Abstract
Thanks to striking progress in both the understanding of anti-tumor immune response and the characterization of several tumor associated antigens (TAA), a more rational design and more sophisticated strategies for anti-tumor vaccination have been possible. However, the effectiveness of cancer vaccines in clinical trial is still partial, indicating that additional studies are needed to optimize their design and their pre-clinical testing. Indeed, anti-tumor vaccination success relies on the choice of the best TAA to be targeted and on the translational power of the pre-clinical model used to assess its efficacy. The chondroitin sulfate proteoglycan-4 (CSPG4) is a cell surface proteoglycan overexpressed in a huge range of human and canine neoplastic lesions by tumor cells, tumor microenvironment and cancer initiating cells. CSPG4 plays a central role in the oncogenic pathways required for malignant progression and metastatization. Thanks to these features and to its poor expression in adult healthy tissues, CSPG4 represents an ideal oncoantigen and thus an attractive target for anti-tumor immunotherapy. In this review we explore the potential of CSPG4 immune-targeting. Moreover, since it has been clearly demonstrated that spontaneous canine tumors mimic the progression of human malignancies better than any other pre-clinical model available so far, we reported also our results indicating that CSPG4 DNA vaccination is safe and effective in significantly increasing the survival of canine melanoma patients. Therefore, anti-CSPG4 vaccination strategy could have a substantial impact for the treatment of the wider population of spontaneous CSPG4-positive tumor affected dogs with a priceless translational value and a revolutionary implication for human oncological patients.
Keywords: Chondroitin sulfate proteoglycan-4 (CSPG4), Cancer immunotherapy, Comparative oncology, DNA vaccination
Background
It is when oncology meets immunology that cancer immunotherapy begins. Strengthen the patient’s own immune response against cancer cells represents one of the most challenging and exciting concept of active cancer immunotherapy [1].
Neoplastic initiation and progression are accompanied by the accumulation of several genetic modifications in somatic cells. The transcriptional and mutational landscape of tumors indicates that there is a clear opportunity for the immune system to distinguish tumor cells from healthy tissue and trigger a specific attack against both conserved and mutated tumor-associated antigens (TAA) [2, 3]. Indeed, a high number of potential TAA has been identified for each individual type of cancer [2, 4], making a more rational design and more sophisticated strategies for targeted anti-tumor vaccination a reality. In this evolving scenario, DNA vaccines represent an attractive and potentially effective tool for antigen-specific immunotherapy. Theoretically any mutated, abnormally expressed or over-expressed TAA could be exploited as a target to design a specific anti-cancer DNA vaccine, however, despite extensive effort by academia and industry, only one cancer vaccine has been approved by the U.S. Food and Drug Administration (FDA) so far [5]. The obstacles in successfully translating the virtually infinite number of potentially targetable TAA into effective anti-cancer vaccines have highlighted that being a TAA does not necessarily mean to be a good target for immunotherapy. Moreover, despite the existence of several successful immunotherapeutic strategies in mouse cancer models, their translation to human malignances fails because of unacceptable toxicity or a lack of efficacy [6, 7].
For these reasons, the clear need for more refined and predictive pre-clinical models becomes apparent. Therefore, in 2003 the National Cancer Institute’s Center for Cancer Research introduced the Comparative Oncology Program in order to foster the study of naturally occurring cancer in pet animals as models of human tumors [8, 9]. A European initiative with a similar purpose—the LUPA project—has also recently been launched [10]. The aim of comparative oncology is to integrate the naturally occurring cancers seen in veterinary patients into a more general study of cancer biology and therapy, to benefit both veterinary and human oncological patients [8, 9].
This review will highlight the role of chondroitin sulfate proteoglycan-4 (CSPG4) as one of the most attracting TAA identified so far for translational immunotherapy studies in both human and veterinary field.
The oncoantigen concept
Displaying a low level of expression in healthy tissues and a high level of expression in tumors, in addition to own a “driving” role in the promotion of cancer development—pushing the progression of a neoplastic lesion from one stage to the next—are the features of “ideal” immunotherapeutic targets.
The term “oncoantigen” was coined to describe those TAA that display the above-mentioned characteristics [11, 12]. Their stable expression throughout the various tumor developmental stages and the key role in tumor growth and survival make oncoantigens normally not susceptible to immunoediting and even if oncoantigen-loss variants might occur, they would have a crippled tumorigenic potential and undergo negative selection [13]. According to their localization, oncoantigens can be divided in three classes: (a) those expressed on the cell surface (Class I oncoantigens; receptors, adhesion molecules, etc.); (b) those present in the tumor microenvironment (Class II oncoantigens; growth factors, angiogenic factors, etc.); and (c) those that are intracellular proteins (Class III oncoantigens; non-receptor tyrosine kinases, transcription factors, cell cycle molecules). Being susceptible to the attack of both T cells and antibodies, Class I oncoantigens are considered the ideal targets for effective anti-cancer immunotherapeutic strategies [12, 14, 15].
The expression level in neoplastic and/or cancer initiating cells (CIC), the cellular localization and the role in oncogenic pathways, together with the percentage of patients with tumors expressing a given TAA, are characteristics also used by the National Cancer Institute of the U.S. to prioritize cancer antigens for the development of focused immunotherapeutic vaccines [16]. The CSPG4 belongs to this list of promising TAA [16].
CSPG4 identification card: structure, function and distribution
CSPG4, high molecular weight-melanoma associated antigen (HMW-MAA) and melanoma chondroitin sulfate proteoglycan (MCSP) are different names to label a cell surface molecule with unique structural characteristics. CSPG4 was first characterized on human melanoma cells more than 30 years ago [17]; in the same period, other studies have identified the nerve/glial antigen 2 (NG2) that is the rat orthologue of CSPG4 [17, 18]. The complete sequence of CSPG4 expressed by human melanoma cells was published in 1996 [19]: the gene, located on chromosome 15:24q2, is composed of 10 exons and the cDNA sequence length is of 8071 base pair (bp) encoding an open reading frame of 2322 amino acids (aa) [20, 21]. CSPG4 is a transmembrane protein that can be expressed on the cells either as a N-linked glycoprotein of ~250 kDa or as a ~450 kDa N-linked glycoprotein associated to a proteoglycan component [21]. The extracellular region of CSPG4 contains the sequences for the glycosylation with the chondroitin sulfate (CS) chains that can influence the distribution of the protein on the cell surface [22]. The large extracellular domain (1–2221 aa) is composed of three subdomains D1–D3 [17], encompassing binding sites for extracellular matrix (ECM) proteins, growth factors, integrins, matrix metalloproteinases and lectins. In particular, the D1 subdomain (1–640 aa) is an N-terminal globular domain consisting of two laminin G-type regions (LGR) and abundant disulfide bonds critical to maintain the tertiary structure. In these regions there are sites for ligand binding, for integrins interactions and cell matrix or cell–cell connections. The D2 subdomain (641–1590 aa) is composed of a succession of 15 “CSPG repeat” motifs, some of which are binding sites for CS chains covalently attached, collagens V and VI, or FGF and PDGF growth factors [23, 24]. The D3 subdomain (1591–2221 aa) is a membrane proximal globular domain with binding sites for the galectin-3, β1 integrins, and other lectins (e.g., p-selectin) [25, 26]. This juxtamembrane region contains also some proteolytic sites for the CSPG4 cleavage. Actually, CSPG4 fragments have been widely described [27, 28]; tryptic products have been detected in sera from healthy donors and cancer patients but their biological and clinical significance is still fairly unknown [26, 29, 30].
The extracellular portion of the CSPG4 is followed by a 25-aa transmembrane sequence (2222–2246 aa) joined to a 75-aa cytoplasmatic domain (2247–2322 aa) encompassing sites critical for CSPG4 function: (a) the PDZ binding domain on the carboxyl terminal portion that is the site for the attachment of scaffold proteins such as MUPP1, syntenin and GRIP1 [31]; (b) two multiple threonine phosphoacceptor sites phosphorylated by PKCα and extracellular signal-regulated kinase (ERK) 1 and 2 [32] and (c) a proline-rich region (PRR) that should promote other protein interactions [33]. A schematic depiction of CSPG4 structure is represented in Fig. 1.
Fig. 1.
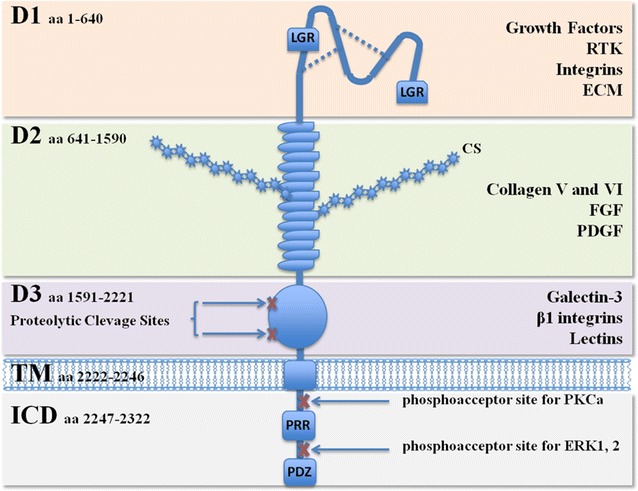
Schematic drawing of CSPG4 protein. CS chondroitin sulfate, D1, D2 and D3 subdomains of the extracellular portion, TM transmembrane domain, ICD intracellular domain, LGR laminin G-type regions, PRR proline-rich region, PDZ PDZ binding domain. The most important molecules interacting with each subdomain of CSPG4 are indicated on the right. RTK receptor tyrosine kinase, ECM extracellular matrix, FGF fibroblast growth factors, PDGF platelet-derived growth factor, PKC protein kinase C, ERK extracellular signal-regulated kinases
As a membrane-spanning molecule, CSPG4 plays an important role in the communication between the outside and the inside compartments of the cell. Notably, CSPG4 is devoid of a catalytic activity [34]; however, it can participate in signal transduction starring as co-receptor. Thanks to its extended extracellular arm, CSPG4 could “capture” and present growth factors to different tyrosine kinase receptors (RTK) or link the molecules of the ECM, including collagen type II, V, and VI, laminin, tenascin and fibronectin to integrins, potentiating and sustaining the activated pathways [17, 35]. Along with enhancing growth factor activity and integrin-mediated pathways, CSPG4 could also serve as a direct cell surface receptor for ECM components [23, 36]. The intracellular domain serves as “central recruitment” for scaffolding proteins linking CSPG4 to intracellular signaling pathways and to the actin cytoskeleton [37]. All these features highlight the central key role of CSPG4 in orchestrating cell proliferation, adhesion, migration and survival.
CSPG4 is involved mainly in tissue development or homeostasis and retains a limited expression in adult healthy tissues [26]. Its expression was initially found in a limited number of normal cell types, including vascular pericytes, articular chondrocytes, and the microglia in the central nervous system. More recently, CSPG4 mRNA expression has been detected in a broader range of tissues, including brain, gastrointestinal tract and endocrine organs, where it appears to be distinctive of precursor/progenitor cells of epithelial and mesodermal origin [38–41]. However, in normal tissues CSPG4 mRNA expression levels do not correlate with the protein expression [41].
CSPG4 as a prototype oncoantigen in human tumors
Marked by a restricted distribution in normal tissues, CSPG4 is over-expressed in several haematological and solid neoplastic conditions besides melanomas, including oligodendrocytomas, gliomas, childhood acute lymphoblastic leukemia and acute myeloid leukemia, renal cell carcinomas, chondrosarcomas, pancreatic and triple-negative breast carcinomas. In neoplastic lesions CSPG4 is highly expressed on both malignant cells and activated pericytes within the tumor microenvironment [42, 43]. The association between CSPG4 expression and patients’ poor prognosis suggests that CSPG4 is directly involved in the neoplastic progression [18, 24, 29, 41, 44–47]. The best-established implication regards the link between CSPG4 and melanoma progression that was first appreciated as a result of its widespread expression in the majority (85% or greater) of human melanomas [21, 48, 49].
As a consequence of its ability to coordinate several intracellular pathways regulating different cell functions, CSPG4 becomes involved in tumorigenesis at multiple levels [41, 46]. Specifically, the over-expression of CSPG4 could sustain a high proliferative phenotype of tumor cells. By means of its ability to act as co-receptor, binding to and presenting growth factors to their cognate RTK, CSPG4 could potentiate the activation of the mitogen-activated protein kinases (MAPK) pathway, resulting in the selective growth and survival advantage of CSPG4-positive tumor cells [50, 51]. Also in those melanomas in which the BRAFV600E mutation determines a constitutive activation of the downstream MAPK pathway positively impacting on cell proliferation, the expression of CSPG4 is required to maximize the tumorigenic effect [52]. CSPG4 over-expression has also been involved in cancer cell progression through the regulation of intracellular pathways implicated in tumor cell adhesion and migration. Several studies have demonstrated that integrin-mediated signaling can be super-activated in the presence of CSPG4 over-expression, as compared to stimulation of integrins alone [52–54]. By binding ECM molecules and interacting with integrins, CSPG4 enhances integrin activation leading to the formation of a complex with signal transduction molecules such as focal adhesion kinase (FAK), which is a key factor for initial cell spreading [55]. Through a FAK-independent mechanism, CSPG4 could increase the activation of ERK 1 and 2 and sustaining cancer cell migration also through this pathway [52]. Activated CSPG4 can as well recruit the tyrosine-phosphorylated p130cas, an adaptor protein involved in the linkage of actin cytoskeleton to the extracellular matrix during cell migration, invasion and transformation [56, 57], contributing in this way to cytoskeletal reorganization and metastatic spread of CSPG4-positive tumor cells. In addition, several studies have demonstrated a role for CSPG4 in mediating multi-drug resistance via stimulation of PI3K/Akt signaling [58, 59]. Finally, it is thus not surprising that CSPG4 is overexpressed on CIC [46, 60] and tumor-derived exosomes [61] that are emerging as major players in cancer development and progression, contributing to recurrences, metastasis formation and chemoresistance [62–64].
CSPG4 oncoantigen: a new star on the stage of comparative oncology
In recent years, the study of naturally occurring tumors in pet animals as models of human cancer, i.e. the comparative oncology, has been on the rise. Indeed, the difficulties in translating into the clinics several immunotherapeutic strategies found to be effective in rodents [6, 7] prompted the National Cancer Institute’s Center for Cancer Research [8] and, later on, a European Group [10] to launch the Comparative Oncology Program in order to find more informative pre-clinical models. In particular, the long history of dogs in biomedical research, their strong anatomical and physiological similarities to humans and the high number of pet dogs that are diagnosed with cancer and managed therapeutically, make these companion animals an attractive comparative model. Canine tumors mimic the progression of human malignancies better than any other pre-clinical model available so far since they grow over long periods of time following the natural evolution of human tumors, give rise to recurrences and metastases, and provide similar response to conventional therapies [9, 65, 66]. As a result, the study of spontaneous tumors developing in dogs as models for human malignancies is a priceless translational tool for accelerating the development of novel immunotherapeutic strategies with a substantial impact on the management of both canine and human oncological patients. Several canine tumors are “under the microscope” of comparative oncology for their translational relevance; among them there are lymphosarcomas, mammary carcinomas, melanomas and osteosarcomas [9, 67–70].
At the light of the comparative oncology concepts and considering the biological role of the CSPG4 oncoantigen in human tumors and its high conservation in structural and functional properties through phylogenetic evolution, the identification of CSPG4 in canine tumors could represent an unprecedented opportunity to pre-clinically test anti-CSPG4 immunotherapies in the veterinary field, highly predictive of their clinical efficacy in the human oncology. With this in mind and considering the well-known link between CSPG4 expression and melanoma progression in humans, other than the urgent need of effective strategies for the treatment of both human and canine melanomas [71–73], we were the first to evaluate CSPG4 expression in canine malignant melanomas [74]. About 60% of canine melanomas stained positive for CSPG4. The staining was mostly restricted to the tumor cell membrane with a different grade (or score) of expression in the different subtypes, being higher in epithelioid and in the more aggressive amelanotic phenotype. These findings labeled CSPG4 as a new potential marker for canine malignant melanoma diagnosis and as a promising candidate antigen for translational immunotherapy studies in dogs.
Moreover, we have evaluated the presence of CSPG4 on different cell lines generated from three canine melanoma patients: one named OLGA, generated starting from the bioptic material obtained from a metastatic lymph node, and two lines named CMM9 and CMM10, derived from primary oral melanomas. CSPG4 expression on tumor cell membrane has been confirmed by flow cytometry analysis in all the canine melanoma cell lines analyzed (unpublished data, Fig. 2a), thus representing an interesting tool for the in vitro study of CSPG4 in a canine melanoma model. Besides, since in human tumors CSPG4 expression has been associated with the highly tumorigenic CIC subpopulation [46], we evaluated the expression of CSPG4 also on CIC-enriched melanospheres obtained from canine cell lines. Preliminary results suggest a CSPG4 over-expression at mRNA level in the first two in vitro passages of OLGA-derived melanospheres (P1 and P2) as compared to the epithelial counterpart (unpublished data, Fig. 2b), confirming CSPG4 as an attractive immunotherapeutic target potentially effective against recurrences and metastases in dogs.
Fig. 2.
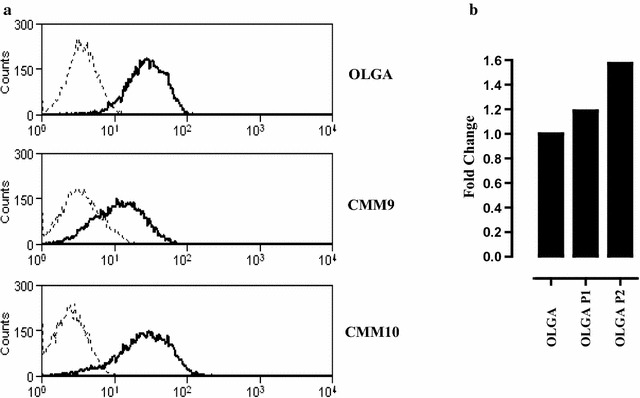
CSPG4 expression in canine melanoma cells and derived-CIC. a CSPG4 expression levels in three canine melanoma cell lines: OLGA [121], CMM9 and CMM10 (kind gift from Dr. Sasaki Nobuo and Dr. Nakagawa Takayuki, Laboratory of Veterinary Surgery, University of Tokyo, Japan). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 50 U/mL penicillin, and 50 μg/mL streptomycin (both from Invitrogen) in humidified incubator at 37 °C under 5% CO2. 2 × 105 cells were incubated with a mix of CSPG4-specific mAb (225.28, VF4-TP108, VF20-TP108 and VF20-VT20; kindly provided by Prof. Soldano Ferrone, Massachusetts General Hospital, Boston, MA, USA) for 1 h at 4 °C. After washing with PBS, cells were incubated with a FITC-conjugated anti-mouse secondary antibodies for 30 min at 4 °C. Flow cytometry was performed with a CyAn ADP (DakoCytomation) and the results were analyzed with Summit 4.2 (DakoCytomation) software. Black lines show CSPG4 expression, while dotted grey lines show the background of cells stained with FITC-conjugated anti-mouse secondary antibody alone. A representative staining of three independent experiments is reported. b For CIC-enrichment, epithelial melanoma cells were detached by using non-enzymatic and mechanical dissociation and plated in ultra-low-attachment flasks at 6 × 104 viable cells/mL in serum-free DMEM-F12 medium supplemented with 20 ng/mL basic fibroblast growth factor (FGF), 20 ng/mL epidermal growth factor (EGF), 5 µg/mL insulin, and 0.4% bovine serum albumin (BSA). Non-adherent spherical clusters of cells (P1), were collected after 7 days and disaggregated using non-enzymatic and mechanical dissociation. P1-derived single-cell suspensions were seeded again at 6 × 104 viable cells/mL to generate non-adherent spherical clusters of cells (P2). 1 μg of RNA extracted from OLGA, P1-OLGA and P2-OLGA was retrotranscribed using RETROscript™ reagents (Ambion) and qPCR was carried out using gene-specific primers (Qiagen). Data were analyzed using SDS software 2.3 (Applied Biosystems). Relative CSPG4 gene expression was quantified using the threshold cycle (CT) value and normalized to housekeeping RNA18S. Relative expression of CSPG4 gene in the P1-OLGA and P2-OLGA compared with OLGA epithelial cells was calculated according to the method of Fold Change (2−(DeltaDelta CT)). Results representative of one out of three independent experiments is reported
Finally, as already shown for human tumors, it is likely that CSPG4 is expressed by other canine tumor histotypes besides malignant melanoma. Indeed, our immunohistochemical analysis of CSPG4 expression performed on a cohort of 29 canine osteosarcoma patients, revealed 23 (79%) positive and 6 (21%) negative cases. Particularly, the positivity score assigned as previously described [74] among the 23 CSPG4-expressing osteosarcomas was distributed as follow: 8 with a score of 8 (34.8%), 5 with a score of 7 (21.7%), 5 with a score of 6 (21.7%), 3 with a score of 5 (13.0%) and 2 with a score of 4 (8.7%). These preliminary data suggest a widespread expression of CSPG4 in appendicular osteosarcomas (unpublished data, Fig. 3), the most common primary canine malignant bone tumor [75].
Fig. 3.
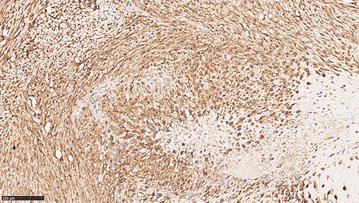
CSPG4 expression in canine osteosarcoma. Tissue samples from 29 canine osteosarcomas collected at the Diagnostic Laboratory of the Department of Animal Pathology of the University of Turin were examined. Data regarding breed, sex, age, tumor localization and clinical TNM staging were available for all dogs. The sample was fixed in 4% neutral buffered formalin, embedded in paraffin, and sectioned at 4 µm. Immunohistochemical analysis for CSPG4 was performed as previously described [74]. Briefly, sections were exposed to high-temperature antigen unmasking by incubation at 98 °C with citric acid buffer, pH 6.0. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 30 min at room temperature. Tissue sections were incubated for 12 h at room temperature with a polyclonal anti-CSPG4 antibody (diluted 1:40, Sigma Aldrich), then 30 min with biotinylated-secondary antibody (Vectastain Elite ABC) and revealed with the ImmPACT DAB kit for peroxidase. A total score considering the proportion of positively stained tumor cells and the average staining intensity was assigned as previously described [74]. Briefly, the score indicating the positivity of tumor cells was assigned as follow: 0 (none); 1 (<1/100 or <1%); 2 (1/100–1/10 or 1–10%); 3 (1/10–1/3 or 10–30%); 4 (1/3–2/3 or 30–70%); and 5 (>2/3 or >70%). The score representing the estimated average staining intensity of positive tumor cells encompass 0 if none, 1 weak, 2 intermediate, 3 strong. The two scores were then added to each other to obtain a final score of CSPG4 expression ranging from 2 to 8. A representative image from a canine appendicular osteosarcoma is shown. Neoplastic cells are characterized by diffuse and strong cytoplasmic and membrane immunolabeling for CSPG4 and the total expression score is 8, resulted by the sum of the percentage of positive cells (=5) and the staining intensity (=3). Magnification 20X
All together, these findings highlight the great value of CSPG4 as a translational immunotherapeutic target in veterinary clinical practice, not only for melanoma but also for other tumor types.
CSPG4 immune-targeting
The development of anti-cancer therapies directed against CSPG4 represents an unprecedented opportunity to simultaneously target tumor cells, CIC and pericytes on tumor vasculature. Moreover, since CSPG4 orchestrates multiple intracellular signaling pathways [35], its targeting could concurrently impair different oncogenic features of tumor cells. For these reasons several immunotherapeutic approaches against CSPG4 for the treatment of melanoma and other CSPG4-expressing tumor histoypes have been tested both in pre-clinical and clinical settings (Table 1).
Table 1.
CSPG4 pre-clinical and clinical studies cited in the text
| References | Cancer type | Methods/therapy | Study phase | Principal evidences |
|---|---|---|---|---|
| Burns et al. [86] | Melanoma | CAR-T cells generated from mAb 225.28S | Pre-clinical, in vitro | CAR-T cells are reactive against CSPG4-expressing cells and explanted human melanomas |
| Geldres et al. [85] | Melanoma, HNSCC, BC | CAR-T cells generated from mAb 763.74 | Pre-clinical, in vitro and in vivo | CAR-T cells are cytotoxic against a variety of CSPG4-expressing cells and inhibit tumor growth |
| Beard et al. [87] | GB, mesothelioma, BC, osteosarcoma, melanoma, GB-derived CIC | CAR-T cells generated from mAb 225.28S, TP41.2, 149.53 and G71.1 | Pre-clinical, in vitro | CAR-T cells demonstrate cytokine secretion and cytolytic function |
| Schmidt et al. [89] | Melanoma | CAR-T cells generated with 61scFv | Pre-clinical, in vitro and in vivo | CAR-T cells specific for CD20+CSPG4+ cells induce tumor eradication through targeted elimination |
| Erfurt et al. [91, 92] | Melanoma | CD4+ T cell isolated from healthy donors and patients | Pre-clinical, in vitro | Identification of CSPG4 peptide-specific CD4+ T cells reactive against melanoma cells |
| Rivera et al. [45] | Mesothelioma | mAb TP41.2 | Pre-clinical, in vitro and in vivo | mAb treatment inhibits adhesion, motility, invasiveness of cancer cells and tumor growth |
| Wang et al. [46] | TNBC | mAb 225.28 | Pre-clinical, in vitro and in vivo | mAb treatment inhibits adhesion and migration of cancer cells and tumor recurrences/metastasis |
| Poli et al. [95] | GB | Combinatorial treatment with mAb9.2.27 and NK cells | Pre-clinical, in vivo | Combination treatment inhibits tumor growth through immunological mechanisms |
| de Bruyn et al. [98] | Melanoma | Bifunctional fusion protein between mAb 9.2.27 and soluble human TRAIL | Pre-clinical, in vitro and in vivo | Bifunctional fusion protein induces the apoptosis of cancer cells and the inhibition of tumor growth |
| Bluemel et al. [99] | human-CSPG4 transected CHO cells | Different mAb for the generation of CSPG4/CD3-bispecific antibodies (BiTE) | Pre-clinical, in vitro | BiTE antibodies redirect the lysis of CSPG4+ cells according to the position of epitope binding domains |
| Torisu-Itakura et al. [100] | Melanoma | CSPG4/CD3-bispecific BiTE | Pre-clinical, in vitro | BiTe antibodies redirect the lysis of melanoma cells engaging patient-derived T cells |
| Amoury et al. [101] | TNBC | CSPG4-specific single-chain mAb 9.2.27 fragment fused to MAP tau | Pre-clinical, in vitro and in vivo | Fusion construct induces cytotoxic effects on TNBC cancer cells and the inhibition of tumor growth |
| Chekenya et al. [58] | GB | CSPG4 sh-induced KD | Pre-clinical, in vitro and in vivo | CSPG4 is associated with multi-drugs resistance and tumor growth through α3β1 integrin/PI3 K signaling |
| Yu et al. [102] | Melanoma | mAb 225.28 | Pre-clinical, in vitro | mAb treatment enhances the in vitro efficacy of Braf-mediated inhibition of cancer cells |
| Mittelman et al. [104] | Melanoma | Vaccination with mouse anti-idiotypic mAb MF11-30 | Clinical, in vivo | MF11-30 is safe, immunogenic and induces minor response in stage IV melanoma patients |
| Mittelman et al. [105] | Melanoma | Vaccination with mouse anti-idiotypic mAb MK2-23 | Clinical, in vivo | MK2-23 is immunogenic and induces survival prolongation and metastasis regression |
| Wang et al. [106] | Melanoma | Vaccination with mouse anti-idiotypic mAb MK2-23 conjugated to IL-2 | Pre-clinical, in vivo | IL-2 conjugation to MK2-23 is critical to induce an effective humoral and cellular response |
| Riemer et al. [107] | Melanoma | Vaccination with mAb 225.28-selected mimotope fused with streptococcal ABP | Pre-clinical, in vitro and in vivo | Mimotope is immunogenic and reactive against CSPG4+ melanoma cells |
| Wagner et al. [108] | Melanoma | Vaccination with mAb 225.28-selected mimotope fused with tetanus toxoid | Pre-clinical, in vitro and in vivo | Mimotope is immunogenic and reactive against CSPG4+ melanoma cells |
| Luo et al. [109] | Melanoma | Vaccination with peptide P763.74 mimicking CSPG4 | Pre-clinical, in vitro and in vivo | P763.74 inhibits melanoma cells migration through immunological and non-immunological mechanisms |
| Piras et al.[96], Riccardo et al. [9] | Melanoma | DNA electrovaccination | Pre-clinical, in vitro and in vivo | Anti-CSPG4 DNA vaccination is immunogenic and clinically effective in canine melanoma patients |
HNSCC head and neck squamous-cell carcinoma, BC breast cancer, GB glioblastoma, TNBC triple negative breast cancer, KD knock-down
One explored strategy in the immunotherapy field, consisting in the stimulation of autologous tumor-specific lymphocytes ex vivo to improve cell mediated immunity before adoptively transferring them back to the patient, were first described almost four decades ago. Since then, several attempts to treat metastatic melanoma were carried out exploiting the adoptive cell transfer (ACT) of autologous lymphocytes derived from the tumor or from the blood of patients [76–80]. Despite the induction of an objective cancer regression in a measurable proportion of treated patients, this strategy has several limitations, including the requirement of pre-existing antitumor-reactive cells that should be expanded ex vivo and the inadequate applicability in other cancer histotypes other than melanoma. The possibility of genetically engineering T cells with conventional T-cell receptors (TCRs) or chimeric antigen receptors (CARs) and consequently redirecting T lymphocytes to recognize and destroy specific TAA has opened up new opportunities for the utilization of ACT to treat different types of cancer patients [81]. Indeed, these T cell-based approaches have been already used in pre-clinical and clinical trials for the treatment of several types of malignancies, including melanoma, myeloma and haematologic malignancies [82–85]. On the wave of these novel approaches, CSPG4 has been considered a suitable target for CAR-T cells. Indeed, several CSPG4-specific CARs have been generated by utilizing monoclonal antibodies (mAbs) reactive against CSPG4 and pre-clinically demonstrated anti-tumor activity against not only melanoma, but also against many other CSPG4-positive cancer histotypes, including breast carcinoma, head and neck squamous cell carcinoma and mesothelioma, as well as against CIC [85–88]. Moreover, Smidth and collaborators interestingly changed the prospective of ACT therapy, demonstrating that the adoptive transfer of CARs directed against CD20 and CSPG4 molecules—co-expressed by less that 2% of melanoma cells—is effective in the eradication of tumor lesions, while the targeting of any other minor subset is less effective [89]. In this way they revealed that melanoma lesions can be efficiently eradicated through the specific T cell-mediated elimination of a definite melanoma cell population, highlighting the fundamental relevance of CSPG4 targeting. Indeed, redirecting T cells to CSPG4 using CARs may represent a robust immunotherapeutic approach to target multiple solid tumors; however, till now, the clinical experience with engineered T cells is limited and some challenges have still to be overcome [90].
The importance of CSPG4 as a promising target for T cell-based immunotherapy has been also supported by Erfurt and colleagues, which demonstrated the presence of a CD4+ T cells reactivity against specific peptides located in the extracellular domain of CSPG4 antigen in the peripheral blood of both healthy donors and melanoma patients, in the absence of clinical signs of autoimmunity. Importantly, these peptide-specific CD4+ T cells could strongly recognize CSPG4 expressing melanoma cells, suggesting that the identified peptides are naturally processed by tumor cells. These findings supported the idea that the activation and the expansion of CSPG4-reactive CD4+ T cells circulating in the blood could be considered for further development of anti-CSPG4 immunotherapeutic strategies [91, 92].
However, since in several malignancies tumor cells have been reported to escape T-cell recognition [93, 94], more and more researchers have focused their attentions on mAb-based therapies and several studies have investigated the potentiality of mAb-based anti-tumor strategies directed against CSPG4. This body of findings solidly demonstrated, in different cancer cells and in various experimental settings, the efficacy of anti-CSPG4 mAb in impairing cancer cell proliferation, migration and invasion. These effects are mediated by multiple mechanisms, including direct mAb activity, such as the induction of CSPG4 down-regulation and a reduced activation of CSPG4-dependent signaling pathways, and mAb dependent recruitment and activation of immune-effector mechanisms [45, 60, 95, 96]. More sophisticated mAb-based approaches, including chimeric anti-CSPG4 antibodies fused to super-antigens, bi- and tri-specific antibodies, or cytolytic fusion protein of CSPG4-specific single-chain antibody fragment genetically fused to microtubule-associated protein (MAP) tau, were also exploited [97–101], confirming the efficacy of the immune-targeting of CSPG4-positive cancer cells. Recent studies have also provided evidences of the positive impact of anti-CSPG4 mAb in improving the efficacy of anti-cancer drugs in glioblastoma and melanoma pre-clinical models, indirectly associating the CSPG4 expression with multidrug resistance in these tumor histotypes [58, 102, 103].
An alternative approach to mAb administration is obviously active immunization, which has the potential to bring about effective and long-lasting anti-tumor responses without significant side effects and the risk of resistance development. First evidences of the effectiveness of active immunization against CSPG4 in melanoma patients were achieved through vaccination with the anti-idiotypic antibody MK2-23, which bears the internal image of the mAb 763.74 against a defined CSPG4 epitope. Interestingly, the induction of CSPG4-specific antibodies in immunized patients was associated with significantly longer survival and metastasis regression [104, 105]. However, this approach never ended up in clinics, due to both the difficulties in standardization of MK2-23 (it has to be conjugated to keyhole limpet hemocyanin as a carrier) and to side effects associated with Bacille Calmette–Guerin administration required to induce an efficient immune response [106]. Alternative strategies evaluating in a pre-clinical setting the impact of the fusion of mAb MK2-23 with IL-2 demonstrated an enhanced immunogenicity of the novel construct and thus the possibility to bypass the requirement for conjugation to a carrier and administration with an adjuvant [106]. Moreover, anti-CSPG4 vaccination was again of interest when the mimotope technology emerged, indeed immunizations with CSPG4 mimotopes resulted in an inhibition of proliferation, migration and invasion of CSPG4-positive melanoma cells through the induction of a specific antibody response responsible for both immunological and non-immunological antitumor functions [107–109].
Overall, these are encouraging data providing a strong rationale for the development of new strategies of active immunization against CSPG4.
DNA vaccination against CSPG4: a strategy for the treatment of canine malignant melanoma with translational implications
Amongst the various possible approaches for active immunization, DNA vaccination has some advantages over other strategies. Among them there are the high stability of DNA and the possibility to generate the vaccine in large amounts in a cheaper and faster manner than other vaccines [110]. DNA vaccines consist in circular DNA constructs (plasmids) that encode one or more TAA. Thanks to its bacterial derivation and to the presence of hypomethylated CpG dinucleotide-containing motifs, the plasmid is able per se to stimulate the innate immune system by interacting with toll-like receptor (TLR)-9 [111] augmenting the antigen-specific immune response. The stimulation of a range of TLR9-expressing cells, including dendritic cells (DC) and B cells, can create an inflammatory milieu for triggering the adaptive immune response [112]. Plasmids administration intradermally, subcutaneously or intramuscularly, results in transfection of resident cells, including DC and other antigen-presenting cells. This allows TAA expression and presentation on both MHC Class I and Class II molecules and stimulation of the cellular and the humoral arms of the immune system against TAA-positive cancer cells (reviewed in [113]).
A large body of studies assessed the efficacy and the safety of the DNA vaccination technique for cancer treatment and prevention in a variety of animal models and even in humans with plasmids encoding different TAA [114–120]. However, overall clinical benefit has been so far limited, despite the high efficacy of DNA vaccines in pre-clinical models [110]. A critical issue linked to the high failure of DNA vaccination approaches in the clinical trials could be related to the way of vaccine administration and design. The intramuscular administration of the DNA vaccine in association with electroporation is one of the most effective way of immunization identified so far [114]. Another major challenge in the development of successful cancer DNA vaccines lies in the fact that most oncoantigens, including CSPG4, are non-mutated and tolerated self-proteins. Therefore, there is the need of identifying a strategy to overcome the immune-tolerance normally existing in patients, in order to induce a proper long-term immune response towards self-antigens. The use of DNA plasmids coding for xenogeneic proteins is one interesting strategy widely investigated in several pre-clinical and clinical studies that have demonstrated its efficacy [121–124]. Moreover, xenovaccination has recently been shown to improve survival in veterinary cancer patients, mainly in dogs affected by spontaneous disease [96, 121, 125, 126]. Positive results obtained in veterinary trials led to the approval by the US FDA of the first xenogeneic DNA vaccine against tyrosinase, ONCEPT (Merial), for the treatment of oral malignant melanomas in dogs [5]. Although the therapeutic efficacy of ONCEPT has been recently questioned [127], its licensing has elicited enthusiasm for this immunization strategy as a potentially effective immunotherapeutic approach. Indeed, the use of a xenogeneic antigen could circumvent the immune tolerance because it preserves a high degree of similarity and, at the same time, is different enough from the targeted self-oncoantigen. Those differences between epitopes of the orthologue and the native protein are responsible for eliciting T and B cell responses against the xenoantigen that may cross-react with the self-target. This makes the DNA vaccine highly immunogenic and consequently more likely effective.
The ability of xenogeneic DNA vaccines to break the immune tolerance and to induce an effective immune response against a self-oncoantigen has been extensively demonstrated also by us [96, 114, 124, 128] and was applied to our anti-CSPG4 vaccine. Indeed, we have recently demonstrated the immunogenicity, safety and therapeutic efficacy of the electroporation of a xenogeneic DNA vaccine coding for the human CSPG4 (Hu-CSPG4) protein in prospectively enrolled client-owned dogs with en bloc surgically resected stage II and III CSPG4-positive spontaneous oral malignant melanoma [96, 121]. The choice to use the human sequence of CSPG4 was due to the high conservation of CSPG4 through evolution, with 82% of homology and 88% of similarity of the Hu-CSPG4 to the canine counterpart (Do-CSPG4). The results obtained in our studies demonstrate the ability of the xenogeneic DNA vaccine against CSPG4 to induce a specific humoral response against the human protein coded by the plasmid and against the canine counterpart. This antibody response relates favorably with the significant prolongation of disease-free and overall survival time in vaccinated dogs with surgically resected malignant melanoma as compared to controls treated with surgery alone [96, 121].
Conclusions
Our results indicate that CSPG4 xenogeneic DNA vaccination associated to electroporation in dogs affected by MM is safe and able to overcome host unresponsiveness to the self-antigen, resulting in significantly increased overall and disease-free survival, thanks to the induction of anti-CSPG4 antibodies. These findings corroborate the clinical impact of CSPG4 immune-targeting for the management of canine melanoma patients. Moreover, the recognition of CSPG4 expression in other aggressive and incurable tumor histotypes may promote the spread of anti-CSPG4 vaccination strategy for the treatment of the wider population of CSPG4-positive tumor affected dogs. Finally, the recognized value of spontaneous tumors in dogs as a priceless model for predicting tumor behavior and response to immunotherapy in humans, highlights the translational potential of active CSPG4 immune-targeting that might be revolutionary for the management of both canine and human CSPG4-positive cancer patients.
Authors’ contributions
VR and GB equally contributed to this paper. FC and FR equally contributed to this paper. FC and FR conducted the background and literature research and drafted the manuscript. FR and RDM performed the experiments on canine MM cell lines. SI performed the IHC analysis on canine osteosarcoma tissues. VR, GB, EQ and PB contributed to the draft of the main text and critically revised the manuscript and provided additional contents. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge financial support from Fondazione Ricerca Molinette Onlus and from Fondazione CRT, Torino, Italy, within the ‘Richieste Ordinarie 2015’ call. FR acknowledges Fondazione Italiana per la Ricerca sul Cancro (FIRC) for the financial support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Funding
This work was supported by grants from Fondazione Ricerca Molinette Onlus and from Fondazione CRT, Torino, Italy, within the ‘Richieste Ordinarie 2015’ call. FR has been supported with a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- TAA
tumor-associated antigens
- FDA
Food and Drug Administration
- CSPG4
chondroitin sulfate proteoglycan-4
- CIC
cancer initiating cells
- HMW-MAA
high molecular weight-melanoma associated antigen
- MCSP
melanoma chondroitin sulfate proteoglycan
- NG2
nerve/glial antigen 2
- bp
base pair
- aa
amino acids
- CS
chondroitin sulfate
- ECM
extracellular matrix
- LGR
laminin G-type regions
- FGF
fibroblast growth factors
- PDGF
platelet-derived growth factor
- ERK
extracellular signal-regulated kinase
- PRR
proline-rich region
- RTK
tyrosine kinase receptors
- MAPK
mitogen-activated protein kinases
- FAK
focal adhesion kinase
- ACT
adoptive cell transfer
- TCRs
T cell receptors
- CARs
chimeric antigen receptors
- mAb
monoclonal antibody
- MAP
microtubule-associated protein
- TLR
toll-like receptor
- DC
dendritic cells
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- BSA
bovine serum albumin
Footnotes
Valeria Rolih, Giuseppina Barutello, Federica Cavallo and Federica Riccardo contributed equally to this work
Contributor Information
Valeria Rolih, Email: valeria.rolih@unito.it.
Giuseppina Barutello, Email: giuseppina.barutello@unito.it.
Selina Iussich, Email: selina.iussich@unito.it.
Raffaella De Maria, Email: raffaella.demaria@unito.it.
Elena Quaglino, Email: elena.quaglino@unito.it.
Paolo Buracco, Email: paolo.buracco@unito.it.
Federica Cavallo, Email: federica.cavallo@unito.it.
Federica Riccardo, Email: federica.riccardo@unito.it.
References
- 1.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelstein B, Kinzler K. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 4.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 6.Kamb A. What’s wrong with our cancer models? Nat Rev Drug Discov. 2005;4:161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- 7.Kamb A, Kamb A, Wee S, Wee S, Lengauer C, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 8.Paoloni MC, Khanna C. Comparative oncology today. Vet Clinics N Am-Small Anim Pract. 2007;37:1023–1032. doi: 10.1016/j.cvsm.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riccardo F, Aurisicchio L, Impellizeri JA, Cavallo F. The importance of comparative oncology in translational medicine. Cancer Immunol Immunother. 2014;64:137–148. doi: 10.1007/s00262-014-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lequarré AS, Andersson L, André C, Fredholm M, Hitte C, Leeb T, et al. LUPA: a European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J. 2011;189:155–159. doi: 10.1016/j.tvjl.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Lollini P-L, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 12.Cavallo F, Calogero RA, Forni G. Are oncoantigens suitable targets for anti-tumour therapy? Nat Rev Cancer. 2007;7:707–713. doi: 10.1038/nrc2208. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. The immune hallmarks of cancer. Cancer Immunol Immunother. 2011;2011:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iezzi M, Quaglino E, Amici A, Lollini PL, Forni G, Cavallo F. DNA vaccination against oncoantigens: a promise. Oncoimmunology. 2012;1:316–325. doi: 10.4161/onci.19127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/A:1025731428581. [DOI] [PubMed] [Google Scholar]
- 18.Campoli M, Ferrone S, Wang X. Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Cancer Res. 2010;109:73–121. doi: 10.1016/B978-0-12-380890-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 19.Pluschke G, Vanek M, Evans A, Dittmar T, Schmid P, Itin P, et al. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/CritRevImmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 22.Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114(Pt 12):2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- 23.Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- 24.Cattaruzza S, Nicolosi PA, Braghetta P, Pazzaglia L, Benassi MS, Picci P, et al. NG2/CSPG4-collagen type VI interplays putatively involved in the microenvironmental control of tumour engraftment and local expansion. J Mol Cell Biol. 2013;5:176–193. doi: 10.1093/jmcb/mjt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooney CA, Jousheghany F, Yao-Borengasser A, Phanavanh B, Gomes T, Kieber-Emmons AM, et al. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011;13:R58. doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushi J-I, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama A, Lin XH, Stallcup WB. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asher RA, Morgenstern DA, Properzi F, Nishiyama A, Levine JM, Fawcett JW. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci. 2005;29:82–96. doi: 10.1016/j.mcn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Keleg S, Titov A, Heller A, Giese T, Tjaden C, Ahmad SS, et al. Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive marker in pancreatic tumors. PLoS ONE. 2014;9:e100178. doi: 10.1371/journal.pone.0100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Y, Makagiansar IT, Fukushi JI, Liu FT, Fukuda MN, Stallcup WB. Molecular basis of interaction between NG2 proteoglycan and galectin-3. J Cell Biochem. 2006;98:115–127. doi: 10.1002/jcb.20768. [DOI] [PubMed] [Google Scholar]
- 31.Stegmüller J, Schneider S, Hellwig A, Garwood J, Trotter J. AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J Neurocytol. 2002;31:497–505. doi: 10.1023/A:1025743731306. [DOI] [PubMed] [Google Scholar]
- 32.Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKC?? helps balance cell proliferation and migration. J Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang X, Burg MA, Barritt D, Dahlin-Huppe K. Nishiyama a, Stallcup WB. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 35.Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, et al. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24:1148–1157. doi: 10.1111/j.1755-148X.2011.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee N, Stegmüller J, Schätzle P, Karram K, Koroll M, Werner HB, et al. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J Biol Chem. 2008;283:8310–8317. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- 38.Midwood KS, Salter DM. Expression of NG2/human melanoma proteoglycan in human adult articular chondrocytes. Osteoarthr Cartil. 1998;6:297–305. doi: 10.1053/joca.1998.0128. [DOI] [PubMed] [Google Scholar]
- 39.Petrini S, Tessa A, Carrozzo R, Verardo M, Pierini R, Rizza T, et al. Human melanoma/NG2 chondroitin sulfate proteoglycan is expressed in the sarcolemma of postnatal human skeletal myofibers: abnormal expression in merosin-negative and Duchenne muscular dystrophies. Mol Cell Neurosci. 2003;23:219–231. doi: 10.1016/S1044-7431(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 40.Kozanoglu I, Boga C, Ozdogu H, Sozer O, Maytalman E, Yazici AC, et al. Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy. 2009;11:527–533. doi: 10.1080/14653240902923153. [DOI] [PubMed] [Google Scholar]
- 41.Nicolosi PA, Dallatomasina A, Perris R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics. 2015;5:530–544. doi: 10.7150/thno.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- 43.Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu NC, Nien PY, Yokoyama KK, Chu PY, Hou MF. High chondroitin sulfate proteoglycan 4 expression correlates with poor outcome in patients with breast cancer. Biochem Biophys Res Commun. 2013;441:514–518. doi: 10.1016/j.bbrc.2013.10.093. [DOI] [PubMed] [Google Scholar]
- 45.Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, et al. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res. 2012;18:5352–5363. doi: 10.1158/1078-0432.CCR-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XX, Wang Y, Yu L, Sakakura K, Visus C, Schwab JH, et al. CSPG4 in cancer: multiple roles. Curr Mol Med. 2010;10:419–429. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- 47.Petrovici K, Graf M, Hecht K, Reif S, Pfister K, Schmetzer H. Use of NG2 (7.1) in AML as a tumor marker and its association with a poor prognosis. Cancer Genom Proteom. 2010;7:173–180. [PubMed] [Google Scholar]
- 48.Wilson BS, Ruberto G, Ferrone S. Immunochemical characterization of a human high molecular weight melanoma associated antigen identified with monoclonal antibodies. Cancer Immunol Immunother. 1983;14:196–201. doi: 10.1007/BF00205360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacomini P, Veglia F, Rehle T, Natali PG, Fei PC, Ferrone S. Level of a membrane-bound high-molecular-weight melanoma-associated antigen and a cytoplasmic melanoma-associated antigen in surgically removed tissues and in sera from patients with melanoma. Cancer Res. 1984;44:1281–1287. [PubMed] [Google Scholar]
- 50.Andres JL, DeFalcis D, Noda M, Massague J. Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J Biol Chem. 1992;267:5927–5930. [PubMed] [Google Scholar]
- 51.Wang J, Svendsen A, Kmiecik J, Immervol H, Skaftnesmo KO, Planagum J, et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS ONE. 2011;6:e23062. doi: 10.1371/journal.pone.0023062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Price MA, Neudauer CL, Wilson C, Ferrone S, Xia H, et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J Cell Biol. 2004;165:881–891. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iida J, Meijne AM, Spiro RC, Roos E, Furcht LT, McCarthy JB. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995;55:2177–2185. [PubMed] [Google Scholar]
- 54.Joo NE, Watanabe T, Chen C, Chekenya M, Stallcup WB, Kapila YL. NG2, a novel proapoptotic receptor, opposes integrin alpha4 to mediate anoikis through PKCalpha-dependent suppression of FAK phosphorylation. Cell Death Differ. 2008;15:899–907. doi: 10.1038/cdd.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 56.Eisenmann KM, McCarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, et al. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- 57.Cabodi S, Tinnirello A, Di Stefano P, Bisarò B, Ambrosino E, Castellano I, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66:4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 58.Chekenya M, Krakstad C, Svendsen A, Netland I, Staalesen V, Tysnes BB, et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signalling. Oncogene. 2008;4157:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svendsen A, Verhoeff JJC, Immervoll H, Brøgger JC, Kmiecik J, Poli A, et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011;122:495–510. doi: 10.1007/s00401-011-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazar I, Clement E, Ducoux-Petit M, Denat L, Soldan V, Dauvillier S, et al. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment Cell Melanoma Res. 2015;28:464–475. doi: 10.1111/pcmr.12380. [DOI] [PubMed] [Google Scholar]
- 62.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 63.Hood JL, San Roman S, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 64.Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6:37151–37168. doi: 10.18632/oncotarget.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 66.Tamburini BA, Trapp S, Phang TL, Schappa JT, Hunter LE, Modiano JF. Gene expression profiles of sporadic canine hemangiosarcoma are uniquely associated with breed. PLoS ONE. 2009;4:e5549. doi: 10.1371/journal.pone.0005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marconato L, Martini V, Aresu L, Sampaolo M, Valentini F, Rinaldi V, et al. Assessment of bone marrow infiltration diagnosed by flow cytometry in canine large B cell lymphoma: prognostic significance and proposal of a cut-off value. Vet J. 2013;197:776–781. doi: 10.1016/j.tvjl.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Rivera P, von Euler H. Molecular biological aspects on canine and human mammary tumors. Vet Pathol. 2010;2011(48):132–146. doi: 10.1177/0300985810387939. [DOI] [PubMed] [Google Scholar]
- 69.Bergman PJ. Canine oral melanoma. Clin Tech Small Anim Pract. 2007;22:55–60. doi: 10.1053/j.ctsap.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Maniscalco L, Iussich S, Morello E, Martano M, Biolatti B, Riondato F, et al. PDGFs and PDGFRs in canine osteosarcoma: new targets for innovative therapeutic strategies in comparative oncology. Vet J. 2013;195:41–47. doi: 10.1016/j.tvjl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Improta G, Leone I, Donia M, Gieri S, Pelosi G, Fraggetta F. New developments in the management of advanced melanoma-role of pembrolizumab. OncoTargets Ther. 2015;8:2535–2543. doi: 10.2147/OTT.S72823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Proulx DR, Ruslander DM, Dodge RK, Hauck ML, Williams LE, Horn B, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44:352–359. doi: 10.1111/j.1740-8261.2003.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 73.Murphy S, Hayes AM, Blackwood L, Maglennon G, Pattinson H, Sparkes AH. Oral malignant melanoma-the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. 2005;3:222–229. doi: 10.1111/j.1476-5810.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 74.Mayayo SL, Prestigio S, Maniscalco L, La Rosa G, Aricò A, De Maria R, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. 2011;190:e26–e30. doi: 10.1016/j.tvjl.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 75.Morello E, Martano M, Buracco P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J. 2011;189:268–277. doi: 10.1016/j.tvjl.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 77.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/00002371-200205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anurathapan U, Chan RC, Hindi HF, Mucharla R, Bajgain P, Hayes BC, et al. Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol Ther. 2014;22:623–633. doi: 10.1038/mt.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2015;30:157–167. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Geldres C, Savoldo B, Hoyos V, Caruana I, Zhang M, Yvon E, et al. T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clin Cancer Res. 2014;20:962–971. doi: 10.1158/1078-0432.CCR-13-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burns WR, Zhao Y, Frankel TL, Hinrichs CS, Zheng Z, Xu H, et al. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 2010;70:3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beard RE, Zheng Z, Lagisetty KH, Burns WR, Tran E, Hewitt SM, et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J Immunother Cancer. 2014;2:25. doi: 10.1186/2051-1426-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Geldres C, Ferrone S, Dotti G. Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen receptor-based T-cell immunotherapy of solid tumors. Expert Opin Ther Targets. 2015;19:1339–1350. doi: 10.1517/14728222.2015.1068759. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uslu U, Schuler G, Dörrie J, Schaft N. Combining a chimeric antigen receptor and a conventional T-cell receptor to generate T cells expressing two additional receptors (TETARs) for a multi-hit immunotherapy of melanoma. Exp Dermatol. 2016;25:672–879. doi: 10.1111/exd.13095. [DOI] [PubMed] [Google Scholar]
- 91.Erfurt C, Sun Z, Haendle I, Heirman C, Thielemans K, Der Bruggen V, et al. Tumor-reactive CD4+ T cell responses to the melanoma-associated chondroitin sulphate proteoglycan in melanoma patients and healthy individuals in the absence of autoimmunity. J Immunol. 2007;178:7703–7709. doi: 10.4049/jimmunol.178.12.7703. [DOI] [PubMed] [Google Scholar]
- 92.Erfurt C, Müller E, Emmerling S, Klotz C, Hertl M, Schuler G, et al. Melanoma-associated chondroitin sulphate proteoglycan as a new target antigen for CD4+ T cells in melanoma patients. Int J Cancer. 2009;124:2341–2346. doi: 10.1002/ijc.24235. [DOI] [PubMed] [Google Scholar]
- 93.Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol Lett. 2014;159:55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 95.Poli A, Wang J, Domingues O, Planagumà J, Yan T, Rygh CB, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–1546. doi: 10.18632/oncotarget.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piras LA, Riccardo F, Iussich S, Maniscalco L, Gattino F, Martano M, et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination. Vet Comp Oncol. 2016. [DOI] [PMC free article] [PubMed]
- 97.Tordsson JM, Ohlsson LG, Abrahmsén LB, Karlström PJ, Lando PA, Brodin TN. Phage-selected primate antibodies fused to superantigens for immunotherapy of malignant melanoma. Cancer Immunol Immunother. 2000;48:691–702. doi: 10.1007/s002620050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Bruyn M, Rybczynska AA, Wei Y, Schwenkert M, Fey GH, Dierckx RA, et al. Melanoma-associated chondroitin sulfate proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo. Mol Cancer. 2010;9:301. doi: 10.1186/1476-4598-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torisu-Itakura H, Schoellhammer HF, Sim M-S, Irie RF, Hausmann S, Raum T, et al. Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific BiTE antibody that engages patient-derived T cells. J Immunother. 2011;34:597–605. doi: 10.1097/CJI.0b013e3182307fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amoury M, Mladenov R, Nachreiner T, Pham AT, Hristodorov D, Di Fiore S, et al. A novel approach for targeted elimination of CSPG4-positive triple-negative breast cancer cells using a MAP tau-based fusion protein. Int J Cancer. 2016;139:916–927. doi: 10.1002/ijc.30119. [DOI] [PubMed] [Google Scholar]
- 102.Yu L, Favoino E, Wang Y, Ma Y, Deng X, Wang X. The CSPG4-specific monoclonal antibody enhances and prolongs the effects of the BRAF inhibitor in melanoma cells. Immunol Res. 2011;50:294–302. doi: 10.1007/s12026-011-8232-z. [DOI] [PubMed] [Google Scholar]
- 103.Pucciarelli D, Lengger N, Takacova M, Csaderova L, Bartosova M, Breiteneder H, et al. Anti-chondroitin sulfate proteoglycan 4-specific antibodies modify the effects of vemurafenib on melanoma cells differentially in normoxia and hypoxia. Int J Oncol. 2015;47:81–90. doi: 10.3892/ijo.2015.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mittelman A, Chen ZJ, Kageshita T, Yang H, Yamada M, Baskind P, et al. Active specific immunotherapy in patients with melanoma: a clinical trial with mouse antiidiotypic monoclonal antibodies elicited with syngeneic anti-high-molecular-weight-melanoma-associated antigen monoclonal antibodies. J Clin Invest. 1990;86:2136–2144. doi: 10.1172/JCI114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mittelman A, Chen GZJ, Wong GY, Liu C, Hirai S, Ferrone S. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody mk2-23: modulation of the immunogenicity in patients with malignant melanoma. Clin Cancer Res. 1995;1:705–713. [PubMed] [Google Scholar]
- 106.Wang X, Ko EC, Peng L, Gillies SD, Ferrone S. Human high molecular weight melanoma-associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: enhancement of immunogenicity of anti-idiotypic monoclonal antibody MK2-23 by fusion with interleukin 2. Cancer Res. 2005;65:6976–6983. doi: 10.1158/0008-5472.CAN-04-2328. [DOI] [PubMed] [Google Scholar]
- 107.Riemer AB, Hantusch B, Sponer B, Kraml G, Hafner C, Zielinski CC, et al. High-molecular-weight melanoma-associated antigen mimotope immunizations induce antibodies recognizing melanoma cells. Cancer Immunol Immunother. 2005;54:677–684. doi: 10.1007/s00262-004-0632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wagner S, Hafner C, Allwardt D, Jasinska J, Ferrone S, Zielinski CC, et al. Vaccination with a human high molecular weight melanoma-associated antigen mimotope induces a humoral response inhibiting melanoma cell growth in vitro. J Immunol. 2005;174:976–982. doi: 10.4049/jimmunol.174.2.976. [DOI] [PubMed] [Google Scholar]
- 109.Luo W, Ko E, Hsu JC, Wang X, Ferrone S. Targeting melanoma cells with human high molecular weight-melanoma associated antigen-specific antibodies elicited by a peptide mimotope: functional effects. J Immunol. 2006;176:6046–6054. doi: 10.4049/jimmunol.176.10.6046. [DOI] [PubMed] [Google Scholar]
- 110.Stevenson FK, Ottensmeier CH, Rice J. DNA vaccines against cancer come of age. Curr Opin Immunol. 2010;22:264–270. doi: 10.1016/j.coi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 111.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 112.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8 + T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 113.Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJM. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. doi: 10.1016/S0065-2776(06)90005-4. [DOI] [PubMed] [Google Scholar]
- 114.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in Her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 115.Senovilla L, Vacchelli E, Garcia P, Eggermont A, Fridman WH, Galon J, et al. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology. 2013;2:e23803. doi: 10.4161/onci.23803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herrada AA, Rojas-Colonelli N, González-Figueroa P, Roco J, Oyarce C, Ligtenberg MA, et al. Harnessing DNA-induced immune responses for improving cancer vaccines. Hum Vaccines Immunother. 2012;8:1682–1693. doi: 10.4161/hv.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arigoni M, Barutello G, Lanzardo S, Longo D, Aime S, Curcio C, et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis. 2012;15:305–316. doi: 10.1007/s10456-012-9263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aurisicchio L, Mancini R, Ciliberto G. Cancer vaccination by electro-gene-transfer. Expert Rev Vaccines. 2013;11:1–11. doi: 10.1586/14760584.2013.836903. [DOI] [PubMed] [Google Scholar]
- 119.Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, et al. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144:1098–1106. doi: 10.1053/j.gastro.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 120.Marchini C, Kalogris C, Garulli C, Pietrella L, Gabrielli F, Curcio C, et al. Tailoring DNA vaccines: designing strategies against HER2-positive cancers. Front Oncol. 2013;3. [DOI] [PMC free article] [PubMed]
- 121.Riccardo F, Iussich S, Maniscalco L, Mayayo SL, La Rosa G, Arigoni M, et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res. 2014;20:3753–3762. doi: 10.1158/1078-0432.CCR-13-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan J, Ku GY, Gallardo HF, Orlandi F, Manukian G, Rasalan TS, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun Arch. 2009;9:5. [PMC free article] [PubMed] [Google Scholar]
- 123.Soong RS, Trieu J, Lee SY, He L, Tsai YC, Wu TC, et al. Xenogeneic human p53 DNA vaccination by electroporation breaks immune tolerance to control murine tumors expressing mouse p53. PLoS One. 2013;8:e56912. doi: 10.1371/journal.pone.0056912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cavallo F, Aurisicchio L, Mancini R, Ciliberto G. Xenogene vaccination in the therapy of cancer. Expert Opin Biol Ther. 2014;14:1427–1442. doi: 10.1517/14712598.2014.927433. [DOI] [PubMed] [Google Scholar]
- 125.Alexander AN, Huelsmeyer MK, Mitzey A, Dubielzig RR, Kurzman ID, MacEwen EG, et al. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol Immunother. 2006;55:433–442. doi: 10.1007/s00262-005-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 127.Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol. 2013;11:219–229. doi: 10.1111/vco.12057. [DOI] [PubMed] [Google Scholar]
- 128.Quaglino E, Mastini C, Amici A, Marchini C, lezzi M, Lanzardo S, et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010;70:2604–2612. doi: 10.1158/0008-5472.CAN-09-2548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


