1b gene locus showed the absence of a classical TATA box which is in agreement with the existence of several mRNA start sites (Figure 2b). Interestingly, the distal major mRNA start site could not be detected in bone marrow cells from vav-Gfi1b transgenic mice. The mapping of the more proximal major start site is in good agreement with the recent description of the human Gfi1b promoter (19) and the 5′ end of the mGfi1b cDNA. A comparison of human, rat and murine sequences showed a high degree of sequence similarity. In particular, a conservation of the GATA-1 and NF-Y binding sites described previously to be important for Gfi1b regulation (19) and of the region around the mRNA start site was evident (Figure 2b). Moreover, four DNase I hypersensitive sites could be mapped using wt splenocytes that overlap significantly with areas that contain Gfi1b-binding sites and regions of high sequence similarity between human, rat and murine loci (Figure 3a). In addition, a number of putative Gfi1/Gfi1b-binding sites containing the ‘AATC’ core (4) were detected upstream of the first exon. In particular, two very closely spaced putative binding sites with high sequence similarity to the ideal Gfi1/Gfi1b-binding site were found directly upstream of the mRNA start site and were conserved in all three species (Figures 2b and 3b).
Figure 2.
Start site mapping and characterization of the Gfi1b promoter. (a) Primer extension on bone marrow and spleen cells from wt and Gfi1b transgenic mice. The primer for the primer extension analysis hybridizes to the sense strand from nucleotides +34 to +13 in the first exon of the Gfi1b gene. Two major start sites were conserved in bone marrow and spleen of wt mice, marked by arrows. The more distal start could not be observed in bone marrow RNA from Gfi1b transgenic mice. No products were observed in the yeast t-RNA control. (b) Analysis of the mouse, rat and human Gfi1b promoter. Nucleotide sequence alignment of the mouse, human and rat Gfi1b core promoter sequences. Numbers above the lines are the nucleotide numbers starting with +1 at the 5′ end of the murine cDNA. Homology of the mouse, rat and human Gfi1b promoter sequences was analyzed using the MultAlin interface software (31). Marked in bold letters in the consensus line are the core sequences of the binding sites for the transcription factors GATA, NF-Y that have been described previously (19) and the tandem Gfi1b-binding site (boxed). The arrow above the mGfi1b sequence marks the shortest primer extension product found in bone marrow and spleen (see Figure 2a). The arrow under the hGfi1b sequence marks the previously described mRNA start site for the human Gfi1b (19). Underlined is the most 5′ end of the published cDNA for Gfi1b. The long arrow marks the localization of the primer used for the primer extension experiments.
Figure 3.
Human–mouse sequence homology, DNase I hypersensitive sites and Gfi1/Gfi1b binding sites overlap around exon 1 of Gfi1b (a) Mapping of DNase I hypersensitive sites. Nuclei of splenocytes from a wild-type mouse (C57Bl/6) were isolated and digested with the indicated amount of DNase I for 15 min on ice. After SmaI digestion, the DNA was separated by 0.8% agarose gel electrophoresis, transferred to a nylon membrane and hybridized with a PCR generated 32P-labeled 285 bp probe covering the most 5′ end directly downstream of the SmaI site. The result was visualized by autoradiography. The sequences for the primers to generate the probe were SmaUS1, cctgggatccttcagtggcagag; and SmaLS1, gtcctggaactccctctatagaac. The positions of the core promoter, exon 1 and exon 2 relative to the 5′-SmaI site were indicated. The detected hypersensitive sites were marked H1–H4. (b) High similarities (S1–S5) between mouse and human sequences overlap with the mapped hypersensitive sites (H1–H4), exon 2, the core promoter sequence (prom) and the regions where putative Gfi1/Gfi1b binding sites (diamonds) reside. These similarities are even higher as within the non-coding exon 1.
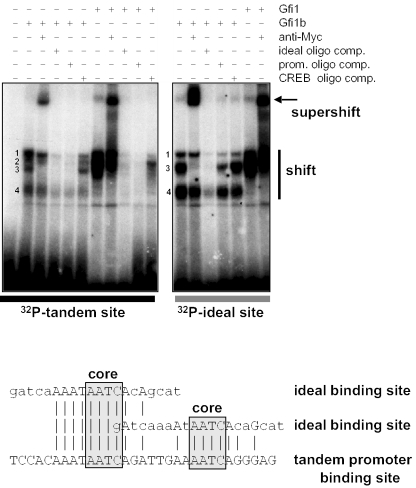
To test whether this tandem Gfi1/Gfi1B-binding site can be recognized by Gfi1 proteins, we performed electrophoretic mobility shift analysis. Oligonucleotides containing the putative tandem binding site from the Gfi1b promoter or the ideal Gfi1 binding site (4) were radioactively labeled and reacted with in vitro translated Myc-tagged Gfi1b and Gfi1. Using the tandem binding site as a probe, four protein:DNA complexes were obtained (Figure 4) that could be almost completely disrupted in the presence of both unlabeled ideal and tandem promoter oligonucleotides (Figure 4). However, only complex 3 could be efficiently supershifted with anti-Myc antibodies although the intensity of the band representing complex 1 was also diminished (Figure 4). Since complexes 1, 2 and 4 but not 3 appear to be affected by treatment with the unrelated CREB oligo, it is likely that only complex 3 represents the true Gfi1b/DNA complex (Figure 4). Complexes 1–3 could also be obtained with Gfi1 and the tandem promoter sequence (Figure 4). Competition with ideal and tandem promoter sites was equally efficient to disrupt these complexes, but competition with an unrelated oligo revealed that, in contrast to Gfi1b, only complex 2 appeared to be specific (Figure 4).
Figure 4.
Gfi1 and Gfi1b bind to the tandem Gfi1-binding site in vitro. Upper panel: gel-shift analysis was performed using in vitro translated, Myc-tagged proteins and 32P-labeled double-stranded oligonucleotides as indicated. Where indicated, a 50-fold excess of unlabeled double-stranded oligonucleotides was added as a competitor to show sequence-specific binding. The anti-Myc antibody (9E10) was used to generate a supershift of specific protein–oligonucleotide complexes and to detect the specific Gfi1/Gfi1b-DNA shift complexes labeled 1–4. Only complex 3 is efficiently supershifted by antibody addition and specifically competed by the Gfi1/Gfi1b promoter- and ideal-oligonucleotides, but not by the unrelated CREB-binding site oligonucleotide. Lower panel: sequence similarity analysis comparing the tandem Gfi1-binding site and an ideal Gfi1-binding site used for the gel-shift analysis.
With the ideal Gfi1 binding site as a probe, only three complexes (1, 3 and 4) were obtained with Gfi1b (Figure 4) and again only complex 3 was efficiently supershifted with anti-Myc antibodies. All complexes were disrupted with an ideal competitor sequence (Figure 4). Strikingly, when Gfi1b was bound to the ideal site, competition with the unlabeled tandem promoter probe was less efficient than with the unlabeled ideal probe, suggesting that the ideal binding site offers a more stable binding to Gfi1b than the tandem promoter sequence (Figure 4). In the presence of the unrelated CREB competitor oligo, all three complexes were still present, suggesting that complexes 1, 3 and 4 represent specific protein:DNA complexes but only complex 3 is recognized by the anti-Myc antibody, possibly because of the accessibility of the epitope tag (Figure 4).
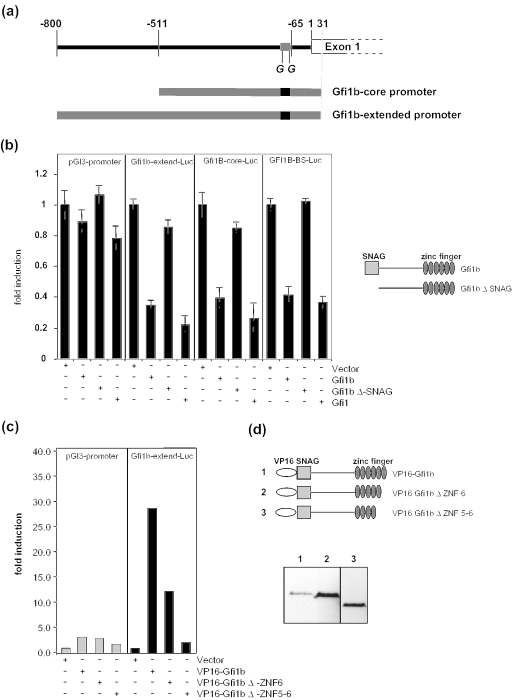
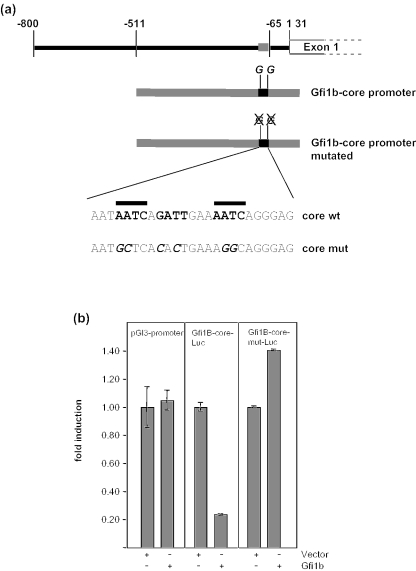
Next, we used reporter gene assays to verify that the Gfi1b gene can be repressed by Gfi1b itself. DNA fragments covering two differently extending regions 5′ of the Gfi1b gene were inserted into the pGL3 basic vector to drive expression of the luciferase gene (Figure 5a). Transfection into NIH 3T3 cells along with Gfi1b expression vectors demonstrated a repression of the Gfi1b promoter by Gfi1b and also by Gfi1 (Figure 5b). Deletion of the SNAG domain abrogated the repression of all reporter constructs (Figure 5b). In addition, constructs that allowed the expression of fusion proteins between Gfi1b and the transactivation domain of the herpes simplex virus protein VP16, transactivated transcription from the distal Gfi1b promoter and this activity was dependent on the presence of all zinc-finger domains (Figure 5c). To test whether the obtained findings reflect a bona fide interaction of Gfi1b with its cognate binding site, we deleted both Gfi1/Gfi1b-binding sites and one potential third site in the core promoter and used this mutated form to drive luciferase in a new reporter gene construct (Figure 6a and b). Clearly, when these sites were mutated, Gfi1b was no longer able to repress transcription from the Gfi1b core promoter (Figure 6b), indicating the specificity of the observed repression shown in Figure 5b.
Figure 5.
Transcription from the Gfi1b promoter can be repressed by Gfi1b and Gfi1. (a) Schematic description of the Gfi1b-promoter luciferase reporter plasmids which extend from +31 to −511 or +31 to −800, respectively, relative to the 5′ end of the mRNA start site (see Figure 2b). (b) Gfi1b-promoter-driven luciferase constructs were cotransfected into NIH3T3 cells with pCDNA3 as control and pCDNA3-expression plasmids for full-length or mutated Gfi1b as indicated (right panel). The pGl3-promoter vector (left) and an SV 40 driven pGl3-contol vector were the Gfi1b tandem binding site from where the Gfi1b promoter was inserted into the polylinker (Gfi1b-BS-Luc, right), which served as a control. Fold inductions were derived by dividing the normalized luciferase activity of each value by the average of the vector control. (c) A Gfi1b-promoter (+31 to −800) driven luciferase construct was cotransfected into NIH3T3 cells with pCDNA3 as control or pCDNA3-expression plasmids for full-length or mutated Gfi1b fused in-frame to an N-terminal VP16 AD. Fold inductions were derived as described in (b). (d) Schematic description of the VP16-Gfi1b/VP16-Gfi1b zinc-finger-deletion fusion proteins. The expression of the VP16-Gfi1b proteins was detected by western blot using an α-VP16 antibody. Deletion of each zinc finger greatly enhanced the expression level. Therefore, lane 3 shows a shorter exposure of the same blot in lanes 1–2.
Figure 6.
Mutations in the Gfi1b promoter that delete Gfi1b-binding sites abrogate repression by Gfi1b. (a) Schematic description of the Gfi1b core promoter luciferase reporter plasmids and the mutations that have been introduced. The sequence represents the tandem promoter binding site shown in Figure 4 (b) Gfi1b core promoter driven luciferase constructs and a construct carrying the mutations indicated in (a) were cotransfected into NIH3T3 cells with pCDNA3 as control (vector) and pCDNA3-expression plasmids for full-length Gfi1b as indicated. The pGl3-promoter vector (left) served as a control. Fold inductions were derived by dividing the normalized luciferase activity of each value by the average of the vector control.
Gfi1b can repress transcription from the Gfi1 promoter in vivo
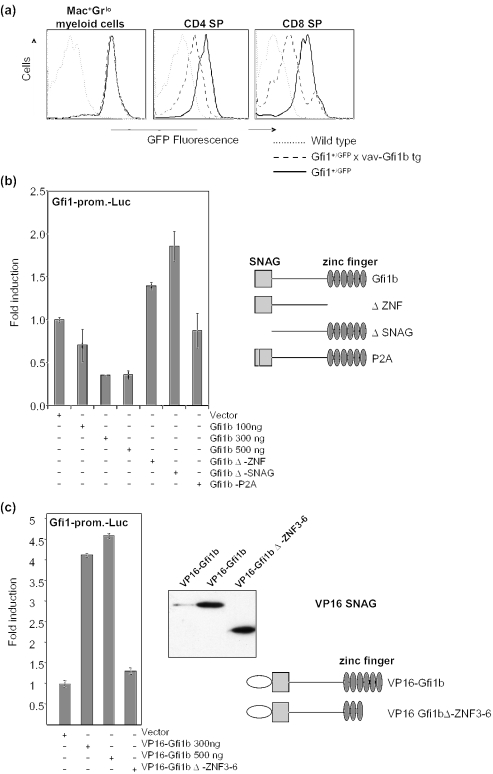
It has previously been established that the Gfi1 gene underlies an auto-regulatory feedback control and that Gfi1 can bind directly to sites in its own promoter (8,16). Moreover, it has also been shown that Gfi1b can cross-repress the expression of Gfi1 by using the cognate binding sites in the Gfi1 promoter (16). To test whether the vav-Gfi1b transgene would be able to repress transcription from the Gfi1 locus in vivo, we generated combinatorial mutant mice that carry a Gfi1:GFP knock-in allele where the coding region of the GFP is under the control of the Gfi1 promoter (8) and the vav-Gfi1b transgene by cross-breeding. Indeed, CD4+ or CD8+ thymocytes from double-mutant mice showed a clear shift in GFP fluorescence which not only confirms the previously described cross-regulation of the Gfi1 gene by Gfi1b (16) (Figure 7a) but also demonstrates the functionality of the vav-Gfi1b transgene. However, no such cross-regulation was observed in myeloid cells from bone marrow that express the surface markers Mac-1 and Gr-1 (Figure 7a). Reporter gene assays with a construct containing the luciferase gene under the control of the Gfi1 promoter described before (8) also demonstrated that Gfi1b can repress transcription of Gfi1 and that this depends on the intactness of the SNAG domain and the presence of all zinc-finger domains (Figure 7b). When a fusion protein of Gfi1b and the transactivation domain of the herpes simplex virus VP16 was used for the same reporter gene assays, transcription was found to be activated and this activation was dependent on the SNAG domain and the DNA-binding zinc-finger domains (Figure 7c).
Figure 7.
Gfi1b/Gfi1 cross-regulation in bone marrow and thymocytes. (a) Bone marrow cells were stained with α-Mac1-PercpCy5.5/α-Gr1-PE. Thymocytes of Gfi1:GFP knock-in mice (Gfi1+/GFP) were stained with α-CD4-PE/α-CD8-PercpCy5.5. GFP fluorescence of gated cells was analyzed by flow cytometry as described previously (8). Histograms show the GFP fluorescence intensity of Mac1+/Gr1lo bone marrow cells, or CD4 single positive or CD8 single positiveT-lymphocytes from wild type, Gfi1+/GFP and Gfi1+/GFP/vav-Gfi1b transgenic mice as indicated. (b) A Gfi1-promoter driven luciferase construct that was described previously (8) was cotransfected into NIH3T3 cells with pCDNA3 as control and pCDNA3-expression plasmids for full-length or mutated Gfi1b as indicated. Transfection efficiency was controlled by cotransfection of beta-galactosidase expression plasmids. Fold inductions were derived by dividing the normalized luciferase activity of each value by the average of the vector control. (c) A Gfi1-promoter driven luciferase construct was cotransfected into NIH3T3 cells with pCDNA3 as control and pCDNA3-expression plasmids encoding full-length or mutated Gfi1b fused in-frame to an N-terminal VP16 AD. Fold inductions were derived as described in (b). Right panel: the expression of the VP16-Gfi1b proteins was detected by western blot using an α-VP16 antibody. Also given is a schematic representation of the VP16 fusion constructs used.
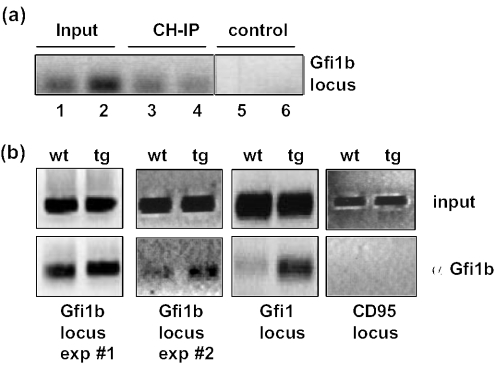
To test whether Gfi1b directly occupies binding sites in the Gfi1b and the Gfi1 promoter, we used ChIP. B220+ cells from wt and vav-Gfi1b transgenic mice were purified and chromatin–DNA complexes were precipitated with anti-Gfi1b antibodies. After reverse cross-linking, primers located in the Gfi1b or Gfi1 promoter region were used for the subsequent PCR reaction. Gfi1b/DNA complexes could not only be detected in wt B-cells but also in B-cells from vav-Gfi1b transgenic mice where they were significantly enriched (Figure 8a and b). With primers located in the CD95 gene locus where no Gfi1/Gfi1b binding sites exist, no PCR product could be obtained indicating the specificity of the reaction (Figure 8b). These findings suggest that Gfi1b directly occupies sites in both the Gfi1b and the Gfi1 promoter.
Figure 8.
Gfi1b binds to the Gfi1 and Gfi1b promoters sequences in vivo. (a) Cells from spleen of two wild-type mice were isolated and CH-IP was performed separately using an α-Gfi1b antibody or no antibody as a control. The Gfi1b-promoter region could be amplified from 0.01% of input DNA (lanes 1 and 2) or immunoprecipitated DNA (lanes 3 and 4) only if the Gfi1b antibody was used for immunoprecipitation, but not without antibody (lanes 5 and 6). (b) B220+ cells from spleen of wild-type and vav-Gfi1b transgenic mice were isolated and ChIP was performed using an α-Gfi1b antibody. The Gfi1b-promoter region (Gfi1b locus) and the Gfi1 promoter region (Gfi1 locus) could be amplified from 0.01% of input DNA or immunoprecipitated DNA. For the Gfi1b locus, two representative experiments are shown. A more prominent band is observed if cells from vav-Gfi1b transgenic mice were used for ChIP. As a negative control, primers from the CD95 gene locus were used.
DISCUSSION
Gfi1 and Gfi1b are similar proteins with functions in hematopoiesis, lymphoid development and a differential, cell type specific expression pattern. By using a transgenic mouse model where Gfi1b is constitutively expressed in all hematopoietic cells, we can show that the Gfi1b gene is under an auto-regulatory feedback control and silences its own transcription. Results from the analysis of endogenous and transgenic mRNA levels in vav-Gfi1b transgenic mice, in vitro gel-shift and reporter gene assays strongly support this auto-regulatory mechanism at the Gfi1b locus. Moreover, the results of ChIP experiments clearly indicated that Gfi1b represses its transcription by binding to its own promoter sequences in vivo. In addition, vav-Gfi1b transgenic mice were used to demonstrate that Gfi1b can regulate the expression of Gfi1 in vivo, which supports earlier findings (16). Hence, the Gfi1b gene locus can be considered as a bona fide target gene of both Gfi1b and Gfi1. Along the same line, we can show that Gfi1 is able to repress expression of reporter genes that depend on the Gfi1b promoter. With these data and our present findings, it has to be concluded that both Gfi1 and Gfi1b are controlled by auto-regulatory feedback loops and can repress expression of each other in a cross-regulatory fashion. In each case, a transcriptional repression by direct binding of Gfi1 or Gfi1b to the respective promoters is very likely to mediate this regulation.
It remains unclear at this point whether cross-regulation of Gfi1 and Gfi1b genes can be generally held responsible for the mutually exclusive expression of both proteins that is seen in some hematopoietic lineages or whether other mechanisms are also involved. Strikingly, our findings and previously published data (16) demonstrate that cross- and auto-repression of both Gfi1 and Gfi1b take place in lymphoid cells but is absent in the myeloid lineage. It is thus unlikely that the specific and exclusive expression of Gfi1 in granulocytes [(10); H. Zeng, unpublished data] and of Gfi1b in erythroid precursors (11,14) is mediated by cross-regulation. In contrast, cross-repression appears the most likely mechanism to assure the specific expression pattern in thymic T-cells, where Gfi1 expression levels are high and Gfi1b is largely absent. Preliminary data suggest upregulation of the Gfi1b gene in Gfi1-deficient thymocytes (K. Fiolka and T. Möröy, unpublished data) and support this hypothesis. Other cell types such as pre-B-cells or HSCs co-express both proteins but the deletion of Gfi1 or Gfi1b is either without large effects as in pre-B-cells, or only the deletion of Gfi1 produces a noticeable phenotype as in HSCs (14,15) and the absence of Gfi1b has no effect (15). Therefore, it is likely that both proteins may have redundant functions in one cell type and specific, nonoverlapping tasks in others. Additional studies with cell type specific deletions of the Gfi1 or Gfi1b gene have to be done to clarify this point.
Deletion of Gfi1 has profound effects on thymic pre-T-cell development (7). If Gfi1b cross-regulates the expression of Gfi1 as was convincingly demonstrated previously and is shown here to be the case, forced expression of a transgenic Gfi1b allele in T-cells should lead to a downregulation of Gfi1 in pre-T-cells and to a recapitulation of the phenotype seen in Gfi1-deficient mice. However, this is not the case in our vav-Gfi1b transgenic mice although robust expression levels of the Gfi1b transgene could be reached both on mRNA and protein levels. There are two possible explanations for this observation. Either the expression level of exogenous Gfi1b is still not high enough to completely downregulate Gfi1 expression and the lower level of Gfi1 is sufficient to maintain pre-T-cell development. Or, alternatively, the concomitant expression of Gfi1b takes over the functions of the suppressed Gfi1. The data presented here from combinatorial mutant mice that harbor both a Gfi1b transgene and the Gfi1:GFP allele suggest that the first explanation is more probable, because the downregulation of GFP fluorescence is partial and not complete indicating that Gfi1 expression is not fully silenced in CD4+ or in CD8+ cells. Since a complete silencing of the Gfi1:GFP allele was reached with an lck-driven Gfi transgene as reported previously (8), it is thus most likely that a higher level of Gfi1b transgene expression has to be reached to observe such a complete downregulation for endogenous Gfi1. In addition, it has been reported that an lck promoter driven Gfi1b transgene with a high-level expression of Gfi1b in the thymus is also able to completely repress Gfi1 expression (9). Both findings would support the conclusion that a rather high level of Gfi1b expression is necessary to completely silence endogenous Gfi1 which is not reached in our transgenic model.
In contrast to the vav-Gfi1b transgenic mice studied here, phenotypic alterations in thymocyte differentiation were observed in lck-Gfi1b transgenic mice (9), which was probably due to the higher expression level in these animals. However, the phenotype in lck-Gfi1b transgenic mice is clearly different from the effects caused by a targeted deletion of the Gfi1 gene (7) leaving the question open whether Gfi1b can replace Gfi1 or whether different expression levels of Gfi1b can obscure such an effect. Since Gfi1 and Gfi1b are clearly different proteins, their ability to act as transcriptional repressors may vary in particular depending on cellular context and cell type specific expression and activity level. In addition, although Gfi1 is expressed at a very high level in thymic pre-T-cells compared with other cells, as for instance early B-cell precursors or even activated T-cells, the level of expression varies significantly during pre-T-cell development at mRNA and protein levels (8), which also suggests the existence of other mechanisms of regulation in addition to cross-repression and auto-regulation. Other experimental models have to be designed to clarify the differences and similarities of Gfi1 and Gfi1b functions and to solve the question of to what degree and under what conditions can Gfi1 and Gfi1b possess mutually redundant or exclusive roles.
A more obvious difference between Gfi1 and Gfi1b is reflected by their role in malignant transformation. A weak oncogenic activity of Gfi1 has been demonstrated in transgenic mouse models where constitutive Gfi1 expression was targeted to the T-cell lineage by the lck promoter (20). However, no evidence exists so far that such a role can also be ascribed to Gfi1b. Clearly, when tumors that arise in mice after infection with MoMuLV are analyzed, the Gfi1 gene is one of the most frequently selected target loci where proviral insertions take place (21–24). Whereas the Gfi1 gene has even been originally discovered as a proviral insertion site (22), the Gfi1b gene is not a common retroviral target selected during lymphomagenesis and has been isolated by virtue of sequence homology to Gfi1 (25,26). Whether this reflects a lower ability of Gfi1b to contribute to malignant transformation remains to be shown, but the transgenic mice that were analyzed in this study did not show any signs of malignant transformation within the first 12 months of life. However, after 1 year, splenomegaly and an uncharacterized type of lympho- or myelo-proliferative disease developed (Vassen and Möröy, unpublished) suggesting a potential participation of Gfi1b in the development of neoplastic diseases. Still, only combinatorial mutant mice bearing the vav-Gfi1b transgene and other mutations in oncogenes or tumor suppressor genes will unequivocally demonstrate whether or not Gfi1b has any oncogenic potential.
Auto-regulation by genetic feedback loops is a common phenomenon among eukaryotic transcription factors and has been described for many other examples (27–30). Here the situation seems to be more complex, since we provide evidence that Gfi1b expression is not only under an auto-regulatory control but also cross-regulates Gfi1 expression. Reporter gene assays also suggest that Gfi1 can regulate the Gfi1b promoter, and previously published findings demonstrated auto- regulation of Gfi1 (8,16). With these data, a picture emerges for a particularly complex lymphoid-specific regulatory network that is built around the two Gfi1 proteins, suggesting a fine-tuned mechanism that ensures precise control over mRNA and protein levels of both Gfi1 proteins. Such a complex regulation may be necessary in lymphoid cells since Gfi1 and Gfi1b control essential steps during lymphoid development as the gene knock-out studies have demonstrated in a most impressive way. In addition, Gfi1 can be a transforming onco-protein and contributes significantly to the malignant transformation of lymphocytes when expressed at high levels. Hence, it is certainly vital for an organism to closely check Gfi1 and Gfi1b expression levels to avoid errors in lymphoid differentiation or a predisposition to lymphoma.
Acknowledgments
We are indebted to Angelika Warda, Wojciech Wegrzyn and Inge Spratte for technical assistance and Petra Plessow and Tomas Civela for excellent animal care. We are indebted to Dr J. Adams, Walter and Eliza Hall Institute for Medical Research, Melbourne, Australia, for providing the vav promoter element. This work was supported by the Deutsche Forschungsgemeinschaft, DFG (grant Mo 435/18-1, 18-2), the ‘Fonds der chemischen Industrie’ and the ‘IFORES Program’ of the University of Essen Medical School. Funding to pay the Open Access publication charges for this article was provided by the University of Essen Medical School.
REFERENCES
- 1.Duan Z., Horwitz M. Gfi-1 oncoproteins in hematopoiesis. Hematology. 2003;5:339–344. doi: 10.1080/10245330310001612116. [DOI] [PubMed] [Google Scholar]
- 2.Möröy T. The transcription factor ‘growth factor independence 1’—Gfi1. Int. J. Biochem. Cell Biol. 2004;37:541–546. doi: 10.1016/j.biocel.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Grimes H.L., Chan T.O., Zweidler-McKay P.A., Tong B., Tsichlis P.N. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol. Cell. Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zweidler-McKay P.A., Grimes H.L., Flubacher M.M., Tsichlis P.N. Gfi1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol. Cell. Biol. 1996;16:4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt T., Karsunky H., Rödel B., Zevnik B., Elsässer H.P., Möröy T. Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with beta-selection. EMBO J. 1998;17:5349–5359. doi: 10.1093/emboj/17.18.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karsunky H., Mende I., Schmidt T., Möröy T. High levels of the onco-protein Gfi-1 accelerate T-cell proliferation and inhibit activation induced T-cell death in Jurkat T-cells. Oncogene. 2002;21:1571–1579. doi: 10.1038/sj.onc.1205216. [DOI] [PubMed] [Google Scholar]
- 7.Yücel R., Karsunky H., Klein-Hitpass L., Möröy T. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J. Exp. Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yücel R., Kosan C., Heyd F., Möröy T. Gfi1:green fluorescent protein knock-in mutant reveals differential expression and auto-regulation of the growth factor independence 1 (Gfi1) gene during lymphocyte development. J. Biol. Chem. 2004;279:40906–40917. doi: 10.1074/jbc.M400808200. [DOI] [PubMed] [Google Scholar]
- 9.Doan L.L., Kitay M.K., Yu Q., Singer A., Herblot S., Hoang T., Bear S.E., Morse H.C., III, Tsichlis P.N., Grimes H.L. Growth factor independence-1B expression leads to defects in T cell activation, IL-7 receptor alpha expression, and T cell lineage commitment. J. Immunol. 2003;170:2356–2366. doi: 10.4049/jimmunol.170.5.2356. [DOI] [PubMed] [Google Scholar]
- 10.Karsunky H., Zeng H., Schmidt T., Zevnik B., Kluge R., Schmid K.W., Dührsen U., Möröy T. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nature Genet. 2002;30:295–300. doi: 10.1038/ng831. [DOI] [PubMed] [Google Scholar]
- 11.Saleque S., Cameron S., Orkin S.H. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 2002;16:301–306. doi: 10.1101/gad.959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallis D., Hamblen M., Zhou Y., Venken K.J., Schumacher A., Grimes H.L., Zoghbi H.Y., Orkin S.H., Bellen H.J. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 13.Hock H., Hamblen M.J., Rooke H.M., Traver D., Bronson R.T., Cameron S., Orkin S.H. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18:109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 14.Zeng H., Yücel R., Kosan C., Klein-Hitpass L., Möröy T. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 2004;23:4116–4125. doi: 10.1038/sj.emboj.7600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hock H., Hamblen M.J., Rooke H.M., Schindler J.W., Saleque S., Fujiwara Y., Orkin S.H. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 16.Doan L.L., Porter S.D., Duan Z., Flubacher M.M., Montoya D., Tsichlis P.N., Horwitz M., Gilks C.B., Grimes H.L. Targeted transcriptional repression of Gfi1 by GFI1 and Gfi1b in lymphoid cells. Nucleic Acids Res. 2004;32:2508–2519. doi: 10.1093/nar/gkh570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogilvy S., Elefanty A.G., Visvader J., Bath M.L., Harris A.W., Adams J.M. Transcriptional regulation of vav, a gene expressed throughout the hematopoietic compartment. Blood. 1998;91:419–430. [PubMed] [Google Scholar]
- 18.McLaughlin J., Chianese E., Witte O.N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc. Natl Acad. Sci. USA. 1987;84:6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D.Y., Kuo Y.Y., Lai J.S., Suzuki Y., Sugano S., Chang Z.F. GATA-1 and NF-Y cooperate to mediate erythroid-specific transcription of Gfi-1B gene. Nucleic Acids Res. 2004;32:3935–3946. doi: 10.1093/nar/gkh719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt T., Karsunky H., Gau E., Zevnik B., Elsässer H.P., Möröy T. Zinc finger protein GFI-1 has low oncogenic potential but cooperates strongly with pim and myc genes in T-cell lymphomagenesis. Oncogene. 1998;17:2661–2667. doi: 10.1038/sj.onc.1202191. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt T., Zörnig M., Beneke R., Möröy T. MoMuLV proviral integrations identified by Sup-F selection in tumors from infected myc/pim bitransgenic mice correlate with activation of the Gfi-1 gene. Nucleic Acids Res. 1996;24:2528–2534. doi: 10.1093/nar/24.13.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilks C.B., Bear S.E., Grimes H.L., Tsichlis P.N. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol. Cell. Biol. 1993;13:1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheijen B., Jonkers J., Acton D., Berns A. Characterization of pal-1, a common proviral insertion site in murine leukemia virus-induced lymphomas of c-myc and Pim-1 transgenic mice. J. Virol. 1997;71:9–16. doi: 10.1128/jvi.71.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zörnig M., Schmidt T., Karsunky H., Grzeschiczek A., Möröy T. Zinc-finger protein GFI-1 cooperates with myc and pim-1 in T-cell lymphomagenesis by reducing the requirements for IL-2. Oncogene. 1996;12:1789–1801. [PubMed] [Google Scholar]
- 25.Tong B., Grimes H.L., Yang T.Y., Bear S.E., Qin Z., Du K., El-Deiry W.S., Tsichlis P.N. The Gfi-1B proto-oncoprotein represses p21WAF1 and inhibits myeloid cell differentiation. Mol. Cell. Biol. 1998;18:2462–2473. doi: 10.1128/mcb.18.5.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rödel B., Wagner T., Zörnig M., Niessing J., Möröy T. The human homologue (Gfi1b) of the chicken GFI gene maps to chromosome 9q34.13-A locus frequently altered in hematopoietic diseases. Genomics. 1998;54:580–582. doi: 10.1006/geno.1998.5601. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.B., Watada H., Scheel D.W., Mrejen C., German M.S. Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J. Biol. Chem. 2000;275:36910–36919. doi: 10.1074/jbc.M005202200. [DOI] [PubMed] [Google Scholar]
- 28.Bateman E. Autoregulation of eukaryotic transcription factors. Prog Nucleic Acid Res. Mol. Biol. 1998;60:133–168. doi: 10.1016/s0079-6603(08)60892-2. [DOI] [PubMed] [Google Scholar]
- 29.Legraverend C., Antonson P., Flodby P., Xanthopoulos K.G. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res. 1993;21:1735–1742. doi: 10.1093/nar/21.8.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serfling E. Autoregulation—a common property of eukaryotic transcription factors? Trends Genet. 1989;5:131–133. doi: 10.1016/0168-9525(89)90049-8. [DOI] [PubMed] [Google Scholar]
- 31.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1998;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]