Abstract
This review deals with the design and application strategies of new antibiotics based on naturally occurring antimicrobial peptides (AMPs). The initial candidate can be designed based on three-dimensional structure or selected from a library of peptides from natural or laboratory sources followed by optimization via structure-activity relationship studies. There are also advanced application strategies such as induction of AMP expression from host cells by various factors (e.g., metals, amino acids, vitamin D and sunlight), the use of engineered probiotic bacteria to deliver peptides, the design of prodrug and peptide conjugates to improve specific targeting. In addition, combined uses of newly developed AMPs with existing antimicrobial agents may provide a practical avenue for effective management of antibiotic-resistant bacteria (superbugs, including biofilm). Finally, we highlight AMPs already in use or under clinical trials.
1. Antimicrobial peptides to combat resistant bacteria
There are now global voices calling for solutions to the antibiotic resistance problem. At the moment, key pathogens such as Clostridium difficile and Staphylococcus aureus cause over 25,000 deaths per year [1]. The deaths due to C. difficile increased from almost none in 1989 to 2.4 per 100,000 populations in 2007 [2]. In addition, the total deaths from methicillin-resistant S. aureus (MRSA) are now comparable to those caused by HIV-1 [3]. It is projected that 10 million people could die of infectious diseases by 2050 if effective measures had not been taken [4]. Such a devastating picture should never become a reality. Therefore, efforts are now being made to achieve a proper use of existing antibiotics on one hand, and to develop new alternatives on the other.
Antimicrobial peptides (AMPs) are important candidates for developing novel antibiotics. They are expressed by the host to eliminate invading pathogens and boost immune response [5–10]. Such beneficial effects of AMPs are determined by their physical properties: short (<50 amino acids), cationic (average net charge +3), and having an average hydrophobic content of 42% [11,12]. Some representatives and their properties are provided in Table 1. The cationicity and hydrophobicity of these peptides are two critical elements for generating the frequently observed amphipathic structure (Figure 1a), which enables cationic AMPs to preferentially target anionic bacterial membranes (Figure 1b) rich in phosphatidylglycerols (PGs) [9,10,13,14]. In contrast, human cell membranes are dominated by zwitterionic phospholipids (PCs) and cholesterol (Figure 1c). Such a membrane composition difference is believed to be one of the major reasons why AMPs are selective [9,13]. The membrane-bound peptides appear to exert their effects via multiple mechanisms. For example, human cathelicidin LL-37 peptides can cause anionic lipid clustering, permeate bacterial membranes, and even damage the membranes [14,15] via carpet/toroidal models [16,17]. In addition, different AMPs can work by different mechanisms. While proline-rich peptides, with a low hydrophobicity of 23% on average [11], target bacterial ribosomes [18,19], lantibiotics and cyclotides can both bind to phosphatidylethanolamines (PEs) [20–22]. The expression of multiple AMPs may not be redundant. Rather, these peptides may work synergistically for optimal outcomes [10]. Finally, AMPs can also boost immune response to further clear invading pathogens [6,7]. All of these mechanisms make it difficult for pathogens to develop resistance. Indeed, some AMPs are already in use and Figure 1d depicts the timeline of their applications. This article highlights current design and application strategies for antimicrobial peptides. Our discussion focuses on the most recent achievements published during 2015–2016. Some important AMP discoveries reported in 2014 can be found in our recent review article [20].
Table 1.
Select antimicrobial peptides, amino acid sequences and properties1
| Class | Peptide | Amino acid sequence | Length | Net charge | Pho | Boman index | Source | APD ID |
|---|---|---|---|---|---|---|---|---|
| I | Gramicidin A | VGALAVVVWLWLWLW | 15 | 0 | 93% | −3.3 | Bacteria | 499 |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | 23 | +3 | 43% | 0.41 | Amphibians | 144 | |
| LL-37 (cathelicidin) | LLGDFFRKSKEKIGKEFKRIVQRIKFLRNLVPRTES | 37 | +6 | 35% | 3.0 | human | 310 | |
| Pyrrhocoricin (PrAMP) | VDKGSYLPRPTPPRPIYNRN | 20 | +3 | 15% | 3.2 | insects | 170 | |
| II | Nisin A (lantibiotic) | ITSISLCTPGCKTGALMGCNMKTATCHCSIHVSK | 34 | +3 | 44% | 0.37 | Bacteri | 205 |
| HNP1 (α-defensin) | ACYCRIPACIAGERRYGTCI YQGRLWAFCC | 30 | +3 | 53% | 1.1 | Human | 176 | |
| TAP (β-defensin) | NPVSCVRNK GICVPIRCPGSMKQIGTCVGRAVKCCRKK | 38 | +9 | 42% | 1.7 | Bovine | 235 | |
| Plectasin | GFGCNGPWD EDDMQCHNH CKSIKGYKGGYCAKGGFVCKCY | 40 | +1 | 32% | 1.4 | Fungi | 549 | |
| III | Colistin | XTXXKLLXXT | 10 | +6 | 20% | 2.9 | Bacteria | 2204 |
| Daptomycin | WNDTGKDADGSEY | 13 | −3 | 15% | 3.5 | Bacteria | 2203 | |
| Microcin J25 (bacteriocin) | VGIGTPIFSYGGGAGHVPEYF | 21 | −1 | 33% | −0.64 | Bacteria | 480 | |
| Alamethicin (peptaibol) | PBABAQBVBGLBPVBBEQ | 18 | −1 | 66% | −0.68 | Fungi | 2197 | |
| IV | Gramicidin S | VKLFPVKLFP | 10 | 2 | 60% | −1.3 | Bacteria | 2243 |
| Subtilosin A (bacteriocin) | NKGCATCSIGAACLVDGPIPDFEIAGATGLFGLWG | 35 | −2 | 51% | −0.46 | bacteria | 928 | |
| Kalata B1 (cyclotide) | GLPVCGETCVGGTCNTPG CTCSWPVCTRN | 29 | 0 | 37% | 0.67 | Plants | 729 | |
| RTD-1 (θ-defensin) | GFCRCLCRRGVCRCICTR | 18 | 5 | 55% | 2.8 | Monkey | 445 |
Data taken from the APD (http://aps.unmc.edu/AP), where Pho = hydrophobic%, B = α-aminoisobutyric acid and X = 2,4-diaminobutanoic acid. Boman index was originally called protein-binding potential by Boman [5]. Additional information, including the original reference, can be found in the APD. A unified peptide classification has been proposed [83] and adopted in the APD. This classification is based on the covalent bonding patterns of polypeptides. Class I (or UCLL) contains all linear peptides where both the backbone and sidechains may be modified but do not lead to a loop structure. Class II (or UCSS) contains peptides with a sidechain-sidechain connection, irrespective of intra or inter-connections. Class III (or UCSB) peptides must contain a covalent bond between backbone and sidechain, while class IV (or UCBB) consists of circular peptides due to a bond between backbone and backbone [83].
Figure 1. Antimicrobial peptides (a–c) and their timeline of applications (d).
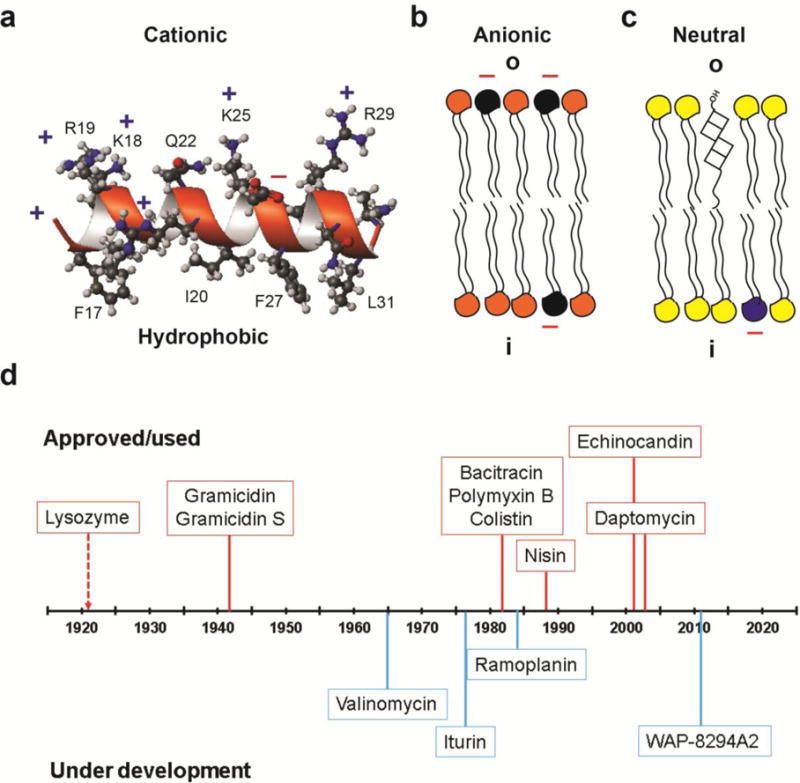
The amphipathic and cationic antimicrobial peptide (a) preferentially binds to anionic bacterial membranes (b) rather than mammalian cell membranes, consisting of phosphatidylcholines (yellow) and cholesterol (square) with minor anionic lipid (blue) in the inner leaf (i) of the bilayer (c). The NMR structure of the major antimicrobial region (residues 17–32) of human cathelicidin LL-37 (a) is used to illustrate the amphipathic feature [14]. In the cartoon view of bacterial inner membranes (b), E. coli is assumed with 30% anionic phophatidylglycerols (black) and 70% phosphatidylethanolamines (orange). (d) Select AMPs in use (orange, top) or under clinical trials (blue, bottom) are depicted. Further examples can be viewed in Table 2.
2. Discovery and design strategies of antimicrobial peptides
Two general strategies are currently in use to obtain a peptide lead. First, a starting candidate can be identified from a peptide library. Second, a peptide candidate can be designed based on the three-dimensional (3D) structure of a target molecule or the peptide itself.
2.1. Library screening
To identify a peptide template, a library of candidates, either from natural or man-made sources, can be screened or searched. Naturally occurring AMPs (currently over 2,800), isolated from the three life domains, are registered in the Antimicrobial Peptide Database (APD) [23]. Such natural peptides appear to have good activity against pathogens, but poor toxicity to host cells [24,25]. By screening the APD database, we have obtained potent antibacterial and anti-HIV peptides [26,27]. One can also use the database filters to design new peptides. For example, frequently occurring amino acids derived from the database are sufficient to design antimicrobial peptides. In addition, most abundant peptide motifs can be searched and combined into new AMPs [23]. Remarkably, it is possible to derive from the database all the parameters needed for ab initio design of a potent peptide against MRSA [25*]. Here a set of AMPs with known activity against Gram-positive bacteria were utilized as templates to extract the most probable peptide parameters such as peptide length, net charge, hydrophobic content and structure. Interestingly, the database-derived parameters have been successfully utilized to synthesize small molecules that closely mimic the database designed peptide [28]. Useful peptides have also been screened directly from uncultivable bacteria by using the i-chip technology, allowing Ling et al. to identify teixobactin as a potentially new peptide antibiotic [29**]. As a different approach, Mongui et al. tried to obtain novel anti-malarial peptides from metagenomic libraries containing candidates originated from diverse microbial communities [30].
Likewise, combinatorial libraries can be used. Masuda et al. screened the library of collagen-like triple-helical peptides to identify RO-A, which is active against Escherichia coli and Bacillus subtilis. The RO-A peptide has stability in human serum and low cytotoxicity to mammalian cells [31]. Chew et al. screened a phage displayed peptide library to identify a novel antiviral peptide gg-ww against dengue virus serotype 2 [32]. In addition, a high-throughput screening system was used to identify a peptoid K15 with modest efficacy against drug resistant pathogens [33].
2.2. Structure-based design
Structure-based design (also called rational design) has been a cornerstone technique for development of traditional antibiotics. In this approach, the 3D structure of the target and drug molecules are normally harnessed to glean the needed clues for molecular design. Interestingly, the major antimicrobial region of human cathelicidin LL-37 for membrane binding was identified via structural studies by NMR spectroscopy [34]. Moreover, the 3D structure of the LL-37 peptide was utilized to enhance antibacterial potency against MRSA [35**].
Not all AMPs target bacterial membranes, however. Proline-rich AMPs (PrAMPs) have recently been shown to bind either the 70S or 50S subunit of ribosomes of Gram-negative bacteria [18,19,36]. Gagnon et al. [37] confirmed this mechanism by determining the crystal structures of several PrAMPs, such as Bac71–35 and pyrrhocoricin (Table 1) in complex with a ribosome. The N-terminal twelve residues of Onc112 are critical for partially occluding the ribosomal exit tunnel and simultaneously overlapping the aminoacyl (A) site, preventing elongation after initiation [38,39]. Furthermore, both potency and activity spectrum of oncocin 18 is enhanced by systematically replacing each amino acid with other 19 residues [40]. Based on the structural information, Goldbach et al. have combined the two types of ribosomal binding PrAMPs by bridging Api137 (an apidaecin analog binding to 50S) and Onc112 (an oncocin binding to 70S) via esterification to ethylene glycol, leading to increased antimicrobial activity against Pseudomonas aeruginosa and E. coli [41*]. Hence, PrAMPs are a promising class of AMPs as a new type of antibiotics. Indeed, Onc72 shows in vivo efficacy comparable to meropenem, although the in vitro activity of meropenem is 44-fold higher [42].
3. Application strategies of antimicrobial peptides
Not all AMPs possess all the required properties (e.g., potency, selectivity, stability, and easy production) for direct uses as antibiotics and additional engineering is usually required to make them drug-like. Alternatively, AMPs may be applied indirectly by inducing gene expression from host cells or by engineering probiotic bacteria as an AMP delivery vehicle.
3.1. Prodrug
The majority of AMPs are gene-encoded and expressed as a precursor protein to neutralize the potential harmful effects of the AMP on the host. The AMP will be released by a protease. A construct that is designed to be similar to the AMP precursor is called prodrug here. This construct may offer advantages such as minimized cytotoxicity and enhanced protease stability. The prodrug form named P-dpMtx, consisting of an anionic peptide, a cephalosporin antibiotic linker, and a delivery peptide (dpMtx), is very effective in clearing mycobacterium residing within the macrophages [43]. Forde et al. described the optimization of the AMP prodrug model for cystic fibrosis (CF) to produce pro-WMR, a peptide with greatly reduced cytotoxicity. The bactericidal activity of pro-WMR is restored in neutrophil elastase-rich bronchoalveolar lavage fluid from the CF patients [44].
The prodrug concept can also be utilized to achieve specific pathogen targeting [45]. Here a prodrug construct can contain a recognition moiety (e.g., antibody), a protease-sensitive peptide linker, and an effector molecule (e.g. rifalogue). Thus, intracellular S. aureus is effectively eliminated by an antibody–antibiotic conjugate [46**]. In another interesting experiment, kanamycin plus a cell penetration peptide (P14LRR) can release the antibiotic in a reduced cellular environment, leading to effective clearing intracellular pathogens (Mycobacterium tuberculosis) within macrophages. It is also effective in vivo against Salmonella in a Caenorhabditis elegans model [47*].
3.2. Conjugation to improve potency or specific targeting
AMP properties may also be improved via conjugation, where two components are merged into one. Different from the prodrug design, there is no cleavage site in such conjugates. The fluoroquinolone antibiotic (levofloxacin) conjugated to a 10-residue CAMP Pep-4 shows enhanced antimicrobial activity even in the presence of an increased salt concentration. However, the antibiotic counterpart is not responsible for the mechanism of action, which was found to be peptide driven through disruption of bacterial membrane integrity [48]. Furthermore, the nonantibiotic moiety dithiocarbazate, conjugated to a cell penetrating peptide, is highly active against a wide range of pathogens, especially S. aureus [49]. Hyperbranched polyglycerol conjugated to aurein 2.2, or conjugation of an AMP to polyphosphoester, yields peptides with better biocompatibility and antimicrobial activity [50–51].
Bacterial targeting and detection of peptide library fragments against Listeria monocytogenes was achieved by binding with the C-terminal fragment (GEAFSAGVHRLANG) of leucocin A, a class IIa bacteriocin [52]. Persister cells of E. coli and S. aureus are effectively targeted by pentobra (cell penetrating peptide conjugated to tobramycin) up to 4–6 logs better than the individual aminoglycoside, tobramycin [53]. Further, chloramphenicol attached to UBI29–41 peptide using a glutaric anhydride linker is selectively targeted at the infection sites with S. aureus and E. coli, and demonstrated efficacy in both in vitro and in vivo mouse models. It also shows reduced toxicity to normal cells [54]. In another study, acridine conjugation to a nuclear localization sequence converts it into a potent broad spectrum AMP, with membrane disruption and DNA binding mechanisms of action [55].
3.3. Combined uses of AMPs with existing antimicrobial agents
Synergy between AMPs could be a fundamental defense strategy to keep different life forms healthy [56*]. In current clinical practice, two or more drugs are frequently used in combination to improve the treatment outcomes. Naturally, a combined use of new AMPs with existing antibiotics can extend the lifetime of traditional antibiotics and reduce the amount of the peptide needed for treatment. This is important considering that the cost of production is a major hurdle in the development of peptide therapeutics.
There are numerous examples demonstrating the advantages of combined use. For instance, synergy between polymyxin and carbapenems or rifampicin can efficiently suppress the development of polymyxin resistance [57]. Also, plectasin NZ2114 is synergistic with new antibiotics like teicoplanin, moenomycin, and dalbavancin against VanA-type vancomycinresistant Enterococcus faecalis [58]. In an important unorthodox combination, colistin with fusidic acid is excellent against multidrug-resistant Acinetobacter baumannii infections [59]. Interestingly, select peptidomimetics are highly active in human blood plasma against a wide range of bacteria. The synergism may result from complement proteins and/or clotting factors [60]. Further, a single combined dose of oligo-acyl-lysyls (OAKs) with rifampin also imparts increased survival chances from 10–20% to 60% against Klebsiella infected mice [61]. In addition, the combination of a lipopeptide bacillomycin D with antifungal amphotericin B leads to excellent antibiofilm and wound-healing properties against Candida albicans [62]. Inexpensive compounds (e.g., ZnCl2, NaF, or EDTA) can also be used as synergistic elements to improve peptide activity [63]. The antimicrobial cell wall hydrolases is synergistic with daptomycin in a murine model of S. aureus bacteremia [64].
Combined uses of AMPs with antibiotics can also enhance their antibiofilm potency [20]. For instance, merecidin (formerly known as 17BIPHE2), a human LL-37 derived peptide, can disrupt the biofilms of S. aureus USA300 under laboratory conditions [65], but is unable to do so in the case of P. aeruginosa. This may result from the complex nature of biofilms with a protective biopolymer coating for the bacterial communities. The preformed biofilms, however, can be dispersed when merecidin is used in combination with existing antibiotics (unpublished).
3.4. Induction of AMP expression in host cells
The observation that AMPs are induced in host cells upon pathogen invasion offers a novel strategy to combat infections. Instead of administrating antibiotics to treat infected patients, doctors may use a proper agent to induce AMP expression at a desired site and at the right time. At present, multiple factors, ranging from amino acids to sunlight, are documented to stimulate the expression of AMPs (reviewed in [66]). The connection between light therapy and the human LL-37 expression provides new insight into this therapy for tuberculosis [67]. In addition, butyrates can work synergistically with vitamin D to induce additional AMPs. Recently, Ottosson et al. [68] found new analogs aroylated phenylenediamines that can induce even more peptides. Importantly, in vivo efficacy of this approach has been demonstrated in a rabbit model for Shigellosis. In the same vein, the maggot therapy is likely related to the expression and secretion of AMPs such as lucifensin into infected wounds by fly larvae that promote healing [69].
3.5. Engineered probiotic bacteria
AMPs may also be expressed by creating an expression vector for use in probiotic bacteria. Multiple lactic acid bacteria (LAB) are regarded as safe by the FDA [70]. Many of these bacteria are symbiotic with their hosts. They confer benefits such as the upregulation of antiinflammatory pathways, the down-regulation of pro-inflammatory cytokine pathways [19,71], protection from enteric pathogens via H2O2 release and species-specific bacteriocin production [72], and attenuation of virulence factor expression by such pathogens [73]. Therefore, LAB are highly desirable as carriers for delivery of AMPs against specific human pathogens that colonize the human intestinal tract [72]. The development of plasmid vectors with AMP insertions should consider an appropriate origin of replication, a co-expressed immunity gene against the inserted AMP, a fused secretory signal recognized by specific ATP-binding cassette (ABC) transporters (or by the more widely used Sec secretion pathway), and a means of inducing expression, if the engineered construct does not enable constitutive expression. A promising construct was made by Themsakul et al. who created an expression secretion vector using new signal peptides (SPs) from Lactobacillus casei ATCC334. The three SPs they chose were used to make green fluorescent protein (GFP) fusions, with the CwhSP showing the highest amount of GFP secretion [74]. CwhSP was then fused and used to express and secrete the M2e:HBc (matrix 2 protein fused to Hepatitis B core antigen) protein in a pLC plasmid. Another LAB vector was made by Jiminez et al. [75] who fused the Sec dependent signal peptide, Usp45, to the enterocin A and the entA immunity gene. Although these systems are promising, there are hurdles to overcome. One such obstacle is that of lateral transfer of these plasmids to other members of the gut microbial community. Another issue stems from unintentional microbiotic imbalances that may be created through administration of these therapeutic LAB, causing unexpected health issues. For example, some commensal bacteria aid in eliciting an immune response through intercellular communication via vesicular release of microbial factors [76]. Prolonged release of AMPs by engineered probiotics may kill commensal bacteria, and thus mitigate their beneficial effects. Nevertheless, as many as seven AMPs have been expressed in a recently reported modular peptide expression system pMPES [77]. In addition, commensal Enterococcus faecalis strain has been engineered to express bacteriocin-21 (a variant enterocin AS-48) via a plasmid with impaired bacterial conjugation ability to de-colonize vancomycin-resistant enterococci in the mouse gut [78**,79].
4. Peptide antibiotics and concluding remarks
After the discovery of human lysozyme, the first antimicrobial protein, in 1922 by Alexander Fleming (refer to the AMP discovery timeline at http://aps.unmc.edu/AP/timeline.php), multiple peptides have been discovered and used (Figure 1d). Some of the peptides in Table 2 are assembled by a multienzyme system. Bacterial gramicidin is the first peptide antibiotic used to treat wound infections [80]. Daptomycin is another FDA-approved AMP for treating Gram-positive bacterial infections [81], adding another example to the list of antimicrobial peptides in clinical use (Table 2). In addition, nisin is the first ribosomally synthesized AMP widely utilized as a food preservative in over 50 countries [82]. Interestingly, these AMPs (Table 1 and its footnote) fall into the four unified peptide classes proposed by Wang [83], implying that a wide range of peptides has a potential to become a new antibiotic.
Table 2.
Select antimicrobial peptides in use or under development1
| Class | Peptide | Source | Activity spectrum | Mechanism of action | Approval/Use | Ref |
|---|---|---|---|---|---|---|
| I | Gramicidin | Bacillus brevis | Gram +/− pathogens | Forms an ion channel in membranes. | Ophthalmic use as solutions/drops (1940s) | [85] |
| II | Nisin | Lactococcus lactis | Gram +pathogens | Binds lipid II and inhibits cell wall synthesis. | Food preservative (1969, WHO; 1988, FDA) | [86] |
| III | Bacitracin | Bacillus subtilis | Gram +/− pathogens | Inhibits cell wall and peptidoglycan synthesis. | Powder form (before 1982) | [87] |
| III | Polymyxin B | Bacillus polymyxa | Gram− pathogens (e.g., P. aeruginosa) | Damages membranes | Aerosporin injection (before 1982); endotoxin removing (2003) | [88] |
| III | Colistin, or polymyxin E | Paenibacillu s polymyxa | Pseudomon as and Acinetobacter sp. | Damages membranes | Suspension/drops (1982) | [89] |
| III | Daptomycin | Streptomyces roseosporus. | Gram+ MRSA | Damages membranes | Treat complicated skin infection (2003, FDA). | [90] |
| III | Echinocandin2 | Glarea lozoyensis; Coleophoma empedra; A. nidulans | Fungi (C. albicans) | Inhibits the synthesis of glucan and cell wall. | FDA approved | [91] |
| IV | Gramicidin S | Bacillus brevis | Gram+/− pathogens | Disrupts the outer membrane. | Wound infections (1942, Soviet Union) | [92] |
| I | Pexiganan (Magainin) | Xenopus laevis | Diabetic foot ulcers | Membranes | Phase III | [93] |
| I | Omiganan (indolicidin) | Bos taurus | Rosacea | Membrane/DNA | Phase II | [94] |
| I | OP-145 (LL-37) | Homo sapiens | Chronic middle ear infection | Membranes | Phase I/II | [94] |
| I | PXL01 (lactoferricin) | Homo sapiens | Prevention of postsurgical adhesion formation | Repressing proinflammatory cytokine secretion and promoting fibrinolysis. | Phase II | [94] |
| I | PAC-113 (histatin 3) | Homo sapiens | Oral candidiasis | Membranes/Inhibiting cytokine production. | Phase II | [94] |
| II | Iseganan/IB-367 (protegrin-1) | Sus scrofa | Oral mucositis | Membranes | Phase III | [94] |
| II | Novexatin/NP-213 (defensin) | Homo sapiens | Fungal nail infection | Membrane lysis | Phase II | [94] |
| III | Iturin A (lipopeptide) | Bacillus subtilis | Fungi; G +/− bacteria | Forms ion conducting pores in membranes. | Preclinical | [95] |
| III | Ramoplanin | Actinoplanes sp. ATCC 33076. | Gram+ bacteria | Inhibits cell wall synthesis. | VRE (Phase III); C. difficile (Phase II). | [96] |
| III | WAP-8294A2 (Lotilibcin) | Lysobacter sp. | Gram+ bacteria (VRE and MRSA) | Interacts with membranes. | Systemic infection (Phase I/II) | [97] |
| IV | Valinomycin | Streptomyces tsusimaensis; S. fulvissimus | Gram+ bacteria; Fungi (C. albicans) | Forms a K+ ion channel in membranes. | Not available | [98] |
Data from http://www.accessdata.fda.gov/scripts/cder/ob/search_product.cfm; https://clinicaltrials.gov/; https://pubchem.ncbi.nlm.nih.gov/; http://aps.unmc.edu/AP.
The FDA-approved echinocandins are seminsynthetic lipopeptides derived from caspofungin, micafungin, and anidulafungin.
By utilizing the design and application strategies in Figure 2, as well as expanding the search space in nature, additional AMPs are anticipated to find practical uses. The prodrug and peptide conjugates are elegant constructions that can improve drug specificity and potency at the expense of cost. Cell-specific and timely expression of AMPs from the host by inducing agents such as light and vitamin D, or via the delivery of engineered probiotic bacteria will be an important antimicrobial strategy actively under development. Finally, understanding the mechanism of commensal bacterial survival or pathogenic bacterial resistance to antibiotics, including AMPs, may provide new clue to the development of novel antibiotics [20]. Indeed, additional antibacterial or antifungal peptides are currently under clinical trials (see select examples in Table 2).
Figure 2. Application strategies of antimicrobial peptides.
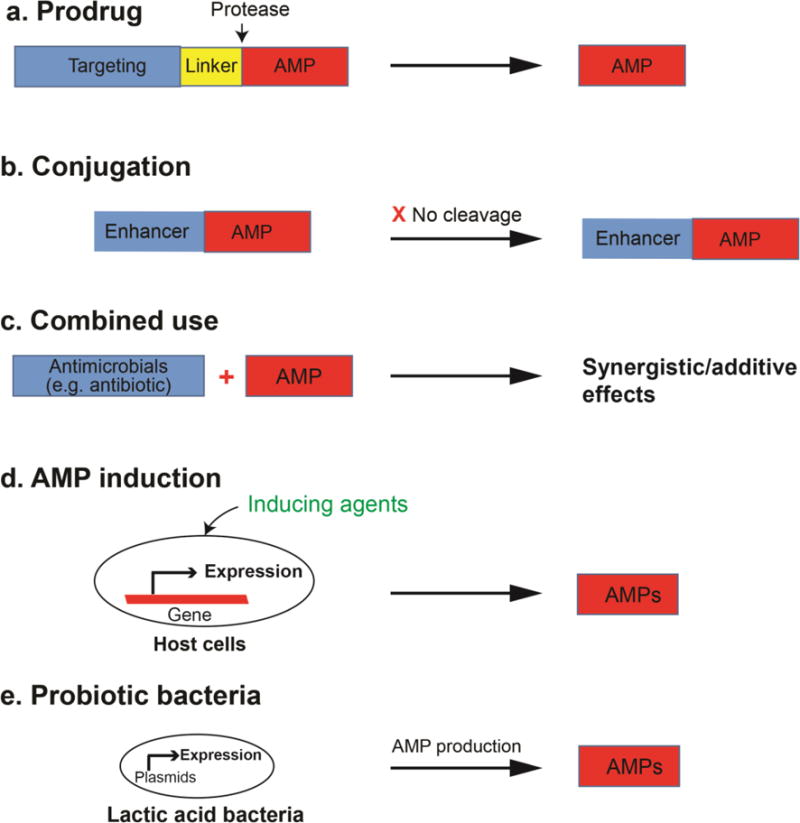
(a) prodrug; (b) conjugation; (c) combined use; (d) induction from the host; and (e) the use of probiotic bacteria to deliver and express the needed antimicrobial peptides. See the text for further details.
In summary, we predict that future peptide antibiotic research will at least cover the following three aspects: First, new treatment possibilities for existing AMPs will be further explored [84]. Second, new AMPs may be developed by utilizing the strategies highlighted in Figure 2 [10] and our search for new peptide candidates from nature will continue. Third, new AMPs and existing antimicrobial agents may be used in combination to better combat antibioticresistant bacteria (i.e., superbugs), especially those in the biofilm form [20]. Cost is regarded as a limiting factor in developing peptide antibiotics. With the development of personalized medicine, however, it may not be always required to produce AMPs at a large scale, thereby removing the cost issue as well.
Highlights.
Antibiotic resistance is a serious problem of our era.
Antimicrobial peptides are important templates for developing new antibiotics.
Both peptide discovery and application strategies are described.
Some AMPs are already in use and more are under development.
Acknowledgments
We are grateful to the NIH funding support (R01 AI105147 and R03 AI128230) to GW. DZ gratefully acknowledges UGC, Govt. of India for the Raman Postdoctoral Fellowship (F.No. 5–133/2016 (IC)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* Papers of interest
** Papers of outstanding interest
- 1.National Action Plan for Combating Antibiotic-resistant Bacteria. the White House; Washington: Mar, 2015. [Google Scholar]
- 2.Hansen V, Oren E, Dennis LK, Brown HE. Infectious Disease Mortality Trends in the United States, 1980–2014. JAMA. 2016;316:2149–2151. doi: 10.1001/jama.2016.12423. [DOI] [PubMed] [Google Scholar]
- 3.http//www.cdc.gov/hiv/surveillance/resources/reports/2005report/.
- 4.O’Neill J. The Review on Antimicrobial Resistance: Tracking drug-resistant infections globally. UK: Dec, 2014. [Google Scholar]
- 5.Boman HG. Antibacterial peptides: Basic facts and emerging concepts. J Inter Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 6.Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 7.Lai Y, Gallo RL. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeaman MR, Yount NY. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Antimicrobial peptides of multicellullar organisms. Nature. 2012;415:359–365. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Wang G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. CABI; Wallingford, UK: 2010. [Google Scholar]
- 11.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Wang G. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Epand RF, Mishra B, Lushnikova T, Thomas VC, Bayles KW, Epand RM. Decoding the functional roles of cationic side chains of the major antimicrobial region of human cathelicidin LL-37. Antimicrob Agents Chemother. 2012;56:845–856. doi: 10.1128/AAC.05637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epand RF, Wang G, Berno B, Epand RM. Lipid segregation explains the antimicrobial activity of fragments of the human cathelicidin LL-37. Antimicrob Agents Chemother. 2009;53:3705–3714. doi: 10.1128/AAC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341(Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 17.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42(21):6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 18.Krizsan A, Volke D, Weinert S, Strater N, Knappe D, Hoffmann R. Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew Chem Int Ed Engl. 2014;53:12236–12239. doi: 10.1002/anie.201407145. [DOI] [PubMed] [Google Scholar]
- 19.Mardirossian M, Grzela R, Giglione C, Meinnel T, Gennaro R, Mergaert P, Scocchi M. The Host antimicrobial peptide Bac7(1–35) binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem Biol. 2014;21:1639–1647. doi: 10.1016/j.chembiol.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Mishra B, Lau K, Lushnikova T, Golla R, Wang X. Antimicrobial peptides in 2014. Pharmaceuticals. 2015;8:123–150. doi: 10.3390/ph8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troeira Henriques S, Craik DJ. Cyclotide Structure and Function: The Role of Membrane Binding and Permeation. Biochemistry. 2017;56:669–682. doi: 10.1021/acs.biochem.6b01212. [DOI] [PubMed] [Google Scholar]
- 22.Hullin-Matsuda F, Makino A, Murate M, Kobayashi T. Probing phosphoethanolaminecontaining lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie. 2016;130:81–90. doi: 10.1016/j.biochi.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra B, Lushnikova T, Golla RM, Wang X, Wang G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017;49:316–328. doi: 10.1016/j.actbio.2016.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Mishra B, Wang G. Ab initio design of potent anti-MRSA peptides based on database filtering technology. J Am Chem Soc. 2012;134:12426–12429. doi: 10.1021/ja305644e. This is the first demonstration of peptide design based entirely on the antimicrobial peptide database, which contains a library of well-curated peptides and a series of information filters. Recently, this database derived design concept has been used successfully to synthesize small molecule mimics [28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menousek J, Mishra B, Hanke ML, Heim CE, Kielian T, Wang G. Database screening and in vivo efficacy of antimicrobial peptides against methicillin-resistant Staphylococcus aureus USA300. Int J Antimicrob Agents. 2012;39:402–406. doi: 10.1016/j.ijantimicag.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Waston K, Peterkofsky A, Buckheit R., Jr Identification of novel human immunodeficiency virus type 1 inhibitory peptides based on the antimicrobial peptide database. Antimicrob Agents Chemother. 2010;54:1343–1346. doi: 10.1128/AAC.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Y, Lushnikova T, Golla RM, Wang X, Wang G. Small molecule mimics of DFTamP1, a database designed anti-Staphylococcal peptide. Bioorg Med Chem. 2017;25:864–869. doi: 10.1016/j.bmc.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. The exploitation of previously uncultivatable bacteria enabled the authors to identify a novel peptide antibiotic that targets bacterial cell wall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongui A, Pérez-Llanos FJ, Yamamoto MM, Lozano M, Zambrano MM, Del Portillo P, Fernández-Becerra C, Restrepo S, Del Portillo HA, Junca H. Development of a genetic tool for functional screening of anti-malarial bioactiveextracts in metagenomic libraries. Malar J. 2015;14:233. doi: 10.1186/s12936-015-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda R, Kudo M, Dazai Y, Mima T, Koide T. Collagen-like antimicrobial peptides. Biopolymers. 2016;106:453–459. doi: 10.1002/bip.22791. [DOI] [PubMed] [Google Scholar]
- 32.Chew MF, Tham HW, Rajik M, Sharifah SH. Anti-dengue virus serotype 2 activity and mode of action of a novel peptide. J Appl Microbiol. 2015;119:1170–80. doi: 10.1111/jam.12921. [DOI] [PubMed] [Google Scholar]
- 33.Fisher KJ, Turkett JA, Corson AE, Bicker KL. Peptoid Library Agar Diffusion (PLAD) Assay for the High Throughput Identification of Antimicrobial Peptoids. ACS Comb Sci. 2016;18:287–91. doi: 10.1021/acscombsci.6b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 35**.Wang G, Hanke ML, Mishra B, Lushnikova T, Heim CE, Chittezham Thomas V, Bayles KW, Kielian T. Transformation of human cathelicidin LL-37 into selective, stable, and potent antimicrobial compounds. ACS Chem Biol. 2014;9:1997–2002. doi: 10.1021/cb500475y. Structure-based design of antimicrobial peptides is rare [10]. This paper reports a true structure-based design. In the 3D structure of the protease stable peptide template derived from the major antimicrobial region of human cathelicidin LL-37 [34], a hydrophobic cavity is detected. By repairing this defect, the peptide merecidin (i.e., 17BIPHE2) gains activity against MRSA as well as other ESKAPE pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krizsan A, Prahl C, Goldbach T, Knappe D, Hoffmann R. Short Proline-Rich Antimicrobial Peptides Inhibit Either the Bacterial 70S Ribosome or the Assembly of its Large 50S Subunit. Chembiochem. 2015;16:2304–8. doi: 10.1002/cbic.201500375. [DOI] [PubMed] [Google Scholar]
- 37.Gagnon MG, Roy RN, Lomakin IB, Florin T, Mankin AS, Steitz TA. Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein synthesis inhibition. Nucleic Acids Res. 2016;44:2439–2450. doi: 10.1093/nar/gkw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seefeldt AC, Nguyen F, Antunes S, Perebaskine N, Graf M, Arenz S, Inampudi KK, Douat C, Guichard G, Wilson DN, Innis CA. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat Struct Mol Biol. 2015;22:470–475. doi: 10.1038/nsmb.3034. [DOI] [PubMed] [Google Scholar]
- 39.Roy RN, Lomakin IB, Gagnon MG, Steitz TA. The mechanism of inhibition of protein synthesis by the proline-rich peptide oncocin. Nat Struct Mol Biol. 2015;22:466–469. doi: 10.1038/nsmb.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knappe D, Ruden S, Langanke S, Tikkoo T, Ritzer J, Mikut R, Martin LL, Hoffmann R. Optimization of oncocin for antibacterial activity using a SPOT synthesis approach: extending the pathogen spectrum to Staphylococcus aureus. Amino Acids. 2016;48:269–80. doi: 10.1007/s00726-015-2082-2. [DOI] [PubMed] [Google Scholar]
- 41*.Goldbach T, Knappe D, Reinsdorf C, Berg T, Hoffmann R. Ribosomal binding and antibacterial activity of ethylene glycol-bridged apidaecin Api137 and oncocin Onc112 conjugates. J Pept Sci. 2016;22:592–599. doi: 10.1002/psc.2905. This paper illustrates structure-based design of a hybrid peptide that covers the two different binding sites (50S and 70S) of the ribosomes, leading to enhanced antimicrobial potency. [DOI] [PubMed] [Google Scholar]
- 42.Knappe D, Adermann K, Hoffmann R. Oncocin Onc72 is efficacious against antibiotic-susceptible Klebsiella pneumoniae ATCC 43816 in a murine thigh infection model. Biopolymers. 2015;104:707–11. doi: 10.1002/bip.22668. [DOI] [PubMed] [Google Scholar]
- 43.Pereira MP, Shi J, Kelley SO. Peptide targeting of an antibiotic prodrug toward phagosome-entrapped mycobacteria. ACS Infect Dis. 2015;1:586–592. doi: 10.1021/acsinfecdis.5b00099. [DOI] [PubMed] [Google Scholar]
- 44.Forde É, Schütte A, Reeves E, Greene C, Humphreys H, Mall M, Fitzgerald-Hughes D, Devocelle M. Differential In Vitro and In Vivo Toxicities of Antimicrobial Peptide Prodrugs for Potential Use in Cystic Fibrosis. Antimicrob Agents Chemother. 2016;60:2813–21. doi: 10.1128/AAC.00157-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forde E, Devocelle M. Pro-Moieties of Antimicrobial Peptide Prodrugs. Molecules. 2015;20:1210–1227. doi: 10.3390/molecules20011210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. This paper demonstrates specific targeting of S. aureus by constructing an antibody-linker-antibiotic. The cleavage of the construct releases the antibiotic that kills the bacteria. This design potentially minimizes unwanted toxic effects. [DOI] [PubMed] [Google Scholar]
- 47*.Brezden A, Mohamed MF, Nepal M, Harwood JS, Kuriakose J, Seleem MN, Chmielewski J. Dual targeting of intracellular pathogenic bacteria with a cleavable conjugate of kanamycin and an antibacterial cell-penetrating peptide. J Am Chem Soc. 2016;138:10945–10949. doi: 10.1021/jacs.6b04831. This paper deals with the delivery of kanamycin by using a cell-penetrating peptide to eliminate intracellular Mycobacterium tuberculosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez CA, Papanastasiou EA, Juba M, Bishop B. Covalent modification of a tenresidue cationic antimicrobial peptide with levofloxacin. Front Chem. 2014;2:71. doi: 10.3389/fchem.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Low ML, Maigre L, Dorlet P, Guillot R, Pages JM, Crouse KA, Policar C, Delsuc N. Conjugation of a new series of dithiocarbazate Schiff base Copper(II) complexes with vectors selected to enhance antibacterial activity. Bioconjug Chem. 2014;25:2269–2284. doi: 10.1021/bc5004907. [DOI] [PubMed] [Google Scholar]
- 50.Kumar P, Shenoi RA, Lai BF, Nguyen M, Kizhakkedathu JN, Straus SK. Conjugation of aurein 2.2 to HPG yields an antimicrobial with better properties. Biomacromolecules. 2015;16:913–923. doi: 10.1021/bm5018244. [DOI] [PubMed] [Google Scholar]
- 51.Pranantyo D, Xu LQ, Kang ET, Mya MK, Chan-Park MB. Conjugation of Polyphosphoester and Antimicrobial Peptide for Enhanced Bactericidal Activity and Biocompatibility. Biomacromolecules. 2016;17:4037–4044. doi: 10.1021/acs.biomac.6b01452. [DOI] [PubMed] [Google Scholar]
- 52.Azmi S, Jiang K, Stiles M, Thundat T, Kaur K. Detection of Listeria monocytogenes with short peptide fragments from class IIa bacteriocins as recognition elements. ACS Comb Sci. 2015;17:156–163. doi: 10.1021/co500079k. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt NW, Deshayes S, Hawker S, Blacker A, Kasko AM, Wong GC. Engineering persister-specific antibiotics with synergistic antimicrobial functions. ACS Nano. 2014;8:8786–8793. doi: 10.1021/nn502201a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, Liu C, Chen D, Madrid K, Peng S, Dong X, Zhang M, Gu Y. Bacteria-targeting conjugates based on antimicrobial peptide for bacteria diagnosis and therapy. Mol Pharm. 2015;12:2505–2516. doi: 10.1021/acs.molpharmaceut.5b00053. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Yang X, Song J, Zheng X, Chen J, Ma P, Zhang B, Wang R. Conjugation with acridines turns nuclear localization sequence into highly active antimicrobial peptide. Engineering. 2015;1:500–505. [Google Scholar]
- 56*.Yu G, Baeder DY, Regoes RR, Rolff J. Combination effects of antimicrobial peptides. Antimicrob Agents Chemother. 2016;60:1717–24. doi: 10.1128/AAC.02434-15. This paper illustrates the importance of synergy of AMPs in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents. 2015;45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Breidenstein EB, Courvalin P, Meziane-Cherif D. Antimicrobial activity of plectasin nz2114 in combination with cell wall targeting antibiotics against vana-type Enterococcus faecalis. Microb Drug Resist. 2015;21:373–379. doi: 10.1089/mdr.2014.0221. [DOI] [PubMed] [Google Scholar]
- 59.Phee LM, Betts JW, Bharathan B, Wareham DW. Colistin and fusidic acid, a novel potent synergistic combination for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2015;59:4544–4550. doi: 10.1128/AAC.00753-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Citterio L, Franzyk H, Palarasah Y, Andersen TE, Mateiu RV, Gram L. Improved in vitro evaluation of novel antimicrobials: potential synergy between human plasma and antibacterial peptidomimetics, AMPs and antibiotics against human pathogenic bacteria. Res Microbiol. 2016;167:72–82. doi: 10.1016/j.resmic.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Jammal J, Zaknoon F, Kaneti G, Goldberg K, Mor A. Sensitization of Gram-negative bacteria to rifampin and OAK combinations. Sci Rep. 2015;5:9216. doi: 10.1038/srep09216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabbene O, Azaiez S, Di Grazia A, Karkouch I, Ben Slimene I, Elkahoui S, Alfeddy MN, Casciaro B, Luca V, Limam F, Mangoni ML. Bacillomycin D and its combination with amphotericin B: promising antifungal compounds with powerful antibiofilm activity and wound-healing potency. J Appl Microbiol. 2016;120:289–300. doi: 10.1111/jam.13030. [DOI] [PubMed] [Google Scholar]
- 63.Walkenhorst WF. Using adjuvants and environmental factors to modulate the activity of antimicrobial peptides. Biochim Biophys Acta. 2016;1858:926–935. doi: 10.1016/j.bbamem.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 64.Wittekind M, Schuch R. Cell wall hydrolases and antibiotics: exploiting synergy to create efficacious new antimicrobial treatments. Curr Opin Microbiol. 2016;33:18–24. doi: 10.1016/j.mib.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Mishra B, Golla R, Lau K, Lushnikova T, Wang G. Anti-Staphylococcal biofilm effects of human cathelicidin peptides. ACS Med Chem Lett. 2016;7:117–121. doi: 10.1021/acsmedchemlett.5b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals. 2014;7:545–594. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jarrett P, Scragg R. A short history of phototherapy, vitamin D and skin disease. Photochem Photobiol Sci. 2017;16:283–290. doi: 10.1039/c6pp00406g. [DOI] [PubMed] [Google Scholar]
- 68.Ottosson H, Nylén F, Sarker P, Miraglia E, Bergman P, Gudmundsson GH, Raqib R, Agerberth B, Strömberg R. Potent Inducers of Endogenous Antimicrobial Peptides for Host Directed Therapy of Infections. Sci Rep. 2016;6:36692. doi: 10.1038/srep36692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Čeřovský V, Bém R. Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals. 2014;7:251–264. doi: 10.3390/ph7030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valeriano VD, Balolong MP, Kang DK. Probiotic Roles of Lactobacillus spp. in Swine: Insights from Gut Microbiota. J Appl Microbiol. 2017;122:554–567. doi: 10.1111/jam.13364. [DOI] [PubMed] [Google Scholar]
- 71.Riaz Rajoka MS, Shi J, Zhu J, Shao D, Huang Q, Yang H, Jin M. Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl Microbiol Biotechnol. 2017;101:35–45. doi: 10.1007/s00253-016-8005-7. [DOI] [PubMed] [Google Scholar]
- 72.Affhan S, Dachang W, Xin Y, Shang D. Lactic acid bacteria protect human intestinal epithelial cells from Staphylococcus aureus and Pseudomonas aeruginosa infections. Genet Mol Res. 2015;14:17044–17058. doi: 10.4238/2015.December.16.5. [DOI] [PubMed] [Google Scholar]
- 73.Yang X, Brisbin J, Yu H, Wang Q, Yin F, Zhang Y, Sabour P, Sharif S, Gong J. Selected lactic acid-producing bacterial isolates with the capacity to reduce Salmonella translocation and virulence gene expression in chickens. PLoS One. 2014;9:e93022. doi: 10.1371/journal.pone.0093022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Themsakul S, Suebwongsa N, Mayo B, Panya M, Lulitanond V. Secretion of M2e:HBc fusion protein by Lactobacillus casei using Cwh signal peptide. FEMS Microbiol Lett. 2016;363:fnw209. doi: 10.1093/femsle/fnw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez JJ, Diep DB, Borrero J, Gutiez L, Arbulu S, Nes IF, Herranz C, Cintas LM, Hernandez PE. Cloning strategies for heterologous expression of the bacteriocin enterocin A by Lactobacillus sakei Lb790, Lb. plantarum NC8 and Lb casei CECT475. Microb Cell Fact. 2015;14 doi: 10.1186/s12934-015-0346-x. 166-015-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M, Badía J, Baldomà L. Activation of Immune and Defense Responses in the Intestinal Mucosa by Outer Membrane Vesicles of Commensal and Probiotic Escherichia coli Strains. Front Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geldart K, Forkus B, McChesney E, McCue M, Kaznessis YN. pMPES: A Modular Peptide Expression System for the Delivery of Antimicrobial Peptides to the Site of Gastrointestinal Infections Using Probiotics. Pharmaceuticals. 2016;9:60. doi: 10.3390/ph9040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Kommineni S, Kristich CJ, Salzman NH. Harnessing bacteriocin biology as targeted therapy in the GI tract. Gut Microbes. 2016;7:512–517. doi: 10.1080/19490976.2016.1233089. This paper demonstrates a possible use of an engineered probiotic bacterium to re-establish the commensal bacteria and replace the unwanted colonization of resistant pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature. 2015;526:719–722. doi: 10.1038/nature15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patrick JW, Gamez RC, Russell DH. The Influence of Lipid Bilayer Physicochemical Properties on Gramicidin A Conformer Preferences. Biophys J. 2016;110:1826–1835. doi: 10.1016/j.bpj.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hofer U. Antimicrobials: The central role of lipids in daptomycin action. Nat Rev Microbiol. 2016;14:729. doi: 10.1038/nrmicro.2016.172. [DOI] [PubMed] [Google Scholar]
- 82.Rai M, Pandit R, Gaikwad S, Kövics G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J Food Sci Technol. 2016;53:3381–3394. doi: 10.1007/s13197-016-2318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol Biol. 2015;1268:43–66. doi: 10.1007/978-1-4939-2285-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berditsch M, Lux H, Babii O, Afonin S, Ulrich AS. Therapeutic Potential of Gramicidin S in the Treatment of Root Canal Infections. Pharmaceuticals. 2016;9:56. doi: 10.3390/ph9030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubos RJ. Studies on a bactericidal agent extracted from a soil bacillus: i. preparation of the agent its activity in vitro. J Exp Med. 1939;70:1–10. doi: 10.1084/jem.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mattick AT, Hirsch A. Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet. 1947;2:5–8. doi: 10.1016/s0140-6736(47)90004-4. [DOI] [PubMed] [Google Scholar]
- 87.Johnson BA, Anker H, Meleney FL. Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science. 1945;102:376–377. doi: 10.1126/science.102.2650.376. [DOI] [PubMed] [Google Scholar]
- 88.Ainsworth GC, Brown AM, Brownlee G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature. 1947;159:263. doi: 10.1038/160263a0. [DOI] [PubMed] [Google Scholar]
- 89.Few AV, Schulman JH. The absorption of polymyxin E by bacteria and bacterial cell walls and its bactericidal action. J Gen Microbiol. 1953;9:454–466. doi: 10.1099/00221287-9-3-454. [DOI] [PubMed] [Google Scholar]
- 90.Eliopoulos GM, Willey S, Reiszner E, Spitzer PG, Caputo G, Moellering RC., Jr In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986;30:532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denning DW. Echinocandins and pneumocandins–a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 92.Gause GF, Brazhnikova MG. Gramicidin S and its use in the treatment of infected wounds. Nature. 1944;154:703. [Google Scholar]
- 93.Fox JL. Antimicrobial peptides stage a comeback. Nat Biotechnol. 2013;31:379–383. doi: 10.1038/nbt.2572. [DOI] [PubMed] [Google Scholar]
- 94.Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Besson F, Peypoux F, Michel G, Delcambe L. Characterization of iturin A in antibiotics from various strains of Bacillus subtilis. J Antibiot (Tokyo) 1976;29:1043–1049. doi: 10.7164/antibiotics.29.1043. [DOI] [PubMed] [Google Scholar]
- 96.Cavalleri B, Pagani H, Volpe G, Selva E, Parenti F. A-16686, a new antibiotic from Actinoplanes. I. Fermentation, isolation and preliminary physico-chemical characteristics. J Antibiot (Tokyo) 1984;37:309–317. doi: 10.7164/antibiotics.37.309. [DOI] [PubMed] [Google Scholar]
- 97.Kato A, Nakaya S, Kokubo N, Aiba Y, Ohashi Y, Hirata H, Fujii K, Harada K. A new anti-MRSA antibiotic complex, WAP-8294A. I. Taxonomy, isolation and biological activities. J Antibiot (Tokyo) 1998;51:929–935. doi: 10.7164/antibiotics.51.929. [DOI] [PubMed] [Google Scholar]
- 98.Harold FM, Baarda JR. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967;94:53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


