Abstract
Many Phoenix dactylifera (date palm) cultivars are grown in the arid and semiarid regions of the world, including Saudi Arabia. P. dactylifera is highly tolerant to salinity stress. To investigate the response of Khalas cultivar of P. dactylifera, two-month-old plants were treated with sodium chloride (50, 100, and 150 mM NaCl) for three months. Our result showed that proline content was higher in all treated plants compared to control plants. Thiobarbituric acid reactive substances (TBARS) were increased at 100 and 150 mM NaCl treatments; however, the result was found nonsignificant between control and plants treated at 50 mM NaCl. Similarly, enzyme activities of catalase (CAT) and superoxide dismutase (SOD) were 0.805 and 0.722 U/mg protein/min, respectively, and were greater at 100 and 150 mM NaCl treatments compared to the control plants. Total chlorophyll content and fresh weight of shoots and roots decreased substantially with the increase of salinity. A cDNA start codon-targeted (cDNA-SCoT) marker showed a variation in different gene expressions profiling between treated and untreated plants under various NaCl concentrations.
1. Introduction
In recent decades, soil salinity has become a global agricultural constraint [1, 2]. Salinity is increasing on Arabic land, and more than 50% would be salinized by the year 2050, if suitable corrections are not made [3]. Furthermore, the salinized areas are increasing every year at a rate of 10% for different reasons including poor cultural practices, irrigation with saline water, weathering of native rocks, high surface evaporation, and low precipitation [4, 5]. Salt stress causes average yield losses of more than 50% in major crops in agriculture-based countries [6]. Reactive oxygen species (ROS) are produced in plant cells under salinity stress [7], which can damage the cells. It also affects many metabolic and physical processes of the plant, and as a result, the growth is hampered [8]. A high salinity stress causes osmotic and ionic stresses in the plant cells, which lead to several physiological and morphological modifications [9].
Phoenix dactylifera (date palm) is the main horticultural fruit tree in many arid and semiarid countries in the Middle East, North Africa, and Central America [10]. P. dactylifera can survive under extreme abiotic stresses, including conditions of drought, high temperature, and relatively high soil salinity levels [11–14]. The salinity stress affected the large area of arid and semiarid regions of agricultural field [15] and has impacted more losses in P. dactylifera and other crop species [16].
The antioxidant enzyme activities such as catalase (CAT) and superoxide dismutase (SOD) increase under salinity stress for scavenging regenerated ROS to protect the cell from damage [17, 18]. The enzyme SOD is found in various compartments of the cell and catalyzes the superoxide radicals (O2−) to H2O2 and O2 [19]. The H2O2 is removed from the cell by peroxidases and catalase [19–22].
The proline, an osmoprotectant, is produced under abiotic and biotic stresses [23] in plants. Heat and cold treatments can result in a significant increase in proline level in the leaves and roots of P. dactylifera [14]. The changes occurred in SOD and chlorophyll a/b-binding protein under salt stress in P. dactylifera [24]. Thiobarbituric acid reactive substances (TBARS), which are produced in the plant cells, act as a potential indicator of damage under induction of stresses [25]. An increase in TBARS content under salinity stress can cause damage to membranes and also to particular cell tissues [26–28]. Usually, osmotic or salt stress induces TBARS accumulation [29]. TBARS accumulation in cowpea leaves under salinity stress depends on exposure time [30]. However, a reduction in TBARS level under salinity stress is poorly reported in the literature [31].
Different methods have been developed for the gene expression study in plants or animals such as cDNA microarray, cDNA-SRAP, cDNA-AFLP, serial analysis of gene expression (SAGE), suppression subtractive hybridization (SSH), representational difference analysis (RDA), and mRNA differential display (DD) [32–42]. All these markers have advantages and disadvantages based on the reproducibility of the results, available resources, technical expertise, and cost of development.
A cDNA start codon-targeted (cDNA-SCoT) marker has been used for the study of gene expression in Saccharum officinarum, Mangifera indica, Phoenix dactylifera, and Dendrobium officinale [43–46]. However, this marker has also been used for the assessment of genetic diversity in various plant species [47–50]. A high degree of variability has been found among the germplasms of P. dactylifera under salinity and drought stresses [51]. Knowledge of molecular mechanisms under salinity and drought conditions in P. dactylifera is limited [52–57]. In the present study, we performed experiments on the Khalas cultivar of P. dactylifera to determine the antioxidant system response and gene expression profiling under salinity stress.
2. Materials and Methods
A pot experiment was conducted in a growth chamber for salinity stress treatments in four replicates. The pots were filled with a mixture of sand and peat moss (3 : 1). The healthy seeds of P. dactylifera were surface sterilized with sodium hypochlorite solution (4.0% available chlorine) for 10 min and washed thoroughly four times with distilled water. The seeds were sown in plastic pots and watered at regular interval to maintain moisture for better germination. Salinity stress treatments were given to the two-month-old plants of Khalas cultivar of P. dactylifera for three months. Three concentrations of NaCl as low (T-50, 50 mM), intermediate (T-100, 100 mM), and high (T-150, 150 mM) were used to treat the plants. Each concentration of salt solution (100 ml) was given to each pot after two-week time intervals. 100 ml of 1/4 strength MS solution was added to each pot after two-week time intervals. The pots were maintained in the growth chamber at 26-27°C, photoperiod 16 h per day, and relative humidity of 72%. The salt-treated and untreated plants were harvested after three months. Biochemical and molecular parameters were subsequently taken to study the antioxidant system response of P. dactylifera under salinity stress.
2.1. Biomass and Morphological Traits
Fresh leaf and root weight and shoot and root length were measured after three months of salinity treatment. Each treatment was compared to control plants for the evaluation of their salt stress responses.
2.2. Proline Estimation
The proline was estimated using the method developed by Hanson et al. [58]. Fresh leaves (0.3 g) were ground in 10 ml of aqueous sulphosalicylic acid (3%). The mixture was centrifuged for 15 min at 9000 ×g. The supernatant (2 ml) from the above step was taken and mixed with an equal volume of acid ninhydrin (1.25 g ninhydrin in 30 ml acetic acid and 20 ml of 6 N H3PO4) and acetic acid. The mixture was placed for 1 h in boiling water for incubation. After incubation, the mixture was taken out from the boiling water and immediately placed in an ice bath. 4 ml of toluene was added in the mixture (4 ml) after taking it from the ice water bath. The mixture was vortexed, and chromatophore-containing toluene was separated from the aqueous phase. The absorbance was taken at 520 nm (Model UB-1800, Shimadzu, Japan) to determine proline content.
2.3. Total Chlorophyll
Total chlorophyll was estimated according to the Arnon method [59]. The leaves were separated and washed with DDW; 0.1 g of chopped leaves was placed in the test tubes for each treatment, and 10 ml of DMSO was added to each test tube. The tubes were kept in an oven at 65°C. After 120 minutes, the tubes were taken out and the absorbance of the solution was recorded immediately at 663 nm and 645 nm on a UV-vis spectrophotometer (Model UB-1800, Shimadzu, Japan). The pigment concentration was calculated in μg/ml for treated and untreated samples.
2.4. Superoxide Dismutase (SOD)
The activity of superoxide dismutase (EC 1.15.1.1) was measured according to the method developed by Dhindsa et al. [60]. A fresh sample (0.05 g) was homogenized in 2 ml of extraction mixture containing phosphate buffer (0.5 M, pH 7.3), 0.3 mM-EDTA, 1% Triton × 100 (w/v), and 1% PVP (w/v). The mixture was centrifuged for 10 min at 4°C at 10,000 ×g. The supernatant was taken after centrifugation for the assay of SOD activity. The assay mixture, consisting of 1.5 ml reaction buffer, 0.2 ml of methionine, 0.1 ml of each (1 M-NaCO3, 2.25 mM-NBT solution, 3 mM-EDTA, riboflavin, and enzyme extract), and 1 ml of DDW, was incubated under the light. The blank mixture containing all substances was kept in the dark. Absorbance of samples along with the blank mixture was read at 560 nm using the UV-vis spectrophotometer (Model UB-1800, Shimadzu, Japan). A 50% reduction in color was considered as one enzyme unit (EU). The activity of SOD was calculated in EU (mg−1 protein min−1).
2.5. Catalase (CAT)
The activity of catalase (EC 1.11.1.6) was determined in the leaves using the method of Aebi [61]. 0.5 g of fresh leaf samples was ground in extraction buffer (5 ml) containing phosphate buffer (0.5 M, pH 7.3), 0.3 mM-EDTA, and 0.3 mM-H2O2. The mixture was centrifuged for 20 min at 10,000 ×g at 4°C. The reaction was carried out in 2 ml of reaction mixture (0.1 ml, 3 mM-EDTA, 0.1 ml of enzyme extract, and 0.1 ml of 3 mM-H2O2) for 5 min. CAT activity was estimated at 240 nm using the UV-vis spectrophotometer with the help of extinction coefficient (R) 0.036 mM−1cm−1 and expressed in EU (mg−1 protein min−1).
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
TBARS content was determined in the leaves using the method developed by Cakmak and Horst [62] with minor modification. The fresh leaf samples (0.5 g) were ground in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA). The centrifugation was performed for 5 min at 12,000 ×g for supernatant collection. The supernatant was taken from the above step, and 1 ml of it was added to 4 ml of 0.5% (w/v) TBA in 20% (w/v) TCA. The mixture was placed for 30 min at 90°C in water bath, and thereafter, the reaction was terminated in an ice bath. The centrifugation was performed for 5 min at 10,000 ×g for supernatant collection. The absorbance of the supernatant was read at 532 and 600 nm wavelengths on a spectrophotometer (Model UB-1800, Shimadzu, Japan). The TBARS content was calculated using the following formula:
| (1) |
where A532 = absorbance at 532 nm, A600 = absorbance at 600 nm, V = extraction volume, and W = fresh weight of tissue.
2.7. RNA Extraction for cDNA-SCoT Marker Profiling
Total RNA was isolated from the control and salinity-stressed plants using the RNeasy plant mini kit (Qiagen) according to the instructions given in the manual. The quantity and quality were measured using the spectrophotometer (Nanodrop 8000, Thermo Scientific). High quality of cDNA was prepared using the QuantiTect Reverse Transcription Kit (Qiagen). The PCR reaction was performed in a total volume of 25 μl using the SCoT primers (Table 1) for the study of expression profiling. These primers were selected from the literature of monocot plant species [63]. The PCR bead (GE Healthcare, UK) was used for PCR amplification. The cDNA was diluted in RNase-free water to working concentration 50 ng for PCR amplification with SCoT primer (20 picomole per reaction). PCR was performed in an AB Veriti 96-well thermal cycler. The cycling profile was 94°C for 3 min, 45 cycles at 94°C for 1 min, 44.5°C for 30 s, 72°C for 1 min, and a cycle of 72°C for 5 min. The amplified products were resolved on 1.3% TBE agarose gel.
Table 1.
List of SCoT primer sequences used in the PCR reaction.
| S.N. | Primer code | Primer sequence (5′-3′) |
|---|---|---|
| 1 | SCoT-1 | CAACAATGGCTACCACCA |
| 2 | SCoT-2 | CAACAATGGCTACCACCC |
| 3 | SCoT-3 | CAACAATGGCTACCACCG |
| 4 | SCoT-4 | CAACAATGGCTACCACCT |
| 5 | SCoT-5 | CAACAATGGCTACCACGC |
| 6 | SCoT-6 | CAACAATGGCTACCACGG |
| 7 | SCoT-7 | CAACAATGGCTACCACGT |
| 8 | SCoT-8 | CAACAATGGCTACCAGCA |
| 9 | SCoT-9 | CAACAATGGCTACCAGCC |
| 10 | SCoT-10 | AAGCAATGGCTACCACCA |
| 11 | SCoT-11 | GCAACAATGGCTACCACC |
| 12 | SCoT-12 | CATGGCTACCACCGGCCC |
| 13 | SCoT-13 | ACCATGGCTACCACCGCA |
| 14 | SCoT-14 | CCATGGCTACCACCGCAG |
| 15 | SCoT-15 | ACCATGGCTACCACCGCA |
| 16 | SCoT-16 | CCATGGCTACCACCGCAG |
| 17 | SCoT-17 | CCATGGCTACCACCGCAC |
| 18 | SCoT-18 | CCATGGCTACCACCGCCT |
2.8. Statistical Analysis
The data recorded in all experiments were statistically analyzed by using IBM SPSS STATISTICS 19. Data from each parameter was subjected to a one-way analysis of variance (ANOVA); the post hoc comparison for the observation was assumed by Duncan's test. The data shown are the averages of four replicates and were statistically significant at the p < 0.05 level.
3. Results and Discussion
Free radicals, or ROS, are produced in plant cells under stress conditions and may react with pigments, lipids, proteins or nucleic acids which leads to membrane damage, lipid peroxidation, and inactivation of enzymes, thus affecting the cell viability [64, 65]. Plant gene expression analysis is very important in agriculture under biotic and abiotic stresses as it promotes genetic improvement of other crops for their yield and quality traits.
Fresh weight of shoot and root of P. dactylifera decreased significantly as the salinity increased (Figures 1 and 2). High salinity stress caused more reduction in the weight of shoot and root (1.392 and 1.160 g) as compared to control plants (2.697 and 2.201 g), respectively. Alkhateeb et al. [66] performed experiments on P. dactylifera under salinity stress and found that growth declined with increasing salinity stress. Excess salinity affects plants severely due to water stress, membrane disorganization, nutritional disorders, ion toxicity, and the expansion and reduction of cell division [67, 68]. The root length was more affected than the shoot length (Figure 3). The more reduction in the root length (26 cm) was observed significantly at 150 mM NaCl when compared to control plants (35.33 cm). The shoot length was less affected under salinity stress, and a nonsignificant variation was found among treated as well as control plants (Figures 4 and 5). There was no effect of salinity observed on the shoot length up to 50 mM NaCl, and the plant grew normally like a normal plant. Ramoliya and Pandey [69] studied on some P. dactylifera varieties under salinity stress and found that some varieties can tolerate high levels of soil salinity (12.8 ds m−1) without a visible effect. Total chlorophyll content decreased significantly in all treated P. dactylifera plants as the salinity increased. A low chlorophyll content (27.241 μg/ml) was observed in plants treated at high salinity stress (Figure 6) compared to control plants (47.873 μg/ml). Similarly, chlorophyll a and b decreased under salinity stress in a date palm [70].
Figure 1.
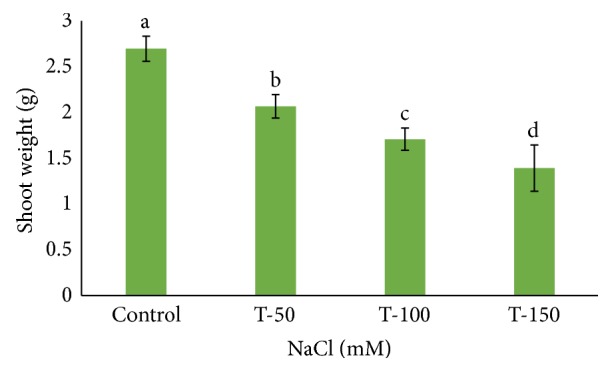
Fresh shoot weight in Phoenix dactylifera grown in the pot at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Figure 2.
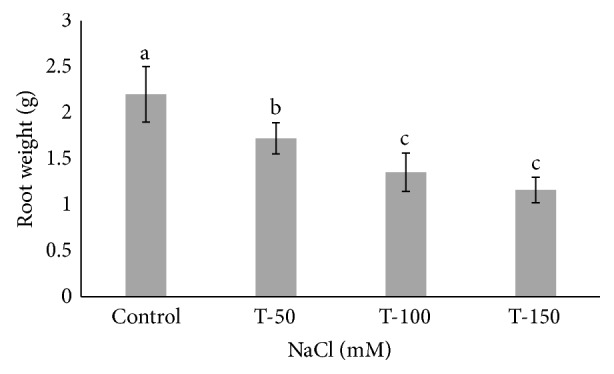
Fresh root weight in Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Figure 3.

Morphological variations in the root and shoot of Phoenix dactylifera grown under different NaCl concentrations (control: 0 mM, T-50: 50 mM, T-100: 100 mM, and T-150: 150 mM) for 3 months.
Figure 4.
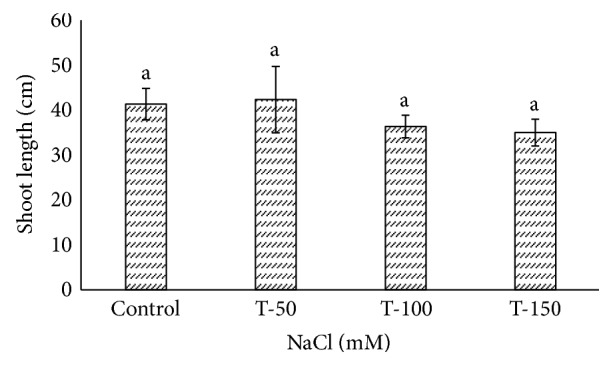
Shoot length variation in Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Figure 5.
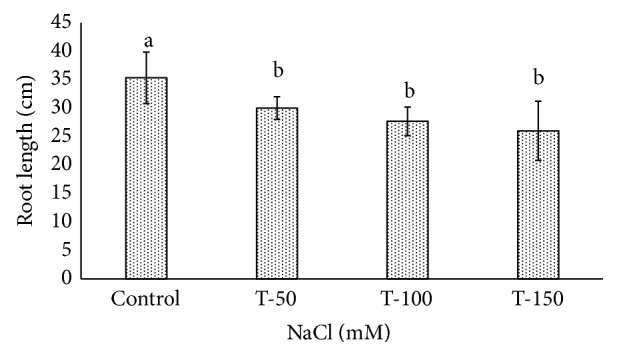
Root length variation in Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Figure 6.
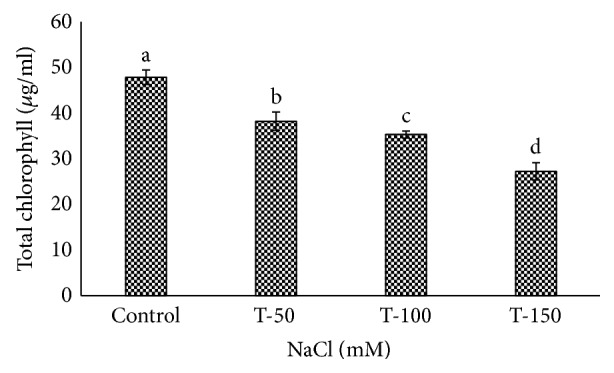
Effect of salinity on chlorophyll content in Phoenix dactylifera. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Catalase activity increased significantly in the leaves of date palm plants under 100 and 150 mM NaCl treatments, and it was 0.481 and 0.805 U/mg protein/min, respectively (Figure 7). However, a very low CAT activity (0.087 U/mg protein/min) was observed at 50 mM NaCl nonsignificantly compared to control plants. CAT activity induced at an application of 100 mM NaCl in wild Lycopersicon pennellii [71]. SOD activity increased under 100 mM NaCl and 150 mM NaCl treatments compared to nontreated plants (Figure 8). A very low SOD activity of 0.138 U/mg protein/min was found in plants treated at 50 mM NaCl. A high SOD enzyme activity of 0.667 and 0.722 U/mg protein/min was found significantly at 100 mM NaCl and 150 mM NaCl treatments compared to the control plants, where SOD activity only reached 0.1 U/mg protein/min. Our results were consistent with earlier findings for P. dactylifera where catalase and peroxidase activities increased under salinity treatments [70]. The CAT and SOD activities also increased in P. dactylifera Hillawi cv [72] under salinity stress. The activities of CAT and SOD were enhanced at 100 mM NaCl in wild Lycopersicon pennellii [71]. The responses of SOD and CAT were high under salinity stress (120 and 240 mM NaCl) in the leaves of two-week-old seedlings of barley, and it was found significant [73]. An increase in antioxidant enzymes under stressful conditions plays an important role to overcome the oxidative stress and often correlates to the type and magnitude of the stress [65].
Figure 7.
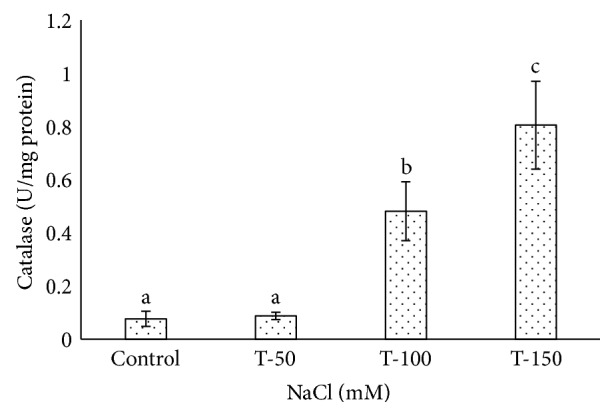
Catalase activity in Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
Figure 8.
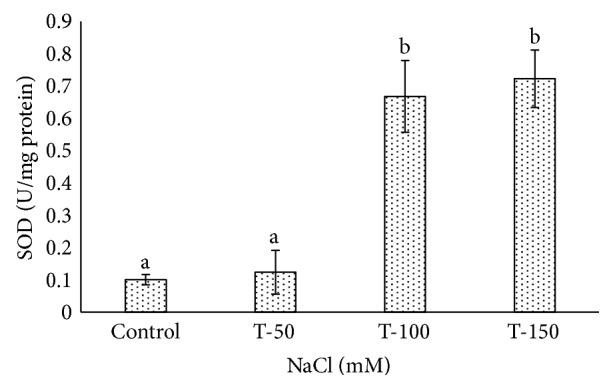
Superoxide dismutase activity in Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
The proline accumulation varies in different plant species and their organs under salinity stress. The proline content increased significantly in the leaves of all treated plants of P. dactylifera as the salinity increased (Figure 9). More accumulation of proline (2106.20 and 2632.99 μg/g FW) was observed significantly under 100 and 150 mM NaCl treatments compared to control plants. Our findings related to proline accumulation were consistent with the results of Abdulwahid [72], who performed experiments on P. dactylifera under salinity stress. The proline was over accumulated in the roots and leaves of a date palm plant under abscisic acid, drought, and extreme temperatures and was remarkably high when leaves were exposed to suboptimum salinity and temperatures stresses [13]. The cultivars of Phaseolus vulgaris (Canario 60 and Pinto Villa) accumulated high proline content in leaves and shoots under 150 mM NaCl [74]. A high accumulation of proline was found under salinity stress in mulberry [75], green gram [76], Jerusalem artichoke [77], and canola [78]. The production of proline under stress conditions play an important role to protect the plant cells as it acts as soluble nitrogen sink, a signal of senescence, an osmoregulator, and an indicator of plant resistance [79].
Figure 9.
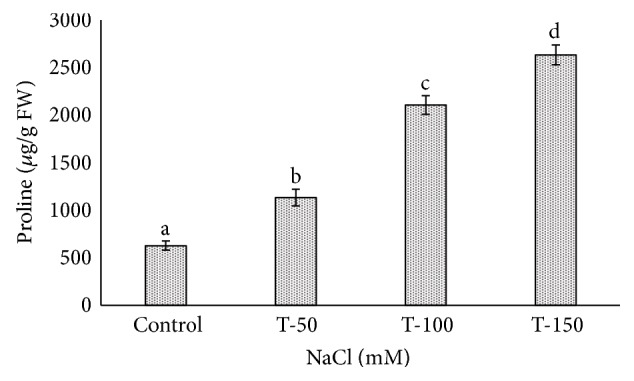
Proline accumulation in the leaf of Phoenix dactylifera grown at different concentrations of NaCl. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
TBARS level increased in P. dactylifera plants under salinity stress as compared to control plants. A high content of TBARS (11.151 nM/g FW) was found in plants treated at 100 mM NaCl (Figure 10), and thereafter, a reduction was observed. However, result was found to be nonsignificant between control and plants treated at 50 mM NaCl. The plant species such as Solanum nigrum [80], Artemisia annua [81], Glycyrrhiza uralensis Fisch [82], and Gypsophila aucheri Boiss [83] showed increased TBARS content under salinity stress.
Figure 10.
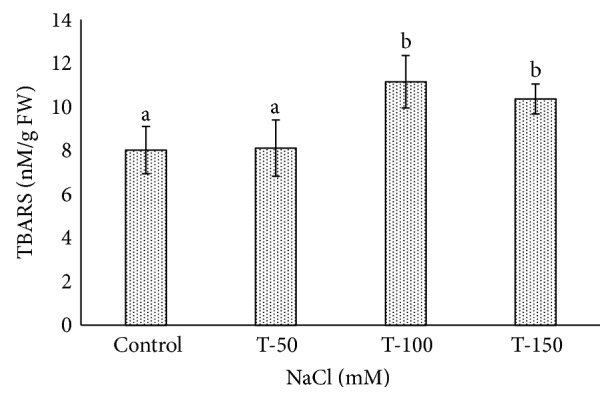
Effect of salinity stress on TBARS accumulation in Phoenix dactylifera. Data represent means of four replicates ± standard deviation. Different letters on bars represent the significant values according to Duncan's test (p < 0.05).
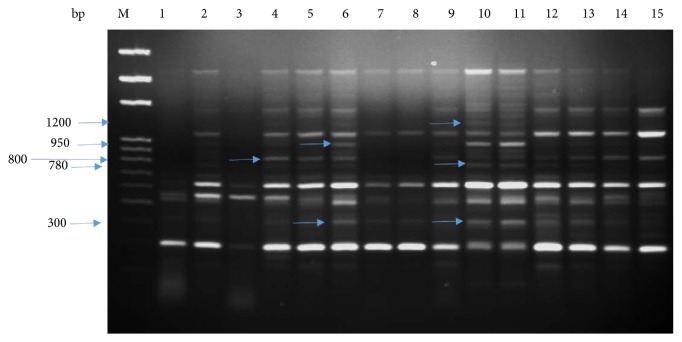
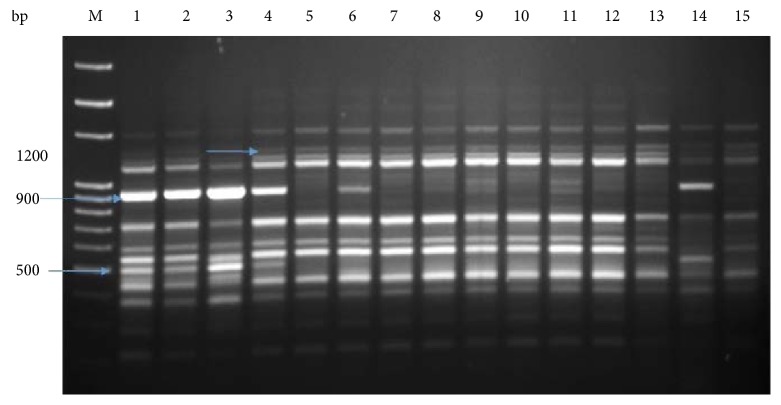
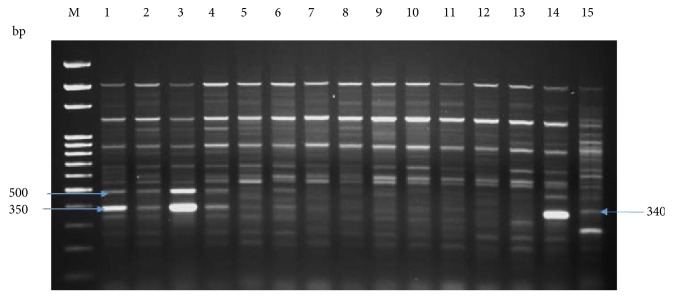
We used cDNA-SCoT marker for the comparison of treated and untreated plants under salinity stress as the antioxidant system response and biomass of P. dactylifera were changed at various concentrations of NaCl stress which could be possible due to the expression of different genes. A single primer cDNA-SCoT technique has been applied to study gene expression in different plant species [43–45, 83–85]. The oligo-dT-anchored cDNA-SCoT was used in M. indica to study gene expression under abiotic stresses [44]. In our study, a different banding pattern was produced between treated and untreated plants using the cDNA-SCoT marker which indicated the expression of different genes under NaCl stress (Figures 11, 12, and 13). Different amplicons of size (1200, 950, 800, 780, and 300 bp) were produced in treated plants whereas were absent in control plants (Figure 11). The amplicon of size 950 bp was produced at 50 and 100 mM NaCl, whereas absent at 150 mM NaCl. Similarly, the amplicon size of 750 bp was produced at 100 mM NaCl and absent at 50 and 150 mM NaCl. The size of 1200 bp amplicon was produced in all treated plants, whereas 900 and 500 bp were produced in control as well as in plants treated at 50 mM NaCl (Figure 12). The size of amplicons 500 and 350 bp were produced in control as well as in plants treated at 50 mM NaCl whereas absent in plants treated at 100 mM NaCl (Figure 13). Similarly, the amplicon of size 340 bp was produced in plants treated at 150 mM NaCl (Figure 13). Thus, different NaCl concentrations impacted the expression profile of various genes which led to change in plant growth, biomass, and antioxidant system response. The cold resistance-related genes have been studied in sugarcane under cold stress using the cDNA-SCoT technique [84]. The differentially expressed genes in sugarcane, induced by Leifsonia xyli subsp. xyli, was studied using the cDNA-SCoT technique [85]. Wu et al. [43] used the cDNA-SCoT technique on sugarcane for the differential expression of gibberellin-induced genes for stalk elongation, which represented the upregulation and downregulation of genes.
Figure 11.
cDNA-SCoT marker profiling generated from individual plant leaf of Phoenix dactylifera at different concentrations of NaCl (SCoT primer 3). Lane M: 100 bp ladder; lanes 1, 2, and 3 (control); lanes 4, 5, 6, and 7 (50 mM NaCl); lanes 8, 9, 10, and 11 (100 mM NaCl); lanes 12, 13, 14, and 15 (150 mM NaCl).
Figure 12.
cDNA-SCoT marker profiling generated from individual plant leaf of Phoenix dactylifera at different concentrations of NaCl (SCoT primer 18). Lane M: 100 bp ladder; lanes 1, 2, and 3 (control); lanes 4, 5, 6, and 7 (50 mM NaCl); lanes 8, 9, 10, and 11 (100 mM NaCl); lanes 12, 13, 14, and 15 (150 mM NaCl).
Figure 13.
cDNA-SCoT marker profiling generated from individual plant leaf of Phoenix dactylifera at different concentrations of NaCl (SCoT primer 4). Lane M: 100 bp ladder; lanes 1, 2, and 3 (control); lanes 4, 5, 6, and 7 (50 mM NaCl); lanes 8, 9, 10, and 11 (100 mM NaCl); lanes 12, 13, 14, and 15 (150 mM NaCl).
Thus, based on the above results, P. dactylifera can be used by plant researchers to uncover the salt tolerant genes and their application in a plant-breeding program.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) and King Abdul Aziz City for Science and Technology, Kingdom of Saudi Arabia, Award no. 11-BIO1943-02.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Rengasamy P. World salinization with emphasis on Australia. Journal of Experimental Botany. 2006;57(5):1017–1023. doi: 10.1093/jxb/erj108. [DOI] [PubMed] [Google Scholar]
- 2.Munns R., Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 3.Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnology Advances. 2009;27(1):84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Tanji K. K. Nature and extent of agricultural salinity. In: Tangi K. K., editor. Agricultural Salinity Assessment and Management. New York: American Society of Civil Engineers; 1990. pp. 1–17. [Google Scholar]
- 5.Szabolcs I. Soil salinization. In: Pessarakli M., editor. Handbook of Plant Crop Stress. New York: Marcel Dekker; 1994. pp. 3–11. [Google Scholar]
- 6.Bray E. A., Bailey-Serres J., Weretilnyk E. Responses to abiotic stresses. In: Buchanan B. B., Gruissem W., Jones R. L., Rockville M. d., editors. Biochemistry and Molecular Biology of Plants. USA: American Society of Plant Biologists; 2000. pp. 1158–1203. [Google Scholar]
- 7.Stanisavljevic N. S., Nikolić D. B., Jovanovic Z. S., Samardzic J. T., Radovic S. R., Maksimovic V. R. Antioxidative enzymes in the response of buckwheat (Fagopyrum esculentum Moench) to complete submergence. Archives of Biological Sciences. 2011;63(2):399–405. doi: 10.2298/ABS1102399S. [DOI] [Google Scholar]
- 8.Di Baccio D., Navari-Izzo F., Izzo R. Seawater irrigation: antioxidant defence responses in leaves and roots of a sunflower (Helianthus annuus L.) ecotype. Journal of Plant Physiology. 2004;161(11):1359–1366. doi: 10.1016/j.jplph.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Jampeetong A., Brix H. Effects of NaCl salinity on growth, morphology, photosynthesis and proline accumulation of Salvinia natans. Aquatic Botany. 2009;91(3):181–186. doi: 10.1016/j.aquabot.2009.05.003. [DOI] [Google Scholar]
- 10.Food and Agriculture Organization of the United Nations. 2005 Worldwide Dates Production Statistics. Rome: Food and Agriculture Organization of the United Nations; 2006. [Google Scholar]
- 11.Furr J. R., Armstrong W. W. Water and salinity problems of Abadan Island date gardens. Annual Date Growers’ Institute Report. 1975;52:14–17. [Google Scholar]
- 12.Zaid A., De Wet P. F. Climatic requirements of date palm. In: Zaid A., editor. Date Palm Cultivation. Rome: Food and Agriculture Organization of the United Nations; 2002. pp. 57–72. (Food and Agriculture Organization Plant Production and Protection Paper No.156). [Google Scholar]
- 13.Yaish M. W. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.) Genetics and Molecular Research. 2015;14(3):9943–9950. doi: 10.4238/2015.August.19.30. [DOI] [PubMed] [Google Scholar]
- 14.Yaish M. W., Antony I., Glick B. R. Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie van Leeuwenhoek. 2015;107(6):1519–1532. doi: 10.1007/s10482-015-0445-z. [DOI] [PubMed] [Google Scholar]
- 15.Pitman M. G., Läuchli A. Global impact of salinity and agricultural ecosystems. In: Läuchliand A., Lüttge U., editors. Salinity: Environment-Plants-Molecules. Dordrecht: Kluwer: Springer Netherlands; 2002. pp. 3–20. [Google Scholar]
- 16.Cookson P., Lepiece A. Could date palm sever disappear from the Batinah Salination of a coastal plaininthe Sultanate of Oman. In: Mahdi K. A., editor. Water in the Arabian Peninsula: Problems and Policies. Reading: Ithaca Press, Garnet Publishing Ltd.; 2001. pp. 221–235. [Google Scholar]
- 17.Jabeen N., Ahmad R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. Journal of the Science of Food and Agriculture. 2013;93(7):1699–1705. doi: 10.1002/jsfa.5953. [DOI] [PubMed] [Google Scholar]
- 18.Gao S., Ouyang C., Wang S., Xu Y., Tang L., Chen F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant, Soil and Environment. 2008;54(9):374–381. [Google Scholar]
- 19.Scandalios J. G. Oxygen stress and superoxide dismutase. Plant Physiology. 1993;101(1):712–726. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kono Y., Fridovich I. Inhibition and reactivation of Mn-catalase. The Journal of Biological Chemistry. 1983;258(22):13646–13468. [PubMed] [Google Scholar]
- 21.Gara L. D., Pinto M. C., Tommasi F. The antioxidant systems vis-á-vis reactive oxygen species during plant-pathogen interaction. Plant Physiology and Biochemistry. 2003;41(10):863–870. doi: 10.1016/S0981-9428(03)00135-9. [DOI] [Google Scholar]
- 22.Jablonski P. P., Anderson J. W. Light-dependent reduction of hydrogen peroxide by ruptured pea chloroplasts. Plant Physiology. 1982;69(6):1407–1413. doi: 10.1104/pp.69.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabados L., Savoure A. Proline: a multifunctional amino acid. Trends in Plant Science. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.El Rabey H. A., Abdulrahman L., Al-Malki K., Abulnaja O., Rohde W. Proteome analysis for understanding abiotic stress (salinity and drought) tolerance in date palm (Phoenix dactylifera L.) International Journal of Genomics. 2015;2015:11. doi: 10.1155/2015/407165.407165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez J. A., Almansa M. S. Short term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiologia Plantarum. 2002;115(2):251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagesh Babu R., Devaraj V. R. High temperature and salt stress response in French bean (Phaseolus vulgaris) Australian Journal of Crop Science. 2008;2(2):40–48. [Google Scholar]
- 27.Dudda A., Herold M., Holzel C., Loidl-Stahlhofen A., Jira W., Mlakar A. Lipid peroxidation, a consequence of cell injury? South African Journal of Chemistry. 1996;49(3-4):59–64. [Google Scholar]
- 28.Gechev T., Gadjev I., Van Breusegem F., et al. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cellular and Molecular Life Sciences CMLS. 2002;59(4):708–714. doi: 10.1007/s00018-002-8459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaparzadeh N., D’Amico M. L., Khavari-Nejad R. A., Izzo R., Navari-Izzo F. Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiology and Biochemistry. 2004;42(9):695–701. doi: 10.1016/j.plaphy.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Cavalcanti F. R., Oliveira J. T. A., Martins-Miranda A. S., Viegas R. A., Silveira J. A. G. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytologist. 2004;163(3):563–571. doi: 10.1111/j.1469-8137.2004.01139.x. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo-Neto A. D., Prisco J. T., Eneas-Filho J., Rolim Medeiros J. V., Gomes-Filho E. Hydrogen peroxide pretreatment induces salt-stress acclimation in maize plants. Journal of Plant Physiology. 2005;162(10):1114–1122. doi: 10.1016/j.jplph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y., Yang F., Tian X., Zhang W., Wang X., Ma G. Different patterns of transcriptomic response to high temperature between diploid and tetraploid Dioscorea zingiberensis CH. African Journal of Biotechnology. 2011;10(44):8847–8854. [Google Scholar]
- 33.Zamharir M. G., Mardi M., Alavi S. M., et al. Identification of genes differentially expressed during interaction of Mexican lime tree infected with "Candidatus Phytoplasma aurantifolia". BMC Microbiology. 2011;11(1):2–9. doi: 10.1186/1471-2180-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillmann A., Dunne E., Kenny D. cDNA amplification by SMART-PCR and suppression subtractive hybridization (SSH)-PCR. Methods in Molecular Biology. 2009;496:223–243. doi: 10.1007/978-1-59745-553-4_15. [DOI] [PubMed] [Google Scholar]
- 35.Wee A. W., Lee S. F., Robin C., Heckel D. G. Identification of candidate genes for fenvalerate resistance in Helicoverpa armigera using cDNA-AFLP. Insect Molecular Biology. 2008;17(4):351–360. doi: 10.1111/j.1365-2583.2008.00809.x. [DOI] [PubMed] [Google Scholar]
- 36.Polesani M., Desario F., Ferrarini A., et al. CDNA-AFLP analysis of plant and pathogen genes expressed in grapevine infected with Plasmopara viticola. BMC Genomics. 2008;9(1):142–151. doi: 10.1186/1471-2164-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nettuwakul C., Pongtongkam P., Thongpan A., Peyachoknagul S. Detection of photoperiod responsive Gene in KDML 105 Rice (Oryza sativa L.) using cDNA-SRAP technique. Kasetsart Journal (Natural Science) 2007;41(4):651–659. [Google Scholar]
- 38.Liang P., Meade J. D., Pardee A. B. A protocol for differential display of mRNA expression using either fluorescent or radioactive labeling. Nature Protocols. 2007;2(3):457–470. doi: 10.1038/nprot.2007.46. [DOI] [PubMed] [Google Scholar]
- 39.Huang X., Li Y., Niu Q., Zhang K. Q. Suppression subtractive hybridization (SSH) and its modifications in microbiological research (version a) Applied Microbiology and Biotechnology. 2007;76(4):753–760. doi: 10.1007/s00253-007-1076-8. [DOI] [PubMed] [Google Scholar]
- 40.Blackshaw S., Croix B. S., Polyak K., Kim J. B., Cai L. Current Protocols in Human Genetics. Maryland: John Wiley & Sons, Inc.; 2007. Serial analysis of gene expression (SAGE): experimental method and data analysis. Chapter 11, Unit 11.7. [DOI] [PubMed] [Google Scholar]
- 41.Bowler L. D. Representational difference analysis of cDNA. Methods in Molecular Medicine. 2004;94(2):49–66. doi: 10.1385/1-59259-679-7:49. [DOI] [PubMed] [Google Scholar]
- 42.Velculescu V. E., Zhang L., Vogelstein B., Kinzler K. W. Serial analysis of gene expression. Science. 1995;270(5235):484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 43.Wu J. M., Li Y. R., Yang L. T., et al. cDNA-SCoT: a novel rapid method for analysis of gene differential expression in sugarcane and other plants [online] Australian Journal of Crop Science. 2013;7(5):659–664. [Google Scholar]
- 44.Luo C., He X. H., Hu Y., Yu H. X., Ou S. J., Fang Z. B. Oligo-dT anchored cDNA–SCoT: a novel differential display method for analyzing differential gene expression in response to several stress treatments in mango (Mangifera indica L.) Gene. 2014;548(2):182–189. doi: 10.1016/j.gene.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Al-Ameri A. A., Al-Qurainy F., Gaafar A. Z., Khan S., Nadeem M. Male specific gene expression in dioecious Phoenix dactylifera (Date palm) tree at flowering stage. Pakistan Journal of Botany. 2016;48(1):131–135. [Google Scholar]
- 46.Li D. B., Gao Y. H., Si J. P. SCoT differential expression of cold resistance related genes in Dendrobium officinale under low temperature stress. Zhongguo Zhong Yao Za Zhi = China Journal of Chinese Materia Medica. 2013;38(4):511–515. [PubMed] [Google Scholar]
- 47.Al-Qurainy F., Khan S., Nadeem M., Tarroum M. SCoT marker for the assessment of genetic diversity in Saudi Arabian date palm cultivars. Pakistan Journal of Botany. 2015;47(2):637–643. [Google Scholar]
- 48.Bhattacharyya P., Kumaria S., Kumar S., Tandon P. Start codon targeted (SCoT) marker reveals genetic diversity of Dendrobium nobile Lindl., an endangered medicinal orchid species. Gene. 2013;529(1):21–26. doi: 10.1016/j.gene.2013.07.096. [DOI] [PubMed] [Google Scholar]
- 49.Satya P., Karan M., Jana S., et al. Start codon targeted (SCoT) polymorphism reveals genetic diversity in wild and domesticated populations of ramie (Boehmeria nivea L. Gaudich.), a premium textile fiber producing species. Meta Gene. 2015;3:62–70. doi: 10.1016/j.mgene.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo C., He X. H., Chen H., Ou S. J., Gao M. P. Analysis of diversity and relationships among mango cultivars using start codon targeted (SCoT) markers. Biochemical Systematics and Ecology. 2010;38(6):1176–1184. doi: 10.1016/j.bse.2010.11.004. [DOI] [Google Scholar]
- 51.Elshibli S. Genetic Diversity and Adaptation of Date Palm (Phoenix dactylifera L.), [Ph.D. Thesis] Helsinki, Finland: University of Helsinki; 2009. [Google Scholar]
- 52.Rizkalla A. A., Badr-Elden A. M., Nower A. A. Protoplast isolation, salt stress and callus formation of two date palm genotypes. Journal of Applied Sciences Research. 2007;3(10):1186–1194. [Google Scholar]
- 53.Darwesh R. S. S., El-Banna A. A. Role of potassium and salinity effects on growth and chemical compositions of date palm plantlets. Arab Universities Journal of Agricultural Sciences. 2011;19(1):233–244. [Google Scholar]
- 54.El-Sharabasy S. F., Wanas W. H., Al-Kerdany A. Y. Date palm cultivars in vitro screening to drought tolerance using isozymes. Arab Journal of Biotechnology. 2008;1:263–272. [Google Scholar]
- 55.Gomez-Vidal S., Salinas J., Tena M., Lopez-Llorca L. V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis. 2009;30(17):2996–3005. doi: 10.1002/elps.200900192. [DOI] [PubMed] [Google Scholar]
- 56.Sghaier-Hammami B., Valledor L., Drira N., Jorrin-Novo J. V. Proteomic analysis of the development and germination of date palm (Phoenix dactylifera L.) zygotic embryos. Proteomics. 2009;9(9):2543–2554. doi: 10.1002/pmic.200800523. [DOI] [PubMed] [Google Scholar]
- 57.Djibril S., Mohamed O. K., Diaga D., et al. Growth and development of date palm (Phoenix dactylifera L.) seedlings under drought and salinity stresses. African Journal of Biotechnology. 2005;4(9):968–972. [Google Scholar]
- 58.Hanson A. D., Nelson C. E., Pedersen A. R., Everson E. H. Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Science. 1979;19(4):489–493. doi: 10.2135/cropsci1979.0011183X001900040015x. [DOI] [Google Scholar]
- 59.Arnon D. Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhindsa R. S., Plumb-Dhindsa P., Thorpe T. A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. Journal of Experimental Botany. 1981;32(1):93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- 61.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 62.Cakmak I., Horst J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiologia Plantarum. 1991;83(3):463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- 63.Collard B. C. Y., Mackill D. J. Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Molecular Biology Reporter. 2009;27(1):86–93. doi: 10.1007/s11105-008-0060-5. [DOI] [Google Scholar]
- 64.Anjum N. A., Sofo A., Scopa A., et al. Lipids and proteins major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research. 2015;22(6):4099–4121. doi: 10.1007/s11356-014-3917-1. [DOI] [PubMed] [Google Scholar]
- 65.Gill S. S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Alkhateeb S. A., Alkhateeb A. A., Solliman M. In vitro response of date palm (Phoenix dactylifera L.) to K/Na ratio under saline conditions. Biological Research. 2015;48(1):p. 63. doi: 10.1186/s40659-015-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munns R. Comparative physiology of salt and water stress. Plant Cell and Environment. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 68.Shabala S., Cuin T. A. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2008;133(4):651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 69.Ramoliya P. J., Pandey A. N. Soil salinity and water status affect growth of Phoenix dactylifera seedlings. New Zealand Journal of Crop and Horticultural Science. 2003;31(4):345–353. doi: 10.1080/01140671.2003.9514270. [DOI] [Google Scholar]
- 70.Darwesh R. S. Improving growth of date palm plantlets grown under salt stress with yeast and amino acids applications. Annals of Agricultural Science. 2013;58(2):247–256. doi: 10.1016/j.aoas.2013.07.014. [DOI] [Google Scholar]
- 71.Mittova V., Guy M., Tal M., Volokita M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. Journal of Experimental Botany. 2004;55(399):1105–1113. doi: 10.1093/jxb/erh113. [DOI] [PubMed] [Google Scholar]
- 72.Abdulwahid A. H. Investigation of the effect of salt stress on the antioxidant enzyme activities on leaves of date palm (Phoenix dactylifera) seedling. Advances in Agriculture & Botanics-International Journal of the Bioflux Society. 2012;1(4):94–102. [Google Scholar]
- 73.Unal A. T., Aktas L. Y., Guven A. Effects of salinity on antioxidant enzymes and proline in leaves of barley seedlings in different growth stages. Bulgarian Journal of Agricultural Science. 2014;20(4):883–887. [Google Scholar]
- 74.Jimenez-Bremont J. F., Becerra-Flora A., Hernández-Lucero E., Rodríguez-Kessler M., Acosta-Gallegos J. A., Ramírez-Pimentel J. G. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biologia Plantarum. 2006;50(4):763–766. doi: 10.1007/s10535-006-0126-x. [DOI] [Google Scholar]
- 75.Surabhi G. K., Reddy A. M., Kumari G. J., Sudhakar C. Modulations in key enzymes of nitrogen metabolism in two high yielding genotypes of mulberry (Morus alba L.) with differential sensitivity to salt stress. Environmental and Experimental Botany. 2008;64(2):171–179. doi: 10.1016/j.envexpbot.2008.04.006. [DOI] [Google Scholar]
- 76.Misra N., Gupta A. K. Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Science. 2005;169(2):331–339. doi: 10.1016/j.plantsci.2005.02.013. [DOI] [Google Scholar]
- 77.Huang Z., Zhao L., Chen D., Liang M., Liu Z., Shao H. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PloS One. 2013;8(4, article e62085) doi: 10.1371/journal.pone.0062085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue X., Liu A., Hua X. J. Proline accumulation and transcriptional regulation of proline biosynthesis and degradation in Brassica napus. Biochemistry and Molecular Biology Report. 2009;42(1):28–34. doi: 10.5483/BMBRep.2009.42.1.028. [DOI] [PubMed] [Google Scholar]
- 79.Hayat S., Hayat Q., Alyemeni M. N., Wani A. S., Pichtel J., Ahmad A. Role of proline under changing environments - a review. Plant Signaling Behavior. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdallah S. B., Aung B., Amyot L., et al. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiologiae Plantarum. 2016;38(3):1–13. doi: 10.1007/s11738-016-2096-8. [DOI] [Google Scholar]
- 81.Qureshi M. I., Israr M., Abdin M. Z., Iqbal M. Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environmental and Experimental Botany. 2005;53(2):185–193. [Google Scholar]
- 82.Pan Y., Wu L. J., Yu Z. L. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch) Journal of Plant Growth Regulation. 2006;49(2-3):157–165. doi: 10.1007/s10725-006-9101-y. [DOI] [Google Scholar]
- 83.Esen A. H. S., Özgür R., Uzilday B., Tanyolaç Z. Ö., Dinc A. The response of the xerophytic plant Gypsophila aucheri to salt and drought stresses: the role of the antioxidant defence system. Turkish Journal of Botany. 2012;36(6):697–706. doi: 10.3906/bot-1201-5. [DOI] [Google Scholar]
- 84.Chen X. L., Li Y. R., Yang L. T., et al. cDNA-SCOT differential display of cold resistance related genes in sugar cane under low temperature stress. Biotechnology Bulletin. 2010;8:120–124. [Google Scholar]
- 85.Chen M. H., Zhang B. Q., Song X. P., et al. cDNASCoT analysis of differentially expressed genes in sugarcane induced by Leifsonia xyli subsp. Xyli. Acta Agronomica Sinica. 2013;39(9):1119–1126. doi: 10.3724/SP.J.1006.2013.01119. [DOI] [Google Scholar]