Abstract
Recent evidence indicates that the traditional syndromes known as renal osteodystrophy, secondary hyperparathyroidism and vitamin D deficiency are related to mortality in persons with chronic kidney disease (CKD). The so-called “Kidney Bone Disease”, also know as “Mineral-and-Bone-Disorders”, is defined to include 3 interrelated entities: bone disorders, mineral disarrays, and vascular calcification. These disorders are common in individuals with moderate to advanced CKD and may be related to cardiovascular disease and risk. We have identified 13 common and clinically relevant conditions of contemporary nature that are related to the Kidney Bone Disease, including: calcitriol (active vitamin D) deficiency, 25(OH)-vitamin D deficiency, biochemical hyperparathyroidism, relatively low parathyroid hormone level, increased serum alkaline phosphatase (hyperphosphatasemia), elevated fibroblast growth factor-23, high turnover bone disease, adynamic bone disease, vascular calcification, hyper- and hypophosphatemia, and hyper- and hypocalcemia. We present a critical review of these 13 conditions with emphasis on CKD patient survival and other pertinent clinical outcomes. We also review unresolved controversies surrounding the administration of nutritional vitamin D, activate vitamin D analogs, calcimimetics and recombinant parathyroid hormone teriparatide; compare mortality predictability of parathyroid hormone and alkaline phosphatase; and examine potential risks of mineral disarrays and abnormally high and low levels of calcium and phosphorus in CKD patients.
Keywords: Hyerparathyroidism, vitamin D, calcimimetic, hypocalcemia, hypophosphatemia, alkaline phosphatase, parathyroid hormone (PTH)
Introduction
Bone disorders, mineral disarrays, and vascular calcification are the 3 closely interrelated and common conditions in individuals with moderate to advanced chronic kidney disease (CKD).1, 2 These conditions appear to be related to progressive deficiency of active vitamin D and worsening secondary hyperparathyroidism (SHPT) that happen with gradual loss of renal function and lead to renal osteodystrophy.3 Some opinion leaders have suggested the designation of “CKD Mineral and Bone Disorder” (CKD-BMD) for the constellation of the foregoing disorders.1, 2 Even though the term CKD-MBD may sound more inclusive than the traditional term “renal osteodystrophy”, many physicians and health care professionals as well as many patients continue to refer to these disorders as “Renal Bone Disease”4, 5 or simply “Kidney Bone Disease”.6, 7 Table 1 includes the list of most commonly encountered conditions related to the Kidney Bone Disease in individuals with CKD. We present a critical review of the 13 conditions listed in Table 1 with emphasis on CKD patient survival and other pertinent clinical outcomes.
Table 1.
Disorders related to kidney bone disease
| Disorder/Condition | Possible Cause | Potential Consequence | |
|---|---|---|---|
| 1 | 1,25 dihydroxy vitamin D (calcitriol) deficiency | insufficient renal 1-alpha hydroxylation, ↑FGF-23, hyperphosphatemia, ↑PTH | probably the main driver of SHPT and renal osteodystrophy, MICS, vascular calcification, atherosclerotic CV disease and death, poor survival |
| 2 | 25(OH) vitamin D deficiency | Nutritional (reflecting background population), MICS | MICS, vascular calcification, atherosclerotic CV disease and death, poor survival |
| 3 | Hyperparathyroidism | ↓cacitriol, hypocalcemia, hyperphosphatemia, ↓ vitamin D receptor, ↓ calcium-sensing receptor, PTH resistance, ↑FGF-23 | renal osteodystrophy, uremic toxin, vascular calcification, atherosclerotic CV disease and death, poor survival, refractory anemia resistant to ESA, |
| 4 | Low PTH | MICS, calcium load, administration of native or active vitamin D or calcimimetics, PTH assay error | questionable primary (causal) link to adynamic bone disease or other outcomes |
| 5 | increased level of circulating alkaline phosphatase (hyperphosphatasemia) | SHPT, calcitriol deficiency, high turnover bone disease | Worsening renal osteodystrophy, vascular calcification, atherosclerotic CV disease and death, poor survival |
| 6 | Elevated FGF-23 | ↓GFR, hyperphosphatemia | Phosphaturic effect (with adequate residual renal function), inhibition of 1-alpha hydroxylation, ↓cacitriol, hypocalcemia |
| 7 | High turnover bone disease | SHPT, calcitriol deficiency, 25(OH) D deficiency (?), uremia per se | Renal osteodystrophy, decreased bone mineral density, osteitis fibrosa, pathological fractures, hyperphosphatesemia |
| 8 | Adynamic bone disease | Calcium load (both high calcium bath and high po calcium intake), diabetes mellitus, peritoneal dialysis, intake of vitamin D and calcimimetic (?), aluminum deposition | Hypercalcemia (cause vs. consequence?), bone fracture (?) |
| 9 | Vascular calcification | MICS, diabetes mellitus, vitamin D deficiency, SHPT | Peripheral vascular disease, CV disease and death, poor survival |
| 10 | Hyperphosphatemia | ↓GFR, increased phosphorus ingestion in protein and preservatives, ↓FGF-23, vitamin D analogs, calcimimetics (in NDD-CKD) | ↑SHPT, ↑FGF-23, ↑vascular calcification, ↑ mortality |
| 11 | Hypophosphatemia | Malnutrition-inflammation complex, protein-energy wasting, low protein intake, imposed (overzealous) dietary restrictions, calcimimetics (in dialysis patients) | Increased death risk (as a surrogate of malnutrition and poor PO intake) |
| 12 | Hypercalcemia | Calcium load, calcium-based binder, dialsyate cakcium, vitamin D, refractory (tertiary) hyperparathyroidism, adynamic bone disease | ↑vascular calcification, ↑ mortality |
| 13 | Hypocalcemia | ↓GFR, calcimimetics | Potential risk of arrhythmias, possible increased risk of death |
I. Calcitriol (Active Vitamin D) Deficiency
The SHPT is engendered, at least in part, as a result of the progressive decline in circulating level of 1,25 dihydroxycholecalciferol (1,25(OH)2D3 or calcitriol), also known as activate vitamin D, across worsening stages of CKD.8–10 Replacement of activate vitamin D has thus become the main strategy in the treatment of SHPT. The introduction of a number of analogues of the activate vitamin D molecule, together also known as vitamin D receptor activators (VDRA), has broadened our therapeutic armamentarium,11, 12 but has also made decisions about which drug to use more complicated.13
We recently examine the rationale for the therapeutic use of activate vitamin D and summarized the available scientific evidence supporting the use of them, alone or in combination with other therapeutic agents such as calcium sensing receptor (CSR) agonists, also known as calcimimetics, and/or nutritional vitamin D, i.e., precursor of 25(OH) vitamin D.3,12 In particular, we reviewed the recent observational data on the association of VDRA and survival in CKD and expanded our discussion on interpretation of such associative data in the setting of clinical practice and current and anticipated treatment guidelines.3, 12 There are increasing number of observational studies published to date indicating survival advantages of active vitamin D in CKD patients, including several studies in maintenance dialysis patients14–21 and 2 additional studies in non-dialysis dependent (NDD) CKD patients.22, 23 To our knowledge, only one observational study has not uniformly confirmed the association of vitamin D agents and CKD survival, in which apparently both active and nutritional vitamin D agents were examined combined as treatment group.24 The consistency of the epidemiologic data is remarkable, making a strong point about the potential of causality given other features of Hill’s causality criteria25 such as biological plausibility and scientific coherence.3
Some experimental studies using animal models have shown an association between active vitamin D and vascular calcification26–28 or myocardial fibrosis,29 whereas other studies have shown salutary effects including improved left ventricular hypertrophy30 or lack of vascular calcification with more selective VDRA that may have less effect on vitamin D receptors of the gastrointestinal tract, leading to less calcium and phosphorus absoption.31 Nevertheless, the survival advantages of VDRA should eventually be tested in randomized controlled trial, not withstanding the fact that many fundamental beliefs of the contemporary medicine and public health, such as the association between smoking and lung cancer, have never been tested in any randomized controlled trial.
Differences in survival-advantages between paricalcitol and calcitriol observed in a large epidemiologic study32 may also be explained by virtue of their differential effects on diverse PTH segments. Monier-Faugere et al.33 found that calcitriol and paricalcitol have trivial but biologically significant differences between their effects on circulating levels of PTH-(1-84), intact PTH, large C-terminal PTH fragments (C-PTH), and the ratio of PTH-(1-84) to C-PTH fragment or PTH-(7-84), i.e., iPTH minus PTH-(1-84). The PTH-(7-84), has been shown to act as a partial antagonist of PTH-(1-84), with opposite biologic activities.34, 35 In CKD patients reduction in renal function is accompanied by a higher increase in C-PTH than in PTH-(1-84).36 The administration of cinacalcet to dialysis patients with SHPT37 or to patients with parathyroid cancer38 does not change the ratio between intact PTH and PTH-(1-84).34 Among VDRAs, paricalcitol has less hypercalcemic and hyperphosphatemic effects and does not induce low bone turnover in a rat model of renal failure.39 Also, the affinity of paricalcitol for vitamin D receptor is three times less than that of calcitriol, whereas its calcemic and phosphatemic effects have been shown to be 10 times lower.33 Biologically plausible mechanisms that can explain survival advantageous of different VDRAs are yet to be discovered and confirmed in clinical trials.
II. 25(OH) Vitamin D Deficiency
The Kidney Disease Outcome Quality Initiative (KDOQI) guidelines recommend measuring 25(OH) D (calcidiol) levels in individuals with CKD and hyperparathyroidism and, if below 30 ng/ml, to replace it with nutritional (inactive) vitamin D2 (ergocalciferol) or D3 (cholecalciferol).40 The recommended ergocalciferol dose is 50,000 units (one pill) per week to per month for 3 to 6 months, according to the severity of vitamin D deficiency.41 Assuming liver function is intact, the administered nutritional (inactive) vitamin D precursor or substrate is expected to be converted to 25(OH) D and ready for further 1α-hydroxilation in the kidney or in non-renal (peripheral) organs.12 Although many studies have shown meaningful epidemiologic or pathophysiologic association between low 25(OH) D level and outcomes in the general population,42 such findings need yet to be confirmed in observational or interventional studies in CKD patient populations.43 A recent prospective cohort study showed that lower levels of 25(OH) D were associated with increased mortality in incident hemodialysis patients, but VDRA administration virtually nullified the mortality-predictability of 25(OH) D deficiency.44
Prior to the VDRA-era, nutritional (inactive) vitamin D agents such as ergocalciferol or cholecalciferol were used with less success in dialysis patients. A controlled trial by Berl et al45 found that vitamin D3 was not effective in decreasing serum PTH level in dialysis patients, whereas calcitriol was. Nine of 12 patients on D3 showed histologic deterioration of bone disease, whereas in six of seven patients who received calcitriol, improved to unchanged bone histology was observed.45 In another study by Malluche et al46 vitamin D3, in doses that normalized intestinal absorption of calcium, failed to restore bone histology to normal, although mineralization and collagen texture of osteoid were consistently improved.
There is ongoing debate as to whether in the absence of renal 1α-hydroxilation in renal failure, the peripheral (non-renal) 1α-hydroxilase is adequate to generate the required magnitude of circulating 1,25(OH)2D, especially if the availability of the substrate, i.e., 25(OH) D is enhanced by increasing the intake of nutritional vitamin D.47 Apart from above unanswered question, 25(OH) vitamin D may have a more widespread role beside being a mere precursor or substrate to activate vitamin D.43 Even though currently there is no convincing data about usefulness of nutritional vitamin D in CCKD,12 there is heightened debate in the pharmaceutical industry related circles as to whether the combination of nutritional D plus calcimimetic can be as good as active vitamin D alone. Despite less encouraging results pertaining to the administration of nutritional D to dialysis patients in the past, it is possible that the combination of nutritional (inactive) vitamin D and a calcimimetic48 is more promising than nutritional D alone, a hypothesis that needs to be tested in well-designed randomized controlled trials.
III. Biochemical Hyperparathyroidism
In individuals with normal renal function, a serum intact PTH of 65 pg/mL or above is considered abnormal and likely a sign of primary hyperparathyroidism. Indeed in the face of normal to high serum calcium level, even PTH levels in mid to high 50’s pg/ml may be already abnormal.49 The most recent version of the KDOQI, however, has recommended higher cutoff levels as the definition of treatment-eligible SHPT, i.e., PTH levels above 75 pg/ml, 110 pg/ml and 300 pg/ml, for CKD stages 3, 4 and 5, respectively.49 Whereas the Kidney Disease Initiative Global Outcome (KDIGO) has proposed the target range within the approximately 2 to 9 times higher than the upper PTH threshold level of each region or country, both KDOQI and KDIGO guidelines recommend PTH target levels that are much higher than normal range of the general US population (<65 pg/ml). Assuming that PTH is a uremic toxin and associated with poor survival in CKD, as recently shown by Kovesdy et al.,50 normalization of PTH levels using active or nutritional vitamin D agents or calcimimetics appears prudent, provided reliable PTH assays can be used.51
A recent study showed that in over 58,000 maintenance hemodialysis patients, PTH levels above 300 pg/ml were associated with increased death risk (Figure 1).15 In this epidemiologic analysis using time-dependent models, intact PTH levels in 300 to 600 pg/ml were incrementally associated with higher death risk when compared to the 150 to 300 pg/ml (reference) group. However, PTH levels above 600 pg/ml, although still associated with high death risk, did not show further increase in mortality as would be expected for a dose-response phenomenon. The aforementioned attenuated mortality with a PTH above 600 pg/ml may be due to the fact that most dialysis patients with very high PTH levels often received higher than usual doses of the active vitamin D analogs (Figure 1).15 Consistent with this notion a recent study showed that the survival advantages of the African American dialysis patients, who usually have higher PTH levels, can also be explained by virtue of their higher likelihood of receiving active vitamin D agents.21 In another recent study, the ratio of paricalcitol dose per unit of PTH was linearly associated with greater survival.20
Figure 1.
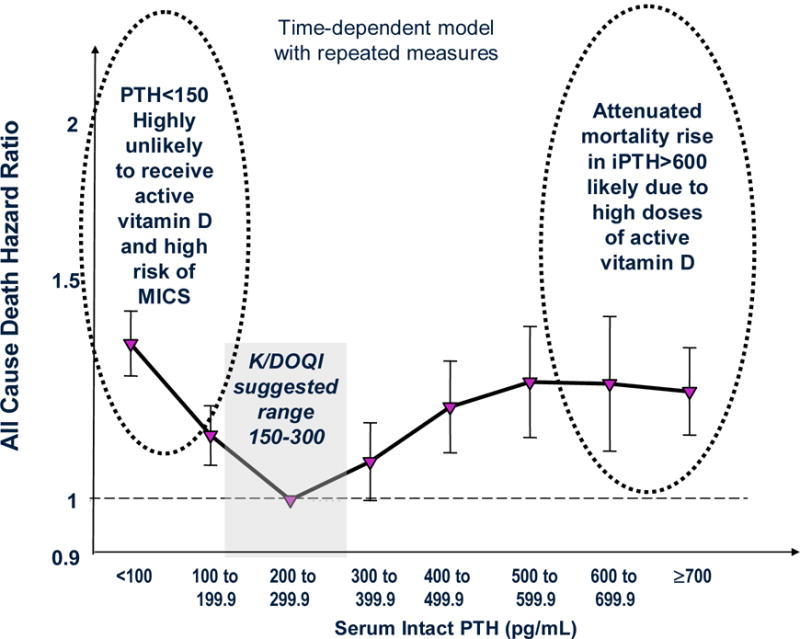
The U-shaped association between serum intact PTH and survival in 58,058 maintenance hemodialysis patients over 2-years (adapted from15).
IV. Syndrome of Apparent Low Serum PTH
The KDOQI guidelines recommend against PTH levels below 150 pg/ml in CKD stage 5 in order to mitigate the risk of adynamic bone disease.40 However, significant discrepancies may exist between the histopathologic diagnosis of adynamic bone and the biochemical detection of “low PTH”. A recent epidemiologic study in NDD-CKD found that the lower the PTH the greater the survival.50 Interestingly, a large epidemiologic study in maintenance hemodialysis patients showed a similar trend in the conventional Cox model using baseline PTH measures at the start of the cohort, whereas time-dependent survival model showed that a low PTH appeared associated with higher death risk, i.e., a so-called U-shaped phenomenon (Figure 1).15 This may be due to several reasons listed in Table 2 and depicted in Figure 2. In particular, the KDOQI guidelines recommend withholding active vitamin D agents and calcimimetics when PTH is below certain range,40 and this very recommendation may indeed lead to increased death risk as discussed above. High calcium load from calcium based binders52 or dialysate bath53 may lead to vascular calcification and cardiovascular death and may also suppress PTH at the same time exaggerating the artificial association between low PTH and poor outcomes. Serum PTH level may also be confounded by such non-bone related factors such as obesity54 or pentosidine mediated carbonyl stress.55 PTH assay discrepancies may lead to seemingly low PTH levels as well.56, 57 Different subtypes and/or fragments of PTH may be measured via different assays, such as the inhibitory (7–84) portion that is related to one of the large the carboxy-terminal and that is measured along with the active (1–84) PTH when the so-called “intact PTH assay” is used.4 Finally, the administration of teriparatide, a synthetic recombinant 1–34 PTH,58 to osteoporotic patients with or without CKD, may increases bone turnover by stimulating osteoblasts more than osteoclasts, leading to elevated serum calcium and suppressed serum phosphorus and circulating (measurable) intact PTH levels. A recent case report indicated that teriparatide was successfully used in a parathyoridectomized CKD patient with severe hypocalcemia due to "hungry bone syndrome.59 Currently there is no data available about the consequenses of teriparatide therapy in uremia.
Table 2.
Causes of biochemically measured, relatively low serum intact PTH level in CKD patients, e.g. PTH <150 pg/ml, using conventional PTH assays
| Calcium based binders |
| Calcium rich diet |
| Higher calcium concentration in dialysate bath |
| Metabolic syndrome |
| Diabetes mellitus |
| Malnutrition-inflammation complex |
| Oxidative stress |
| Carbonyl (pentosidine) stress |
| Peritoneal dialysis |
| Advanced age |
| Caucasian race |
| PTH assay errors |
| Administration of nutritional or active vitamin D agents |
| Administration of calcimimetics |
| Administration of recombinant PTH (Teriparatide)* |
| Adynamic bone disease |
Footnote: Administration of recombinat PTH, known as Teriparatide (injectable Forteo™) may suppress the measurable naïve PTH level based of the PTH assay employed.
Figure 2.
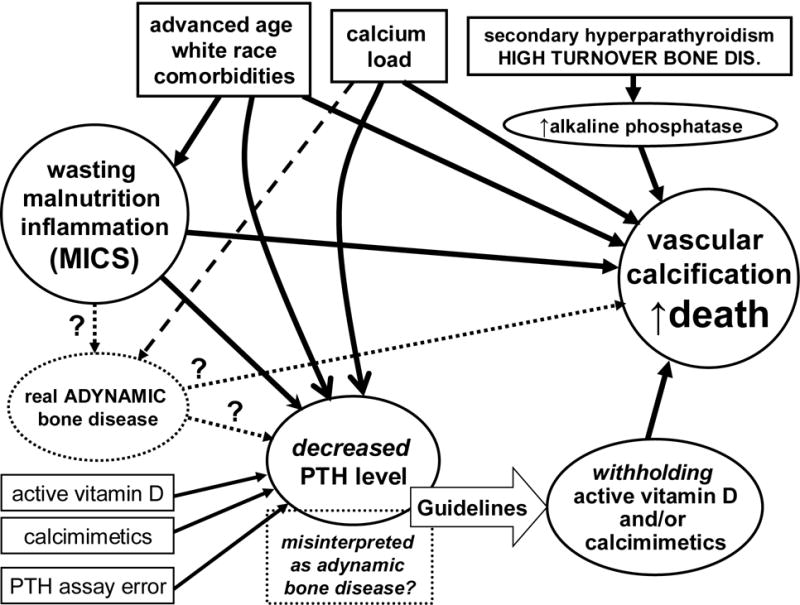
Schematic representation of the links between components of Kidney Bone Disease and vascular calcification. Note potential contributors of low PTH level and its confounded association with poor outcomes.
A recent epidemiologic study found that even among dialysis patients with an intact PTH below 150 pg/ml a high serum alkaline phosphatase (>120 IU/L) was associated with higher death risk compared to lower alkaline phosphatase levels.60 In a large cohort of Japanese dialysis patients, low PTH (<150 pg/ml) was associated with the greatest survival.61 We have recently found that low PTH is yet another facet of the malnutrition-inflammation complex and that after adjusting for this confounder, the PTH in 100 to 150 pg/ml range is associated with best 5-year survival in a cohort of hemodialysis patients.62
V. Increased Serum Alkaline Phosphatase
Hyperphosphatasemia or hyperphosphatasia refers to disorders that feature elevated serum alkaline phosphatase activity in the blood.63 High serum alkaline phosphatase level in CKD patients is usually from excesses of the bone isoforms of the enzyme, but it may also happen in certain types of liver disorders and biliary obstruction.64, 65 Whereas serum alkaline phosphatase used to be a traditional measure for the management of kidney bone disease, in recent years it appeared to have fallen out of favor, probably since the KDOQI guidelines did not include it in its recommendations, nor did they suggest any cutoff levels or target ranges for it.40 A recent epidemiologic study has shown robust associations between higher serum alkaline phosphatase (esp. if > 120 U/L) and poor survival in hemodialysis patients.60 Indeed, compared to serum PTH, which has a U-shaped association with mortality (Figure 1), serum alkaline phosphatase appears to have a linear and incremental association with both all-cause and cardiovascular mortality (Figure 3) and this association appears to hold across different PTH strata including PTH below 150 pg/ml.15
Figure 3.
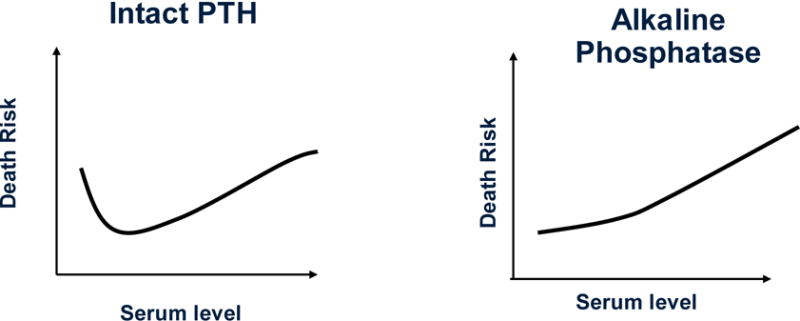
A comparison between survival predictability of serum PTH (as shown in Figure 1) and serum alkaline phosphatase in dialysis patients.
Since lower alkaline phosphatase is associated with lower PTH (and ultimately with hypOdynamic bone), some might expect a higher, rather than lower, mortality associated with low serum levels. Our epidemiologic findings15, 60 are contrary to this expectation, suggesting that alkaline phosphatase may be more than a mere marker of bone turnover. Higher alkaline phosphatase has indeed been shown to result in increased hydrolysis of pyrophosphate,66, 67 which is a potent inhibitor of vascular calcification.68–70 The effect of alkaline phosphatase on pyrophosphate could be the link that explains why lower levels of the former are associated with a linear decrease in mortality.71 Indeed, a recent epidemiologic study found that higher levels of alkaline phosphatase, and no other biomarkers such as PTH or minerals, were associated with coronary artery calcification in hemodialysis patients.72 Another possible explanation for the observed association is a link between higher alkaline phosphatase levels and lower 25(OH) vitamin D level,73–75 which is per se associated with increased mortality.44 Alkaline phosphatase is a marker of bone resorption, and as such it is closely associated with PTH levels.
Alkaline phosphatase can be effectively lowered by both active vitamin D products12, 76, 77 and calcimimetics.78 Indeed a recent meta-analysis, which questioned the PTH lowering effect of active vitamin D analogs, showed that these agents can decrease serum alkaline phosphatase effectively.79 Recent data indicate a link between pyrophosphate and tissue-nonspecific alkaline phosphatase as a causative pathway to vascular calcification.66, 71 A recent study suggested that the lower serum alkaline phosphatase the better is the response of dialysis patients to erythropoietin stimulating agents during anemia management.80 The consistency of epidemiologic and experimental data on alkaline phosphatase and the fact that vitamin D and calcimimetics can both lower its circulating level makes this traditional marker a promising tool for the management of Kidney Bone Disease, notwithstanding that lack of adequate recommendations by current KDOQI guidelines.
VI. Fibroblast Growth Factor-23
Similar to PTH, FGF-23 has phosphaturic properties, but it also inhibits 1-α hydroxylation and, hence, may aggravate calcitriol deficiency leading to further PTH production and release.81, 82 It is unclear to what extent the different pathophysiologic mechanisms, i.e., intrinsic loss of enzymatic activity vs. suppression by FGF-23, contribute to the lower 1-alfa hydroxylase activity, but the net effect is a progressive decline in activate vitamin D levels with advancing stages of CKD. It is speculated that VDR activation may increase whereas calcimimatic administration may decrease FGF-23.82 The latter may explain why calcimimetics lead to paradoxical hyperphosphatemia in patients with CKD stages 3 and 4 and some urine output, whereas in dialysis patients with no significant residual renal function serum phosphorus tends to decrease similar to hungry bone syndrome after parathyroidectmy.82 Similar to hyperglycemia associated increase in A1c level,83 persistent hyperphosphatemia may lead to higher FGF-23 levels, which per se independent of serum phosphorus is associated with both increased death risk and increased serum alkaline phosphatase in maintenance dialysis patients.84 Further epidemiologic and clinical studies are needed to examine the association between FGF-23 with vascular calcification and mortality in CKD patients.
VII. High Turnover Bone Disease and Osteoporosis
Even though changes in bone structure in histopathologic examinations are the hallmarks of the high turnover bone disease, such biomarkers as increased serum PTH and/or alkaline phosphatase are often used as screening and detection tools.4, 85 To date no reliable biomarker of bone histopathology has been found. Diminished bone mineral density may be observed with this or other types of renal osteodystrophy.86, 87 Indeed low bone mineral density and osteoporosis is often associated with increased risk of aortic calcification in the general population.88 A recent study showed that diminished bone mineral density was related to protein-energy wasting and poor survival in dialysis patients.89 Additional studies to examine the association between the nature of bone disease or its histopathology and survival in CKD patient population are needed to show consistency of such findings. Nevertheless, emerging studies that indicate robust and incremental association between serum alkaline phosphatase and mortality (see above) may indicate deleterious effect of high turnover bone disease on survival.
VIII. Adynamic (Low Turnover) Bone
Notwithstanding the pathognomonic histopathologic features of adynamic bone disease in CKD, recent evidence implies that adynamic bone status might indeed be a secondary phenomenon and a consequence of the malnutrition-inflammation-complex syndrome (MICS), which is per se associated with hypoalbuminemia, increased serum levels of pro-inflammatory cytokines, protein-energy wasting90 and increased cardiovascular disease and death in maintenance dialysis patients.91 A recent study in 44 chronic peritoneal dialysis patients showed that low serum albumin was associated with adynamic bone.92 Although we are not aware of any study that indicates a direct causal effect of inflammation on adynamic bone disease in CKD, in vitro PTH secretion is suppressed by interleukin-6,93 a strong pro-inflammatory cytokine that is associated with poor outcome in maintenance dialysis patients. Interleukin-1 beta (IL-1β), another pro-inflammatory cytokine, may also suppress PTH secretion; in another in vitro study, PTH secretion from cultured parathyroid tissue slices was significantly inhibited in media containing IL-1β.94 This effect may be mediated through the specific IL-1 receptors that upregulate calcium-sensing receptor mRNA leading to apparent low bone turnover.94 Indeed in the foregoing study, the inhibitory effect of IL-1β could be counteracted by the IL-1 receptor antagonist (IL-1ra),94 indicating that the inflammation induced suppression of PTH can potentially be overcome by treatment of MICS in individuals with CKD. Hence, interventions that can improve hypoalbuminemia and kidney disease wasting by correcting malnutrition and/or by mitigating inflammation, e.g., via agents that counteract tumor-necrosis-factor-alpha or other pro-inflammatory cytokines,95 may be more promising approaches for the management of adynamic bone disease rather than decreasing the dose of or withholding activated vitamin D analogs or calcimimetics.
IX. Vascular Calcification
The recent literature is oversaturated with exponential numbers of studies indicating a high prevalence of vascular calcification in CKD96, 97 and its association with death risk.98 Medial calcification, which happens more frequently in CKD, is associated with increased death risk as compared to no vascular calcification, but intimal calcification has the strongest association with death risk.99 There are mixed data about the association of calcium based phosphorus binders and vascular calcification.100, 101 A recent controlled trial did not show any conclusive association between type of binder and short-term survival, even though a trend towards better survival was observed with non-calcium binder sevelamer.102, 103 Another trial showed no advantage of sevelamer over calcium acetate in conjunction with statin, but both treatment arms were associated with increased calcification.104 There are conflicting data and opinions about the association of vitamin D or calcimemtics with worsening or amelioration of vascular calcification.13 The highly competitive nature of the binder marketing in the current environment and a lack of conclusive data has lead to major confusion among both physicians and patients as to whether any medication or intervention is associated with significant vascular calcification and if so, whether the calcification that is engendered in this way is related to poor survival.13
X. Hyperphosphatemia
The link between phosphorus retention and SHPT has been known for decades.105 Recent evidence puts more emphasis on the FGF-23 pathway as the link between hyperphosphatemia and active vitamin D deficiency due to inhibition of 1-α hydroxylation of 25(OH) vitamin D. Several recent epidemiologic studies have found an incremental association between phosphorus levels above 6 or 7 mg/dL and increased death risk.15, 106 As to which cutoff level of serum phosphorus is accepted in which stage of CKD, is a matter of ongoing debate, despite the clear-cut cutoff levels recommended by KDOQI guidelines.40 A recent study suggested that dietary protein restriction to lower serum phosphorus may cause more harm, i.e., increased mortality, than good in dialysis patients.107 A similar concern appears to exist with regard to restricting or discontinuing active vitamin D medications to lower serum phosphorus, esp. in the current era of ongoing performance pressure of achieving “good phosphorus level” at all costs.
XI. Hypophosphatemia
Two large epidemiologic studies from Fresenius106 and DaVita national databases15 showed that even after extensive multivariate adjustment including for surrogates of malnutrition, serum phosphorus levels below 3.0 g/dL were associated with increased death risk in maintenance hemodialysis patients. Indeed in unadjusted survival models, consistent with the real world of dialysis patients, a low serum phosphorus, which is often observed in patients with malnutrition-inflammation complex, is a by far stronger marker of poor survival than normal to high serum phosphorus.108 Because of the high risk of death associated with low appetite109 and poor protein intake,110 it is possible that imposing protein restriction to control phosphorus may cause more harm in CKD patient populations.105 Hence, more diligent use of potent phosphorus binders and more emphasis on restricting non-protein sources of phosphorus such as in preservatives or fast food may be the preferred future approaches to this end.
XII. Hypercalcemia
Whereas several epidemiologic studies have shown an incremental association between high serum mineral level and mortality in dialysis patients,106, 111 a recent epidemiologic study found somewhat different calcium threshold levels above which mortality starts to increase, i.e., 8.5 mg/dL vs. 10.5 mg/dL, when conventional Cox survival model with fixed covariates and time-dependent survival models using calendar quarterly varying values where used, respectively.15 As shown in Figure 4, in conventional (non-time-dependent model) only baseline values at the start of the cohort are used, whereas changes over time are ignored. It is not clear which of these two models are more consistent with the real world scenario, but conventional models that ignore changes in biochemical value over time and that assume that baseline values represent subsequent values may be far fetched.
Figure 4.
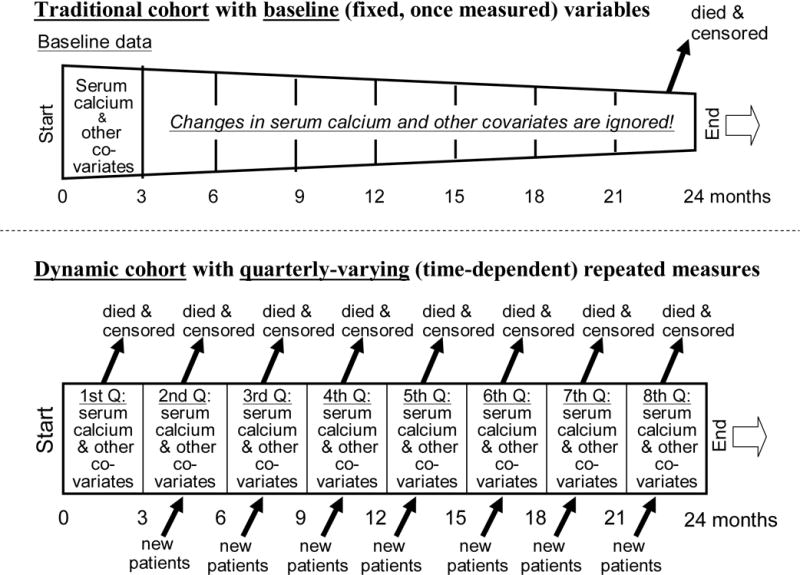
Comparing conventional (fixed covariate) survival models vs. time-dependent (repeated measure) models in examining the association between serum calcium and survival in CKD patients.
XIII. Hypocalcemia
Much more controversy exists as to whether a low serum calcium, e.g. below the KDOQI recommended level of 8.4 mg/dL is harmful or not.112, 113 In the post-calcimimetic era such low calcium values in dialysis patients are no rarities.,114–116 not to mention a lowering trend of dialysate calcium concentration (bath) from 3.5 mEq/L in 1990’s to 2.5 or even 2.25 mEq/L in recent years. Lowered calcium levels may lead to more frequent use of calcium based binders as shown in some controlled trials where more calcium based binders are in use in the calcimimetic arm by the end of the study.117 Time-dependent survival analyses recently showed that such low calcium levels, similar to high calcium values, may be associated with increased death risk.15 Furthermore, a decline in serum calcium over 6 months was associated with increased death risk in the same study.15 Low serum calcium may be associated with cardiac arrhythmias.118 A recent epidemiologic study showed that low calcium level showed a tendency towards higher rates of CKD progression in a group of male NDD-CKD patients.119 Nevertheless, calcimimetics also offer a number of important advantages including reduction of fracture rate and parathyrdoidectomy.120 The concomitant use of active vitamin D analogs with calcimimetics may reduce the risk of severe hypocalcemia and reinforce their beneficial effect. Future studies are required to better address the issue of hypocalcemia in CKD.
Epilogue
The SHPT, vitamin D deficiency and mineral disarrays are associated with multiple skeletal and cardiovascular disorders in CKD. There is a large volume of epidemiologic evidence suggesting a broader beneficial effect from treatment of the Kidney Bone Disease by modulating vitamin D and calcium sensing receptors. Whereas there are insufficient well-designed randomized controlled trials in the field, this shortcoming should not lead to a nihilistic approach to the relevant clinical problems of CKD patients. Nevertheless, due to insufficient clinical data, a single treatment modality, be it nutritional or activate vitamin D, calcimimetics, phosphorus binders, or recombinant PTH, may not claim to be uniformly superior to the others, and a wider therapeutic window often prompts the use of a combination of these agents. Since the ultimate goal is improving the poor survival of CKD patients, any suggested approach to the management of Kidney Bone Disease should be tested to this end.
Acknowledgments
Funding Source:
Dr. Kalantar-Zadeh’s has been funded by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01 DK078106, R21DK078012, and R21 DK077341), American Heart Association grant (0655776Y), a research grant from DaVita and a philanthropic grant from Mr. Harold Simmons.
Footnotes
Relevant Potential Conflict of Interest:
Dr. Kalantar-Zadeh and/or Dr. Kovesdy hav received grants and/or honoraria from Genzyme, Inc, the manufacturer of Sevelamer (Renagel™ and RenVela™) and doxercalciferol (Hectoral™), Abbott laboratories, the manufacturer of Paricalcitol (Zemplar™) and calcitriol (Calcijex™), Shire Phramaceutical, the manufacturer of lanthanum carbonoate (Fosrenol™), and/or Amgen, Inc, the manufacturer of Cinacalcet hydrochloride (Sensipar™).
Other coauthors have declared no conflict of interest.
References
- 1.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Kalantar-Zadeh K. Bone and mineral disorders in pre-dialysis CKD. Int Urol Nephrol. 2008;40:427–440. doi: 10.1007/s11255-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 4.Drueke TB. Is parathyroid hormone measurement useful for the diagnosis of renal bone disease? Kidney Int. 2008;73:674–676. doi: 10.1038/sj.ki.5002800. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Stodell M, Fowler JC. Renal bone disease. J R Soc Med. 2005;98:165–166. doi: 10.1258/jrsm.98.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GH, Benner D, Regidor DL, Kalantar-Zadeh K. Impact of Kidney Bone Disease and Its Management on Survival of Patients on Dialysis. J Ren Nutr. 2007;17:38–44. doi: 10.1053/j.jrn.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Proceedings of the 18th Annual Meeting of the Japanese Society for Kidney Bone Disease, February 24, 2007, Tokyo, Japan. Ther Apher Dial. 2007;11(Suppl 1):S1–66. [PubMed] [Google Scholar]
- 8.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 9.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 10.Andress DL, Coyne DW, Kalantar-Zadeh K, Molitch ME, Zangeneh F, Sprague SM. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract. 2008;14:18–27. doi: 10.4158/EP.14.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Brown AJ, Slatopolsky E. Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007;3:134–144. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1529–1539. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Battleground: chronic kidney disorders mineral and bone disease–calcium obsession, vitamin d, and binder confusion. Clin J Am Soc Nephrol. 2008;3:168–173. doi: 10.2215/CJN.03850907. [DOI] [PubMed] [Google Scholar]
- 14.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr, Thadhani R. Activated injectable vitamin d and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 16.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 17.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 18.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 19.Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, Cannata-Andia JB. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–1078. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]
- 20.Shinaberger CS, Kopple JD, Kovesdy CP, McAllister CJ, van Wyck D, Greenland S, Kalantar-Zadeh K. Ratio of paricalcitol dosage to serum parathyroid hormone level and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1769–1776. doi: 10.2215/CJN.01760408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, Gutierrez O, Camargo CA, Jr, Melamed M, Norris K, Stampfer MJ, Powe NR, et al. Impact of Activated Vitamin D and Race on Survival among Hemodialysis Patients. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 23.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of Oral Calcitriol with Improved Survival in Nondialyzed CKD. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, Akiba T, Greenwood RN, Kimata N, Levin NW, Piera LM, Saran R, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009;24:963–972. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- 25.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederhoffer N, Bobryshev YV, Lartaud-Idjouadiene I, Giummelly P, Atkinson J. Aortic calcification produced by vitamin D3 plus nicotine. J Vasc Res. 1997;34:386–398. doi: 10.1159/000159247. [DOI] [PubMed] [Google Scholar]
- 27.Fleckenstein-Grun G, Thimm F, Frey M, Matyas S. Progression and regression by verapamil of vitamin D3-induced calcific medial degeneration in coronary arteries of rats. J Cardiovasc Pharmacol. 1995;26:207–213. doi: 10.1097/00005344-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kingma JG, Jr, Roy PE. Ultrastructural study of hypervitaminosis D induced arterial calcification in Wistar rats. Artery. 1988;16:51–61. [PubMed] [Google Scholar]
- 29.Repo JM, Rantala IS, Honkanen TT, Mustonen JT, Koobi P, Tahvanainen AM, Niemela OJ, Tikkanen I, Rysa JM, Ruskoaho HJ, Porsti IH. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007;72:977–984. doi: 10.1038/sj.ki.5002458. [DOI] [PubMed] [Google Scholar]
- 30.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 32.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 33.Monier-Faugere MC, Mawad H, Malluche HH. Opposite effects of calcitriol and paricalcitol on the parathyroid hormone-(1–84)/large carboxy-terminal-parathyroid hormone fragments ratio in patients with stage 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1255–1260. doi: 10.2215/CJN.03461006. [DOI] [PubMed] [Google Scholar]
- 34.Drueke TB, Fukagawa M. Whole or fragmentary information on parathyroid hormone? Clin J Am Soc Nephrol. 2007;2:1106–1107. doi: 10.2215/CJN.03140707. [DOI] [PubMed] [Google Scholar]
- 35.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH. Administration of PTH-(7–84) antagonizes the effects of PTH-(1–84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–1138. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 36.Donadio C, Ardini M, Lucchesi A, Donadio E, Cantor T. Parathyroid hormone and large related C-terminal fragments increase at different rates with worsening of renal function in chronic kidney disease patients. A possible indicator of bone turnover status? Clin Nephrol. 2007;67:131–139. doi: 10.5414/cnp67131. [DOI] [PubMed] [Google Scholar]
- 37.Martin KJ, Juppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary LC, Guo MD, Turner SA, et al. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int. 2005;68:1236–1243. doi: 10.1111/j.1523-1755.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 38.Rubin MR, Silverberg SJ, D’Amour P, Brossard JH, Rousseau L, Sliney J, Jr, Cantor T, Bilezikian JP. An N-terminal molecular form of parathyroid hormone (PTH) distinct from hPTH(1 84) is overproduced in parathyroid carcinoma. Clin Chem. 2007;53:1470–1476. doi: 10.1373/clinchem.2007.085506. [DOI] [PubMed] [Google Scholar]
- 39.Slatopolsky E, Cozzolino M, Lu Y, Finch J, Dusso A, Staniforth M, Wein Y, Webster J. Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int. 2003;63:2020–2027. doi: 10.1046/j.1523-1755.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 40.National Kidney Foundation I, Kidney Disease-Dialysis Outcome Quality Initiative. K/DOQI Clinical Practice Guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S202. [PubMed] [Google Scholar]
- 41.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105:c132–138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 42.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 43.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 44.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 45.Berl T, Berns AS, Hufer WE, Hammill K, Alfrey AC, Arnaud CD, Schrier RW. 1,25 dihydroxycholecalciferol effects in chronic dialysis. A double-blind controlled study. Ann Intern Med. 1978;88:774–780. doi: 10.7326/0003-4819-88-6-774. [DOI] [PubMed] [Google Scholar]
- 46.Malluche HH, Ritz E, Werner E, Meyer-Sabellek WA. Long-term administration of vitamin D steroles in incipient and advanced renal failure: effect on bone histology. Clin Nephrol. 1978;10:219–228. [PubMed] [Google Scholar]
- 47.Dusso AS, Finch J, Brown A, Ritter C, Delmez J, Schreiner G, Slatopolsky E. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72:157–164. doi: 10.1210/jcem-72-1-157. [DOI] [PubMed] [Google Scholar]
- 48.Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, Bushinsky DA. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn026. [DOI] [PubMed] [Google Scholar]
- 49.Irvin GL, 3rd, Carneiro DM. Management changes in primary hyperparathyroidism. Jama. 2000;284:934–936. doi: 10.1001/jama.284.8.934. [DOI] [PubMed] [Google Scholar]
- 50.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 51.Salusky IB, Juppner H. New PTH assays and renal osteodystrophy. Pediatr Nephrol. 2004;19:709–713. doi: 10.1007/s00467-004-1433-0. [DOI] [PubMed] [Google Scholar]
- 52.Galassi A, Spiegel DM, Bellasi A, Block GA, Raggi P. Accelerated vascular calcification and relative hypoparathyroidism in incident haemodialysis diabetic patients receiving calcium binders. Nephrol Dial Transplant. 2006;21:3215–3222. doi: 10.1093/ndt/gfl395. [DOI] [PubMed] [Google Scholar]
- 53.Spasovski G, Gelev S, Masin-Spasovska J, Selim G, Sikole A, Vanholder R. Improvement of bone and mineral parameters related to adynamic bone disease by diminishing dialysate calcium. Bone. 2007;41:698–703. doi: 10.1016/j.bone.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Obesity is associated with secondary hyperparathyroidism in men with moderate and severe chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1024–1029. doi: 10.2215/CJN.01970507. [DOI] [PubMed] [Google Scholar]
- 55.Panuccio V, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Asahi K, Miyata T, Zoccali C. Low parathyroid hormone and pentosidine in hemodialysis patients. Am J Kidney Dis. 2002;40:810–815. doi: 10.1053/ajkd.2002.35693. [DOI] [PubMed] [Google Scholar]
- 56.Cantor T. Parathyroid hormone assay drift: an unappreciated problem in dialysis patient management. Semin Dial. 2005;18:359–364. doi: 10.1111/j.1525-139X.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 57.Melamed ML, Eustace JA, Plantinga LC, Jaar BG, Fink NE, Parekh RS, Coresh J, Yang Z, Cantor T, Powe NR. Third-generation parathyroid hormone assays and all-cause mortality in incident dialysis patients: the CHOICE study. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm849. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Hilker S, Martin KJ, Sugimoto T, Slatopolsky E. Biologic activities of parathyroid hormone (1–34) and parathyroid hormone-related peptide (1–34) in isolated perfused rat femur. J Lab Clin Med. 1992;119:738–743. [PubMed] [Google Scholar]
- 59.Mahajan A, Narayanan M, Jaffers G, Concepcion L. Hypoparathyroidism associated with severe mineral bone disease postrenal transplantation, treated successfully with recombinant PTH. Hemodial Int. 2009 doi: 10.1111/j.1542-4758.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 60.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, Van Wyck D, Kopple JD, Kalantar-Zadeh K. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakai S, Akiba T, Kazama J, Yokoyama K, Fukagawa M, Tominaga Y, Iseki K, Tsubakihara Y. Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial. 2008;12:49–54. doi: 10.1111/j.1744-9987.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 62.Dukkipati R, Kovesdy CP, Kim Y, Colman S, Budoff MJ, Nissenson AR, Sprague SM, Kopple JD, Kalantar-Zadeh K. Association of Relatively Low Serum Parathyroid Hormone with Malnutrition-Inflammation Complex and Survival in Maintenance Hemodialysis Patients. Am J Nephrol. 2009 doi: 10.1053/j.jrn.2009.10.006. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reust CE, Hall L. Clinical inquiries. What is the differential diagnosis of an elevated alkaline phosphatase (AP) level in an otherwise asymptomatic patient? J Fam Pract. 2001;50:496–497. [PubMed] [Google Scholar]
- 64.Torres PU. Bone alkaline phosphatase isoforms in chronic renal failure. Kidney Int. 2002;61:1178–1179. doi: 10.1046/j.1523-1755.2002.00241-1.x. [DOI] [PubMed] [Google Scholar]
- 65.Jorge C, Gil C, Possante M, Silva E, Andrade R, Santos N, Cruz A, Teixeira R, Ferreira A. Bone alkaline phosphatase besides intact parathyroid hormone in hemodialysis patients–any advantage? Nephron Clin Pract. 2005;101:c122–127. doi: 10.1159/000086682. [DOI] [PubMed] [Google Scholar]
- 66.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989–991. doi: 10.1038/ki.2008.104. [DOI] [PubMed] [Google Scholar]
- 68.Lomashvili KA, Khawandi W, O’Neill WC. Reduced plasma pyrophosphate levels in hemodialysis patients. J Am Soc Nephrol. 2005;16:2495–2500. doi: 10.1681/ASN.2004080694. [DOI] [PubMed] [Google Scholar]
- 69.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O’Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 70.Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 71.O’Neill WC. Pyrophosphate, alkaline phosphatase, and vascular calcification. Circ Res. 2006;99:e2. doi: 10.1161/01.RES.0000234909.24367.a9. [DOI] [PubMed] [Google Scholar]
- 72.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 74.Sahota O, Masud T, San P, Hosking DJ. Vitamin D insufficiency increases bone turnover markers and enhances bone loss at the hip in patients with established vertebral osteoporosis. Clin Endocrinol (Oxf) 1999;51:217–221. doi: 10.1046/j.1365-2265.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 75.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients [see comments] N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 76.Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis. 2001;38:S45–50. doi: 10.1053/ajkd.2001.28114. [DOI] [PubMed] [Google Scholar]
- 77.Martin KJ, Gonzalez E, Lindberg JS, Taccetta C, Amdahl M, Malhotra K, Llach F. Paricalcitol dosing according to body weight or severity of hyperparathyroidism: a double-blind, multicenter, randomized study. Am J Kidney Dis. 2001;38:S57–63. doi: 10.1053/ajkd.2001.28112. [DOI] [PubMed] [Google Scholar]
- 78.Belozeroff V, Goodman WG, Ren L, Kalantar-Zadeh K. Cinacalcet lowers serum alkaline phosphatase in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:673–679. doi: 10.2215/CJN.03790808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–853. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- 80.Kalantar-Zadeh K, Lee GH, Miller JE, Streja E, Jing J, Robertson JA, Kovesdy CP. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53:823–834. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 82.Kalantar-Zadeh K, Kovesdy CP. Is it worth correcting hyperparathyroidism if hyperphosphatemia and hypocalcemia worsen? A cinacalcet story. Am J Kidney Dis. 2009;53:183–188. doi: 10.1053/j.ajkd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Kalantar-Zadeh K, Kopple JD, Regidor DL, Jing J, Shinaberger CS, Aronovitz J, McAllister CJ, Whellan D, Sharma K. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 84.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MW, Larsson L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 86.Lobao R, Carvalho AB, Cuppari L, Ventura R, Lazaretti-Castro M, Jorgetti V, Vieira JG, Cendoroglo M, Draibe SA. High prevalence of low bone mineral density in pre-dialysis chronic kidney disease patients: bone histomorphometric analysis. Clin Nephrol. 2004;62:432–439. doi: 10.5414/cnp62432. [DOI] [PubMed] [Google Scholar]
- 87.Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 88.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 89.Matsubara K, Suliman ME, Qureshi AR, Axelsson J, Martola L, Heimburger O, Barany P, Stenvinkel P, Lindholm B. Bone mineral density in end-stage renal disease patients: association with wasting, cardiovascular disease and mortality. Blood Purif. 2008;26:284–290. doi: 10.1159/000126925. [DOI] [PubMed] [Google Scholar]
- 90.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 91.Kalantar-Zadeh K, Kopple JD. Response to ‘Adynamic Bone Disease and MICS’. Kidney Int. 2007;71:1327. [letter reply]; [Google Scholar]
- 92.Sanchez-Gonzalez MC, Lopez-Barea F, Bajo MA, Selgas R. Serum albumin levels, an additional factor implicated in hyperparathyroidism outcome in peritoneal dialysis: a prospective study with paired bone biopsies. Adv Perit Dial. 2006;22:198–202. [PubMed] [Google Scholar]
- 93.Carlstedt E, Ridefelt P, Lind L, Rastad J. Interleukin-6 induced suppression of bovine parathyroid hormone secretion. Biosci Rep. 1999;19:35–42. doi: 10.1023/a:1020146023812. [DOI] [PubMed] [Google Scholar]
- 94.Nielsen PK, Rasmussen AK, Butters R, Feldt-Rasmussen U, Bendtzen K, Diaz R, Brown EM, Olgaard K. Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with an up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Commun. 1997;238:880–885. doi: 10.1006/bbrc.1997.7207. [DOI] [PubMed] [Google Scholar]
- 95.Kovesdy CP, Kalantar-Zadeh K. Novel targets and new potential: developments in the treatment of inflammation in chronic kidney disease. Expert Opin Investig Drugs. 2008;17:451–467. doi: 10.1517/13543784.17.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalpakian MA, Mehrotra R. Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial. 2007;20:139–143. doi: 10.1111/j.1525-139X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 97.Mehrotra R. Disordered mineral metabolism and vascular calcification in nondialyzed chronic kidney disease patients. J Ren Nutr. 2006;16:100–118. doi: 10.1053/j.jrn.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Shantouf RS, Budoff MJ, Ahmadi N, Flores F, Tiano J, Gopal A, Kalantar-Zadeh K. Total and Individual Coronary Artery Calcium Scores as Independent Predictors of Mortality in Maintenance Hemodialysis Patients. Nephrol Dial Transplant. 2009 doi: 10.1159/000294405. [submitted] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 100.Nolan CR, Qunibi WY. Calcium salts in the treatment of hyperphosphatemia in hemodialysis patients. Curr Opin Nephrol Hypertens. 2003;12:373–379. doi: 10.1097/00041552-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Block G, Port FK. Calcium phosphate metabolism and cardiovascular disease in patients with chronic kidney disease. Semin Dial. 2003;16:140–147. doi: 10.1046/j.1525-139x.2003.160301.x. [DOI] [PubMed] [Google Scholar]
- 102.Suki WN. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients: results of a randomized clinical trial. J Ren Nutr. 2008;18:91–98. doi: 10.1053/j.jrn.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 104.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 105.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 106.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 107.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amanzadeh J, Reilly RF., Jr Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2:136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 109.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 110.Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kid Dis. 2006;48:37–49. doi: 10.1053/j.ajkd.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 111.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 112.Foley RN, Parfrey PS, Harnett JD, Kent GM, Hu L, O’Dea R, Murray DC, Barre PE. Hypocalcemia, morbidity, and mortality in end-stage renal disease. Am J Nephrol. 1996;16:386–393. doi: 10.1159/000169030. [DOI] [PubMed] [Google Scholar]
- 113.Spodick DH. Classic chronic renal failure: hyperkalemia and hypocalcemia. Am J Geriatr Cardiol. 2005;14:336–337. doi: 10.1111/j.1076-7460.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 114.Nowack R, Wachtler P. Hypophosphatemia and hungry bone syndrome in a dialysis patient with secondary hyperparathyroidism treated with cinacalcet–proposal for an improved monitoring. Clin Lab. 2006;52:583–587. [PubMed] [Google Scholar]
- 115.Lazar E, Hebert K, Poma T, Stankus N. Long-term outcomes of cinacalcet and paricalcitol titration protocol for treatment of secondary hyperparathyroidism. Am J Nephrol. 2007;27:274–278. doi: 10.1159/000101727. [DOI] [PubMed] [Google Scholar]
- 116.Lazar ES, Stankus N. Cinacalcet-induced hungry bone syndrome. Semin Dial. 2007;20:83–85. doi: 10.1111/j.1525-139X.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- 117.Arenas MD, Alvarez-Ude F, Gil MT, Moledous A, Malek T, Nunez C, Devesa R, Carreton MA, Soriano A. Implementation of ‘K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease’ after the introduction of cinacalcet in a population of patients on chronic haemodialysis. Nephrol Dial Transplant. 2007;22:1639–1644. doi: 10.1093/ndt/gfl840. [DOI] [PubMed] [Google Scholar]
- 118.Spodick DH. Hypocalcemia, hyperkalemia, and junctional or sinoventricular rhythm. Am J Geriatr Cardiol. 2005;14:273. doi: 10.1111/j.1076-7460.2005.04221.x. [DOI] [PubMed] [Google Scholar]
- 119.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP. Association of Disorders in Mineral Metabolism with Progression of Chronic Kidney Disease. Clin J Am Soc Neph. 2006;1:825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- 120.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]


