Abstract
Copper(II) oxide nanoparticles (NPCuO) have many industrial applications, but are highly cytotoxic because they generate reactive oxygen species (ROS). It is unknown whether the damaging ROS are generated primarily from copper leached from the nanoparticles, or whether the nanoparticle surface plays a significant role. To address this question, we separated nanoparticles from the supernatant containing dissolved copper, and measured their ability to damage plasmid DNA with addition of hydrogen peroxide, ascorbate, or both. While DNA damage from the supernatant (measured using an electrophoresis assay) can be explained solely by dissolved copper ions, damage by the nanoparticles in the presence of ascorbate is an order of magnitude higher than can be explained by dissolved copper and must therefore depend primarily upon the nanoparticle surface. DNA damage is time-dependent, with shorter incubation times resulting in higher EC50 values. Hydroxyl radical is the main ROS generated by NPCuO/hydrogen peroxide as determined by EPR measurements; NPCuO/hydrogen peroxide/ascorbate conditions generate ascorbyl, hydroxyl, and superoxide radicals. Thus, NPCuO generate ROS through several mechanisms, likely including Fenton-like and Haber-Weiss reactions from the surface or dissolved copper ions. The same radical species were observed when NPCuO suspensions were replaced with the supernatant containing leached copper, washed NPCuO, or dissolved copper solutions. Overall, NPCuO generate significantly more ROS and DNA damage in the presence of ascorbate than can be explained simply from dissolved copper, and the NPCuO surface must play a large role.
Keywords: Nanoparticles, nano-surfaces, nanotoxicology, DNA damage
Introduction
Copper(II) oxide nanoparticles (NPCuO) are used as antimicrobial agents in textiles (Ren et al. 2009) and paints (Cooney 1995), as catalysts in organic synthesis (Alves et al. 2009), in the oxidation of pollutants (Moshe et al. 2009), and they are also generated from electronics waste. Unfortunately, industrial use of NPCuO represents a potential health and environmental concern because the particles are toxic and mutagenic. While copper ion toxicity is attributed to reactive oxygen species (ROS) generation, (Angelé-Martínez 2014; Gaetke 2014) nanoparticle toxicity mechanisms could differ due to surface chemistry and differences in uptake and distribution at the organismal and cellular levels.
Hydrogen peroxide (H2O2), superoxide (O2•−), hydroxyl radical (•OH), and singlet oxygen (1O2) are common ROS, and their interactions with DNA, proteins, and lipids cause oxidative damage and cell death (Bondarenko et al. 2013; Maurer-Jones et al. 2013). Oxidative DNA damage is the primary cause of cell death and mutation in aging, cancer, neurodegeneration, and cardiovascular disease (Burgess et al. 2012; Cooke et al. 2003; Ide et al. 2001; Keyer et al. 1995; Luijsterburg and Van Attikum 2011). Nanoparticles are internalized into bacteria and human cells where they localize in mitochondria and the nucleus (Cronholm et al. 2013; Wang et al. 2012) and potentially damage DNA. Reviews on nanoparticle toxicity call for immediate research to 1) understand the uptake, metabolism, accumulation, and secretion of nanoparticles; 2) develop predictive toxicity models and classify nanoparticles according to their toxicity; and 3) prevent health issues caused by nanoparticle exposure (Bondarenko et al. 2013; Rim et al. 2013).
NPCuO are among the most toxic nanoparticles (Bondarenko et al. 2013). In a comparative toxicity assay, NPCuO caused significant mitochondrial depolarization (Karlsson et al. 2009) and increased DNA damage compared to carbon nanotubes and nanoparticulate TiO2, ZnO, CuZn, Fe3O4, and Fe3O4 (Karlsson, Cronholm, et al. 2008). Many factors influence NPCuO toxicity, including pH, exposure time, dose, zeta potential, solubility, size, porosity, morphology and surface area (Cho et al. 2012; Grassian 2008; Karlsson et al. 2009; Luyts et al. 2013). Although a few reports indicate minimal toxicity upon NPCuO exposure under certain conditions (Karlsson, Cronholm, et al. 2008; Karlsson et al. 2009; Wang et al. 2012), NPCuO are more toxic to cells than bulk CuO (Wang et al. 2012) or polymeric CuO (Thit et al. 2013).
NPCuO can generate DNA-damaging ROS by two primary mechanisms: at the nanoparticle surface or in solution by copper dissolved from the nanoparticle surface. In both cases, the site of ROS generation must be in close proximity to damage DNA due to the short lifetimes of these ROS. Although these two mechanisms are known (Karlsson, Cronholm, et al. 2008; Studer et al. 2010), the amount of damage contributed by each component and the details that control these mechanisms are not well understood.
Dissolved copper ions are reportedly more toxic to aquatic organisms than the same number of copper atoms in a copper oxide nanoparticle (Blinova et al. 2010; Bondarenko et al. 2013; Jo et al. 2012) since many copper atoms reside within the particle core. Nonetheless, NPCuO are highly toxic, in part because the large surface-area-to-volume ratio allows rapid copper dissolution from NPCuO, especially compared to bulk CuO (Bondarenko et al. 2013; Kasemets et al. 2009; Shi et al. 2011), and because the NPCuO surface can also generate ROS (Cho et al. 2012). In a Trojan horse effect (Wang et al. 2012), NPCuO uptake results in orders-of-magnitude greater copper uptake and accumulation in mammalian cells and correspondingly greater DNA damage and cell death than for dissolved copper (Cronholm et al. 2013). NPCuO uptake depends strongly upon nanoparticle size and surface chemistry, including binding and adsorption to biomolecules (Maurer-Jones et al. 2013). Generally, smaller nanoparticles are more toxic, due to a combination of increased surface area, increased copper dissolution rates, and/or increased nanoparticle uptake (Karlsson et al. 2009). Increased toxicity with decreased size is observed in crustaceans (Blinova et al. 2010) and duckweed treated with NPCuO and bulk CuO (Shi et al. 2011).
Most research on NPCuO toxicity has been performed in bacteria and mammalian cells or whole organisms to examine cell growth inhibition, DNA damage, and apoptosis. No in vitro studies have directly assessed the chemical mechanisms of NPCuO-induced toxicity. Our in vitro analysis of NPCuO-mediated DNA damage focuses specifically on oxidative DNA damage as an endpoint, directly relating to mechanisms responsible for mutagenesis, oncogenesis, and cell-death processes, without confounding effects from cellular oxidative stress responses, nanoparticle internalization processes, and adsorption of cellular molecules. This work presents the analysis of DNA damage caused by NPCuO and its undissolved (wCuO) and dissolved (lCuO) fractions in the presence of H2O2 and/or ascorbate to determine the damaging effects of NPCuO, dissolved copper, and NPCuO surface reactions. Electron paramagnetic resonance (EPR) spectroscopy was used to detect ROS generation by NPCuO or dissolved copper in the presence of H2O2 and/or ascorbate. Our results indicate that NPCuO and dissolved copper generate ROS by different mechanisms and that the NPCuO surface plays a significant role in ROS generation.
Materials and Methods
Materials
Water was purified using a Barnstead NANOpure DIamond Life Science water deionization system. 3-Morpholinopropane-1-sulfonic acid (MOPS; Alfa Aesar), CuSO4 (Fisher), L-(+)-ascorbic acid (99+%; Alfa Aesar), Chelex 100 resin (Sigma-Aldrich), and disodium dihydrogen ethylenediaminetetraacetate (EDTA; TCI America) were used as received. CuO nanoparticles (50% weight, U1121W Nanophase Technologies Corporation, distributed through Alfa Aesar/Sigma-Aldrich) were used as received to prepare diluted suspensions. These particles were selected because they are formed by plasma oxidation of copper, which provides a high-purity product, and the same particles were used in several toxicity assays (Kartal et al. 2009; Selvakumar and Suresh 2012) and in studies of heat transfer fluids (Selvakumar and Suresh 2012; Vajjha et al. 2010). The NPCuO suspensions also contained a proprietary dispersant added by the manufacturer. Microcentrifuge tubes were rinsed in 1 M HCl, triply rinsed in deionized H2O, and dried prior to use. Buffered solutions were treated with Chelex resin (2 g/80 mL buffer) for 24 h prior to use. CuSO4 and ascorbate solutions were prepared prior to each experiment and used immediately.
Characterization of CuO nanoparticles
Transmission electron microscope (TEM) images of NPCuO were acquired using a Hitachi TEM H7600 microscope under 115 kV and 300,000× direct magnification. The NPCuO crystal domain size was calculated from its X-ray diffraction spectrum measured by a Rigaku Ultima IV X-ray diffractometer with Kα1(Cu) radiation with a tube voltage and current set at 40 kV and 40 mA, respectively. The average hydrodynamic diameter and zeta potential of NPCuO in MOPS (pH 7) buffer and deionized water were determined using dynamic light scattering with a Malvern Zetasizer Nano ZS instrument.
Determination of dissolved copper using the bathocuproine method
NPCuO (50% wt. in water) was diluted in MOPS buffer (35 mM, pH 7) to make 5 mM NPCuO. The suspension was sonicated for 5 min, centrifuged (13000 rpm/~18000 g RCF for 10 min), and the leachate was separated. The leachate was centrifuged at least three times to ensure NPCuO were removed, and then diluted 10× before mixing with Cu(II) standards (1:1 ratio) and bathocuproine reagents (Eaton et al. 2001) with a scale-down ratio of 3/50. The resulting orange copper-bathocuproine complex absorbance was measured in triplicate using an Agilent 8453UV-vis spectrophotometer. The concentration of dissolved copper in the NPCuO leachate was determined using standard addition with Cu(II) standard solutions of 0.5, 0.25, 0.125, and 0.0625 mg/L (Tables S1, S2 and Figure S1). The bathocuproine method was validated using flame atomic absorption spectroscopy, which gave results for several samples within 10%.
Transfection, amplification, and purification of plasmid DNA
Plasmid DNA (pBSSK) was purified from E. coli strain DH1 using a PerfectPrep Spin kit (Fisher), then dialyzed at 4 °C against EDTA (1 mM) and NaCl (50 mM) for 24 h and then against NaCl (130 mM) for 24 h to remove metal ions. Absorbance ratios for DNA solutions were A250/A260 ≤ 0.95 and A260/A280 ≥ 1.8.
Plasmid DNA damage assays with NPCuO, ascorbate and H2O2
A solution containing NaCl (130 mM), MOPS (pH 7, 10 mM), and ethanol (10 mM) as a radical scavenger (Henle et al. 1999) was combined with NPCuO, lCuO, or wCuO (1.0 – 1000 μM) and ascorbate (0.00125 – 1250 μM) as indicated in Table 1. MOPS buffer was used since it does not chelate copper, and 1.25 molar equivalents of ascorbate were used to ensure that all Cu2+ was reduced to •OH-generating Cu+. Buffer pH was essentially unaffected even at the highest ascorbate concentrations. After 5 min, plasmid DNA (pBSSK, 0.1 pmol in 130 mM NaCl) was added, and the solution was allowed to stand for 5 min before H2O2 (50 μM) addition to give a 10 μL total volume. After 30 or 150 min, EDTA (200 mM, 0.5 μL) and loading dye (2 μL) were added. Dissolved copper gels were performed with CuSO4 solutions instead of NPCuO suspensions.
Table 1.
Concentrations required to cause 50% DNA damage (EC50, μM) for solutions of CuO nanoparticles (NPCuO), washed nanoparticles (wCuO), leachate of NPCuO (lCuO), and dissolved (free) copper (values in parentheses; μM)
| Component | 150 Minutes | 30 Minutes | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| H2O2 | Ascorbate (1.25 equiv) | H2O2 + Ascorbate (1.25 equiv) | Other Conditions | H2O2 | Ascorbate (1.25 equiv) | H2O2 + Ascorbate (1.25 equiv) | |
| NPCuO | 324 ± 29 (1.54) | 39 ± 3 (0.13–0.39)b | 27.8 ± 0.5 (0.09–0.28)b | 52 ± 3a (0.17–0.53)b | > 1000 | ND | 223 ± 60 |
| wCuO | ND | 170 ± 27 (0.22–0.82)b | 69 ± 20 (0.09–0.34)b | - | ND | 253 ± 8 (0.33–0.45)b | 318 ± 37 (0.41–0.57)b |
| lCuO | ND | ND | 321 ± 30 (1.53) | 690 ± 130c (3.3) | ND | > 1000 | 434 ± 83 (2.1)d |
| Cu2+ | 1.5 ± 0.1 | 5.3 ± 0.2 | 1.6 ± 0.2 | 2.3 ± 0.2d | 4.4 ± 0.1 | 10.3 ± 0.9 | 2.3 ± 0.2 |
Constant ascorbate concentration (50 μM), no H2O2.
A range is observed because copper concentrations change during these experiments.
Ascorbate concentration was ~250× the concentration of dissolved copper in lCuO.
Cu2+, ascorbate, and H2O2 were added to DNA samples with lCuO from which the dissolved copper was removed; lCuO concentration corresponded to the same dilution factor for 1000 μM NPCuO.
ND = not determined.
Gel electrophoresis was run on a 1% agarose gel in TAE buffer for 60 min at 140 V to separate nicked (damaged) and supercoiled (undamaged) plasmid DNA. Gels were stained with ethidium bromide for 5 min and washed in water for an additional 10 min before imaging under UV light. Intensities of the damaged and undamaged DNA bands were quantified using UVIproMW software (Jencons Scientific, Inc.). Ethidium bromide stains supercoiled DNA less efficiently than nicked DNA, so supercoiled DNA band intensities were multiplied by 1.24 prior to comparison (Hertzberg and Dervan 1982). Intensities of the nicked and supercoiled bands were normalized for each lane so that % nicked + % supercoiled = 100 %.
CuO nanoparticle treatment for plasmid DNA damage assays
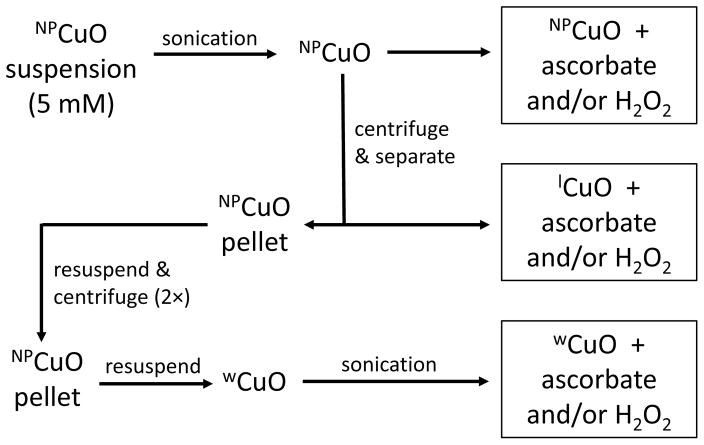
Separation of undissolved and dissolved fractions of NPCuO is described in Figure 1. Briefly, freshly prepared NPCuO stock solution (5.0 mM in MOPS buffer) was sonicated for 10 min. An aliquot (4 mL) of the NPCuO suspension was centrifuged (13000 rpm, ~18000 g, 10 min) to separate the leachate (lCuO) from the solid. The leachate was removed, and the solid was resuspended in deionized water (at the same volume as the lCuO) and centrifuged again. The supernatant was discarded, and the wCuO were resuspended in deionized water and re-sonicated (5 min). All fractions (NPCuO, lCuO, and wCuO) were diluted based upon the original concentration of NPCuO (5.0 mM) and shaken for three seconds to ensure homogeneity before use in DNA damage assays.
Figure 1.
Flowchart illustrating separation of NPCuO components to evaluate DNA damage. NPCuO: whole suspension of CuO nanoparticles, wCuO: washed CuO nanoparticles, lCuO: leachate of CuO nanoparticles.
Removal of dissolved copper from the leachate of CuO nanoparticles (lCuO)
CuO nanoparticles were separated from the suspensions by centrifugation at 14,000 rpm (30,074 RCF) for 45 min. The supernatant was removed and re-centrifuged ~10 times to ensure complete removal of NPCuO. A saturated (NH4)2CO3 solution (200 μL) was mixed with NPCuO supernatant (1 mL), and the resulting mixture was agitated for ~1 min using a vortex mixer. The deep-blue-colored solution was then heated until most of the dissolved copper precipitated, and the supernatant was separated by filtration (Europe 25 mm syringe filter with a 0.2 μm PTFE membrane). Any remaining dissolved copper was removed by treating the supernatant with Chelex resin for 24 h.
Statistical Analysis
Percent DNA damage was plotted with respect to NPCuO, lCuO, wCuO, or Cu2+ concentrations on a semi-log plot and fit to a sigmoidal dose-response curve with maximum damage set to 100%. Data are reported as average values with standard deviations from three independent experiments. EC50 values were calculated by fitting all points of three trials with a single curve (the mean of the EC50 fits from each trial gives similar results to the pooled data, 0–3% difference, but the pooled data should be less sensitive to noise). EC50 value standard deviations were calculated from the three trials’ individual EC50 values. Data in Table S17, line 7 represent the average of two values, since the third gel showed an outlier value and was discarded. The relative standard deviation for the EC50 results was around 11% (average for 20 experiments with reported EC50) and the largest relative standard deviation was 28%. Since the triplicate studies used for calculating standard deviation were performed at close to the same time, uncertainty may be larger in comparing different reaction conditions acquired at different times. Finally, for some curve shapes, the three-parameter fit can be especially sensitive to single points and there are cases where the standard deviation of three trials may underestimate the noise. Based upon these considerations, we consider that the standard deviations somewhat overestimate the accuracy, and we generally do not consider average EC50 differences of < 33% to be significant and chemically important.
Electron paramagnetic resonance (EPR) spectroscopy
EPR spectra were acquired on a Bruker EMX spectrometer using a quartz flat cell at room temperature using a 2,2-diphenyl-1-picrylhydrazyl (DPPH; g = 2.0036 (Mani et al. 2004)) standard centered at 3500 G with a sweep width of 100 G. The modulation amplitude was between 0.50 and 1.00 G, time and conversion constants were 81.92 s; and microwave power and frequency were 20.02 mW and 9.752 GHz, respectively. Samples (500 μL) were prepared in a MOPS buffer solution (10 mM, pH 7) containing NPCuO, wCuO, or lCuO (300 μM) with ascorbate (375 μM), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO, 30 mM) as a spin trap, and H2O2 (22.5 mM, added last) and measured in less than 5 min.
Results
CuO nanoparticles were first characterized by dynamic light scattering/zeta potential, electron microscopy, and X-ray diffraction. We also measured the dissolved copper concentration in the suspensions. The whole NPCuO suspension, the supernatant alone, or washed and resuspended NPCuO were then incubated with DNA, and electrophoresis was performed to determine the percentage of damaged DNA for different nanoparticle concentrations with or without addition of hydrogen peroxide and/or ascorbate (Figure 1). Finally, EPR spectroscopy was performed to determine the ROS generated by NPCuO under various conditions and correlated to the observed DNA damage.
CuO Nanoparticle Characterization
NPCuO were characterized with transmission electron microscopy (TEM), X-ray diffraction (XRD), dynamic light scattering (DLS), and zeta potential analyses. The amount of copper dissolved from NPCuO was measured by UV-vis absorption using the bathocuproine method (Eaton et al. 2001). TEM images show that NPCuO are roughly spherical, with a diameter of 50 – 60 nm (Figure S1). The crystal domain size of NPCuO, calculated from its XRD spectrum (Figure S2) using the Scherrer equation (Scherrer 1918), is 20 – 30 nm. XRD results also confirm that the NPCuO contained no crystalline impurities. The average hydrodynamic diameter of NPCuO in MOPS buffer (pH 7) measured by DLS is ~200 nm weighted by intensity, 146 nm weighted by volume, and ~98 nm weighted by particle number (Table S1 and Figure S3). NPCuO appear to be moderately well-dispersed in water with a zeta potential of −28 mV (Figure S4). A proprietary dispersant, likely similar to a polyethylene glycol as determined by infrared spectroscopy (data not shown), was added to the NPCuO suspensions by the manufacturer.
Concentrations of dissolved copper in the nanoparticle leachate (lCuO) were determined using the standard addition method. A representative calculation for copper release from NPCuO in MOPS buffer is shown in Table S2 and Figure S4. Time dependence of dissolved copper concentrations from wCuO in buffer and from NPCuO suspension in buffer with ascorbate are presented in Figure S4C. The dissolved copper concentration is linear up to 150 min, and dissolved copper from wCuO is about half that of NPCuO. The concentration of dissolved copper measured using the bathocuproine method (0.5% the concentration of NPCuO) is consistent with previous reports (Atha et al. 2012; Gunawan et al. 2011). Dissolved copper concentrations increase with time (Kasemets et al. 2009; Studer et al. 2010) and with lower pH (Bondarenko et al. 2013; Cho et al. 2012; Grassian 2008; Studer et al. 2010); ascorbate may increase dissolved copper concentrations by lowering pH and chelating copper from the NPCuO surface.
DNA damage by CuO nanoparticles under oxidative stress conditions
We performed an in vitro plasmid DNA damage assay to measure CuO-mediated damage since DNA damage is intimately related to cell mutagenesis and death (Keyer et al. 1995; Luijsterburg and Van Attikum 2011). Plasmid DNA damage conditions were selected to produce single-strand nicks in the DNA backbone, resulting in closed, circular plasmids in distinct bands that are easily separated from undamaged, supercoiled DNA by gel electrophoresis. This technique is simpler than lipid and protein oxidation experiments, which require longer treatment times, more rigorous separation techniques, and identification of multiple oxidation products.
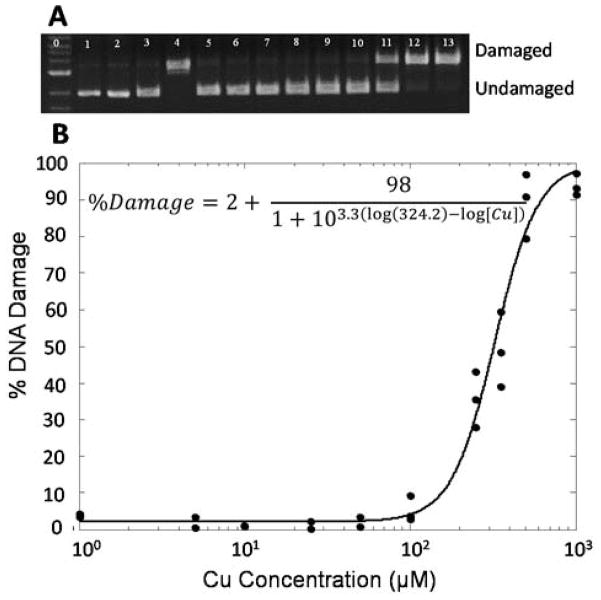
To compare DNA damage from NPCuO suspension, washed NPCuO suspension (wCuO), or leachate solution (lCuO; Figure 1), each of these components was combined with plasmid DNA, H2O2 and/or ascorbate for either 30 or 150 min. Electrophoresis was then performed to separate damaged from undamaged DNA. Figure 2A shows the gel electrophoresis image of plasmid DNA treated with H2O2 and increasing concentrations of NPCuO. DNA is undamaged upon treatment with H2O2 or NPCuO alone (lanes 2–3), and DNA treated with CuSO4 (6 μM, lane 4), ascorbate (7.5 μM), and H2O2 (50 μM) produces damaged DNA in the positive control. As NPCuO concentration increases with a fixed H2O2 concentration (50 μM; lanes 5 to 13), DNA damage increases until essentially all plasmids are damaged. The percentage DNA damage was quantified by integrating the gel band intensities. By fitting NPCuO concentration vs. DNA damage percentage with a sigmoidal dose-response curve (Figure 2B), the EC50 value for NPCuO-mediated DNA damage was calculated as 324 μM (Table 1). At least 21 different DNA damage conditions were tested, each in triplicate, and EC50 values are shown in Table 1. DNA damage data tables and representative gels for each experiment are shown in the supporting information (Tables S5–25 and Figures S5–25).
Figure 2.
A) Gel electrophoresis image of plasmid DNA (p) treated with NPCuO (1–1000 μM) and H2O2 (50 μM) for 150 min at pH 7 (MOPS, 10 mM). Lane 0: 1 kb molecular weight ladder; 1: p; 2: p + H2O2 (50 μM), 3: p + NPCuO (1000 μM); 4: p + Cu2+ (6 μM) + ascorbate (7.5 μM) + H2O2 (50 μM); lanes 5–13: p + H2O2 (50 μM) + increasing concentrations of NPCuO (1, 5, 10, 25, 50, 100, 250, 500, and 1000 μM, respectively). B) Dose-response curve fitting for the gel data in A to obtain an EC50 value.
Table 1 shows both the EC50 values for and the estimated dissolved copper in each sample. Separate concentrations are given for unwashed NPCuO suspensions (that have stabilized after long-term incubation in solution) and for the supernatant (lCuO, where no nanoparticles are present to leach copper). In conditions where we observed continuous copper leaching into the solution (i.e., immediately after nanoparticle washing, or after addition of ascorbate), we give a range corresponding to the smallest initial and largest final concentration we measured during incubation (Figure S4). Copper dissolution rates were approximately the same at 30 and 60 μM ascorbate (where the EC50 was observed), but there is concentration dependence, e.g., copper dissolution rates are slower at very high or low concentrations.
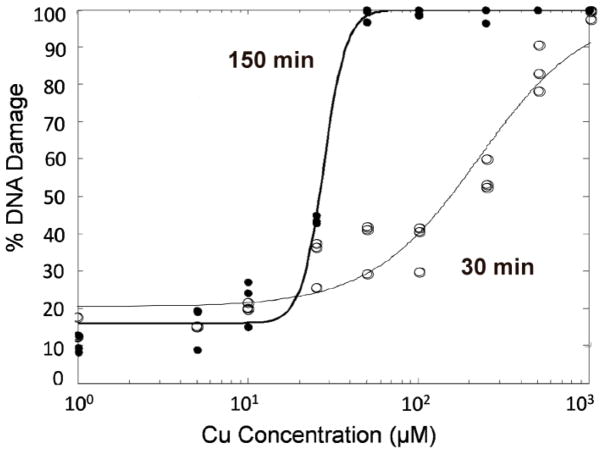
For several reaction conditions, DNA damage was measured at both 30 and 150 minutes (Figure 3). Figure 4 shows the EC50 curves for NPCuO trials at 30 and 150 minutes. The EC50 value for DNA damage decreased with incubation time for all cases with the same initial conditions at 30 and 150 min. However, damage was not generally proportional to time, indicating higher order reaction rates (also supported by the Hillslope being >1 for all 21 reaction conditions). Experiments with wCuO + H2O2, lCuO + H2O2, or lCuO + H2O2 + ascorbate were not performed as they were unnecessary to establish the effects of both nanoparticle components, and the resulting EC50 values for these conditions are expected to be well above expected physiological and environmental copper concentrations (Stockel et al. 1998) based on the trends observed for EC50 values determined for NPCuO + H2O2, NPCuO + ascorbate/H2O2, and wCuO + ascorbate + H2O2 conditions.
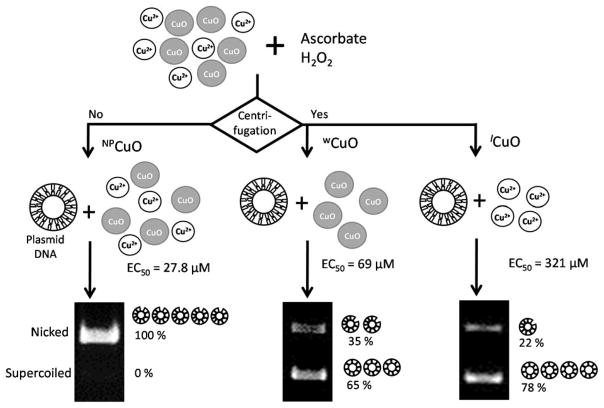
Figure 3.
Comparative scheme of DNA damage (shown in gel images) caused by NPCuO, wCuO, and lCuO fractions (50 μM) with ascorbate and H2O2 for 150 min.
Figure 4.
Comparison of the EC50 plots for DNA damage caused by NPCuO, ascorbate (1.25 equiv; 1.25 – 1250 μM), and H2O2 (50 μM) for 30 min (open circles) and 150 min (filled circles).
EPR detection of radicals
Electron paramagnetic resonance (EPR) spectroscopy was used to detect and identify ROS generated by NPCuO, wCuO, and lCuO under conditions similar to those used in the DNA damage assays (i.e. with H2O2, ascorbate, and both components together). Due to the short lifetime of ROS, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was added as a spin trap, since DMPO adducts of superoxide (O2•−) and hydroxyl radical (•OH) are readily distinguishable (Bartosz 2006; Villamena and Zweier 2004). Ascorbyl radical can be directly observed, and to detect singlet oxygen (1O2), the 2,2,6,6-tetramethyl-piperidine (TEMP) spin trap was used (Fufezan et al. 2002).
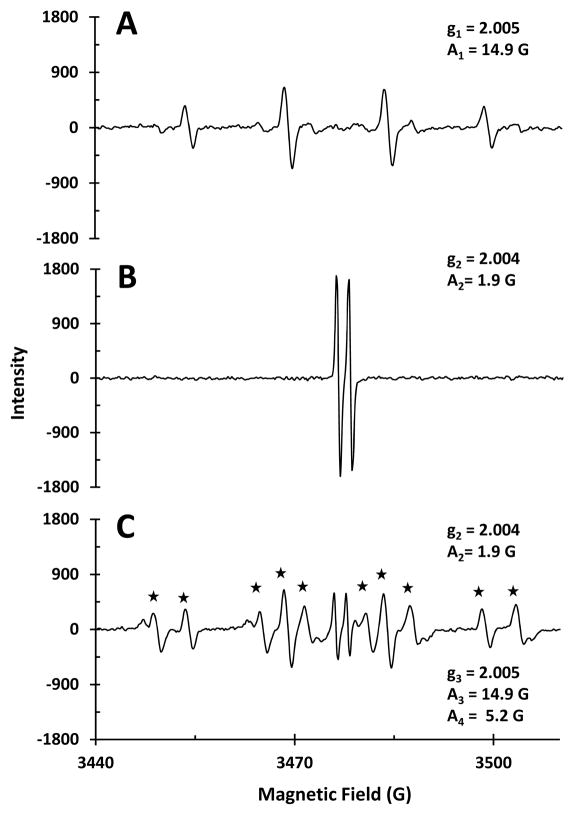
The EPR spectrum of wCuO with H2O2 (Figure 5A) exhibits the characteristic quartet resonance of the DMPO-OH adduct (Villamena and Zweier 2004), indicating •OH formation. Combining wCuO and ascorbate (Figure 5B) results in an EPR spectrum with only the ascorbyl radical resonance observed (A = 1.9 G) (Mouithys-Mickalad et al. 1998). Adding both ascorbate and H2O2 to wCuO, yields an EPR spectrum with resonances for the DMPO-OH adduct, ascorbyl radical, and a DMPO-OOH adduct derived from reaction with superoxide (Figure 5C). The DMPO-O2 adduct decomposes rapidly to DMPO-OOH, which in turn decomposes to generate DMPO-OH (Clément et al. 2004).
Figure 5.
EPR spectra of wCuO (300 μM) with A) H2O2 (22.5 mM), B) ascorbate (375 μM), and C) H2O2 (22.5 mM) and ascorbate (375 μM). All samples in buffer at pH 7 (MOPS, 10 mM) with DMPO (30 mM) as a spin trap. Asterisks indicate DMPO-OOH resonances. A1 and g1; A2 and g2; and g3 and A3 correspond to DMPO-OH, AscH•, and DMPO-OOH resonances, respectively. A4 is the second hyperfine coupling constant for the DMPO-OOH resonance.
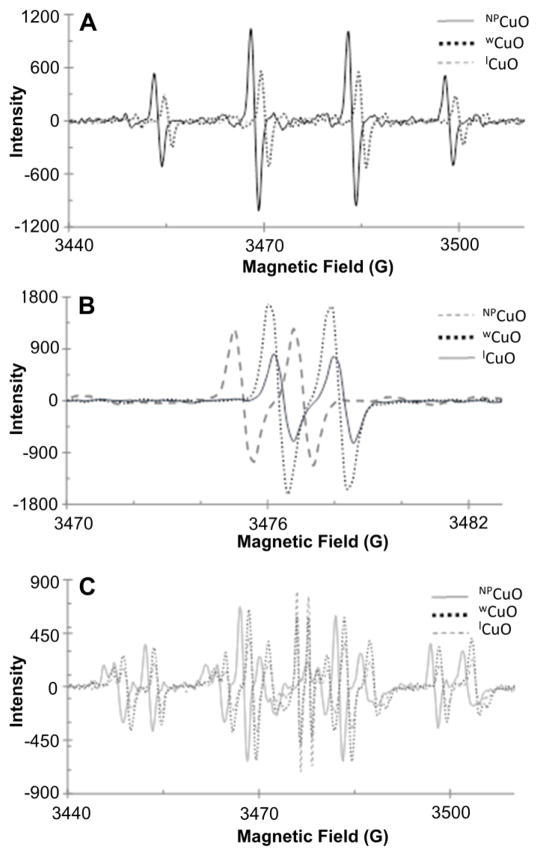
Comparing results from the three CuO fractions (NPCuO, wCuO, and lCuO), we find that the type of ROS detected depends upon whether H2O2, ascorbate, or both are added, but not upon which nanoparticle fraction is added (Figure 6). The EPR instrument displayed day-to-day drift in the magnetic field, causing minor shifts in peak positions, and signal intensities varied somewhat according to sample placement and instrument drift. However, changes in the shape of the spectra are significant and due to changes in relative amounts of each radical detected.
Figure 6.
Comparison of EPR spectra with CuO fractions (NPCuO, wCuO, or lCuO; 300 μM) and A) H2O2 (22.5 mM), B) ascorbate (375 μM), or C) H2O2 (22.5 mM) and ascorbate (375 μM). All samples in buffer at pH 7 (MOPS, 10 mM) with DMPO (30 mM) as a spin trap.
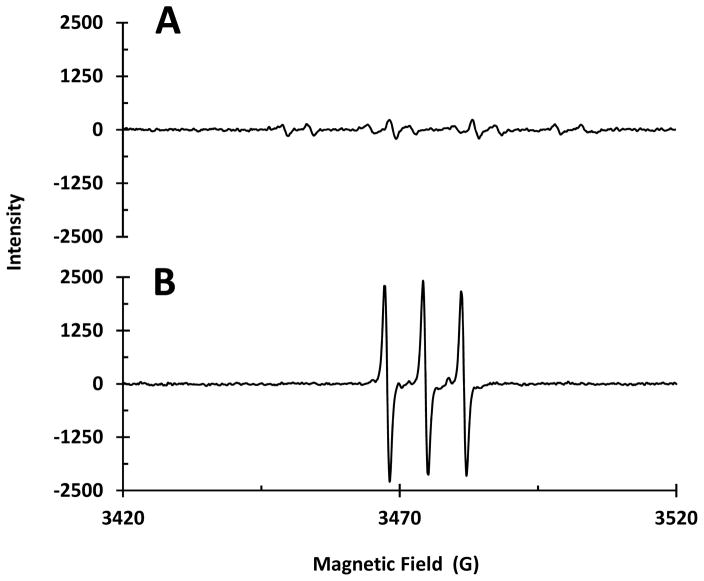
To investigate whether superoxide was generated, the EPR spectrum of K2O (a superoxide salt) was acquired under the same conditions. The EPR spectrum shows only the DMPO-OH resonance (data not shown), indicating rapid superoxide decomposition to •OH. In addition, the EPR spectrum of Cu2+ + H2O2 with DMPO also shows a very low-intensity DMPO-OOH adduct resonance (Figure 7A), confirming superoxide generation under these conditions. Although singlet oxygen formation was confirmed in Cu2+ + H2O2 + ascorbate samples using the TEMP spin trap (Figure 7B), similar experiments conducted on Cu2+ + ascorbate, Cu2+ + H2O2, or nanoparticle-containing samples with TEMP showed no evidence of 1O2 generation. These results indicate that although 1O2 is detected in positive controls using our EPR conditions, the NPCuO samples do not generate 1O2 in detectable concentrations.
Figure 7.
EPR spectra of CuSO4 (300 μM),H2O2 (22.5 mM), and ascorbate using A) DMPO (30 mM) and B) TEMP (30 mM) as a spin trap.
Discussion
Experiments were designed to determine to what extent the nanoparticle surface plays a role in nanoparticle-mediated damage. Figure 3 shows the general approach, where the nanoparticles, washed particles, and supernatant were separately tested for DNA damaging ability. It also shows one of the most striking results: in the presence of ascorbate and hydrogen peroxide, the EC50 was an order of magnitude higher for the NPCuO than could be explained by dissolved copper. At the EC50 concentration, dissolved copper in the NPCuO suspensions ranged from 0.09 μM at the start of the reaction to ~0.27 μM by the end; this range in dissolved copper is due to the gradual dissolution of copper oxide in the presence of ascorbate (Figure S4). In comparison, for dissolved copper from CuSO4, the EC50 value was 1.6 μM, implying the NPCuO is approximately an order or magnitude more damaging than would be expected from the dissolved copper in the sample. To confirm this effect, we repeated similar experiments under multiple conditions (Table 1).
Dissolved copper from CuSO4 and lCuO
Copper is well known to generate ROS and damage DNA through Fenton-like and other reactions (Angelé-Martínez 2014). We observe that Cu2+ damages DNA in presence of H2O2, ascorbate, or both (Table 1). In the presence of both ascorbate and hydrogen peroxide, copper is reduced to Cu+ that then reacts with H2O2 to generate hydroxyl radical in the Fenton-like reaction (Reaction 1). With only a reductant present (ascorbate), Cu2+ is less damaging than in the presence of H2O2 or both H2O2 + ascorbate (Table 1).
| [1] |
To compare the effects of the nanoparticles and the dissolved copper in the nanoparticle suspensions, the nanoparticles were removed, leaving a supernatant containing dissolved copper and an organic dispersant (lCuO). The EC50 for these lCuO samples, based upon dissolved copper measured in the supernatant, was expected to be close to the values for CuSO4-derived dissolved copper, or slightly higher if the dispersant was a mild antioxidant. Indeed, the EC50 value for lCuO with ascorbate and H2O2 was 1.6 ± 0.2 μM at 150 minutes incubation (compared to 1.6 ± 0.2 μM for CuSO4; Table 1) and 2.1 ± 0.2 μM at 30 minutes (compared to 2.3 ± 0.2 μM for CuSO4). We also removed copper from the supernatant, and then spiked CuSO4 back in (Table 1, Cu2+/Other Conditions). Under these conditions, the EC50 value was 2.3 μM, similar to, but somewhat higher than, the value for CuSO4 without the supernatant (1.6 μM). Taken together, these results establish that DNA damage from lCuO can be accounted for by the amount of dissolved copper in solution. Therefore, significant additional damage observed for NPCuO suspensions must be caused directly by the nanoparticles, not copper leached from the nanoparticles.
Colloidal suspension (NPCuO) and washed nanoparticles (wCuO)
From the data presented in Table 1, the DNA damage from NPCuO + H2O2 at 150 min (EC50 = 324 ± 29 μM) is similar to the damage expected from the dissolved copper measured in solution (1.54 μM dissolved copper in NPCuO, nearly identical to the EC50 value of 1.5 μM for Cu2+). At only 30 min incubation, no significant DNA damage is observed under these conditions, and it was therefore not possible to test the contributions of wCuO and lCuO under similar conditions. In contrast, DNA damage by NPCuO in the presence of either ascorbate alone or ascorbate + H2O2 is an order of magnitude greater than can be explained by the dissolved copper in the NPCuO suspensions for both time points (Table 1).
To determine the ability of the nanoparticles alone to damage DNA, NPCuO were separated from the supernatant by centrifugation and washed to remove dissolved copper in the supernatant (Figure 1). These washed nanoparticles had less than half the dissolved copper compared to NPCuO suspensions, although dissolved copper from wCuO increased during incubation with ascorbate at a similar rate to NPCuO (Figure S4C). The NPCuO were consistently more damaging than wCuO, although this effect is smaller at 30 minutes (Table 1). Both NPCuO and wCuO generated significantly higher DNA damage compared to the amount of dissolved copper measured in solution in the presence of ascorbate or ascorbate + H2O2. In both cases, the EC50 value was far lower with ascorbate alone than with H2O2 alone. Adding both H2O2 and ascorbate gave EC50 values similar to but generally lower than ascorbate alone. There is one exception to this rule: for wCuO, the EC50 value at 30 minutes is 25% higher with H2O2 than without it; however, this is likely due to experimental error, since the EC50 curve with ascorbate and H2O2 (Figure S20 and Table S20) is especially noise-sensitive and the “true value” may be lower. Although H2O2 and ascorbate generally appear to be more damaging than either on their own, we cannot determine from these data to what extent the effect is synergistic or additive.
Possible Mechanisms
To elucidate mechanisms behind differences in DNA damaging ability, ROS produced by both the nanoparticles and dissolved copper was determined by EPR spectroscopy under conditions similar to electrophoresis experiments. All CuO fractions (lCuO, NPCuO, and wCuO) produce radicals under DNA-damaging conditions, including •OH in the presence of H2O2, ascorbyl in the presence of ascorbate, both species when both ascorbate and H2O2 are added, and a DMPO-OOH adduct derived from superoxide formation.
H2O2
NPCuO and lCuO have similar EC50 values in the presence of H2O2 (Table 1), and most of the DNA damage can be accounted for by reaction of H2O2 with dissolved copper to generate DNA-damaging •OH (Reaction 1) (Angelé-Martínez 2014). EPR spectra detect •OH consistent with this mechanism (Figures 5 and 6).
Ascorbate
The EC50 values for NPCuO and wCuO are about an order of magnitude lower than expected from the dissolved copper in the supernatant, and need to be explained by additional mechanisms relating to the nanoparticle surface. It is unlikely that DNA adsorbs on the NPCuO surface due to their negative zeta potential (−28 mV), so ROS generated on the nanoparticle surface would likely damage DNA close to the nanoparticle. EPR spectra show that ascorbyl radical (AscH•) was produced. Since AscH• is a weak oxidant, it is unlikely that it directly damages DNA (Iyanagi et al. 1985; Valko et al. 2005). However, AscH• is a better reducing agent than ascorbate (Cadena 1997) and may generate other radicals, including superoxide (Reaction 2).
| [2] |
Only AscH• was observed in the EPR spectrum (not superoxide, •OH, or other species; Figure 5B), but our instrument is not sensitive enough to detect low radical concentrations that may cause DNA damage. For example, 500-fold more concentrated H2O2 was used for EPR studies than in the gel electrophoresis studies to generate enough radicals to be easily identified. In contrast, ascorbate concentrations were similar (depending on the reaction time).
Alternatively, H2O2 generation from a two-electron reduction of O2 has been proposed (Morgan et al. 1976), as well as reduction of Cu2+ by ascorbate to initiate the Fenton-like reaction (Reaction 1). H2O2 generation also may occur from ascorbate oxidation catalyzed by Cu2+ (Jameson and Blackburn 1982). Ascorbate oxidation by O2•− to produce H2O2 and ultimately •OH (Lowry and O’Neill 1992) occurs with a high rate constant (k = 1020) (Sawyer and Valentine 1981) and is reported in human lymphoma (U937) cells cultured with erythrocytes or fibroblasts (Sestili et al. 1996).
H2O2 and ascorbate
In the presence of H2O2 and ascorbate, the EC50 values for NPCuO and wCuO were generally lower than with ascorbate or H2O2 alone. The damage was also greater than could be explained from dissolved copper, although the difference was less dramatic than with ascorbate (because dissolved copper with H2O2 causes more damage than with ascorbate). EPR spectra show, OH•, and O2•−; superoxide was not observed when H2O2 or ascorbate were added individually. However, we cannot rule out generation of low •OH, AscH•, or O2•− concentrations that might explain the DNA damage results.
Hydroxyl radical (•OH) may also be generated by Cu2+ + O2•− + H2O2 in the Haber-Weiss process (Reactions 2–4) (Kehrer 2000). Theoretical models describe formation of O2•−, which disproportionates in protic solvents to yield H2O2 (K(pH 7) = 4 × 1020) (Sawyer and Valentine 1981), with a reduction potential at pH 7 of 0.94 ± 0.02 V (Wood 1974) and formation of •OOH as an intermediate (Bielski 1978). Detection of •OOH in our EPR experiments supports this model, and •OOH can cause DNA nicks, alone (Dix et al. 1996) or bound to Cu+ (Yamamoto and Kawanishi 1989; Schweigert et al. 2000). The reduction potential for O2•− formation from O2 is a thermodynamically unfavorable −0.33 V (Koppenol 1990; Wood 1974), but taking into account O2 solubility (195 μM at 37 °C, 21 kPa at an ionic strength of 0.15 M), this reduction potential increases to −0.18 V (Koppenol et al. 2010), making O2•− generation from O2 more likely. Since NPCuO (20 – 30 nm diameter) reduction potentials range between −4.12 and −4.84 V (Atha et al. 2012), O2•− formation is even more favorable. Adsorption of O2 on NPCuO surfaces may also facilitate electron transfer from the conduction band to form O2•− under conditions similar to our EPR experiments.
| [3] |
| [4] |
Both prooxidant and antioxidant activity is observed for ascorbate in lCuO + ascorbate + H2O2-mediated DNA damage assays. Low concentrations of ascorbate (0.0125 – 12.5 μM) reduce Cu2+ to Cu+, resulting in •OH formation and DNA damage (EC50 = 337 and 514 μM for 30 and 150 min treatment, respectively). However, ascorbate at high concentrations (1.25 – 1250 μM) acts as an antioxidant, likely by quenching its own radical, preventing DNA damage and increasing the EC50 value (Table 1). In the presence of ascorbate or ascorbate + H2O2, AscH• is also observed (Figures 5B and 5C). AscH• may donate one electron to dioxygen to generate O2•− (reaction 2) and, in the presence of copper, H2O2 and •OH (reactions 3 – 4) (Cross et al. 2003; Li, Zhu, et al. 2012). High ascorbate concentrations make this reaction potential positive and thermodynamically favorable (Zhao and Jung 1995). DNA damage and O2•−, 1O2, and •OH formation by treatment with ascorbate and O2 is reported (Morgan et al. 1976). In addition, ROS may be generated by other mechanisms, including electron transfer from the nanoparticle conduction band to ascorbate, as proposed for redox cycling of glutathione and catalase by NPCuO (Atha et al. 2012).
Prooxidant behavior of ascorbate and AscH•-derived products can cause DNA damage (Kimoto et al. 1993) and deoxyribose degradation by •OH (Zhao and Jung 1995). Cu2+ with ascorbate and O2 more effectively kills Bacillus globigii spores than the Fenton-like reaction (reaction 1), and killing effectiveness is reduced in the absence of O2 (Cross et al. 2003). Ascorbate oxidation is also inhibited without O2 (Mystkowski 1942).
Other proposed DNA-damaging mechanisms include formation of a DNA/Cu2+/H2O2 complex or Cu2+-bound •OH as the damaging species (Yamamoto and Kawanishi 1989). 1O2 may form in the presence of NPCuO under oxidative stress conditions (Jose et al. 2011; Li, Zhang, et al. 2012), and this ROS also decomposes into •OH (Lion and Van De Horst 1980). We detected 1O2 generated from Cu2+ + ascorbate + H2O2 using high Cu2+ concentration (300 μM); thus, it is possible that 1O2 also forms from dissolved copper of NPCuO but in amounts undetectable by EPR spectroscopy with our concentrations of dissolved copper. However, 1O2 generation from O2•− is reported, and might also be occurring under our DNA damage conditions (Khan and Kasha 1994; Ueda et al. 2003). These reports indicate •OH generation by different pathways, and support ROS generation by the nanoparticle core (Karlsson, Cronholm, et al. 2008; Atha et al. 2012; Cronholm et al. 2013; Karlsson et al. 2009; Karlsson, Holgersson, et al. 2008; Kasemets et al. 2009; Studer et al. 2010), consistent with our results.
Relative effect from the surface
NPCuO toxicity assayed in human cells, E. coli, rainbow trout, and crustaceans has been primarily attributed to dissolved copper, but toxicity from the NPCuO surfaces has also been reported (Karlsson, Cronholm, et al. 2008; Blinova et al. 2010; Gunawan et al. 2011; Heinlaan et al. 2008; Isani et al. 2013). Many factors affect toxicity of NPCuO in cells and organisms, including uptake rate, compartmentalization in lysozomes or other organelles, changes in pH, redox status of the cell or organelle, and interactions with copper-binding or redox-active biomolecules such as glutathione. Our in-vitro measurements avoid these confounding factors while still measuring DNA damage as a biologically relevant endpoint.
Our results demonstrate that the nanoparticle surface generates DNA-damaging ROS, since DNA is damaged by wCuO + ascorbate + H2O2 (EC50 = 69 μM). NPCuO is more DNA-damaging than wCuO under the same conditions. Moreover, only a small portion of the difference between wCuO and NPCuO DNA-damaging capacities can be explained by removal of dissolved copper. Since approximately 4% of the copper ions in NPCuO are on the surface (calculation in Figure S29), the concentration of surface copper is significantly lower than nanoparticle concentrations (Table 1). In fact, 4% of the EC50 values for 150 min treatment with NPCuO + ascorbate + H2O2 (27.8 μM) or wCuO + ascorbate + H2O2 (69 μM) are 1.1 and 2.8 μM, respectively, similar to the EC50 value of dissolved copper (1.6 μM) under these conditions. This calculation treats all surface sites equally and does not address whether some crystal facets or corner sites may be more catalytically active than others. Overall, the results indicate that in the presence of ascorbate (or ascorbate and H2O2) the average surface site is approximately as damaging to DNA as dissolved copper, and overall damage depends upon the amount of dissolved copper and nanoparticle surface area.
Conclusions
NPCuO cause DNA damage by •OH generation on the surface of CuO nanoparticles (wCuO) and from dissolved copper (lCuO) fractions by reaction mechanisms that involve O2•− and ascorbyl radical in addition to •OH generation. This DNA damage is time-dependent and increases upon addition of ascorbate and/or H2O2. Only a portion of the observed DNA damage can be explained by dissolved copper in the nanoparticle solution, so the surface of the NPCuO must contribute significantly to the observed damage. Knowing the capacity of different NPCuO components to cause DNA damage that leads to cellular toxicity and apoptosis may facilitate development of techniques and therapies to reduce the adverse effects of NPCuO exposure (or enhance antimicrobial properties) and allow us to take better advantage of this material in a wide variety of industrial and other applications.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIH-NIBIB 1R15EB014560) for financial support. Electron microscopy characterization was supported The South Carolina Bioengineering Center of Regeneration and Formation of Tissues (BioCRAFT) center funded under NIGMS of the National Institutes of Health, award number 5P20GM103444-07. C.A.M and K.V.T.N. thank the Clemson University Chemistry Department for graduate fellowships. C.A.M. thanks the Department of Science of the Government of Costa Rica for a graduate fellowship. K.V.T.N. received support from the Vietnam Education Foundation fellowship.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alves D, Santos CG, Paixao MW, Soares LC, De Souza D, Rodrigues OED, Braga AL. CuO nanoparticles: An efficient and recyclable catalyst for cross-coupling reactions of organic diselenides with aryl boronic acids. Tetrahedron Lett. 2009;50:6635–6638. [Google Scholar]
- Angelé-Martínez CGC, Brumaghim JL. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics. 2014;6:1358–1381. doi: 10.1039/c4mt00057a. [DOI] [PubMed] [Google Scholar]
- Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol. 2012;46:1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- Bartosz G. Use of spectroscopic probes for detection of reactive oxygen species. Clin Chim Acta. 2006;368:53–76. doi: 10.1016/j.cca.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Bielski BHJ. Reevaluation of the spectral and kinetic properties of HO2 and O2− free radicals. Photochem Photobiol. 1978;28:645–649. [Google Scholar]
- Blinova I, Ivask A, Heinlaan M, Mortimer M, Kahru A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ Pollut. 2010;158:41–47. doi: 10.1016/j.envpol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RC, Misteli T, Oberdoerffer P. DNA damage, chromatin, and transcription: The trinity of aging. Curr Opin Cell Biol. 2012;24:724–730. doi: 10.1016/j.ceb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena E. Basic mechanisms of antioxidant activity. BioFactors. 1997;6:391–397. doi: 10.1002/biof.5520060404. [DOI] [PubMed] [Google Scholar]
- Cho WS, Duffin R, Thielbeer F, Bradley M, Megson IL, Macnee W, Poland CA, Tran CL, Donaldson K. Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol Sci. 2012;126:469–477. doi: 10.1093/toxsci/kfs006. [DOI] [PubMed] [Google Scholar]
- Clément JL, Ferré N, Siri D, Karoui H, Rockenbauer A, Tordo P. Assignment of the EPR spectrum of 5,5-dimethyl-1-pyrroline-N-oxicdde (DMPO) superoxide spin adduct. J Org Chem. 2004;70:1198–1203. doi: 10.1021/jo048518z. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Cooney TE. Bactericidal activity of copper and noncopper paints. Infect Control Hosp Epidemiol. 1995;16:444–450. doi: 10.1086/648361. [DOI] [PubMed] [Google Scholar]
- Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, Wallinder IO, Moller L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small. 2013;9:970–982. doi: 10.1002/smll.201201069. [DOI] [PubMed] [Google Scholar]
- Cross JB, Currier RP, Torraco DJ, Vanderberg LA, Wagner GL, Gladen PD. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl Environ Microbiol. 2003;69:2245–2252. doi: 10.1128/AEM.69.4.2245-2252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix TA, Hess KM, Medina MA, Sullivan RW, Tilly SL, Webb TL. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4783. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- Eaton AD, Ciesceri LS, Rice EW, Greenberg AE. Standard Methods for the Examination of Water and Wastewater. Washington, D.C: American Public Health Association; 2001. [Google Scholar]
- Fufezan C, Rutherford AW, Krieger-Liszkay A. Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett. 2002;532:407–410. doi: 10.1016/s0014-5793(02)03724-9. [DOI] [PubMed] [Google Scholar]
- Gaetke LMC-J, HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014;88:1929–1938. doi: 10.1007/s00204-014-1355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian VH. When size really matters: Size-dependent properties and surface chemistry of metal and metal oxide nanoparticles in gas and liquid phase environments. J Phys Chem C. 2008;112:18303–18313. [Google Scholar]
- Gunawan C, Teoh WY, Marquis CP, Amal R. Cytotoxic origin of copper(II) oxide nanoparticles: comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano. 2011;5:7214–7225. doi: 10.1021/nn2020248. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J Biol Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- Hertzberg RP, Dervan PB. Cleavage of double helical DNA by methidium-propyl-EDTA-iron(II) J Am Chem Soc. 1982;104:313–315. [Google Scholar]
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Isani G, Falcioni ML, Barucca G, Sekar D, Andreani G, Carpene E, Falcioni G. Comparative toxicity of CuO nanoparticles and CuSO4 in rainbow trout. Ecotoxicol Environ Saf. 2013;97:40–46. doi: 10.1016/j.ecoenv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Iyanagi T, Yamazaki I, Anan KF. One-electron oxidation-reduction properties af ascorbic acid. Biochim Biophys Acta. 1985;806:255–261. [Google Scholar]
- Jameson RF, Blackburn NJ. The copper-catalysed oxidation of ascorbic acid by dioxygen. Part 4. The effect of chloride ions on the kinetics and mechanism. J Chem Soc, Dalton Trans. 1982:9–13. [Google Scholar]
- Jo HJ, Choi JW, Lee SH, Hong SW. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J Hazard Mater. 2012;227–228:301–308. doi: 10.1016/j.jhazmat.2012.05.066. [DOI] [PubMed] [Google Scholar]
- Jose GP, Santra S, Mandal SK, Sengupta TK. Singlet oxygen mediated DNA degradation by copper nanoparticles: potential towards cytotoxic effect on cancer cells. J Nanobiotechnology. 2011;9(1–8):9. doi: 10.1186/1477-3155-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Gustafsson J, Cronholm P, Moller L. Size-dependent toxicity of metal oxide particles--a comparison between nano- and micrometer size. Toxicol Lett. 2009;188:112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Holgersson A, Moller L. Mechanisms related to the genotoxicity of particles in the subway and from other sources. Chem Res Toxicol. 2008;21:726–731. doi: 10.1021/tx7003568. [DOI] [PubMed] [Google Scholar]
- Kartal SN, Green F, III, Clausen CA. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int Biodeterior Biodegradation. 2009;63:490–495. [Google Scholar]
- Kasemets K, Ivask A, Dubourguier HC, Kahru A. Toxicity of nanoparticles ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol In Vitro. 2009;23:1116–1122. doi: 10.1016/j.tiv.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Keyer K, Gort AS, Imlay JA. Superoxide and the production of oxidative DNA damage. J Bacteriol. 1995;177:6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Kasha M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci USA. 1994;91:12365–12367. doi: 10.1073/pnas.91.26.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto E, Tanaka H, Ohmoto T, Choami M. Analysis of the transformation products of dehydro-L-ascorbic acid by ion-pairing high-performance liquid chromatography. Anal Biochem. 1993;214:38–44. doi: 10.1006/abio.1993.1453. [DOI] [PubMed] [Google Scholar]
- Koppenol WH. Oxyradical reactions: From bond-dissociation energies to reduction potentials. FEBS Lett. 1990;264:165–167. doi: 10.1016/0014-5793(90)80239-f. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Stanbury DM, Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic Biol Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164–5173. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu T, Zhao J, Xu B. Interactive enhancements of ascorbic acid and iron in hydroxyl radical generation in quinone redox cycling. Environ Sci Technol. 2012;46:10302–10309. doi: 10.1021/es301834r. [DOI] [PubMed] [Google Scholar]
- Lion Y, Van De Horst A. On the production of nitroxide radicals by singlet oxygen reaction. Photochem Photbiol. 1980;31:305–309. [Google Scholar]
- Lowry JP, O’Neill RD. Homogeneous mechanism of ascorbic acid interference in hydrogen peroxide detection at enzyme-modified electrodes. Anal Chem. 1992;64:453–456. doi: 10.1021/ac00028a022. [DOI] [PubMed] [Google Scholar]
- Luijsterburg MS, Van Attikum H. Chromatin and the DNA damage response: The cancer connection. Mol Oncol. 2011;5:349–367. doi: 10.1016/j.molonc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyts K, Napierska D, Nemery B, Hoet PHM. How physico-chemical characteristics of nanoparticles cause their toxicity: complex and unresolved interrelations. Environm Sci Processes Impacts. 2013;15:23–38. doi: 10.1039/c2em30237c. [DOI] [PubMed] [Google Scholar]
- Mani RG, Smet JH, Von Klitzing K, Narayanamurti V, Johnson WB, Umansky V. Demonstration of a 1/4-cycle phase shift in the radiation-induced oscillatory magnetoresistance in GaAs/AlGaAs devices. Phys Rev Lett. 2004;92:146801–146805. doi: 10.1103/PhysRevLett.92.146801. [DOI] [PubMed] [Google Scholar]
- Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL. Toxicity of engineered nanoparticles in the environment. Anal Chem. 2013;85:3036–3049. doi: 10.1021/ac303636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AR, Cone RL, Elgert TM. The mechanism of DNA strand breakage by vitamin C and superoxide and the protective roles of catalase and superoxide dismutase. Nucleic Acids Res. 1976;3:1139–1149. doi: 10.1093/nar/3.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe TB, Dror I, Berkowitz B. Oxidation of organic pollutants in aqueous solutions by nanosized copper oxide catalysts. Appl Cat, B. 2009;85:207–211. [Google Scholar]
- Mouithys-Mickalad A, Deby C, Deby-Dupont G, Lamy M. An electron spin resonance (ESR) study on the mechanism of ascorbyl radical production by metal-binding proteins. BioMetals. 1998;11:81–88. doi: 10.1023/a:1009265625781. [DOI] [PubMed] [Google Scholar]
- Mystkowski EM. The oxidation of ascorbic acid in the presence of copper. Biochem J. 1942;36:494–500. doi: 10.1042/bj0360494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009;33:587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rim KT, Song SW, Kim HY. Oxidative DNA Damage from Nanoparticle Exposure and Its Application to Workers’ Health: A Literature Review. Saf Health Work. 2013;4:177–186. doi: 10.1016/j.shaw.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DT, Valentine JS. How Super is Superoxide. Acc Chem Res. 1981;14:393–400. [Google Scholar]
- Scherrer P. Bestimmung der grösse und der inneren struktur von kolloidteilchen mittels röntgenstrahlen. Göttinger Nachrichten Gessell. 1918;2:98–100. [Google Scholar]
- Schweigert N, Acero JL, Von Gunten U, Canonica S, Zehnder AJ, Eggen RI. DNA degradation by the mixture of copper and catechol is caused by DNA-copper-hydroperoxo complexes, probably DNA-Cu(I)OOH. Environ Mol Mutagen. 2000;36:5–12. doi: 10.1002/1098-2280(2000)36:1<5::aid-em2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Selvakumar P, Suresh S. Conective performance of CuO/ater nanofluid in an electronic heat sink. Exp Thermal Fluid Sci. 2012;40:57–63. [Google Scholar]
- Sestili P, Brandi G, Brambilla L, Cattabeni F, Cantoni O. Hydrogen peroxide mediates the killing of U937 tumor cells elicited by pharmacologically attainable concentrations of ascorbic acid: Cell death prevention by extracellular catalase or catalase from cocultured erythrocytes or fibroblasts. J Pharmacol Exp Ther. 1996;277:1719–1725. [PubMed] [Google Scholar]
- Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut. 2011;159:1277–1282. doi: 10.1016/j.envpol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockel J, Safar J, Wallace AC, Cohen FE, Prusiner SB. Prion protein selectively binds copper(II) ions. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- Studer AM, Limbach LK, Van Duc L, Krumeich F, Athanassiou EK, Gerber LC, Moch H, Stark WJ. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol Lett. 2010;197:169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Thit A, Selck H, Bjerregaard HF. Toxicity of CuO nanoparticles and Cu ions to tight epithelial cells from Xenopus laevis (A6): Effects on proliferation, cell cycle progression and cell death. Toxicol In Vitro. 2013;27:1596–1601. doi: 10.1016/j.tiv.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Ueda J, Takeshita K, Matsumoto S, Yazaki K, Kawaguchi M, Ozawa T. Singlet oxygen-mediated hydroxyl radical production in the presence of phenols: whether DMPO-*OH formation really indicates production of *OH? Photochem Photobiol. 2003;77:165–170. doi: 10.1562/0031-8655(2003)077<0165:somhrp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vajjha RS, Das DK, Kulkarni D. Development of new correlations for convective heat transfer and friction factor in turbulent regime for nanofluids. Int J Heat Mass Transfer. 2010;53:4607–4618. [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Villamena FA, Zweier JL. Detection of reactive oxygen and nitrogen species by EPR spin trapping. Antioxid Redox Signal. 2004;6:619–629. doi: 10.1089/152308604773934387. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li N, Zhao J, White JC, Qu P, Xing B. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem Res Toxicol. 2012;25:1512–1521. doi: 10.1021/tx3002093. [DOI] [PubMed] [Google Scholar]
- Wood PM. The redox potential of the system oxygen-superoxide. FEBS Lett. 1974;44:22–24. doi: 10.1016/0014-5793(74)80297-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kawanishi S. Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem. 1989;264:15435–15440. [PubMed] [Google Scholar]
- Zhao MJ, Jung L. Kinetics of the competitive degradation of deoxyribose and other molecules by hydroxyl radicals produced by the Fenton reaction in the presence of ascorbic acid. Free Radic Res. 1995;23:229–243. doi: 10.3109/10715769509064036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.