Abstract
Associative learning can enable environmental cues to signal food and stimulate feeding, independent of physiological hunger. Two forebrain regions necessary in cue driven feeding, the basolateral area of the amygdala and the medial prefrontal cortex, communicate via extensive, topographically organized connections. The basolateral nucleus (BLA) sends extensive projections to the prelimbic cortex (PL), and our aim here was to determine if this pathway was selectively recruited during cue-food associative learning. The anterior and posterior basolateral nuclei are recruited during different phases of cue-food learning, and thus we examined whether distinct pathways that originate in these nuclei and project to the PL are differently recruited during early and late stages of learning. To accomplish this we used neuroanatomical tract tracing combined with the detection of Fos induction. To identify projecting neurons within the BLA, prior to training, rats received a retrograde tracer, Fluoro-Gold (FG) into the PL. Rats were given either one or ten sessions of tone-food presentations (Paired group) or tone-only presentations (Control group). The Paired group learned the tone-food association quickly and robustly and had greater Fos induction within the anterior and posterior BLA during early and late learning compared to the Control group. Notably, the Paired group had more double-labeled neurons (FG + Fos) during late training compared to the Control group, specifically in the anterior BLA. This demonstrates selective recruitment of the anterior BLA-PL pathway by late cue-food learning. These findings indicate plasticity and specificity in the BLA-PL pathways across cue-food associative learning.
1. Introduction
Cues that signal food can increase the motivation to procure and consume food in the absence of hunger across species (e.g., Weingarten, 1983; Birch et al., 1989; for reviews see Petrovich & Gallagher, 2003; Holland & Petrovich, 2005; Petrovich, 2013). Environmental cues can gain this ability through associative learning, such as during Pavlovian appetitive conditioning. In this preparation, a neutral cue from the environment (conditioned stimulus, CS) is repeatedly followed by food (unconditioned stimulus, US), which innately evokes feeding behaviors (unconditioned response, UR). The CS then becomes the predictor of the US and ultimately drives the same behaviors (conditioned response, CR). These acquired abilities are well established behaviorally; however, much less is known about the neural plasticity, particularly at a circuit level, that underlies cue-food learning.
The amygdala, specifically the basolateral area, is important for appetitive associative learning and subsequent behaviors (Corbit & Balleine, 2005; Cole et al., 2013; for reviews see Gallagher & Schoenbaum, 1999; Everitt et al., 2003; Holland & Petrovich, 2005; Crombag et al., 2008; Wassum & Izquierdo, 2015), and its function is conceptualized to involve ‘tagging’ biologically relevant incoming stimuli and then informing other brain systems via complex and distributed connectional networks (e.g., Weiskrantz, 1956; Swanson & Petrovich, 1998). The amygdala is a heterogeneous structure (Swanson & Petrovich, 1998), and recent work found that distinct nuclei within the basolateral area (containing the lateral, basolateral [BLA] and basomedial nuclei) were differentially recruited during early and late cue-food learning (Cole et al., 2013). Specifically, the anterior basolateral nucleus (BLAa, Swanson, 2004; also known as the magnocellular division based on its morphology, Savander et al., 1995; Pitkänen et al., 1997) was the only amygdalar nucleus that displayed a significant increase in activation (measured with Fos induction) during early learning, which was maintained throughout training. The posterior basolateral nucleus (BLAp, Swanson, 2004; also known as the parvocellular division based on its morphology, Savander et al., 1995; Pitkänen et al., 1997) was recruited during late training along with other amygdalar nuclei that are connected with the BLAa. These results demonstrate specificity in the recruitment of amygdalar nuclei, and the differential recruitment across early and later learning suggests plasticity within the BLAa and, potentially, with its connectional targets.
The BLA has extensive connections with the medial prefrontal cortex (Kita & Kitai, 1990; Hoover & Vertes, 2007; Reppucci & Petrovich, 2016), which is important for the executive function and control of feeding and other motivated behaviors (Swanson & Petrovich, 1998; Dalley et al., 2004; O’Doherty, 2011). Specifically, the ventromedial prefrontal cortex, including the prelimbic (PL) and infralimbic (ILA) areas, is critical in appetitive cue learning (Ashwell & Ito, 2014; Baldwin et al., 2000, 2002; Burgos-Robles et al., 2013; Cole et al., 2015a; Corbit and Balleine, 2003). This area is necessary for feeding driven by learned food cues (Petrovich et al., 2007; Cole et al., 2015b), can be stimulated to drive food intake (Blasio et al., 2014; Land et al., 2014; Mena et al., 2011) and alters activity in downstream neural regions mediating feeding behaviors (Mena et al., 2013). Furthermore, disruption of the BLA-mPFC pathway attenuates reward-seeking driven by learned contextual and discrete cues (Fuchs et al., 2007; Mashhoon et al., 2010; Stefanik et al., 2013). Nevertheless, the functional connectivity of the BLA-PL pathways has not been investigated during the acquisition of cue-food associations.
Within the medial prefrontal cortex, the BLA most densely innervates the PL, with topographically distinct pathways originating in the BLAa and BLAp (Kita & Kitai, 1990; Hoover & Vertes, 2007; Reppucci & Petrovich, 2016). The BLAa and BLAp are recruited during different phases of cue-food learning (Cole et al., 2013), suggesting that the BLAa-PL and BLAp-PL pathways may also be differently engaged. The goal of the current study was to determine whether the BLA neurons that send direct projections to the PL are selectively activated during cue-food learning and whether distinct pathways that originate in the BLAa and BLAp are differentially recruited during early and late learning of cue-food associations.
2. Methods
In order to identify BLA-to-PL projecting neurons, rats were iontophoretically injected with the retrograde tracer Fluoro-Gold (FG) into the PL. After recovery, rats received either one training session (early learning; S1) or ten training sessions (late learning; S10) of Pavlovian appetitive conditioning. Each training session included eight presentations of a tone CS that for the Paired condition co-terminated with the delivery of two food pellets (US). Rats in the Control group received the CS presentations in the behavioral chambers followed by the US delivery in their home cage at a random interval after each session. The primary measure of learning was the percentage of time rats expressed food cup behavior during the CS. Rats were perfused 90 minutes after the cessation of S1 or S10 session for brain tissue collection. The Control groups did not receive the US on perfusion day. The brain tissue was processed for double-label fluorescence immunohistochemistry for FG and Fos detection (see Supplemental Material for details).
3. Results
3.1. Behavior
During early training (Session 1), the Paired group displayed increasingly more food cup behavior during CSs throughout the session compared to the Control group, signifying learning (Figure 1A). Repeated measures ANOVA (Training group × CS) found a significant effect of CS (F(1,18)=2.713, P<0.05), but no effect of training group (F(1,18)=2.793, P>0.05), or interaction (F(1,18)=1.239, P>0.05). To assess learning during the session, further analysis compared behavior between the first half and the second half of the session (four CSs each). The Paired group displayed more food cup behavior during the last four CSs compared to their responding during the first four CSs (P<0.05) and compared to the Control group (P<0.05; Figure 1B). There were no differences between the groups during the first four CSs (P>0.05) or during pre-CS intervals (P>0.05).
Figure 1.
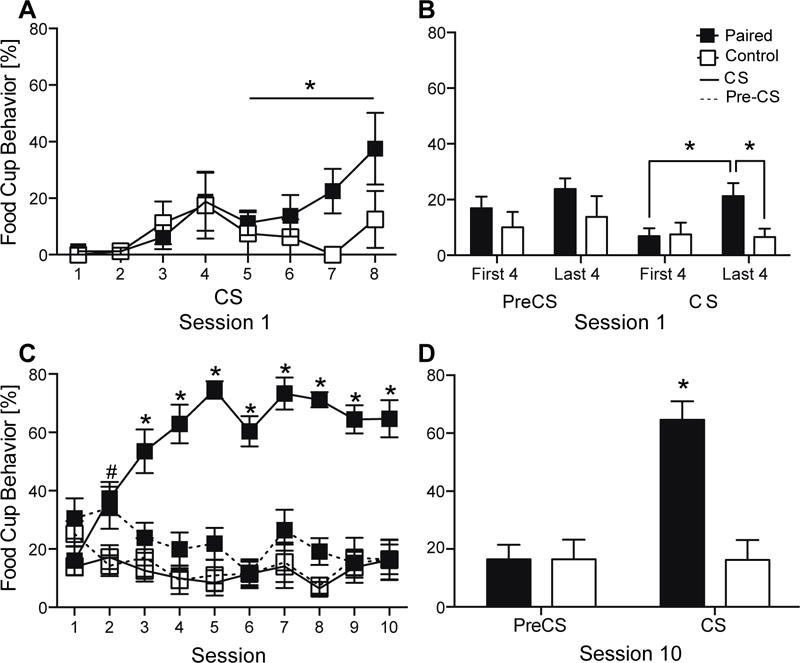
Conditioned responses during training. Percentage of time rats expressed food cup behavior (mean ± SEM) during each CS presentation (A) and during the first and last four pre-CSs and CS (B) during session 1. Expression of food cup behavior during the pre-CS and CS across ten sessions of training (C) and during session 10 (D). *P<0.05; #P<0.05 Paired pre-CS = Paired CS > Control CS.
Over ten sessions of training, the Paired group showed an increase in food cup behavior during the CSs, while the Control group displayed minimal and non-specific food cup behavior throughout training. Repeated measures ANOVA (Training group × Session) revealed a significant effect of training group (F(1,14)=139.018, P<0.0001), a significant effect of session (F(1,14)=6.968, P<0.001) and a significant interaction across sessions (F(1,14)=9.781, P<0.001). During session 2, the Paired group had higher food cup responding compared to the Control group (P<0.05; Figure 1C), but similar responding during the pre-CS and CS intervals (P>0.05). Throughout sessions 3–10, the Paired group showed high responding specifically to the CS compared to their pre-CS responding (P<0.05) and compared to the behavior of the Control group during the CS (P<0.05). During the last session of training (session 10), repeated measures ANOVA (Training group × Time period [CS or pre-CS]) found a significant effect of training group (F(1,14)=8.287, P<0.05), a significant effect of CS vs Pre-CS time period (F(1,14)=63.816, P<0.0001), and a significant interaction (F(1,14)=64.858, P<0.0001). The Paired group showed higher food cup behavior during the CS than the Control group (P<0.001) with no difference in pre-CS behavior between the groups (P>0.05; Figure 1D).
3.2 Neural Analysis
The location and spread of FG injection sites were analyzed throughout the rostro-caudal extent of the prelimbic cortex (PL) based on the Swanson brain atlas (Swanson, 2004). Acceptable injections (see Supplemental Materials) were confined predominantly within the PL (n=36) and were centered within the mid rostro-caudal extent of the PL (Figure 2; Levels 6, 7 and 8; +4.2, +3.6, and +3.2mm from bregma, respectively). The final group numbers were S1 Paired (n=10), S1 Control (n=10), S10 Paired (n=8), and S10 Control (n=8). Importantly, the total numbers of retrogradely-labeled neurons were similar across groups (Figure 3B), confirmed by two-way ANOVAs (Training group × Session) in the BLAa (Training group: F(1,32)=2.477, P>0.05; Session: F(1,32)=0.585, P>0.05) and BLAp (Training group: F(1,32)=0.542, P>0.05; Session: F(1,32)=0.119, P>0.05), signifying that any differences found in the number of double-labeled (FG + Fos) neurons are not due to variances in the number of FG-labeled neurons.
Figure 2.
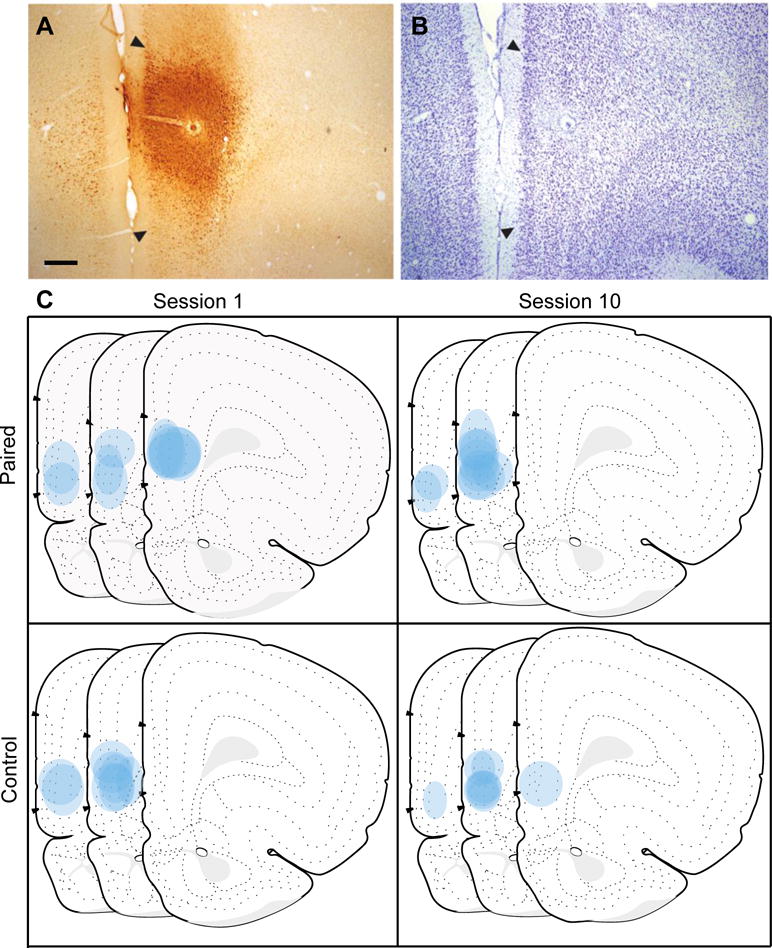
Fluoro-Gold (FG) injection sites in the prelimbic cortex (PL). A photomicrograph of a representative FG injection in the PL (A) with adjacent thionin-stained section (B) used to demarcate PL borders based on a rat atlas (Swanson, 2004). Illustration of all FG injections in the PL for each training group shown on modified Swanson atlas templates (atlas Levels 6, 7 and 8; +4.2, +3.6, and +3.2mm from bregma respectively; C). Scale bar = 100 μm.
Figure 3.
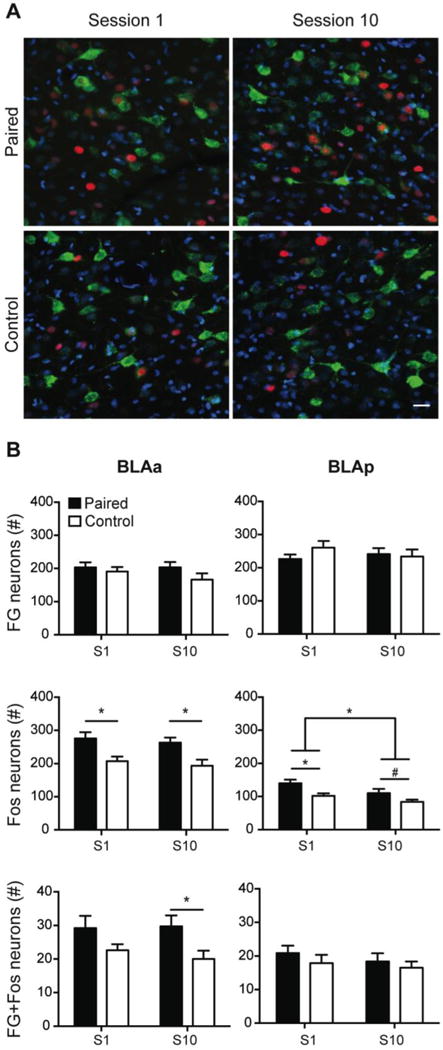
Fos induction in BLA-PL projecting neurons during early and late cue-food learning. Representative images from the BLAa from each training group depicting FG-positive neurons (green), Fos-positive neurons (red), and DAPI, a nuclear counterstain (blue). Scale bar = 25μm (A). Total number of FG-positive neurons, Fos-positive neurons, and double-labeled (FG+Fos) neurons (mean ± SEM) during the first (Session 1; S1) and last (Session 10; S10) training sessions in the BLAa (left) and BLAp (right; B). *P<0.05; #P=0.087.
Representative images of Fos and FG labeled neurons in the BLAa are shown in Figure 3A. Fos induction in the BLA neurons was examined during early (session 1; S1) and late (session 10; S10) tone-food conditioning. Within the BLAa, the Paired group had more Fos-positive neurons than the Control group during S1 and S10 (Figure 3B). The two-way ANOVA (Training group × Session) revealed a significant effect of training group (F(1,32)=16.722, P<0.01), but no effect of session (F(1,32)=0.609, P>0.05), or interaction (F(1,32)<0.000, P>0.05). Post hoc analysis confirmed the Paired group had significantly more Fos-positive neurons than the Control group during S1 (P<0.01) and S10 (P<0.05), replicating previous findings using this protocol (Cole et al., 2013).
There was a similar pattern within the BLAp of higher Fos induction in the Paired group compared to Control group, but there was also an overall decrease in Fos induction across training (Figure 3C). Within the BLAp, a two-way ANOVA (Training group × Session) of Fos induction found a significant effect of training group (F(1,32)=11.279, P<0.01) and a significant effect of session (F(1,32)=6.369, P<0.05), but no interaction (F(1,32)=0.378, P>0.05). The Paired group had more Fos-positive neurons than the Control group during S1 (P<0.001) and a trend towards significance during S10 (P=0.087). Overall, there were more Fos-positive neurons in the S1 groups compared to the S10 groups (P<0.05).
To examine the activation of the BLA-PL pathways, the total number of double-labeled neurons (FG + Fos) within the BLAa and BLAp was quantified (see Supplemental Materials for specifications) and compared across groups and sessions. We found selective Fos induction in the PL projecting BLAa neurons, but not BLAp neurons, in the Paired group during S10. In the BLAa, two-way ANOVA (Training group × Session) of Fos induction in BLAa neurons that project to the PL revealed a significant effect of training group (F(1,32)=7.818, P<0.01), but no effect for session (F(1,32)=0.123, P>0.05), or interaction (F(1,32)=0.290, P>0.05). Post hoc analysis confirmed the Paired S10 group had more double-labeled neurons than the Control S10 group (P<0.05), but a difference between S1 groups was not statistically significant (P>0.05; Figure 3D). In the BLAp, there were no differences in the number of activated projecting neurons between the Paired and Control groups during S1 or S10 (Training group: F(1,32)=1.127, P>0.05; Session: F(1,32)=0.730, P>0.05; Figure 3E).
4. Discussion
In the current study, we examined the functional activation of the BLA-PL pathways during the acquisition of Pavlovian appetitive conditioning. We found significantly more Fos induction in BLAa-to-PL projecting neurons in the Paired group compared to the Control group. This effect was statistically reliable specifically during the late training, but not during the early training. This finding demonstrates recruitment of the BLAa-PL pathway across training, suggesting plasticity during cue-food associative learning. Interestingly, Fos induction in projecting neurons within the BLAp was similar between training groups throughout tone-food conditioning, demonstrating activation of the BLAp-PL pathway was similar in the Paired and Control groups throughout learning. Together, these results show that only the BLAa-PL pathway, but not the BLAp-PL pathway, is activated during well-learned cue-food associations. Additionally, we analyzed total Fos induction in the BLAa and BLAp and found higher overall induction in the Paired groups compared to the Control groups during both phases of learning. This difference between overall activation and the activation of specific BLA-to-PL projecting neurons highlights the importance of identifying how specific neurons are recruited within a critical neural circuitry underlying behavior.
Here, the retrograde tracer injections were aimed at the PL, an area substantially innervated by the BLAa. Our focus was on the BLAa, because that was the only amygdalar cell group recruited during early cue-food training, suggesting it is informing its connectional targets during appetitive conditioning (Cole et al., 2013). Nevertheless, the BLAa and BLAp have distinct connections with the medial prefrontal cortex, and while the BLAa has dense connections with the PL and the anterior cingulate area, the BLAp is connected more heavily with the ILA compared to the PL (Sesack et al., 1989; Kita & Kitai, 1990; Swanson & Petrovich, 1998; Hoover & Vertes, 2007; Little & Carter, 2013; Reppucci & Petrovich, 2016). In accordance, our injections in the PL resulted in labeling and analysis only within the rostral half of the BLAp, and thus the current study did not capture the more substantial projections from the BLAp to the ILA. Given the ILA was also recruited during late learning of cue-food associations, similar to the PL (Cole et al., 2015a), it is possible the BLAp-ILA pathway may be important during appetitive associative learning. Furthermore, the current study found more overall Fos induction in the BLAp (total Fos induction in both projecting and non-projecting neurons) during early and late training in the Paired group, whereas Cole and colleagues (2013) found recruitment of the BLAp only during late learning. A methodological difference in sampling is a potential reason why these results differ. Cole and colleagues (2013) examined the entire extent of the BLAp (the entire dorso-ventral and rostro-caudal area within the nucleus), while in the current study the total Fos was counted within the area of substantial PL projection (only the rostral portion of the BLAp).
In addition to the functional differences found in the current study and aforementioned distinct connections with the mPFC, the BLAa and BLAp also differ in their connections with other forebrain areas. Within the amygdala, the BLAp sends substantial direct pathways to the central amygdala, while the BLAa reaches it indirectly through its connections to the BLAp (Savander et al., 1995; Swanson & Petrovich, 1998). Based on its forebrain connections, the BLAa was characterized as a part of the frontotemporal system, and it projects to the nucleus accumbens and caudoputamen, as well as to the medial frontal and adjacent somatomotor cortical areas (Kita & Kitai, 1990; Swanson & Petrovich, 1998). Importantly, it does not send direct projections to hippocampal formation, the hypothalamus, or the bed nuclei of the stria terminals (Swanson & Petrovich, 1998; Dong et al 2001; Petrovich et al., 2001). The BLAp was characterized as a part of the main olfactory system, and it projects to the nucleus accumbens and the substantia innominata, as well as the hippocampal formation, the hypothalamus, and bed nucleus of the stria terminalis, (Swanson & Petrovich, 1998; Petrovich et al., 2001; Reppucci & Petrovich, 2016).
The findings from the current study and previous work support the notion that the BLAa is a critical early ‘processor’ during appetitive associative learning. Here, we found recruitment of the BLAa during early learning in agreement with Cole and colleagues (2013). The BLAa was the only forebrain region to show selective activation during early learning, while the amygdalar and forebrain targets of its inputs were recruited during late training (Cole et al., 2013, 2015a). Furthermore, electrophysiological recordings also provide evidence that the BLA precedes and influences other cortical processing. Single-unit recordings found that the BLA is activated prior to the activation of the gustatory cortex during palatability processing (Grossman et al., 2008), and BLA inactivation can alter gustatory cortex responses (Piette et al., 2012). This early processing function of the BLA may capture its role in tasks with reward predictive cues, including cue-potentiated eating (Holland et al., 2002), discriminative stimulus responding (Ishikawa et al., 2008), second-order conditioning (Hatfield et al., 1996), devaluation (Hatfield et al., 1996), and Pavlovian to instrumental transfer (Blundell et al., 2001; for review see Wassum & Izquierdo, 2015). The current study suggests that the BLAa processing is relayed to the PL during acquisition, potentially enabling this pathway to later control cue driven reward behaviors. Indeed, inhibition of the BLA-PL pathway decreased conditioned reward seeking (Fuchs et al., 2007; Mashhoon et al., 2010; Stefanik et al., 2013), and BLA inactivation caused a disinhibition of the PL activity during reward seeking, resulting in a deficit in conditioned place preference for morphine (Sun & Laviolette, 2012).
The BLAa is a cortical part of the amygdala (Swanson & Petrovich, 1998), and its output from pyramidal neurons can influence the PL through monosynaptic (McDonald, 1992; Sotres-Bayon et al., 2012) and polysynaptic pathways involving inhibitory interneurons (Perez-Jaranay & Vives, 1991; Gabbott et al., 2006; Floresco & Tse, 2007; Sun & Laviolette, 2012; Dilgen et al., 2013). Inactivation of the BLA decreased PL pyramidal neuron activity, suggesting a monosynaptic pathway (Sotres-Bayon et al., 2012). Alternatively, BLA stimulation increased activity within interneurons, which inhibited PL pyramidal neurons, suggesting a polysynaptic pathway (Dilgen et al., 2013). Through these pathways the BLA input can critically control PL activity, either through excitation or inhibition, and ultimately control behavioral outcomes.
5. Conclusions
In conclusion, we found plasticity and selectivity within the BLA-PL pathways across Pavlovian appetitive conditioning. The BLAa-PL, and not the BLAp-PL, pathway was selectively recruited during cue-food learning and, importantly, this recruitment suggests plasticity in BLAa-PL communication across training. These results suggest the BLA is important during initial appetitive learning, and its communication with the medial prefrontal cortex increases throughout learning as a cue becomes predictive of food to control behavior.
Supplementary Material
Highlights.
Examined BLA-PL pathway activation during early and late cue-food learning
Identified activation of BLA-PL projecting neurons with a retrograde tracer and Fos
Determined BLA-PL pathways differently activated during early and late learning
Specifically the anterior BLA-PL pathway was recruited during late learning
Acknowledgments
We would like to thank Christina Reppucci, Heather Mayer, Marissa Marotta, and Megan Ebner for technical assistance. This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant DK 085721 to GDP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashwell R, Ito R. Excitotoxic lesions of the infralimbic, but not prelimbic cortex facilitate reversal of appetitive discriminative context conditioning: the role of the infralimbic cortex in context generalization. Frontiers in behavioral neuroscience. 2014;8:63. doi: 10.3389/fnbeh.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behavioral Neuroscience. 2000;114(1):84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. Journal of Neuroscience. 2002;22(3):1063–71. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addiction biology. 2014;19(4):652–662. doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforce representation in rats. Journal of Neuroscience. 2001;21(22):9018–26. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ. Prelimbic and Infralimbic Neurons Signal Distinct Aspects of Appetitive Instrumental Behavior. PLoS One. 2013;8(2):1–7. doi: 10.1371/journal.pone.0057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015a;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Scientific reports. 2015b;5 doi: 10.1038/srep16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, Petrovich GD. Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learning & Memory. 2013;20:1–7. doi: 10.1101/lm.031070.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. The Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Bravo R, Müller R. Transient induction of c-fos and c-myc in an immediate consequence of growth factor stimulation. Cancer Surveys. 1985;4(4):655–81. [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dilgen J, Tejeda HA, O’Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. Journal of neurophysiology. 2013;110(1):221–229. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences. 2003;985:233–250. [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. Journal of Neuroscience. 2007;27(8):2045–57. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. European Journal of Neuroscience. 2007;26(2):487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139(3):1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Schoenbaum G. Functions of the amygdala and related forebrain areas in attention and cognition. Annals of the New York Academy of Sciences. 1999;877(1):397–411. doi: 10.1111/j.1749-6632.1999.tb09279.x. [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala–cortical ensembles. The Journal of neuroscience. 2008;28(11):2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforce devaluation effects. The Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feedings by conditioned stimuli. Physiology of Behavior. 2005;86(5):747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain structure & function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155(3):573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Projections to the Frontal Cortex and the Striatum in the Rat. The Journal of Comparative Neurology. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, et al. Medial prefrontal D1 dopamine neurons control food intake. Nature neuroscience. 2014;17(2):248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP, Carter AG. Synaptic Mechanisms Underlying Strong Reciprocal Connectivity between the Medial Prefrontal Cortex and Basolateral Amygdala. The Journal of Neuroscience. 2013;33(39):15333–15342. doi: 10.1523/JNEUROSCI.2385-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacology Biochemistry and Behavior. 2010;96(3):347–353. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain research bulletin. 1992;28(2):179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of Hyperphagia and Carohydrate Intake by μ-Opiod Receptor Stimulation in Circumscribed Regions of Frontal Cortex. The Journal of Neuroscience. 2011;31(9):3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Selleck RA, Baldo BA. Mu-Opioid Stimulation in Rat Prefrontal Cortex Engages Hypothalamic Orexin/Hypocretin-Containing Neurons, and Reveals Dissociable Roles of Nucleus Accumbens and Hypothalamus in Cortically Driven Feeding. The Journal of Neuroscience. 2013;33(47):18540–18552. doi: 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Bravo R, Burckhardt J, Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984;312(5996):716–20. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Annals of the New York Academy of Science. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Perez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain research. 1991;564(1):97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiology & Behavior. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38(1–2):247–89. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Annals of the New York Academy of Sciences. 2003;985(1):251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial Prefrontal Cortex is Necessary for an Appetitive Contextual Conditioned Stimulus to Promote Eating in Sated Rats. The Journal of Neuroscience. 2007;27(24):6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. The Journal of Neuroscience. 2012;32:9981–9991. doi: 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neuroscience. 1997;20(11):517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Structure and Function. 2016;221(6):2937–62. doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361(2):345–68. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical Organization of the Efferent Projections of the Medial Prefrontal Cortex in the Rat: An Anterograde Tract-Tracing Study With Phaseolus vulgaris Leucoagglutinin. Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Frontiers in Behavioral Neuroscience. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Laviolette SR. Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharmacology. 2012;222(4):645–661. doi: 10.1007/s00213-012-2665-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neuroscience. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain A laboratory guise with printed and electronic templates for data, models and schematics. Amsterdam: Elsevier; 2004. [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neuroscience & Biobehavioral Reviews. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten AE. Conditioned cues elicit feeding in sated rats: A role for learning to meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. Journal of Comparative and Physiological Psychology. 1956;49(4):381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


