Abstract
Objective
The extended face network contains clusters of neurons that perform distinct functions on facial stimuli. Regions in the posterior ventral visual stream appear to perform basic perceptual functions on faces, while more anterior regions, such as the ventral anterior temporal lobe and amygdala, function to link mnemonic and affective information to faces. Anterior and posterior regions are interconnected by a long-range white matter tracts however it is not known if variation in connectivity of these pathways explains cognitive performance.
Methods
Here, we used diffusion imaging and deterministic tractography in a cohort of 28 neurologically normal adults ages 18–28 to examine microstructural properties of visual fiber pathways and their relationship to certain mnemonic and affective functions involved in face processing. We investigated how inter-individual variability in two tracts, the inferior longitudinal fasciculus (ILF) and the inferior fronto-occipital fasciculus (IFOF), related to performance on tests of facial emotion recognition and face memory.
Results
Results revealed that microstructure of both tracts predicted variability in behavioral performance indexed by both tasks, suggesting that the ILF and IFOF play a role in facilitating our ability to discriminate emotional expressions in faces, as well as to remember unique faces. Variation in a control tract, the uncinate fasciculus, did not predict performance on these tasks.
Conclusions
These results corroborate and extend the findings of previous neuropsychology studies investigating the effects of damage to the ILF and IFOF, and demonstrate that differences in face processing abilities are related to white matter microstructure, even in healthy individuals.
Keywords: diffusion imaging, faces, emotion, prosopagnosia, face memory, hypoemotionality
INTRODUCTION
Over the past 100 years, several neuropsychological syndromes have been attributed to disruption or disconnection of visual fiber pathways. For instance, associative visual agnosia, visual amnesia (a deficit in storing visual experiences in memory, but not experiences from other sensory modalities), and visual hypo-emotionality (difficulties feeling emotions for visual stimuli but not stimuli registered in non-visual modalities) have at one time or another been attributed to disconnection (reviewed by Catani et al., 2003). The logic underlying this is that brain regions involved in processing perceptual aspects of visual stimuli must interact with limbic regions that process emotion and memory for normal functioning and that disruption of the fiber pathways linking these systems can give rise to disordered processing.
One tract that is geographically positioned to connect visual perceptual processes with limbic emotion and memory processes is the inferior longitudinal fasciculus (ILF). The ILF is a monosynaptic pathway connecting ventral extrastriate regions, and in some cases portions of the inferior parietal lobe, to the anterior temporal lobe (superior, middle, and inferior gyri, as well as the uncus/parahippocampal gyrus), the hippocampus, and the amygdala.
Damage to this tract can result in visual-emotion disconnection syndromes. In one of the earliest studies, Horel and Misantone (1974) transected white matter running along the basolateral portion of the temporal lobes in three monkeys. These monkeys became hypoemotional and lost the ability to retain newly learned visual information. However, this finding must be interpreted cautiously because complete deafferentation modifies the functions of the deafferented areas, and at times causes marked neuronal atrophy (Sprague, 1966), underscoring the fact that regionally dispersed gray matter and white matter work in concert.
A small number of related studies exist in humans. First, Bauer (1982) reported on a patient with traumatic lesion affecting inferior temporal –occipital regions bilaterally. The patient had severe prosopagnosia and visual memory deficits. The patient spontaneously complained that he no longer became emotionally aroused by visual stimuli including beautiful vistas, pretty girls, and erotic images. Upon further research, it was found that he lacked skin conductance responses to emotional images presented in the visual modality, but had persevered functioning in the auditory realm. Bauer (1982) concluded that this patient’s hypoemotionality was most likely due to visual-limbic disconnection (for a similar case study see Habib, 1986). Second, a study of individuals with focal lesions involving both gray and white matter found that damage to the ILF (as well as surrounding gray matter) correlated with impairments in facial emotion recognition (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009; see also Baggio et al., 2012). This finding was recently replicated in a sample population consisting of individuals with traumatic brain injury (TBI; Genova et al., 2015).
These findings suggest that the ILF may serve as a conduit for visual-limbic interactions. The goal of this study was to test this hypothesis in a neurologically normal population using diffusion tensor imaging (DTI) along with deterministic tractography. DTI utilizes diffusion-weighted MR imaging (DW-MRI) to index the degree of diffusion among water molecules within human brain tissue. White matter tracts can be imaged by exploiting the diffusion properties of the myelinated axons that make up the fiber pathways. In myelinated axons, the direction of diffusion is restricted due to the presence of myelin sheaths. DW-MRI captures the degree of restriction, called anisotropy, and provides measures of the microstructural properties of white matter such as the orientation and magnitude of diffusion within each voxel of the brain (Alexander et al., 2011; Alexander, Lee, Lazar, & Field, 2007a; Jones, 2008; Tournier, Mori, & Leemans, 2011a). Tractography allows for the visualization of white matter tracts and can be used in combination with DTI to calculate microstructural indices specific to particular white matter tracts.
We hypothesized that individual differences in facial emotional processing and face memory should correlate with ILF microstructure given that the ILF contains a streamline between ventral extrastriate cortex and temporal limbic regions. We predicted that this relationship would not be found in a control tract, the uncinate fasciculus (UF). Like the ILF, the UF connects temporal limbic regions; however, it does not originate in the extrastriate cortex. Instead, it forms a monosynaptic pathway between the anterior and medial temporal lobes and the orbitofrontal cortex (Catani & Thiebaut de Schotten, 2008). Given its geographic location, the UF has been implicated in processes related to episodic memory (Metzler-Baddeley et al., 2011; Diehl et al., 2008; McDonald et al., 2008; Niogi et al., 2008; also see Table 1 in Olson et al., 2015), and our own working hypothesis suggests that the UF is critical for integrating memory representations stored in the temporal lobes with feedback history computed in the OFC in order to facilitate memory-based decisions (Von Der Heide et al., 2013; Alm et al., 2015).
As a control task, we used a face perception task and predicted that this task would not correlate with microstructure of the ILF.
In addition to the ILF, we examined the inferior fronto-occipital fasciculus (IFOF). Like the ILF, the IFOF begins in ventral occipital lobe. However it terminates in ventral and lateral aspects of frontal cortex (Catani & Thiebault de Schotten, 2008). The IFOF is a mysterious tract; it has not been identified in non-human primates (Schmahmann & Pandya, 2007) but has been identified in humans using diffusion imaging techniques. This has led some researchers to suggest that it may be specific to humans (Catani, 2007). Electrical stimulation of this tract during neurosurgery consistently results in semantic errors (Duffau, Peggy Gatignol, Mandonnet, Capelle, & Taillandier, 2008) providing strong evidence that this tract plays a significant role in semantic memory. However, there is a hint that it may also play some role in face processing as one study reported reduced structural integrity of the right IFOF in congenital prosopagnosia (Thomas et al., 2008). In addition, two studies found a relationship between damage to the right IFOF and emotion recognition impairments (Philippi et al., 2009; Genova et al., 2015). Given these findings, combined with the general absence of a mature literature on this tract, we decided to conduct an exploratory analysis of the IFOF.
METHODS
Study participation occurred in two separate testing sessions. During the behavioral session, participants completed computerized tasks in the laboratory. Participants were tested individually. Computerized tasks were programmed in E-Prime (Version 2.0 Professional) and presented on Dell computers. During a separate session, diffusion-weighted MRI data, as well as high-resolution anatomical scans were acquired at Temple University Hospital.
Participants
Twenty-eight healthy individuals (19 females, 9 males) between the ages of 18 and 28 (M = 21.52, SD = 2.55) volunteered for this experiment. Participants were right-handed with normal or corrected-to-normal vision and no personal history of neurological or psychological disorders as well as no such history in their first-degree relatives (ascertained via self-report). This sample was recruited from the Temple University student body.
Three substantial outliers were found across white matter indices and thus, three participants were excluded from further analyses leaving a study sample of 25 participants. Informed consent was obtained according to the guidelines of the Institutional Review Board of Temple University and participants received monetary compensation for participation in the experiment.
Tasks
1. Face Emotion Recognition
Emotion recognition abilities tend to be at ceiling in the normal population; therefore, we designed a task using morphed emotional faces to achieve a higher level of difficulty. Morphed faces were made using Fantamorph software. A neutral face and a face very clearly showing an emotion were morphed together in different proportions to create emotional faces that were progressively more difficult to perceive. For example, a face that is 60% neutral and 40% happy is more difficult to judge as happy than a 30% neutral and 70% happy face. On each trial, participants were presented with three faces of the same identity. Participants were asked to pair the target face at the top of the screen with the face showing the same amount of expression at the bottom of the screen. For each identity, there was an easy control trial which all participants completed correctly. The task consisted of 200 total trials.
2. Cambridge Face Memory Test (CFMT)
This task is used to assess the ability to store and retrieve long-term memories of faces (Duchaine & Nakayama, 2006). Participants were first familiarized with six different facial identities, each presented at 3 viewpoints during an initial encoding phase. After this, participants completed 72 3-alternative forced choice trials in which they had to identify a target face amongst two foils. The test became progressively more difficult; in the first portion, target faces are presented in the same view as seen during training; in the second portion however, target faces are presented from novel views or lighting conditions. Finally, in the third portion of the task, target faces are presented from novel views with added visual noise. Participants completed 72 total trials.
3. Face Identity Perception
Stimuli and task were taken from the Philadelphia Face Perception Battery (Thomas et. al 2008). This task consisted of morphed faces created using GenHead software (www.genemation.com/). This software creates highly realistic artificial faces across 114 parameters, each an eigenvector derived from a principal component analysis of a large database of face photographs. Additional parameters allow for control of gender, age, and ethnicity. On each trial, one sample stimulus was presented above two side-by-side probe faces. Stimuli were morphed faces that varied along the dimension of facial identity. The task was to choose the probe stimulus that was most similar to the sample stimulus. Accuracy was emphasized, and there was an 8-second time limit per trial. On easy trials, the two stimuli were quite different from one another, while on difficult trials, the two probe stimuli were very similar. The dimension of interest was varied continuously but was later compressed into a single number that indexed one’s ability to perceive small differences in face identity. The side of the “match” stimulus was counterbalanced across trials. There were 100 total trials.
Imaging
Image Acquisition
MRI scanning was conducted at Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a Siemens twelve-channel phased-array head coil. DTI data was collected using a diffusion-weighted echo-planar imaging (EPI) sequence covering the whole brain with the following imaging parameters: 55 axial slices (with no gap between slices), 2.5 mm slice thickness, voxel size = 1.967 mm × 1.967 mm × 2.5 mm, TR = 9,900 ms, TE = 95 ms, FOV = 240 mm2, b values of 0 and 1000 s/mm2 (one b=0 volume), 64 non-collinear directions. These parameters yielded a DTI scan lasting approximately 11 minutes.
In addition to diffusion-weighted images, high-resolution anatomical images (T1-weighted 3D MPRAGE) were also collected for each participant with the following parameters: 160 axial slices, 1 mm slice thickness, TR = 1,900 ms, TE = 2.93 ms, inversion time = 900 ms, flip angle = 9°, FOV = 256 mm. These anatomical images were co-registered to the diffusion images and used to draw regions of interest (ROIs).
DTI preprocessing
Diffusion-weighted images were pre-processed to correct for eddy currents and subject motion using an affine registration model. The b-vector matrix was adjusted based on rigid body registration, ensuring a valid computation of the tensor variables. Non-brain tissue was removed using an automated brain extraction tool (BET), and a standard least squares diffusion tensor fitting model was then applied to the data using FSL. The diffusion tensor fitting provided estimates of fractional anisotropy (FA) and mean diffusivity (MD) as well as three eigenvectors and eigenvalues. FA was computed using the following equation: , where λ1, λ2, and λ3 represent the three eigenvalues respectively. MD was calculated by averaging the three eigenvalues. Axial diffusivity (AD) was represented by the principal eigenvalue (λ1). Finally, radial diffusivity (RD) was calculated by averaging the second and third eigenvalues. Microstructural estimates were computed on individual voxels using a three-dimensional Gaussian distribution model that yielded a single mean ellipsoid for each voxel. All preprocessing was performed using FSL (Smith et al., 2004).
Whole brain deterministic tractography was performed in subject native space using the Diffusion Toolkit and TrackVis software packages (Wang, Benner, Sorensen, & Wedeen, 2007). This software uses a fiber assignment continuous tracking (FACT) algorithm (Mori, Crain, Chacko, & Van Zijl, 1999) to determine the branching and curving of the fiber tracts. The orientation of the principal eigenvector was estimated for each voxel, and nearest-neighbor interpolation was used to step along that direction. Step length was fixed at 0.1 mm, and an angle threshold of 35 degrees was used to determine the termination point of the fiber tracts. A spline filter was used to smooth the tractography data. A multiple region of interest (ROI) based axonal tracking approach (Mori et al., 2002; C. Thomas, Humphreys, Jung, Minshew, & Behrmann, 2010; Wakana et al., 2007) was then used to delineate three fiber pathways bilaterally: the ILF, IFOF, and UF. ROIs were drawn in subject native space using the high-resolution anatomical T-1 images and the methods outlined by Thomas and colleagues (2010) and used by our lab in a prior study (Alm, 2015). Briefly, each white matter tract, two ROIs were drawn and a Boolean AND term was used to select only the fibers that passed through both of these two regions. For the ILF, one ROI was drawn in the ventral occipito-temporal cortex inferior to the lateral ventricles, while the other ROI consisted of the portion of the temporal cortex that is anterior to the point at which the fornix descends to the mammillary bodies. For the IFOF, the same ventral occipito-temporal cortex ROI was used along with an ROI in the frontal lobe, consisting of the portion located anterior to the rostrum of the callosum. Finally, for the UF, the anterior temporal lobe ROI was used along with the frontal lobe ROI (used for defining the IFOF). Mean MD, FA, AD, and RD indices were subsequently extracted from the tracts of interest.
The ILF and IFOF originate in the same region and follow similar path trajectories until the ILF curves ventrally, ending in the ATL and the IFOF curves dorsally, into the frontal lobe. There has been some debate as to whether the two are, in fact, separate fiber pathways, or rather, make up the same white matter tract (Johansen-Berg & Behrens, 2009). For these reasons, we excluded any voxels that overlapped in our tract data for the ILF and IFOF, ensuring dissociation between these fiber pathways.
Statistical Analyses
Statistical analyses were performed using SPSS (Version 21.0). Regression analyses were used to examine the relationship between microstructure of the tracts of interest and performance on the tasks described above. Accuracy was the dependent measure of interest for each of the behavioral tasks. To control for multiple comparisons, family-wise error rate was adjusted using a Bonferroni correction to adjust for simultaneous predictors in each regression model (critical p = .05/2; Mundfrom, Piccone, Perrett, Schaffer, & Roozeboom, 2006). All regression p-values reported were Bonferroni-corrected.
RESULTS
Behavioral data
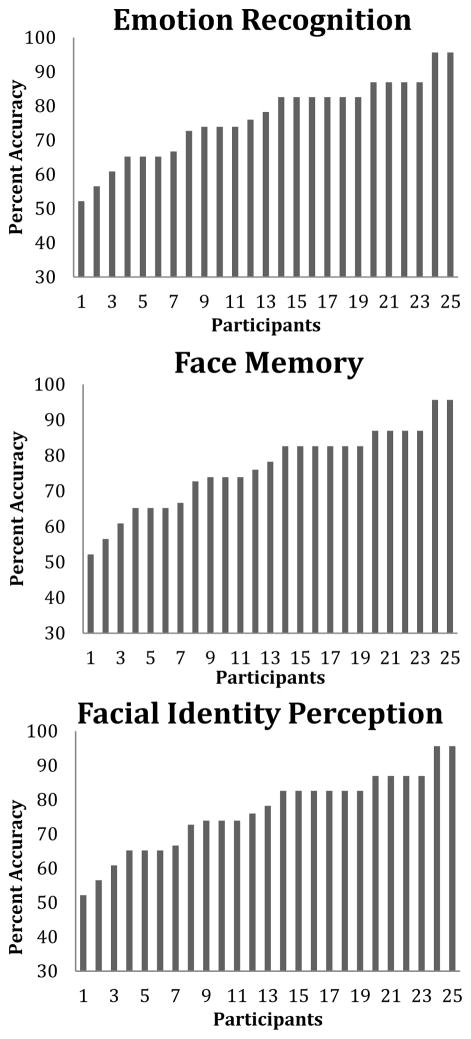
Individual differences in accuracy across the three tasks are presented in Figure 1. Average accuracy on the face emotion task, face memory task and face identity tasks were 72.6%, 63.2%, and 76.5%, respectively. There was sufficient variability to examine individual differences, as shown in Figure 1.
Figure 1.
Variability in scores for each task. On the x-axis is an arbitrary subject number. On the y-axis is performance accuracy. From left to right is the lowest performing participant to the highest performing participant.
DTI data
Regression models were constructed to predict participant accuracy on the three face tasks. Previous studies have shown diffuse changes in white matter microstructure throughout the whole brain as a function of age as well as general gender differences in white matter connectivity throughout the brain (Thomas et. al, 2008; Gong et. al, 2011; Lebel et al., 2012; Ingalhalikar et al., 2014). Therefore we examined the relationship between the white matter measures in our sample with age and gender. To investigate the possibility that age could systematically vary in this dataset, each of the white matter indices measured (bilateral MD, AD, RD and FA) in the ILF and IFOF were correlated with age of the participants. To investigate possible gender differences, t-tests were performed. No significant differences were observed in any of the white matter indices (all p’s > .05), thus we did not control for age or gender in subsequent analyses.
Regression models were constructed to examine whether white matter microstructure of the ILF and/or IFOF significantly predicted behavioral performance across our tasks. Each regression model consisted of bilateral white matter microstructure measurements as predictors and task performance as the dependent measure. The results of the regression analyses are presented in Table 1.
Table 1.
Summary of multiple linear regression models predicting individual differences in performance on the emotional recognition, face memory, and facial identity perception tasks. Bold font and asterisks denote p < .05 (after controlling for multiple comparisons). FA: fractional anisotropy AD: axial diffusivity, β: standardized regression coefficient
| Dependent Variable | Predictor Variables | Inferior Longitudinal Fasiculus | Inferior Fronto-Occipital Fasiculus | Uncinate Fasiculus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| β | t | F | R2 | β | t | F | R2 | β | t | F | R2 | ||
| Emotion Recognition | 1.34 | .11 | 4.2* | .29* | 1.02 | .09 | |||||||
| Right FA | −.38 | 1.56 | −.83* | 2.89* | −.27 | 1.33 | |||||||
| Left FA | .1 | .4 | .87 | 2.81 | .08 | .37 | |||||||
| Emotion Recognition | 3.54* | .25* | .27 | .03 | .4 | .044 | |||||||
| Right AD | −.68* | 2.66* | −.14 | .43 | −.22 | .95 | |||||||
| Left AD | .46 | 1.82 | −.23 | .07 | .16 | .71 | |||||||
| Face Memory | 6.88* | .39* | 8.64* | .44* | 1.85 | .14 | |||||||
| Right FA | −.61* | 2.67* | 1.88* | 4.13* | .04 | .19 | |||||||
| Left FA | −.01 | .044 | −1.69* | 3.72* | .38 | 1.92 | |||||||
| Face Memory | .8 | .07 | .22 | .02 | 4.29 | .28 | |||||||
| Right AD | −.31 | 1.12 | −.04 | .11 | −.35 | 1.95 | |||||||
| Left AD | .08 | .3 | .17 | .43 | .41 | 2.27 | |||||||
| Facial Identity Perception | .02 | .21 | .28 | .03 | .21 | .02 | |||||||
| Right FA | .05 | .19 | −.15 | .42 | −.001 | .004 | |||||||
| Left FA | −.16 | .63 | .25 | .71 | .14 | .65 | |||||||
| Facial Identity Perception | .14 | 1.7 | .1 | .01 | .024 | .26 | |||||||
| Right AD | −.24 | .88 | .14 | .44 | −.07 | .3 | |||||||
| Left AD | .49 | 1.79 | −.1 | .31 | .17 | .72 | |||||||
Emotion Recognition
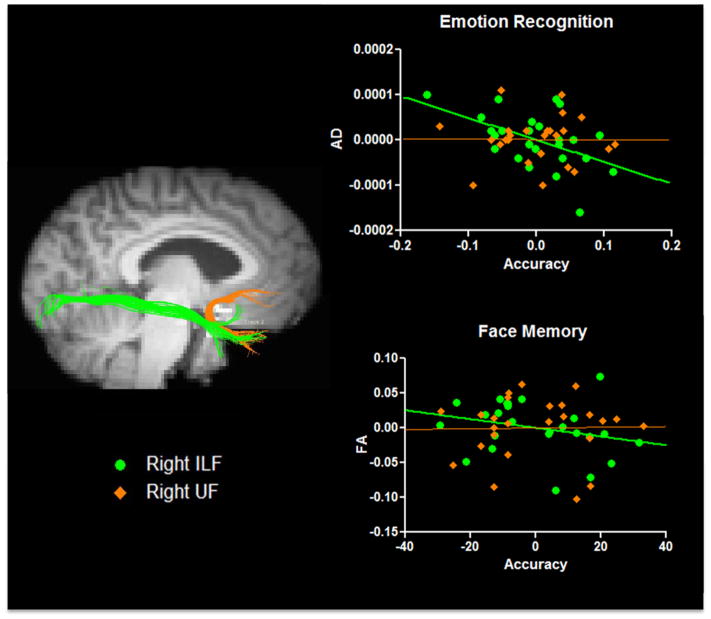
Regression analyses revealed a significant relationship between microstructure of the ILF and emotion recognition. Specifically, AD in the right ILF significantly predicted emotional recognition performance (β = −.7, t (22) = 2.7, p = .03) such that higher AD values were associated with lower accuracy (see Figure 2). AD in the left ILF significantly predicted emotional recognition accuracy before controlling for multiple comparisons, but did not survive after stringently controlling for multiple comparisons (β = .46, t (22) = 1.82, p = .14). Given our sample size, it is possible that a true effect exists in the left ILF but requires greater power to be revealed. No other microstructural indices (MD, RD or FA) in the ILF predicted differences in task performance.
Figure 2.
Unstandardized residuals are graphed. Individual differences in ILF microstructure (green tracts and circles) predicted emotional discrimination accuracy and face memory accuracy. Variation in a control tract, the UF (orange tracts and diamonds), did not significantly predict behavioral performance in either measure.
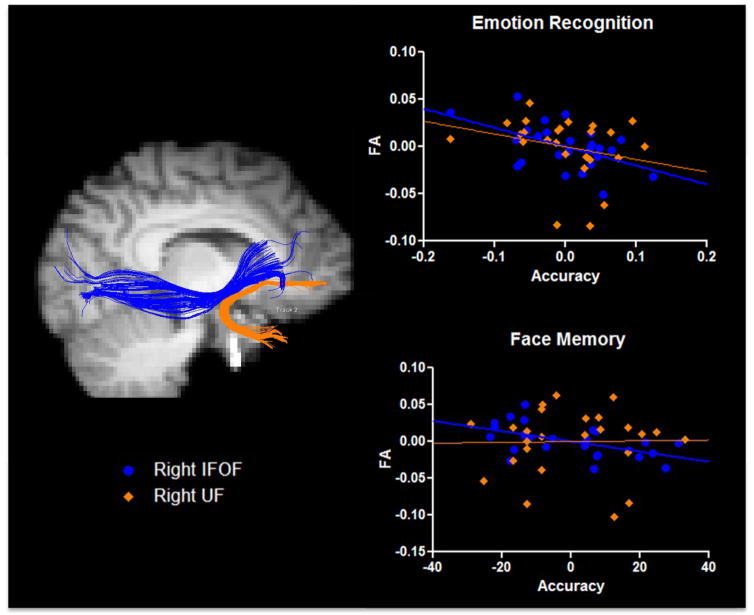
Individual differences in emotion recognition were also related to variability in IFOF microstructure. FA in both the left and right IFOF significantly predicted performance (β = .93, t (22) = 2.87, p = .02 and β = −.91, t (22) = 2.8, p = .02 respectively). This relationship is depicted in Figure 3 (top). AD, RD and MD in the IFOF predicted task performance.
Figure 3.
Unstandardized residuals are graphed for face memory and emotion recognition tasks. Individual differences in IFOF microstructure (blue tracts and circles) predicted emotion recognition accuracy and face memory accuracy. Variation in a control tract, the UF (orange tracts and diamonds), did not significantly predict behavioral performance in either measure.
Individual differences in emotional recognition ability were not related to microstructural differences in a control tract, the UF (p’s > .05).
Face Memory
Face memory accuracy was significantly predicted by microstructural properties of the right ILF. Regression analyses revealed a negative relationship between FA in the right ILF and face memory accuracy (β = −.54 t (22) = 2.17, p = .03; See Figure 2, bottom), such that lower FA was associated with higher face memory performance. There was no significant relationship with the left ILF and individual differences in face memory performance (p’s > .05). MD, RD and AD in the right and left ILF did not predict face memory performance.
Similarly, FA of both left and right IFOF significantly predicted face memory performance. A positive relationship emerged between the left IFOF and face memory accuracy (β = 1.87, t (22) = 3.49, p = .004), while a negative relationship emerged for the right IFOF and face memory accuracy (β = −1.61, t (22) = 3.49, p = .01). Figure 3 (bottom) depicts this relationship. No other white matter indices measured in the IFOF predicted face memory performance.
No significant effects were observed between microstructure of the UF and face memory performance (all p’s > .05).
Facial Identity Perception
One’s ability to perceive small differences in facial identity was not predicted by microstructural differences in any of the tracts examined (all p’s > .05).
DISCUSSION
Theoretical discussions about the involvement of visual fiber pathways in higher-order cognition have existed for years but only recently with the advent of as diffusion-weighted MRI have we been able to test these ideas. Two rare clinical disorders, visual amnesia and visual hypo-emotionality, have been attributed to white matter disconnection (reviewed by Catani et al., 2003). Using this framework, we tested the hypothesis that individual differences in inferior longitudinal fasciculus (ILF) microstructure predicted inter-individual variability in face emotion recognition and face memory. In addition we examined two other long-range white matter tracts: the inferior fronto-occipital fasciculus (IFOF) since there is a small literature linking this tract to face processing (Thomas et al., 2008), and the uncinate fasciculus (UF) as a control fiber pathway, given that our recent literature reviews found no evidence linking this tract to any of the behaviors investigated in this study (Von Der Heide et al., 2013). Our results revealed that microstructure of the right ILF and bilateral IFOF significantly predicted variability in facial emotion recognition performance, as well as face memory performance in healthy young adults. Importantly, there is some specificity to our findings since the microstructural properties of these tracts were not related to performance on a control task of facial identity perception, nor was a control fiber pathway, the UF, related to performance on any of our tasks of interest.
Like the ILF, the UF connects temporal limbic regions; however, it does not originate in the extrastriate cortex. Instead, it forms a monosynaptic pathway between the anterior and medial temporal lobes and the orbitofrontal cortex (Catani & Thiebaut de Schotten, 2008). Based on our reviews of the literature as well as empirical findings (Von Der Heide et al., 2013; Alm et al., 2015), we believe that UF function is specifically related to tasks that involve an interaction between value encoding/updating and long-term memory. Therefore, we did not expect the UF to be recruited in simple, perceptually-driven memory tasks, such as the Cambridge Face Memory Test or in face perception tests without a memory component, such as emotion recognition. Our findings are consistent with these predictions and suggest that performance on such tasks rely on fiber pathways connecting visual regions, in particular the ILF and IFOF.
Cognitive Functions Attributed to the ILF
It is important to bear in mind that white matter does not produce behavior; the electrical activity of neurons produce behavior. White matter provides the communication system that links groups of neurons to other groups of neurons. Bearing this caveat in mind, the small literature on the function of the ILF invariably links this tract to high-level vision. Consistent with the results reported here, two prior studies reported that in humans, damage to the ILF correlates with impairments in facial emotion recognition (Genova et al., 2015; Philippi et al., 2009). In addition, our study is the first to link the ILF to face memory abilities.
However other studies have implicated the ILF in perceptual aspects of face processing, which we did not find. ILF scarring or degeneration in multiple sclerosis and/or fronto-temporal dementia correlates with face identification deficits (Grossi et al., 2012; Yamasaki et al., 2004). Also, individuals with developmental or congenital prosopagnosia have reduced structural integrity of the right ILF (Thomas et al., 2008; Grossi et al., 2012) which predicts face-recognition impairments (Thomas et al., 2008). This raises the question of why we did not observe any effects in our face identity task. One possibility is that there simply isn’t enough microstructural variance in a normative sample to observe a relationship.
The communicative functions of the ILF extend beyond face processing to include functions linked to object recognition (Braem, Honoré, Rousseaux, Saj, & Coello, 2014; Shinoura et al., 2013). Two studies using intraoperative electrical stimulation have reported changes in object naming or object recognition after stimulating the ILF (Coello, 2008; Mandonnet, 2009). Naming and recognizing common objects involves integrating perceptual representations with higher-order conceptual knowledge, potentially stored in lateral regions of the anterior temporal lobe.
Finally, the ILF has been linked to visual aspects of reading. The cell bodies in extrastriate cortex from which the ILF is derived may include the visual word form area thus there is great interest in this tract among reading researchers (see Epelbaum et al., 2008; Gill-Robles et al., 2013; Yeatman et al., 2012). Zemmoura and colleagues (2015) reported that resection of the posterior portion of the ILF, connecting ventral visual cortex to the visual word form area, induced severe reading impairments. However resection of the anterior portion of the ILF, connecting the anterior and medial temporal lobes to the visual word form area, had no effect on reading. These findings should be interpreted in the context of the anatomy of the ILF, which has U-shaped fibers entering and exiting at different waypoints. As such, it is possible that different portions of the ILF have distinct functional roles.
An unexplored topic in this literature is the lateralization of function in the ILF. Coello and colleagues (2013) drew on a single case study to speculate that the left ILF is somewhat more specialized for visual tasks requiring access to language, such as object naming while the right ILF may have functions more aligned with strictly visuospatial functions. Consistent with this, our findings showed that individual differences in right, but not left, ILF microstructure predicted the ability to discern face emotions and remember faces.
Cognitive Functions Attributed to the IFOF
The literature surrounding the functionality of the IFOF is quite small, due in large part to the fact that this tract has not been identified in non-human primates (Schmahmann & Pandya, 2006). Given that this tract may be uniquely human (Catani, 2007) some researchers have posited that its function is related to a uniquely human skill: language. Almairac and colleagues (2014) reported that patients with lesions in areas consistent with the IFOF exhibited impairments on a verbal fluency task. Participants were patients who had undergone surgery for left lateralized low-grade gliomas. Voxel-based lesion-symptom mapping (VLSM) was used to map out the spatial location of lesions across patients. When a mask of the left IFOF was overlaid onto the VLSM maps, the degree of IFOF damage correlated significantly with impairment on a verbal fluency task. This finding was specific to semantic verbal fluency, i.e. generating words belonging to a category of animals. This finding provides corroborating evidence for earlier studies during which electrical stimulation of the left IFOF during neurosurgery produced semantic paraphasias during object naming (Duffau et al., 2005; Duffau et al., 2008; Mandonnet et al., 2007).
These findings have led some researchers to conclude that the IFOF plays an important role in semantic memory. In the diffusion imaging literature, de Zubicaray and colleagues (2011) examined the relationship between IFOF microstructure and semantic memory across a cohort of healthy older adults. A whole brain white matter analysis revealed a significant positive correlation between a composite semantic memory score and FA in the left IFOF, suggesting that the IFOF is involved in semantic access.
Yet some other findings link aspects of face processing to the IFOF. Compared to healthy controls, individuals with congenital prosopagnosia exhibit significantly decreased FA in both right and left IFOF. Furthermore, the degree of face recognition impairment in these individuals correlates with reduction in the percentage of fibers in the right IFOF (Thomas et al., 2009). Damage to the IFOF, along with the ILF, has also been reported in case studies of severe prosopagnosia (e.g. Valdés-Sosa et al., 2011). In a sample of neurologically normal participants ranging in age from 18–86 years, a reduction in the number of fibers and FA of the right IFOF was related to individuals’ abilities to discriminate between faces (Thomas et al., 2008).
Finally, the IFOF has also recently been implicated in emotion recognition. Philippi and colleagues (2009) investigated emotional recognition deficits using Ekman faces (Ekman, 1976) across a large sample of patients with focal brain lesions. Disconnection of the right IFOF predicted impairment on the emotion recognition task. The authors replicated this finding in a case study of a patient with damage restricted to the IFOF. Our findings are consistent with this data. Similarly, Baggio et al. (2012) reported a significant positive relationship between emotion recognition scores and FA values in the right and left IFOF. Of note, these findings were specific to sadness recognition and participants consisted of Parkinson’s patients and healthy controls.
Like the ILF, it is possible that different portions of the IFOF have distinct functional roles. A recent study demonstrated that the IFOF consists of fiber bundles that terminate in various frontal and temporal regions (Sarubbo et al, 2013). The authors posit that differential termination points may yield distinct functional subcomponents of the IFOF, which potentially support discrete functions.
Limitations
The ILF and IFOF are large white matter tracts linking functionally discrete brain regions. As noted earlier, it is possible that both tracts consist of sub-tracts that serve to link domain-specific visual processing areas performing different computational metrics along the ventral stream, and that the face-network constitutes one of these sub-tracts. Consistent with this possibility, one study has shown that different portions of the ILF are associated with recognition ability for different stimulus classes (Tavor et al., 2013). The same logic applies to the IFOF. Our study was not designed to test the finer-grained question of whether white matter from particular cortical subregions, such as the fusiform face area to the amygdala, underlies the observed associations. Future studies should consider functionally localizing cortical subregions like the visual word form area and the fusiform face area in order to discern whether subregions of the ILF participate in distinct cognitive functions.
Second, significant findings were not apparent in all DTI measures. The only relationship between white matter measures and the face memory task were found in FA. This is true for both the IFOF and ILF. In the IFOF, the only relationship between emotion recognition and white matter was also with FA. However, in the ILF, AD was the measure that predicted performance on the emotion recognition task. These results are not terribly surprising, however, as many studies report significant findings in only some white matter indices (e.g. Thomas et al., 2008).
Also, although we observed variability in participants’ behavioral performance, our cohort was more homogenous than the U.S. population at large, since our participant pool consisted largely of university undergraduate and graduate students. The study of individual differences is reliant on sufficient variability in both brain and behavior, which is limited in a healthy, young population. Nevertheless, we found robust brain-behavior correlations that were consistent with the existence literature. We find this encouraging since much of the theory building in cognitive neuroscience is pursued in neurologically normal sample populations similar to the one we tested.
Finally, due to the analysis method we use in this paper (diffusion tensor imaging), we cannot conclude that some participants had better white matter connectivity than others, nor can we conclude that better or worse white matter connectivity was the cause of better performance on our tasks (Jones, 2008). Indeed, many studies report that increased microstructural measures are related to increased task performance (e.g. Alm et. Al, 2015); however, others report that decreased microstructural measures are related to increased task performance (e.g. Hoeft et. Al, 2006,). Thus, in this report we can only conclude that microstructural differences in white matter are related to task performance differences.
Conclusions
The vast majority of DTI studies investigate the functionality of white matter in clinical populations who suffer from a range of deficits and often have widespread brain pathology. Here, we examined microstructural differences in a sample of healthy young adults in order to provide a baseline for future investigators interested in understanding the behavioral significance of variability in structural connectivity. Our results show that the ILF and IFOF both play an important role in facilitating one’s ability to discern facial emotions as well as one’s ability to remember new faces.
Supplementary Material
Acknowledgments
We would like to thank Hyden Zhang, Vanessa Troiani, Lauren Harris, and Tehila Nugiel for assistance with participant testing and tract tracing. This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors declare no competing or conflicting financial interests.
References
- Alexander AL, Hurley Sa, Samsonov Aa, Adluru N, Hosseinbor AP, Mossahebi P, … Field AS. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1(6):423–46. doi: 10.1089/brain.2011.0071. http://doi.org/10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. 2007a;4(July):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2007b;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. http://doi.org/10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Mohamed FB, Olson IR. Fronto-temporal white matter connectivity predicts reversal learning errors. Frontiers in Neuroscience. 2015;9(343):1–11. doi: 10.3389/fnhum.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almairac F, Herbet G, Moritz-Gasser S, de Champfleur NM, Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Structure & Function. 2014 doi: 10.1007/s00429-014-0773-1. http://doi.org/10.1007/s00429-014-0773-1. [DOI] [PubMed]
- Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti MJ, Compta Y, … Junqué C. Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia. 2012;50(8):2121–8. doi: 10.1016/j.neuropsychologia.2012.05.020. http://doi.org/10.1016/j.neuropsychologia.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Bauer RM. Visual hypoemotionality as a symptom of visual-limbic disconnection in man. Archives of Neurology. 1982;39(11):702–8. doi: 10.1001/archneur.1982.00510230028009. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7126000. [DOI] [PubMed] [Google Scholar]
- Braem B, Honoré J, Rousseaux M, Saj A, Coello Y. Integration of visual and haptic informations in the perception of the vertical in young and old healthy adults and right brain-damaged patients. Neurophysiologie Clinique = Clinical Neurophysiology. 2014;44(1):41–8. doi: 10.1016/j.neucli.2013.10.137. http://doi.org/10.1016/j.neucli.2013.10.137. [DOI] [PubMed] [Google Scholar]
- Catani M. From hodology to function. Brain: A Journal of Neurology. 2007;130(Pt 3):602–5. doi: 10.1093/brain/awm008. http://doi.org/10.1093/brain/awm008. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain: A Journal of Neurology. 2003;126(Pt 9):2093–107. doi: 10.1093/brain/awg203. http://doi.org/10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):1105–32. doi: 10.1016/j.cortex.2008.05.004. http://doi.org/10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Coello AF, Duvaux S, De Benedictis A, Matsuda R, Duffau H. Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: a brain stimulation mapping study. Journal of Neurosurgery. 2013;118(1):202–5. doi: 10.3171/2012.10.JNS12527. http://doi.org/10.3171/2012.10.JNS12527. [DOI] [PubMed] [Google Scholar]
- Coello Y, Bartolo A, Amiri B, Devanne H, Houdayer E, Derambure P. Perceiving what is reachable depends on motor representations: evidence from a transcranial magnetic stimulation study. PloS One. 2008;3(8):e2862. doi: 10.1371/journal.pone.0002862. http://doi.org/10.1371/journal.pone.0002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zubicaray GI, Rose SE, McMahon KL. The structure and connectivity of semantic memory in the healthy older adult brain. NeuroImage. 2011;54(2):1488–94. doi: 10.1016/j.neuroimage.2010.08.058. http://doi.org/10.1016/j.neuroimage.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. http://doi.org/10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44(4):576–85. doi: 10.1016/j.neuropsychologia.2005.07.001. http://doi.org/10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain: A Journal of Neurology. 2005;128(Pt 4):797–810. doi: 10.1093/brain/awh423. http://doi.org/10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109(3):461–71. doi: 10.3171/JNS/2008/109/9/0461. http://doi.org/10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Measuring facial movement. Environmental Psychology and Nonverbal Behavior. 1976;1(1):56–75. http://doi.org/10.1007/BF01115465. [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, … Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):962–74. doi: 10.1016/j.cortex.2008.05.003. http://doi.org/10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Genova HM, Rajagopalan V, Chiaravalloti N, Binder A, Deluca J, Lengenfelder J. Facial affect recognition linked to damage in specific white matter tracts in traumatic brain injury. Social Neuroscience. 2014;10(1):27–34. doi: 10.1080/17470919.2014.959618. [DOI] [PubMed] [Google Scholar]
- Gil-Robles S, Carvallo A, Jimenez MDM, Gomez Caicoya A, Martinez R, Ruiz-Ocaña C, Duffau H. Double dissociation between visual recognition and picture naming: a study of the visual language connectivity using tractography and brain stimulation. Neurosurgery. 2013;72(4):678–86. doi: 10.1227/NEU.0b013e318282a361. http://doi.org/10.1227/NEU.0b013e318282a361. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain connectivity: gender makes a difference. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2011;17(5):575–91. doi: 10.1177/1073858410386492. http://doi.org/10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Grossi D, Soricelli A, Ponari M, Salvatore E, Quarantelli M, Prinster A, Trojano L. Structural connectivity in a single case of progressive prosopagnosia: the role of the right inferior longitudinal fasciculus. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2014;56:111–20. doi: 10.1016/j.cortex.2012.09.010. http://doi.org/10.1016/j.cortex.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Habib M. Visual hypoemotionality and prosopagnosia associated with right temporal lobe isolation. Neuropsychologia. 1986;24(4):577–82. doi: 10.1016/0028-3932(86)90101-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3774141. [DOI] [PubMed] [Google Scholar]
- Horel JA, Misantone LJ. The Klüver-Bucy syndrome produced by partial isolation of the temporal lobe. Experimental Neurology. 1974;42(1):101–112. doi: 10.1016/0014-4886(74)90010-7. http://doi.org/10.1016/0014-4886(74)90010-7. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. 1992 Retrieved from https://scholar.google.com/scholar?hl=en&as_sdt=0,39&q=Pyramids+and+Palm+Trees+(Howard+%26+Patterson,+1992#0.
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, … Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):823–8. doi: 10.1073/pnas.1316909110. http://doi.org/10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ. Diffusion MRI. Diffusion MRI. Elsevier; 2009. http://doi.org/10.1016/B978-0-12-374709-9.00022-5. [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):936–52. doi: 10.1016/j.cortex.2008.05.002. http://doi.org/10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test (exp ed) Philadelphia: Lea & Febiger; 1978. Retrieved from https://scholar.google.com/scholar?q=Kaplan.%2C+1978+Boston+Naming+Test&btnG=&hl=en&as_sdt=0%2C39&as_ylo=1978&as_yhi=1979#3. [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic assessments of language processing in aphasia (PALPA): An introduction. Aphasiology. 1996;10(2):159–180. http://doi.org/10.1080/02687039608248403. [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–52. doi: 10.1016/j.neuroimage.2011.11.094. http://doi.org/10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain: A Journal of Neurology. 2007;130(Pt 3):623–9. doi: 10.1093/brain/awl361. http://doi.org/10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(5):691–9. doi: 10.1016/j.cortex.2009.07.015. http://doi.org/10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Girard HM, Pung C, Ahmadi ME, Holland D, … Dale aM. Changes in fiber tract integrity and visual fields after anterior temporal lobectomy. Neurology. 2010;75(18):1631–8. doi: 10.1212/WNL.0b013e3181fb44db. http://doi.org/10.1212/WNL.0b013e3181fb44db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O’Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(37):13236–45. doi: 10.1523/JNEUROSCI.2317-11.2011. http://doi.org/10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. http://doi.org/10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, … van Zijl PCM. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;47(2):215–23. doi: 10.1002/mrm.10074. http://doi.org/10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Mundfrom DJ, Piccone A, Perrett JJ, Schaffer J, Roozeboom M. Bonferroni Adjustments in Tests for Regression Coefficients. Multiple Linear Regression Viewpoints. 2006;32:1–6. [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, … McCandliss BD. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain: A Journal of Neurology. 2008;131(Pt 12):3209–21. doi: 10.1093/brain/awn247. http://doi.org/10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Olson IR, Von der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.06.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(48):15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. http://doi.org/10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure & Function. 2013;218(1):21–37. doi: 10.1007/s00429-011-0372-3. http://doi.org/10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The Complex History of the Fronto-Occipital Fasciculus. Journal of the History of the Neurosciences. 2007 doi: 10.1080/09647040600620468. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/09647040600620468#.VRSDUPnF-1s. [DOI] [PubMed]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. http://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science (New York, NY) 1966;153(3743):1544–7. doi: 10.1126/science.153.3743.1544. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/5917786. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compedium of neuropsychological tests. A Compedium …. 1991 Retrieved from https://scholar.google.com/scholar?q=Spreen+%26+Strauss,+1991&hl=en&as_sdt=0,39#1.
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. NeuroImage. 2014;86(2014):123–30. doi: 10.1016/j.neuroimage.2013.07.085. http://doi.org/10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Thomas AL, Lawler K, Olson IR, Aguirre GK. The Philadelphia Face Perception Battery. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2008;23(2):175–87. doi: 10.1016/j.acn.2007.10.003. http://doi.org/10.1016/j.acn.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nature Neuroscience. 2009;12(1):29–31. doi: 10.1038/nn.2224. http://doi.org/10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;47(7):863–73. doi: 10.1016/j.cortex.2010.07.006. http://doi.org/10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson Ma, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. Journal of Cognitive Neuroscience. 2008;20(2):268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011a;65(6):1532–56. doi: 10.1002/mrm.22924. http://doi.org/10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011b;65(6):1532–56. doi: 10.1002/mrm.22924. http://doi.org/10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Sosa M, Bobes MA, Quiñones I, Garcia L, Valdes-Hernandez PA, Iturria Y, … Asencio J. Covert face recognition without the fusiform-temporal pathways. NeuroImage. 2011;57(3):1162–76. doi: 10.1016/j.neuroimage.2011.04.057. http://doi.org/10.1016/j.neuroimage.2011.04.057. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain: A Journal of Neurology. 2013;136(Pt 6):1692–707. doi: 10.1093/brain/awt094. http://doi.org/10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, … Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–44. doi: 10.1016/j.neuroimage.2007.02.049. http://doi.org/10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proceedings of the 15th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; 2007. p. 3720. [Google Scholar]
- Yamasaki T, Taniwaki T, Tobimatsu S, Arakawa K, Kuba H, Maeda Y, … Kira J. Electrophysiological correlates of associative visual agnosia lesioned in the ventral pathway. Journal of the Neurological Sciences. 2004;221(1–2):53–60. doi: 10.1016/j.jns.2004.03.024. http://doi.org/10.1016/j.jns.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain and Language. 2013;125(2):146–55. doi: 10.1016/j.bandl.2012.04.010. http://doi.org/10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmoura I, Herbet G, Moritz-Gasser S, Duffau H. New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Human Brain Mapping. 2015 doi: 10.1002/hbm.22766. n/a–n/a. http://doi.org/10.1002/hbm.22766. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.