Abstract
Social groups across species rapidly self-organize into hierarchies, where members vary in their level of power, influence, skill, or dominance. In this review we explore the nature of social hierarchies and the traits associated with status in both humans and nonhuman primates, and how status varies across development in humans. Our review finds that we can rapidly identify social status based on a wide range of cues. Like monkeys, we tend to use certain cues, like physical strength, to make status judgments, although layered on top of these more primitive perceptual cues are socio-cultural status cues like job titles and educational attainment. One's relative status has profound effects on attention, memory, and social interactions, as well as health and wellness. These effects can be particularly pernicious in children and adolescents. Developmental research on peer groups and social exclusion suggests teenagers may be particularly sensitive to social status information, but research focused specifically on status processing and associated brain areas is very limited. Recent evidence from neuroscience suggests there may be an underlying neural network, including regions involved in executive, emotional, and reward processing, that is sensitive to status information. We conclude with questions for future research as well as stressing the need to expand social neuroscience research on status processing to adolescents.
Keywords: status, dominance, adolescence, popularity, neural
1. Introduction
From childhood sports competitions and spelling bees, to grade point averages and prom kings and queens, we learn early in life to view our social world in terms of who is better, smarter, or more favored than everyone else. Even as adults, we are quick to identify status symbols such as foreign cars, big houses, and career titles. The ease with which we perceive status cues and assign rank to others reflects a general preference for a hierarchical social organization (Zitek & Tiedens, 2012), perhaps because understanding where we stand relative to others is essential for defining social roles and promoting successful social interaction (Halevy, Chou, & Galinsky, 2011; Savin-Williams, 1979). We undoubtedly vary in the skills and traits we possess, and when choosing the appropriate person to listen to, follow, or emulate, we want someone with the skills and traits we consider the most desirable or important. Thus, organizing social groups in a hierarchical manner is an efficient way to maximize group cohesion and productivity, and the ability to readily perceive status cues in others is an important social skill.
The purpose of social hierarchies is to organize social groups in order to allocate limited resources, such as mates and food (Sapolsky, 2005), facilitate social learning (Henrich & Mcelreath, 2003), and maximize individual motivation (Halevy et al, 2011; Magee & Galinsky, 2008). By definition, some individuals within the hierarchy – those at the top - will be afforded more resources and benefits than others, thus affecting morbidity and mortality. Despite that fact that there are always losers in this scenario, social hierarchies are highly pervasive across human cultures (Sidanius & Pratto, 1999) and they appear to emerge naturally in social groups (Anderson, John, Keltner, & Kring, 2001; Berger, Rosenholtz, & Zelditch, 1980; Chase, Tovey, Spangler-Martin, & Manfredonia, 2002; Gould, 2002; Magee & Galinsky, 2008). Further, this group organization is not strictly a product of human cognition, as almost every group-living species demonstrates a natural tendency to organize into a social hierarchy (Sapolsky, 2004; 2005) where the higher-ranking members possess more power, influence, and advantages than the lower-ranking members (Fragale, Overbeck, & Neale, 2011; Mazur, 1985; Zitek & Tiedens, 2012).
The prevalence of hierarchies and their similarities across species suggest an innate preference, or utility, in the differentiation of power and a possible evolutionary origin (Mazur, 1985). We would expect, then, that humans are equipped with mechanisms for rapidly perceiving status information and recognizing their relative status roles, and that sensitivity to status information is present early in life. However, the neural mechanisms underlying status perception and judgment in humans are only recently gaining attention, and findings remain relatively vague. Even sparser is research regarding the effects of neural maturation on status perception, and how developmental changes in status processing influence the value and impact of social rank.
In this review, we integrate findings from animal, human social psychology, and cognitive neuroscience to argue that status is a prominent feature in social perception, and that understanding how we process status information on a neural and cognitive level is essential to understanding the profound impact status has on social attention and behavior. Additionally, we suggest exploring developmental changes in status perception to better understand adolescent behaviors like peer pressure and bullying.
This review has three aims. First, we address the evolutionary origins of social hierarchies by examining status cues across non-human primate species. Second, we review the current literature on the neural mechanisms underlying the perception and processing of social rank in humans, and discuss the relevance of this work to understanding the impact social status has on social cognition. Finally, we discuss the development of social hierarchies and propose that the neural basis of status processing contributes to developmental changes in hierarchy formation and the value placed on status, and that this has important consequences. We draw from the current literature on brain development and peer relations to propose that developmental changes in neural sensitivity to social feedback can provide insight into social status processing in children and teens.
1.1. Definitions of Terms
We recognize that a variety of disciplines have conducted research on social status from different approaches, and thus a variety of terms appear in the literature. Here we briefly define the status terms referenced in this review.
To begin, hierarchy refers to the ranking of members in social groups based on the power, influence, or dominance they exhibit, whereby some members are superior or subordinate to others (Fiske, 2010; Magee & Galinsky, 2008; Mazur, 1985; Zitek & Tiedens, 2012). Rank is a term often used to objectively refer to where one falls within the hierarchy, and is conceptually similar to the ordinal ranking of numerical values along a number line (Chiao, Bordeaux, & Nalini Ambady, 2004; Chiao et al., 2009). Status, on the other hand, can be measured through social opinion or reputation (Gould, 2002), and is generally associated with admiration and respect (Anderson, Beer, Spataro, & Chatman, 2006; Fiske, 2010; Fragale, Overbeck, & Neale, 2011). The terms “status” and “rank” are often used interchangeably or in conjunction (e.g., Mazur, 1985), as they both represent the higher positions in a social hierarchy.
A number of studies discussed in this review measure or manipulate power or dominance, rather than objective rank. This is because power and dominance are frequently associated with status and are often used to infer the status of others (e.g. Fragale et al., 2011). Power implies control over resources or other group members and is distinguished from dominance as a contextual trait (Anderson & Berdahl, 2002; Fragale et al., 2011). Dominance is associated with the ability to acquire resources (e.g., (Hawley, 1999), and therefore can be a predictor of power (Anderson & Berdahl, 2002) and a means of establishing status across species (Anderson & Berdahl, 2002; Chase, 1980; 1982; Mazur, 1985). Thus, dominance can be viewed as a behavior or personality trait that leads to inequalities in power among group members, and thereby differentiates status.
Dominance can be divided into sociable and aggressive subscales (Kalma, Visser, & Peeters, 1993). These two subscales emphasize that power, or access to resources, can be obtained through aggressive, dominant behaviors, or through prosocial, cooperative actions. Similarly, dominance is contrasted with another dimension of social status – prestige. The correlation between status and prestige may have emerged to facilitate social learning, whereby group members display deference to those who possess qualities deemed valuable (Cheng, Tracy, Foulsham, Kingstone, & Henrich, 2013). Thus, individuals with high prestige maintain influence over the group, but without displaying the force or threat implied by dominance. This distinction will be revisited later, when we discuss status cues and attainment.
2. The Nature of Social Hierarchies
2.1. Structure, Formation and Function
A wealth of evidence indicates social hierarchies are endemic, innate, and most likely, evolved to support survival within a group-living context. While social hierarchies can vary in their specific details, there are shared, definitive features that can be discussed more broadly. Specifically, hierarchies across species are characterized by (a) the ranking of group members who vary in physical or intellectual capacities; (b) rapid and spontaneous formation; and (c) functional and adaptive value to the existence of the social group. Despite some cross-species variability, there is strong evidence that hierarchies arise out of necessity and their existence is beneficial to social groups.
First, inherent to the definition of a social hierarchy is the stratified ranking of group members along a valued dimension, with some members being superior or subordinate to others, and fewer members occupying the highest positions (Magee & Galinsky, 2008). This ‘valued dimension’ can be a behavior signifying dominance, such as overpowering an opponent in a conflict interaction (Mazur, 1985; Zumpe & Michael, 1986), or a skill or trait considered ideal by the group (Anderson & Kilduff, 2009; Hogg, 2001). Both individual characteristics and the outcomes of interactions among group members appear to influence hierarchy establishment (Chase, 1980; Chase et al., 2002), as well as asymmetries in dominance and affiliation (Newton-fisher, 2004; Pellegrini & Bartini, 2001; de Waal, 1986). The underlying concept, however, is that hierarchy formation is the result of individual variation in influence or power and the most valued member achieves the highest status (e.g, Berger, Rosenholtz, & Zelditch, 1980; Henrich & Gil-White, 2001). Additionally, social ranking can occur on a small or large scale, within or across groups (Sidanius & Pratto, 1999). Particularly in humans, rank is not limited to the actual observation of a dominant or valued trait, but is often the product of group consensus, or reputation (Gould, 2002). As a result, the structure of human hierarchies is multidimensional, largely context or group dependent, and self-reinforcing.
Second, hierarchies form quickly and spontaneously among group-living animals. Several findings suggest that positions of superiority or deference are rapidly identified through asymmetrical displays of dominance. For instance, a nonverbal cue as simple as gaze aversion indicates status roles immediately among monkeys meeting for the first time (Deaner, Khera, & Platt, 2005). Similarly, unfamiliar humans automatically display asymmetries in dominance behaviors in paired interactions, adopting either a dominant or submissive posture complementary to that exhibited by the other person (Markey, Funder, & Ozer, 2003; Tiedens & Fragale, 2003). Further evidence suggests that humans rapidly attribute status information to others (Moors & Houwer, 2005), and they spontaneously organize into a hierarchical structure (e.g., Berger et al. 1980; Gould, 2002). Humans also demonstrate high levels of consistency when making status judgments about both themselves and group members (Anderson et al., 2006). Finally, there is often a decline in aggressive interactions and increase in cooperative behaviors following the establishment of a hierarchy (La Freniere & Chariesworth, 1983; Strayer & J. Strayer, 1976) suggesting that status roles are quickly determined and accepted. These findings highlight the salience of status cues and the rapid acknowledgement of where one falls within the developing social hierarchy.
Importantly, the organization of social groups into a hierarchy serves an adaptive function that benefits the group as a whole. When essential resources are limited, individual skills vary, and reproductive fitness determines survival, hierarchies are an efficient way to divide goods and labor among group members. Thus, an important function of the hierarchy may be to define social roles (Halevy et al., 2011) and allocate limited resources (Sapolsky, 2005). A second function may be to increase the survival of high-status members, who possess the most favored traits of the group, and provide them with greater influence over other members. In nonhuman primates specifically, hierarchies favor the reproductive success of the highest-ranking members (Dewsbury, 1982). In humans, hierarchies encourage everyone to defer to the individuals who possess a skill or trait valued by the group, which may be an adaptive component of social learning (Henrich & McElreath, 2003). For instance, we tend to learn from and follow those who know more or have more than we do, because we revere their success. In this sense, deferring to ‘successful’ individuals in higher positions of status is advantageous to the group as a whole, given that these individuals presumably possess ideal knowledge or characteristics. Finally, in favor of the overall well-being of the group, the advantages afforded to higher-ranking members, and the prestige, may serve as a powerful motivating factor resulting in an increase in productivity of the group as a whole (Halevy et al, 2011; Magee & Galinsky, 2008).
2.2. Status Characteristics in Monkeys and Human Adults
Social primates are sensitive to a similar suite of behaviors and traits that serve to establish and convey status (see Table 1), although some factors are uniquely human. These cues can be neatly divided into two categories: perceptual cues and knowledge-based cues. The across-species commonalities suggest an evolved origin for attaining high status and recognizing status in others, and they hint at a potential for status-dedicated neural processing networks. Here we describe traits associated with both actual status and perceptions of status in nonhuman primates and human adults.
Table 1.
Common predictors of status in nonhuman primates and human adults.
| Status Cues | ||
|---|---|---|
|
| ||
| Nonhuman Primates | Humans | |
| Physical Features | ||
| Size | ✓ | ✓ |
| Body weight | ✓ | |
| Height | ? | ✓ |
| Gender | ✓ | ✓ |
| Attractiveness | ? | ✓ |
| Age | ✓ | ✓ |
| Hormonal/Chemical* | ||
| Testosterone | ✓ | ✓ |
| Behavioral | ||
| Dominance/Submission | ✓ | ✓ |
| Gaze patterns | ✓ | ✓ |
| Posture | ✓ | ✓ |
| Aggression | ✓ | ✓ |
| Impulsivity | ✓ | ? |
| Vocal and speech characteristics | ? | ✓ |
| Social/Reputation | ||
| Career title, income, etc. | n/a | ✓ |
| Intelligence | n/a | ✓ |
Hormone levels may increase as a result of status attainment, rather than predict it.
2.2.1. Traits Correlated with Status in Nonhuman Primates
Among nonhuman primates, the relative display of dominant or submissive actions serves to establish where one ranks in a group. Dominant actions, such as biting, hitting, chasing, and open-mouth threats, (Zumpe & Michael, 1986), and physical characteristics, such as body size and gender, are often associated with high rank in primate social groups (Fairbanks, Jorgensen, & Huff, 2004; Morgan et al., 2000). In fact, body size may be one of the most reliable predictors of status attainment upon introduction to a group-living environment, particularly when status is assessed based on the outcomes of antagonistic encounters (Morgan et al., 2000). Though other factors such as birth order or familial lineage might predict status, particularly among females (reviewed in Cheney & Seyfarth, 2007), body weight remains a more reliable predictor (Fairbanks et al., 2004). Gender may influence status as well, depending on the particular species (reviewed in Cheney & Seyfarth, 2007), but developmental growth patterns and the relative size of males to females influence the rates at which one sex dominates the other (Pereira, 1995). Physical size provides an obvious advantage when seeking to dominate or overpower an opponent, and thus offers an advantage in status attainment. However, it is important to note that in some species, affiliative behaviors and alliances are also important to maintaining status positions (Newton-fisher, 2004; de Waal, 1986).
In addition to physical size, variations in neurotransmitter and hormone levels may predispose one to attain higher status. Interestingly, a longitudinal study following captive male vervet monkeys from adolescence to adulthood showed that males with lower levels of serotonin and dopamine metabolites prior to immigration into adult breeding groups were more likely to achieve higher status in a group (Fairbanks et al., 2004). Further, administration of cocaine to increase dopamine levels also predicts status attainment in monkeys (Morgan et al., 2000), suggesting baseline levels of certain neurotransmitters might influence rank. There is also evidence that higher-status males have higher testosterone levels than their low-status counterparts (Mazur, 1985; Beehner, Bergman, Cheney, Seyfarth, & Whitten, 2006; but also see Morgan et al., 2000).
It is possible that neurochemicals affect status through their influence on specific behaviors. For instance, lower levels of serotonin and dopamine metabolites are associated with a higher rate of aggressive, dominant, and impulsive behaviors (Fairbanks et al., 2004; Kaplan, Manuck, Fontenot, & Mann, 2002). Adolescent impulsivity may lead to high status attainment upon introduction to a group-living environment because a lack of inhibition provides an advantage in agonistic encounters (Fairbanks et al., 2004). Conversely, an increase in the hormone oxytocin promotes more affiliative behavior (Chang, Barter, Ebitz, Watson, & Platt, 2012), which may promote status attainment in some species (Newton-fisher, 2004; de Waal, 1986).
However, it should be cautioned that the associations between status and neurotransmitter and hormone levels are complicated, and not necessarily causal. Research has shown an increase in dopamine D2 receptors among dominant but not subordinate macaques when housed in social groups, but not individually (Morgan et al., 2002), and other findings suggest serotonin may mediate dominance behaviors when the hierarchy is uncertain (Raleigh, Mcguire, Brammer, & Yuwiler, 1991). Finally, testosterone levels rise as a result of successfully challenging a group member (Rose, Gordon, & Berstein, 1975), suggesting that group interactions that serve to establish status may have downstream consequences on hormone levels.
2.2.2. Traits Correlated with Status in Humans
Similar to monkeys, body size is correlated with higher status in humans. For instance, male height is associated with a number of factors predictive of status, with taller men being perceived as more dominant and more preferred as reproductive partners (Buunk, Park, Zurriaga, Klavina, & Massar, 2008). The positive relationship between height and dominance may underlie the finding that taller U.S. Presidential candidates are both more likely to receive popular votes and to be reelected (Stulp, Buunk, Verhulst, & Pollet, 2012). Similarly, taller men are more likely to earn more than short men (Harper, 2000; Lundborg, Nystedt, & Olof-Rooth, 2014). Testosterone may also be associated with high status in humans, though its direct role is unclear; baseline level serves as a predictor of dominant or aggressive behaviors, but as previously noted, levels also fluctuate in response to status competitions (Mazur & Booth, 1998). However, testosterone may influence status-seeking behaviors, such as dominance and social aggression (Mazur & Booth, 1998; Terburg & van Honk, 2013).
Many of the cues discussed thus far are associated with dominance. However, like monkeys, prosocial and affiliative behaviors may also serve to successfully exert influence and obtain resources (Charlesworth, 1996). Dominance may be best divided into two subscales: sociable dominance and aggressive dominance (Kalma et al., 1993). Individuals who score higher on sociable dominance tend to have better peer relations and are more socially oriented in their behavior, while those who score high on aggressive dominance are more selfish and exhibit more antisocial behaviors (see Table 2). It is important to note that, while positive social behavior may help a group or an individual (via association with others) to achieve status, it does not necessarily imply that dominant behavior is not present; in fact, scoring high on measures of both sociable and aggressive dominance may be optimal (Kalma et al., 1993).
Table 2.
Observed behaviors and self-reported strategies used to obtain power in social interactions for sociable and aggressive dominance subtypes (Kalma & Peeters, 1993). An unchecked box means this trait is not significantly correlated with dominance type.
| Adult High Status Subtypes | ||
|---|---|---|
|
| ||
| Sociable Dominance | Aggressive Dominance | |
| General | ||
| Exhibits expertise | ✓ | ✓ |
| Exhibits eye contact while speaking | ✓ | |
| Exhibits eye contact while listening | ✓ | |
| Prolongs gaze | ✓ | |
| Bargains | ✓ | |
| Disagrees | ✓ | ✓ |
| Antisocial Behavior | ||
| Interrupts others | ✓ | |
| Deceitful | ✓ | |
| Evasive | ✓ | |
| Uses threats | ✓ | |
| Dominance | ||
| Gives opinions freely | ✓ | ✓ |
| Assertive | ✓ | ✓ |
| Persuasive | ✓ | |
| Persistent | ✓ | |
Alternatively, aggression might not be necessary at all; rather, the individuals who attain high status can do so by making themselves appear valuable to the group, and acting confidently and generously toward others (Anderson & Kilduff, 2009; Henrich & Gil-White, 2001). For instance, there may be two opposing means of establishing status among humans: through displays of dominance, or displays of prestige. Contrary to the aggression and force characteristic of social dominance, individuals demonstrating prestige attain status by demonstrating their competence, knowledge, or skills (Mazur & Booth, 1998; Terburg & van Honk, 2013). This may be why non-dominant individuals with valuable knowledge or skills attain high status, such as college professors. Similar to those who display sociable dominance (Kalma et al., 1993), those who attain status through prestige are rated as more likable than those who use traditional dominance (Cheng et al., 2013). Importantly, humans occupy many different social hierarchies, and different strategies may be used to obtain resources, or high status. This may explain variability in the current literature on human status cues (see Hall, Coats, & Lebeau, 2005). It is also an idea that will be revisited in the developmental section, where there is a distinction between the characteristics of high status, popular teens who are well liked, and those who are not.
Interestingly, physical attractiveness is an additional trait that may help in attaining status in humans, though the literature on this association is mixed (reviewed in Anderson et al., 2001). For instance, there are findings showing that attractiveness predicts sorority recruitment among high-status but not low-status houses (Krendl, Magoon, Hull, & Heatherton, 2011), while other studies suggest attractiveness predicts status for university males but not females (Anderson et al., 2001). Still, other research shows physical appearance provides an advantage for both males and females in the job market, with higher wages associated with higher levels of physical attractiveness (Harper, 2000). The relative value of status cues may vary depending on the context of the social group (e.g., high-status sorority girls care much more about appearance), or whether information other than physical appearance is available when making status judgments.
2.2.3. Cues Used to Perceive Status in Others
There is also a great deal of overlap in the cues used by monkeys and humans to gauge status in others (see Table 1). Like our primate ancestors (Fairbanks et al., 2004; Morgan et al., 2000), physical attributes associated with body size (Keating, Mazur & Segall, 1981; Mueller & Mazur 1996) and upper body strength (Von Rueden, Gurven, & Kaplan, 2008) are often used to recognize the status of others.
A number of behavioral traits associated with dominance also signify rank. As previously noted, monkeys can use dominant gestures, such as open mouth threats, to perceive the status of another monkey (Zumpe & Michael, 1986). Similarly, in humans, asymmetries in status can be inferred through body positions that convey dominance or submission. In particular, an open and expanded physical posture is associated with dominance and status (Hall, Coats, & Lebeau, 2005; Schwartz, Tesser, Powell, Schwartz, & Tesser, 1982; Weisfeld & Beresford, 1982) and may even serve to elicit submissive, enclosed posture in paired interactions (Tiedens & Fragale, 2003). In fact, when the behaviors of actual couples (Sadler & Woody, 2003) and experimentally manipulated partnerships (Markey et al., 2003) are observed during one-on-one interactions, the resulting behaviors tend to be complementary. In other words, one partner expresses more dominant behaviors and the other is more submissive. Further, both partners of the paired interaction express more positive views of their other half when complementary behavior is displayed (Tiedens & Fragale, 2003), suggesting that status cues function to facilitate behavioral synchrony in social interactions.
In humans, dominance can also be conveyed through language and speaking style. For instance, speaking quickly and confidently, avoiding nonstandard speech and too much politeness, and utilizing sophisticated vocabulary and proper enunciation can communicate high status (reviewed in Fiske, 2010). Conversational nonverbal behavior, such as level of eye contact, can convey status as well. Participants judge confederates displaying more eye contact when talking than listening to have more power (Dovidio & Ellyson, 1982). However, other research reports findings suggesting a perceived, but not actual, association between “visual dominance ratio” (the ratio of looking while talking versus while listening) and high status (Hall et al., 2005). Some findings even suggest the opposite, where high status individuals make less eye contact (Ellyson, Dovidio, & Fehr; Hall et al., 2005) and are more disengaged (Kraus & Keltner, 2009) during conversation. Finally, high status speakers tend to be more facially expressive and relaxed, and they interrupt more frequently and maintain a smaller interpersonal distance during communication (Hall et al., 2005).
Facial cues associated with seniority/maturity and masculinity are often used when evaluating facial dominance in humans. For example, physical traits associated with seniority, such as a receding hairline, thin lips, and wide jaw, are associated with dominance across cultures (Keating et al., 1981), suggesting that age is a powerful predictor of status. Facial masculinity is also a prominent cue used to assess rank, and it is associated with both high social status and perceived dominance in men (Mueller & Mazur, 1996; Watkins et al., 2010). Surprisingly, females perceived as having more masculine features also tend to score higher on dominance scales, even when controlling for other features, such as make-up use (Quist, Watkins, F. G. Smith, Debruine, & B. C. Jones, 2011). Additionally, both male and female faces rated higher in masculinity elicit stronger gaze-cuing effects (Jones et al. 2010a), and both men and women with lower pitched voices are perceived as more dominant by both genders (Borkowska & Pawlowski, 2011; Jones, Feinberg, DeBriune, Little, & Vukovic, 2010; Wolff & Puts, 2010). This ‘masculinity effect’ might be due to a societal preference for males in general, or due to male-characteristic emotional expressions. In reference to the latter point, perceived dominance is related to the disposition to show emotions like anger, disgust and contempt, but not fear, sadness, or happiness (Hess, Adams, & Kleck, 2005) and males are socialized to avoid expressing emotions such as fear and sadness (Brody, 2000).
Physical attractiveness is another status cue that seems contrary to findings identifying age and masculinity as status cues. Attractiveness can be positively (Quist et al., 2011; Townshend, 1993) or negatively correlated with dominance in both genders (Keating & Doyle, 2002; Rhodes, Hickford, & Jeffrey, 2000), but in females it is also associated with youth and femininity (reviewed in Rhodes, 2006). As previously noted, the literature on the value of attractiveness to status is mixed (Anderson et al., 2001). It might be the case that status cues like age and masculinity are better predictors of dominance than status per se, or they might be more important status cues in males (Karafin, Tranel, & Adolphs, 2004).
Finally, unlike nonhuman primates, humans use a range of non-perceptual cues, largely based on accrued knowledge, to assess social status. Income, occupation, intelligence, popularity, and prestige are commonly used to assess status (Cheng et al., 2013; Dalmaso, Pavan, Castelli, & Galfano, 2012; Hymel et al., 2011; Zink, Tong, Chen, Bassett, & Stein, 2008). Reputations and labels tend to carry status information and sometimes the only information used to make status judgments is what we learn about someone from others. For instance, Dalmaso and colleagues (2011) presented images of unfamiliar people along with fictive curriculum vitae with either high- or low-status information. This elicited a gaze-cuing effect, where participants more readily followed the gaze of high-status individuals. This shows that information regarding skills and career success is used to rapidly learn the status of unfamiliar individuals when nothing else is known about these people. Similarly, when research participants make decisions to help someone who has a high status career title (lawyer) or low status title (gas station attendant), they favor the high status people (Goodman & Gareis, 1993).
2.2.4. One's Own Status Affects Perception of Other's Status
The accuracy with which one perceives dominance and status cues appears to depend on one's own status. Men who score higher in self- reported dominance are worse at perceiving facial cues of dominance when looking at other men, compared to men who score lower in dominance (Watkins, Jones, & Debruine, 2010). One explanation for this is that lower-status individuals have trained themselves – perhaps unconsciously - to perceive subtle dominance cues because they will incur greater costs than higher-status members if they perceive these cues incorrectly (Watkins et al., 2010). This ‘status training’ may have its roots in our tendency to allocate more attention to high status conspecifics (Deaner, Khera, & Platt, 2005; Jones et al., 2010; Shepherd, Deaner, & Platt, 2006).
Additional work suggests that subjective and objective measures of relative status affect how individuals process social information. Specifically, low subjective social rank correlates with greater neural activity in mentalizing regions when viewing social images and accompanying information (Muscatell et al., 2012). Similarly, objective status – or socioeconomic status – also correlates with activity in the mentalizing network when viewing threatening facial expressions. Thus, one's relative status may affect multiple elements of social perception.
2.3. Summary
Social hierarchies appear to form automatically in both human and nonhuman primate groups. Interestingly, monkeys rapidly and automatically recognize and acknowledge status cues without the benefits of language or human-level reasoning, suggesting there is something reflexive and primitive about the perception of many status cues. Further, they perceive status cues when observing third-party social interactions, and recall these cues as stable traits that generalize to novel interactions (Paxton, Basile, & Hampton, 2011), emphasizing that status cues are salient and embedded into the identity of different group members, even in monkeys. In adult humans, where hierarchies are more complex and status cues are more context dependent, group processes like popular consensus, reputation, and cultural values favoring career success can also dictate status, in addition to a number of the cues associated with status in primates.
Importantly, the overlap in status cues across species suggests a common origin, with the complex cognitive capacities of humans adding a unique socio-cultural and linguistic element to status hierarchies. Further, the nature in which hierarchies form and the fluidity with which status cues are perceived across species motivate the evaluation of underlying cognitive and neural mechanisms, so that we can better understand how status shapes our social perception. These processes will be discussed in the next section.
3. How Social Status Modulates Adult Cognition
In this section, we discuss evidence from behavioral research suggesting the relevance of status to social cognition (see Table 3). This section will primarily focus on human adults, though we present relevant studies with similar findings in monkeys. Next, we will discuss the primary brain regions believed to be involved in, or influenced by, status processing.
Table 3.
Effects of social status on adult cognition.
| Effects of Social Status on Adult Cognition | ||
|---|---|---|
|
| ||
| Status Group | ||
|
| ||
| High-status | Low-status | |
| How much people look at and attend to them | More | Less |
| How much people follow their gaze | More | Less |
| How well they are remembered | Better | Worse |
| How well they overcome audience effects on performance | Better | Worse |
| How much empathy they display to other's pain | Less | More* |
| How well they take another's perspective | Worse | Better* |
This may not be the case for more sociable, high-status individuals.
3.1. Effects of Social Status on Cognition
Here we review how status affects cognition, from two perspectives. The first two subsections discuss how we think about and perceive high and low status individuals, and the second two subsections discuss how our own status influences our cognitive processes.
3.1.1. Selective Attention and Gaze Following
High social status attracts visual attention across species. Both monkeys and humans prefer to look at higher compared to lower ranked group members. Monkeys will forgo a juice reward to view high ranking members of the group, but require juice over-payment to view lower ranking members (Deaner et al., 2005; see Figure 1). This suggests that viewing high-status group members has a high value to the degree that doing so can trump intrinsic caloric rewards. Humans demonstrate a similar bias as shown by our tendency to fixate speakers rated as high-influence and high-status, as compared to those rated as low-influence and low-status, more often and for longer periods of time (Foulsham, Cheng, Tracy, Henrich, & Kingstone, 2010). There is evidence from humans (La Freniere & Chariesworth, 1983; Vaughn & Waters, 1981) and monkeys (Klein, Shepherd, & Platt, 2009) that might even suggest visual regard and attention play a role in determining rank within a group, such that those who are more attended achieve higher status. Another explanation is that high status group members hold more power, and individuals with less power are more motivated to attend to the powerful because they possess more control (Fiske, 1993).
Figure 1.
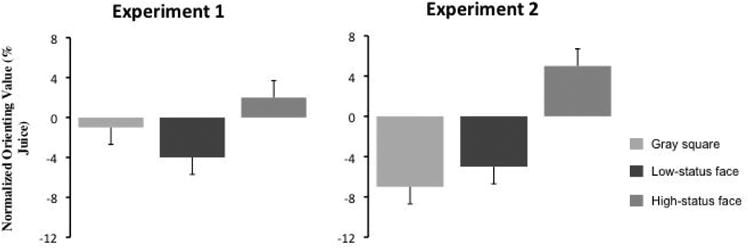
Two male rhesus monkeys had to option to fixate one location on a screen and receive a juice reward, or another location to view an image of a high or low status male. Monkeys sacrificed juice to view images of high status males, but required juice overpayment to view low-status males. The graphs show mean normalized orienting values for gray squares, low-status faces, and high-status faces. Values are sign-reversed, such that a positive value means juice was sacrificed. Reproduced from the work of Deaner, Khera, & Platt, 2005.
Attentional biasing effects of status are also revealed in dynamic aspects of attention, e.g. gaze-cuing. Gaze patterns serve as rapid indicators of status in monkeys and humans and are perceived differently depending on rank (Deaner et al., 2005; Jones et al., 2010; Shepherd et al., 2006). Chance (1967) proposed that the more submissive member of a dyad should readily attend to and even seek out the higher-status member. Recent evidence has shown this to be true (McNelis & Boatright-Horowitz, 1998; Shepherd et al., 2006). For instance, low-status monkeys follow the gaze of other high and low-status members of their social group, whereas high-status monkeys only follow the gaze of other high-status monkeys (Shepherd et al., 2006). Further, in both humans (Jones et al., 2010) and monkeys (Shepherd et al., 2006), gaze-cuing effects appear to be involuntary, reflecting an automatic tendency to attend to the gaze cues of higher-ranking individuals.
Interestingly, in humans, the adverted gaze of high-status individuals influences subtle and immediate shifts in attention even when faces are unfamiliar and status is implied via fictional career information (Dalmaso et al., 2011). Humans also preferentially allocate their attention to individuals who are high-status by virtue of reputation. This gaze-cuing effect may reflect some combination of heightened levels of interest, trust, or respect for high-status individuals.
3.1.2. Memory
Given that high-status faces receive more attention, it is no surprise that they occupy a privileged position in memory. In one of the only studies on this topic, Ratcliff, Hugenberg, Shriver, and Bernstein (2011) required study participants to view a series of faces presented with either a high- or low-status job title. Later, participants were more likely to recognize the high-status faces. Subsequent manipulations altered the status information associated with the faces (e.g., a doctor versus mechanic uniform) and tested for spatial memory of high- and low-status individuals in a spatial matching paradigm. The findings were consistent: those paired with high-status cues were more quickly and accurately recognized and remembered. Further research is needed on this topic as the effect sizes were relatively small and the finding has not been replicated.
3.1.3. Executive Processes
One nefarious consequence of low status is that it is akin to a cognitive load, impairing executive processes. For instance, when ranked within a small group based on intelligence (IQ) scores, lower-status participants showed impaired performance on subsequent IQ test items (Kishida, Yang, Quartz, & Quartz, 2012). These findings suggest an awareness of one's subordinate standing within a group can interfere with cognitive abilities. Interestingly, though all participants initially showed a reduction in performance in a group setting (e.g. an audience effect) higher-ranked members more readily overcame this deficit (Kishida et al., 2012). It is important to note that these effects may simply be due to self-fulfilling prophecy rather than status per se.
It is also worth noting that the causal relationship between status and performance is unclear, as high levels of cognitive functioning may contribute to high social status; for instance, we previously discussed career achievement as a marker of high status (Dalmaso et al., 2011). However, other findings have shown that individuals primed to feel subordinate or low-power are less effective at updating, inhibition, and planning than those primed to feel superior or high-power (Smith, Jostmann, Galinsky, & Dijk, 2008). The lack of power associated with low status might impair executive function, making improvement among low-status members and movement between ranks difficult. Future studies are needed to explicitly determine the effect of social status on one's cognition and performance.
3.1.4. Moral Decisions and Perceptions of Competency
Several studies show that high status, or upper-class individuals are more likely to make unethical decisions, break the law while driving, and even lie or cheat to get their way (Piff, Stancato, Côté, Mendoza-denton, & Keltner, 2012). This may stem from the power and benefits associated with high status. Ironically, the tendency to lie and cheat does little to malign their character; high status individuals are more likely to be perceived as competent, both in the domain in which they are highly ranked and in other, irrelevant domains (Fiske, Cuddy, Glick, & Xu, 2002), and they are more likely to be perceived as trustworthy (Lount & Pettit, 2012). Similarly, deviation from expectations is more likely to be accepted in high status individuals, yet likely to be punished in low status individuals (reviewed by Fiske, 2010).
Another possible explanation is that the subjective perception of power associated with status alters social cognition. A series of studies by Galinsky, Magee, Inesi, and Gruenfeld (2006) showed that participants primed to feel high-power were less likely to adopt the perspective of another individual, less likely to recognize that others do not hold the same knowledge as they did, and less accurate at interpreting the emotional expressions of others. This suggests that individuals with high-power might fail to understand the perspectives, intentions, and emotions of others, and thus make poor moral decisions. These status-related differences in emotional perception might emerge because it is more important for lower-status members to engage in greater perspective taking, given that they tend to be more susceptible to threat and must follow the direction of higher ranked members (Galinsky et al., 2006). However, reaching and maintaining a high status position requires social skills, and these effects should be interpreted with caution. More research is needed to understand the influence of status on moral decisions and social cognition.
In sum, our relative positions within a social hierarchy alter how we perceive, think about, and react to others, as well as how others perceive us. These effects may occur due to the benefits afforded to high status individuals, or due to power and prestige associated with high rank. Notably, these effects are found in response to both observable status cues and reputation based status titles, suggesting status cues are a highly salient. These findings both explain and emphasize the need for the ability to rapidly process status information, and support both the function and impact of social hierarchies. For instance, being able to attend to, remember, and predict the intentions of high-status members might be an important element of a well-functioning hierarchy, given the power, influence, and knowledge associated with these individuals. However, the influence that rank has on cognition may contribute to the self-reinforcing nature of hierarchies, in that status itself activates cognitions and behaviors that serve to maintain the structure, and this may have important implications for societal values like social mobility and equality.
3.2. Neural Underpinnings of Social Status Processing
Given the complex nature of social status processing, it is likely that it is subserved by a spatially distributed neural network. Moreover certain nodes in the network will likely come online or go offline, depending on the type of cue being processed. For instance, perceptual regions, such as the posterior superior temporal sulcus, are undoubtedly important for processing perceptual aspects of status cues, such as direction of gaze, mouth movements, and dynamic facial expressions indicating dominance and submission (e.g., Chiao et al., 2008). However higher-level regions are likely needed to serve as interpreters and integrators of status information. Here we describe the small literature relevant to the higher-level effects, in the context of three status-relevant neural components: (1) rank and magnitude; (2) affective/value/reward processing; and (3) executive processing.
First, a few studies have shown that a brain region involved in magnitude and number processing responds to the rank ordering of group members. It has been shown that activity increases in the inferior intraparietal sulcus (IPS), a region implicated in magnitude judgments and number processing, when military personnel made rank judgments about other military personnel, when participants made rank judgments about cars (Chiao, et al., 2004; Chiao et al., 2009; see Figure 2), or when participants made self-referential financial status judgments (Cloutier, Ambady, Meagher, & Gabrieli, 2012; Cloutier & Gyurovski, 2013). Similar effects in the IPS were found when participants made decisions about the relative status of individuals in static images portraying social interactions that implied status inequality and when making relative weight judgment (Mason, Magee, & Fiske, 2014). This region appears to serve a general function of computing rank order since there were overlapping activations to social rank and non-social rank.
Figure 2.
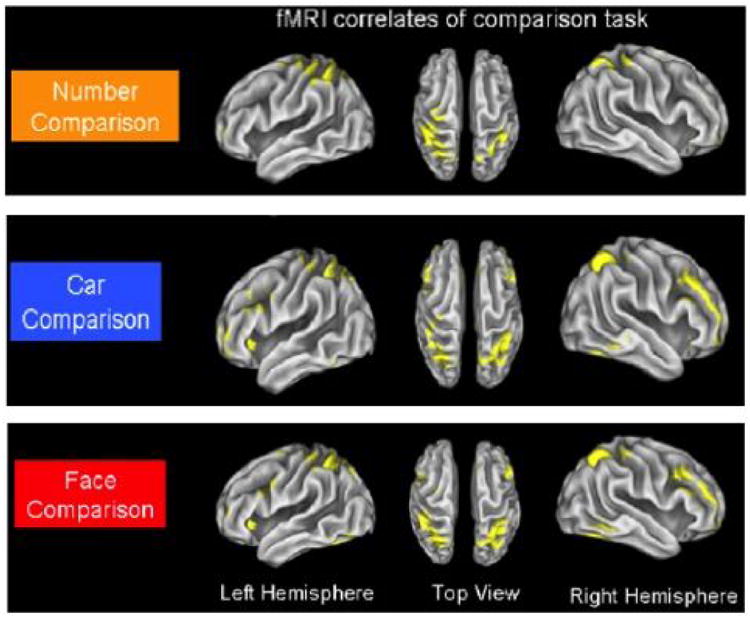
Activity increases in the inferior intraparietal sulcus (IPS) for number magnitude judgments (top), rank comparison judgments of cars (middle), and rank comparison judgments of military personnel (bottom; Chiao et al., 2009).
Second, limbic and paralimbic brain regions implicated in emotion generation and interpretation and reward processing regions like the ventral striatum (reviewed in Delgado, 2007) are sensitive to the presence of high-status individuals. There is evidence from older studies in monkeys that lesions to limbic (the amygdala and ventral anterior cingulate) and paralimbic regions (the temporal pole, orbitofrontal cortex) can cause a dramatic loss of social status as well as the ability to react appropriately to the social status of others (reviewed in Olson, Plotzker and Ezzyat, 2007). Similarly, in humans, the rate at which we learn about other's social status correlates with volumetric differences in the human amygdala, anterior hippocampus, as well as the temporal pole (Kumaran, Melo, & Duzel, 2012).
Limbic regions are strongly interconnected by white matter with key reward processing regions such as the striatum, which is part of the basal ganglion (Ghashghaei, Hilgetag, & Barbas, 2007). A recent study in group-living macaques reported that grey matter volume in the amygdala positively correlated with dominance rank, while grey matter volume in the anterior dorsal striatum positively correlated with subordinance (Noonan et al., 2014). Further, functional coupling among these regions correlated with status rank, where more subordinate group members showed stronger positive coupling. Interestingly, these effects were exclusively related to status – not social network size, suggesting these neural variations are associated with rank, and not other aspects of social behavior. A separate study of macaques found distinct groups of neurons in a subregion of the dorsal striatum called the caudate nucleus that fired either in response to reward outcomes in a food-grabbing task, or to the social state – competitive success compared to submission – with a competing monkey (Santos, Nagasaka, Fujii, & Nakahara, 2012).
As noted earlier, behavioral studies suggest that viewing high-status group members is rewarding (Deaner et al., 2005). Viewing higher-status monkeys correlates with activity in orbitofrontal cortex (OFC), a region in involved in value computations as well as inhibitory control, and the amygdala, which is involved in the emotive coding of the stimulus. These regions are monosynaptically connected (Timbie & Barbas, 2014) and their combined activity may guide visual orienting towards motivationally salient stimuli, such as high-status groups members (Klein et al, 2006). While a direct comparison has not been made in humans, there is evidence that areas known to be involved in reward processing, such as the ventral striatum, are active when making status judgments about others. These effects are modulated by ones relative status and the ability to move up in the hierarchy (Ly, Haynes, Barter, Weinberger, & Zink, 2011; Zink et al., 2008). The monkey literature suggests greater value is assigned to high-status monkeys, and activation of the reward circuitry in human implies a similar rewarding value associated with higher ranked individuals. However, the absence of behavioral data showing a preference for high-status individuals over other rewarding stimuli makes such an interpretation difficult to make. It is unclear whether these responses suggest that viewing higher-status members is rewarding, or just that they hold a more salient presence and command more attention than subordinate members.
Third, brain regions involved in executive processes (e.g. frontal regions) are sensitive to status cues, but it's unclear whether they play a role specifically in status processing, or in social cognition more generally. Various findings link activity in the ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC), and medial prefrontal cortex (MPFC) to the perception or judgment of social status (Cloutier & Gyurovski, 2014; Farrow et al., 2011; Marsh, Blair, Jones, Soliman, & Blair, 2009; Mason et al., 2014; Zink et al., 2008). The anatomical interpretations of these findings are not grounded in a well-established lesion literature. Patients with large frontal lobe lesions often have gross personality and social deficits. Yet at least one study reported that patients with lesions to the DLPFC show impairment in making general social judgments, but not status judgments (Mah, Arnold, & Grafman, 2004).
Because this literature is small, there are several unanswered questions. For instance, one study reported higher activity in the ventral striatum in response to high-status individuals (Zink et al., 2008) while another study showed higher activity in the same brain region to same status individuals (Ly et al., 2011), making the relationship between reward activation and status in humans unclear. In addition, findings suggesting a reduction in activity in brain regions involved in mentalizing and emotions among low status individuals (see Muscatell et al., 2012) are difficult to interpret in the absence of behavioral data. Separate studies have shown that high-powered individuals are not very good at understanding the perspectives and emotions of others and at times, chose not to engage in the same degree of metalizing (Galinsky et al., 2006). However, in monkeys, grey matter volume in the rostral and dorsal PFC and superior temporal sulcus -- regions similar to the human mentalizing network -- positively correlates with both status and social network size in monkeys (Noonan et al., 2014). A greater ability to understand and predict the intentions of others might help to maintain high social status. Future work should combine behavioral and imaging measures to examine how these status-related variations in neural activation correlate with reward-driven or mentalizing behaviors.
In sum, the present literature suggests that brain regions involved in rank ordering, attention, and emotions and reward are responsive to social status information. However, a lack of supporting behavioral and neuropsychological data make some conclusions questionable, and there are other questions regarding the neural processing of status cues that have not yet been explored. For instance, it remains unclear whether status information becomes embedded in our semantic representations of others, thus influencing our social attention and behavior on a more implicit level. This appears to be the case in behavioral studies in both the cognitive and social psychology literature. It is also unclear how emotion, reward and person processing regions of the human brain interact when perceiving status information. Further research on the neural underpinnings of status processing, and the behavioral correlates, is needed.
4. Social Hierarchies and Status Cognition in Children and Adolescents
The formation of social hierarchies varies across different developmental periods, though even very young children are sensitive to perceptual cues signifying dominance. Here we discuss the developmental origins of social hierarchies and the factors contribute to age related changes in status processing, and how these relate to the animal and human adult literature.
4.1. The Nature of Social Hierarchies
By the end of the first year of life, infants possess the belief that size is associated with strength or dominance, and they use this information to anticipate the outcome of a conflict interaction. For instance, 10-13 month old infants look longer when they see a larger, animated block yielding to a smaller block after bumping into one another, as though they recognize this outcome is unexpected (Thomsen, Frankenhuis, Ingold-Smith, & Carey, 2011). By 12-15 months of age, infants not only recognize that one agent will overpower another during a conflict interaction, but that this asymmetric relationship remains consistent from one conflict to another (Mascaro & Csibra, 2012). In real-life social settings, 1-2 year old children display dyadic dominance relations themselves, from which members of a group can be ranked along a linear hierarchy (Strayer & Trudel, 1984). By 3 years, children recognize asymmetries in dominance in an experimental setting by using a variety of cues, including body size, age, power, and possession of resources (Charafeddine et al., 2014). Thus, around one year of age, children recognize that dominance achieves a stable position of power, which is the foundation upon which a social hierarchy forms. This highlights the inherent nature of social hierarchies, and an early respect for socially dominant behavior. As they get closer to pre-school, children appear to expand their knowledge of status to include a variety of status cues.
By pre-school, children are spending more time in social groups, and this presents a point in development where social hierarchies can emerge. In fact, 4-5 year olds display asymmetries in dominance behaviors, and begin to demonstrate a preference for interacting with high-status group members (F. F. Strayer & Trudel, 1984). Dominant members of the social group are also more often watched and imitated than their less dominant peers (Abramovitch & Grusec, 1978; La Freniere & Charlesworth, 1983; Vaughn & Waters, 1981), suggesting a visual preference for high status group members, similar to what is found in primates (e.g., Deaner et al., 2005). Longitudinal studies spanning the school year show that asymmetric social relationships remain stable over time and increase in number across the school year (La Freniere & Charlesworth, 1983), and the stability of the emerging dominance hierarchy increases as children approach elementary school age (Strayer & Trudel, 1984). By the time children enter elementary school, they rapidly form dominance hierarchies (Petit, Bakshi, Dodge, & Coie, 1990; Strayer & Trudel, 1984), and the linearity and stability of the group structure continue to increase with age (Petit et al., 1990). These findings suggest both an early recognition of the unequal distribution of power within a group and an early preference for a hierarchy.
It is important to note that social rank in pre-school children is often based on third party observations of dominance interactions, while in elementary school, children nominate peers in high status positions. Many early studies evaluating social status used measures of social preference, or likability (e.g., Coie et al., 1990; Coie et al., 1982; Dodge, 1983; reviewed in Cairns, 1982; Hymel, Closson, Caravita, & Vaillancourt, 2011). This measure may be less indicative of social reputation and dominance than measures of popularity (e.g., de Bruyn & Cillessen, 2006; Cillessen & Mayeaux, 2004; Parkhurst & Hopmeyer, 1998), and less similar to traditional measures of social hierarchies (Cairns, 1982). While dominance is associated with social preference among younger children (Strayer & Trudel, 1984; Petit et al., 1990), there is a distinction among older children (reviewed in Babad, 2001; Cairns, 1982), teens (de Bruyn & Cillessen, 2006; Lease, Musgrove, & Axelrod, 2002; Parkhurst & Hopmeyer, 1998; Sandstrom & Cillessen, 2006) and emerging adults (Lansu & Cillessen, 2011). Popularity, rather than social preference or acceptance, more closely resembles the dominance hierarchies in humans and nonhuman primates (Coie et al., 1982; Petit et al., 1990), and it has become the more widely accepted metric for social status in childhood in adolescence (reviewed in Cillessen & Marks, 2011).
By early adolescence, peer groups become more differentiated in terms of status rating by peers and the salience and importance of social status beyond other achievements peaks (Coie, Dodge, & Kupersmidt, 1990; LaFontana & Cillessen, 2009). Same-sex female hierarchies are somewhat less stable, showing more frequent fluctuations in rank among mid-ranking and top-ranking members compared to male groups, yet the salience and function of the hierarchy is comparable across genders (Savin-Williams, 1979). Experimental evidence supports the visible role of high-status group members, regardless of gender. Similar to findings in adults and primates discussed earlier, adolescents tend to look longer at more popular, or higher status, class members (Lansu, Cillessen, & Karremans, 2014). Though social status research replicating findings from adults and primates in teens and children is sparse, this study indicates that status begins to influence perception and attention prior to adulthood. It is not clear how early these effects emerge, but these findings suggest that status roles have a powerful impact on cognition and behavior in adolescence.
There is some indication that the importance of popularity subsides in the later teen years (Lafontana & Cillessen, 2009), but there is also evidence that characteristics of popularity persist into early adulthood (Lansu & Cillessen, 2011). Importantly, position in the adolescent social hierarchy, or one's adjustment to this position, may impact adult behavior and wellness (Sandstrom & Cillessen, 2013; Zettergen, Bergman, & Wångby, 2006).
4.2. Status Cues
High status children and teens are those who are more often nominated by their peers as popular, though they are not necessarily nominated as well-liked (e.g., Babad, 2001; de Bruyn & Cillessen, 2006; Cillessen & Rose, 2005; Parkhurst & Hopmeyer, 1998; Witvliet et al., 2010). In the following section, we discuss the cues associated with reputation-based status, or popularity, in children and teens.
As previously noted, a number of studies show that dominance is highly correlated with popularity, beginning with studies in preschool (e.g., Strayer & Trudel, 1984). Social dominance plays an important role in establishing status and may persist as a reliable marker of status into adulthood. Dominance displays among children as early as pre-school function to define the roles within a new social group, so that aggressive behaviors are frequent at the beginning of the school year, then decline as group structure become established (La Freniere & Charlesworth, 1983; Pellegrini & Bartini, 2001; Petit et al., 1990). Pre-school boys tend to have more frequent displays of aggression than girls (Strayer & Strayer, 1976), and aggression is highly correlated with teacher ratings of social dominance (Pellegrini & Bartini, 2001). While this suggests that aggression is an important cue in establishing status within this age group, it is not necessarily a correlate of dominance or status in girls (Strayer & Strayer, 1976). Girls are more likely to engage in ‘relational aggression’ aimed at damaging interpersonal relationships rather than causing physical harm, but this behavior is often correlated with social rejection rather than high status at this age (Crick, Casas, & Mosher, 1997; Crick & Grotpeter, 1995). Thus, there might be early gender differences in the type of aggression used to establish dominance but both genders engage in behaviors used to establish a hierarchy (Hawley, 1999).
Regardless of gender, between the ages of 1 and 5 years agonistic behaviors toward others decline as a function of age while affiliative behaviors become more prevalent (Strayer & Trudel, 1984). One explanation for this developmental change is that aggression declines as group members weigh the costs and benefits of such behavior (Pellegrini & Bartini, 2001) and as social hierarchies stabilize (Savin-Williams, 1979). However, there is also evidence that social dominance in the form of overt or relational aggression is an important predictor of status, or popularity, during the late elementary and teen years (Cillessen & Mayeaux, 2004; Ellis & Zarbatany, 2007; Lansu & Cillessen, 2011; Lease et al., 2002; Shoulberg, Sijtsema, & Murray-Close, 2011; Witvliet et al., 2010). Further, body size and testosterone levels in adolescent males are associated with dominance and status (Schaal, Tremblay, Soussignan, & Susman, 2006; Tremblay et al., 1998), similar to what is found in monkeys and adult humans (e.g., Keating, Mazur & Segall, 1981; Morgan et al., 2000; Mueller & Mazur 1996).
These findings suggest dominance itself remains linked with status across age groups. It may be that dominance manifests itself differently during the later stages of development, such that overt displays of aggression are replaced with more productive, assertive behaviors (La Freniere & Charlesworth, 1983) or coercive and cooperative behaviors (reviewed in Hawley, 1999). Or, as we note in the section on adult status, a distinction between social dominance and aggressive dominance begins to emerge, and both are associated with high status. Within the group of teens nominated as popular, there are those who are not well liked, and those who are (de Bruyn & Cillessen, 2006; Parkhurst & Hopmeyer, 1998). One analysis examined the specific traits associated with popular teens and well-liked popular teens (see Table 4), identified as populistic and prosocial popular respectively (de Bruyn & Cillessen, 2006). The former group was rated as antisocial, arrogant, and having poor academic performance, while the latter was rated as affiliative and academically engaged. However, both groups were rated as attractive and hip, and as having a lot of friends. Thus, likeability is not essential to be considered popular, and popular teens vary in the level of prosocial and dominant behaviors they exhibit, similar to adults (see Table 2). Interestingly, the aforementioned study also found that populistic and prosocial teens were equally listened to, but populistic teens were rated higher on power and leadership than prosocial teens. These findings suggest differences in dominance, or perhaps “being listened to” refers to a more social form of dominance whereas “power” has a negative connotation.
Table 4.
Traits associated with populistic (popular) and prosocial popular (well-liked and popular) adolescents (de Bruyn & Cillessen, 2006). An unchecked box means this group is less likely to be rated by peers as presenting the listed trait.
| Adolescent High Status (Popular) Subtypes | ||
|---|---|---|
|
| ||
| Prosocial Popular | Populistic | |
| General | ||
| Likeable | ✓ | |
| Academically engaged | ✓ | |
| Many friends | ✓ | ✓ |
| Friendly | ✓ | |
| Attractive | ✓ | ✓ |
| Hip | ✓ | ✓ |
| Athletic | ✓ | ✓ |
| Antisocial Behavior | ||
| Bullies/mean and insulting | ✓ | |
| Verbally and physically aggressive | ✓ | |
| Relationally aggressive | ✓ | |
| Exhibits deviant behavior | ✓ | |
| Dominance | ||
| Voices opinions | ✓ | ✓ |
| Powerful | ✓ | |
| Leadership qualities | ✓ | |
| Listened to by others | ✓ | ✓ |
Other studies point to bullying as another manifestation of dominance during development. The dyadic relationship between bully and victim is similar to the dominant-submissive relationships discussed in social status literature. Within the same gender, bullies tend to be popular and aggressive whereas victims are unpopular (Rodkin & Berger, 2008). Despite being disliked by their peers, bullies perceived as powerful and popular (Farmer, Estell, Bishop. O'Neal, & Cairns, 2003; Vaillancourt, Hymel, & McDiugall, 2003) are also rated by their peers as having a number of status correlates found in high-status adults, including power, leadership, competence, and valued assets (Vaillancourt et al., 2003). In contrast, victims of bullies are often vulnerable and have low social status (Berger & Rodkin, 2009; Veenstra, Lindenberg, Zijlstra, De Winter, Verhulst, & Ormel, 2007). As is the case in adults, possessing power or displaying dominance, or a combination of both, can be associated with higher status depending on the context.
In sum, a number of traits associated with status in monkeys and human adults, including dominance, power, and influence, are also relevant to child and teen social hierarchies. However, systematic research regarding the extent to which these factors influence status perception and cognition is lacking in these age groups, so it's unclear whether status impacts social information processing to the extent it does in adults. Given the value placed on social status during adolescence, it is possible that the effects of status are even more prominent. In the next section, we discuss this potential for greater status sensitivity among adolescents in the context of social rejection neuroscience literature.
4.3. Status-Related Neural Circuits
Research on developmental changes in neural sensitivity to social status information is nearly nonexistent. However, evidence that the brain continues to undergo maturational changes and that social information is particularly salient during the teenage years suggests that adolescents will also be more sensitive to social status cues.
First, a number of regions involved in social status processing in adults undergo developmental changes between childhood and adulthood. Recall that our discussion of the neural underpinnings of status processing in adults included regions involved in magnitude judgment (the IPS), prefrontal areas (VLPFC, DLPFC) and emotion processing areas (amygdala, anterior cingulate). The prefrontal cortex continues to develop throughout adolescence, with the DLPFC being one of the latest regions to mature (Gogtay et al., 2004). Additionally, functional imaging studies suggest age-related changes in activation of the IPS when making non-symbolic magnitude judgments (Ansari & Dhital, 2006), and in the OFC, anterior cingulate, and amygdala, there are age-related changes in activations to various social stimuli (reviewed in Burnett, Sebastian, Kadosh, & Blakemore, 2011). Thus, the general developmental state of the adolescent brain suggests there will be age-related differences in status processing.
Second, there is a wide body of research on neural development and social relationships focusing on peer feedback, and specifically peer rejection. The developmental literature on social status groups often identifies those who are rejected as those who receive little to no peer nominations (e.g., Coie et al., 1982). Thus, peer rejection corresponds to low social status, since high peer status is based on favorable peer nominations. Importantly, there is overlap between the brain networks sensitive to rejection and those involved in status perception in adults. We therefore borrow from the peer rejection literature to suggest that status processing in adolescents differs from in adults, and suggest there are plausible neural models to explain this relationship: (1) affective brain regions may be more sensitive, or even hyperactive, to social information during adolescence; (2) inhibitory brain regions may be immature, or underactive, during adolescence; or (3) affective brain regions are overactive and inhibitory regions are underactive during adolescence.
First, there is evidence that adolescents are more sensitive to peer acceptance and rejection. Behavioral data suggests social rejection is more salient during adolescence than childhood (Silk et al. 2012), and adolescents report more anxiety and distress following exclusion than younger children and adults (Sebastian, Viding, Williams, & Blakemore, 2010). Adolescents also show increased activity in brain regions involved in reward and affective processing in response to peer feedback. Specifically, adolescents show greater activation in the anterior cingulate cortex (ACC) in response to exclusion relative to adults (Bolling et al., 2011; Masten et al., 2009), and greater ventral striatal activation in response to peer acceptance (Guyer, Choate, Pine, & Nelson, 2011). It is believed that one function of the ACC is to signal emotional pain resulting from social rejection (Eisenberger, Lieberman, & Williams, 2007) and activity in this region is positively correlated with self-reports of rejection sensitivity in adults (Burklund, Eisenberger, & Lieberman, 2007) and subjective distress in teens (Masten et al., 2009). The ventral striatum, part of the basal ganglion, is associated with both reward processing (reviewed in Delgado, 2007) and emotional regulation (Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). Thus adolescents are more sensitive to both the affective response and social reward associated with peer acceptance. Importantly, the opposite of peer acceptance -- peer rejection -- is associated with low social status, and thus these data may reflect increased sensitivity to status cues that determine where one stands within a social group. The ACC and ventral striatum also show greater activation during affective face processing (Monk et al., 2003; Pfeifer et al., 2011), suggesting teenagers may also be more reactive to facial cues representing dominance and status.
Second, this heightened sensitivity in adolescence may be due to immature frontal cortices and impaired emotional regulation. Adolescents show an overall increase in activity in the VLPFC (Bolling et al., 2011; Masten et al., 2009) in response to social exclusion relative to inclusion. Compared to adults, adolescents show greater activation in the dorsal medial prefrontal cortex (DMPFC; reviewed in Sebastian et al., 2010) and ventral ACC (Bolling et al., 2011), but less VLPFC activation (Bolling et al., 2011; Sebastian et al., 2011). These findings suggest age related changes in both affective processing of social information and top-down regulation (see Figure 3). Interestingly, activity in the VLPFC, DMPFC, and ventral striatum is negatively correlated with subjective feelings of distress among rejected adolescents and with activity in affective regions including the ACC and insula (Masten et al., 2009). This suggests that the VLPFC and DMPFC may be involved in regulating the emotional consequences of social rejection, and failure to do so effectively may lead to a stronger affective response to peer feedback. Functional connectivity between the VLPFC and ventral ACC increases from adolescence to adulthood (Bolling et al., 2011), which may further explain the age-related decline in rejection sensitivity.
Figure 3.
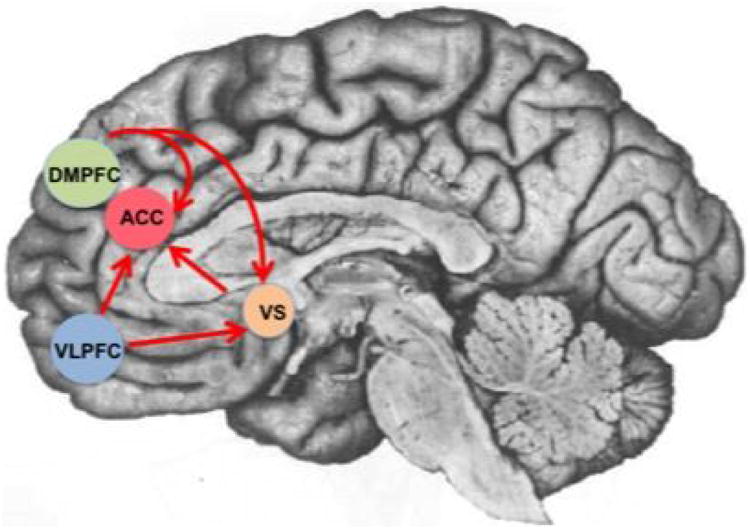
Brain regions sensitive to status information in adolescents. Dorsal medial prefrontal cortex (DMPFC), ventral lateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), and ventral striatum (VS). Direction of arrows represents regulatory pathways.
Further insight into this top-down regulatory approach comes from evidence that adults primed to feel socially excluded subsequently fail to recruit DMPFC when viewing negative, but not positive, social scenes (Powers, Wagner, Norris, & Heatherton, 2011). Adolescents, on the other hand, show an increase in activity in this region in response to social distress (Masten et al., 2009). It may be that teenagers are unable to successfully divert their attention from negative social information, and only attend to what is emotionally salient. Interestingly, only teenagers demonstrate a negative correlation between MPFC activity and resistance to peer influence (RPI) scores (Sebastian et al., 2011), suggesting that teens who are less affected by peers also show more adult-like levels of frontal activity. Similarly, teens who report more time spent with friends show less activity in the ACC and insula in response to rejection (Masten, Telzer, Fuligni, Lieberman, & Eisenberger, 2012), suggesting adolescents with positive social environments and social skills may be less sensitive to social rejection or more successful at regulating emotions.
Together, these data suggest that adolescents show increased sensitivity to status-relevant information from peers, either due to hyperactive affective centers or underactive inhibitory centers of the brain, or both. The data does not come directly from studies on social status, but peer acceptance is a crucial determinant of popularity within this age group. Additionally, we previously discussed the role of similar emotion generation and reward regions, as well as frontal regions including the MPFC and VLPFC, in status processing in adults. It is unclear whether adolescents would demonstrate similar age-related differences in the activation of these regions in the status judgment paradigms used in adults as they do in social rejection paradigms. Further research is needed to compare changes in status processing across age groups.
5. Summary and Conclusions
Social hierarchies are extremely prevalent, existing across species and present early in human development. From the extensive literature regarding hierarchies in nonhuman primates and humans, there are several important conclusions that can be drawn: (1) social hierarchies are a natural and necessary part of social groups; (2) status has a profound effect on thought and behavior; (3) the neural basis of status processing, particularly during development, is only beginning to be understood, and many questions remain unaddressed in the current literature.
In the following paragraphs, we propose a few directions for future research in the emerging field of developmental social neuroscience on status processing.
First, there have been no neuroimaging studies in which social status is examined in the context of other forms of person-specific conceptual knowledge such as name and occupation. There has been a great deal of research on ‘person identity’ nodes (Bruce & Young, 1986), the theoretical place in the brain where knowledge about individuals – their face, voice, and gait, as well as conceptual knowledge about the person – is brought together to form a conceptual representation of an individual that can be accessed through numerous distinctive retrieval cues. It is unclear how status information becomes embedded in these representations, and whether this consequently affects social perception and behavior. There is a well-studied network of inferior temporal regions associated with face processing, with regions in the ventral anterior temporal lobes that are particularly sensitive to person-related semantic information (Von der Heide, Skipper, & Olson, 2013; Collins & Olson, in press) and neurons in the orbitofrontal cortex that are potentially responsive to the rewarding aspects of other people (Tsao, Moeller, & Freiwald, 2008). It will be important for future researchers to examine how social status modifies and interacts with these person processing neural regions, and how these regions interact with salience and valuation regions when viewing high or low status individuals. These findings might explain the preferential attention high-status individuals receive.
Second, little is known about how maturational changes in the social brain affect how children and teens perceive and understand status, and what the consequences of these effects may be. On one hand, neural maturation might influence sensitivity to status information. For instance, research with younger children suggests they understand the basic concepts of dominance and power from a young age (Mascaro & Csibra, 2012; Thomsen et al., 2011), but children have difficulty understanding the concept of popularity and its correlates until fourth grade (Xie, Y. Li, Boucher, Hutchins, & Cairns, 2006). Thus, there is reason to believe the effects of status on cognition and perception vary throughout development. Researchers should further address the role of brain development in the emergence of an understanding of the social hierarchy and what it means to be popular.
Additionally, imaging studies similar to those assessing social status processing in adults have not been conducted in children or teens. Compared to children and adults, adolescents show different patterns of activation in reward, affective, and frontal regions in response to various forms of social feedback. It would be interesting to see whether this age group also shows a greater neural sensitivity in reward and salience regions when making status judgments, or viewing high or low status peers. Similarly, it is uncertain whether adolescent status modulates the neural responses to social feedback and rejection. For instance, teenagers who spend more time with friends show less activity in regions of the brain involved in social pain in response to peer rejection (Masten, Telzer, Fuligni, Lieberman, & Eisenberger, 2012), suggesting some social factors may buffer the negative effects of social exclusion. Future research could examine how status processing in teens relates to the experience, both cognitively and on a neural basis, of negative social events, and whether being high-status buffers the response to rejection.
Understanding developmental changes in status processing might also help to explain other teenage behaviors, like risk-taking. A number of studies suggest adolescent social status is associated with risky and deviant behavior (Allen, Chango, Szwedo, Schad, & Marston, 2012; Allen, Porter, Mcfarland, P. Marsh, & Mcelhaney, 2005). Recent work in our lab suggests that the presence of high status or low status peers can promote risky decision making on a computerized driving task, but this effect depends on whether participants report a desire to be a member of the same social group as the observer (Koski, Smith, Chein, Steinberg, & Olson, 2014; see Figure 4). Thus, the effects of popularity may depend more on the extent to which teenagers value status, rather than actual status or the status of their peer group. Future work should aim to understand how status biases judgments, attention, and cognition in children and teens, and how these effects might contribute to decision making in a social context. For instance, research should address whether age-related changes in the social brain affect how adolescents attend to and process status information, and how this potentially contributes to adolescents' greater propensity for risk-taking in a peer context.
Figure 4.
Late adolescents performed the Stoplight driving task alone and in the presence of a peer. The data show the proportion of risk actions (running yellow light) when participants report high or low interest in joining the social crowd of the observing peer. The interaction is significant (F (1, 66) = 4.17, p = .04), suggesting that teens take more risks when around peers whose status they would like to attain.
Future research should also explore whether one's status during development modulates brain maturation. For example, it is unclear how the experience of high status (e.g., receiving a lot visual attention and being emulated) might influence social processing networks in the brain. We see that in monkeys, grey matter volume and functional coupling in social processing regions of the brain correlates with status and social network size (Noonan et al., 2014). Though the direction of the effect is uncertain, there appears to be a relationship between the structure of this neural network and the social environment. In humans, it is important to examine how developmental experience in different social environments may be related to neural function. For instance, future longitudinal studies could investigate the dynamic relations between children's and adolescents' experience of different levels of social status (e.g., popularity) and the development of their neural network structure, and determine if there are age-related differences in such processes.
Another example, if children are primed to believe they are higher or lower status compared to their peers and then asked to subsequently perform a cognitive task, we predict that those who feel-high status will perform better, and performance will correlate with variations in neural activity in frontal brain regions, similar to findings in adults (Kishida et al., 2012). A potential confound with the aforementioned study was that IQ performance was used as both the ranking variable and performance measure. Future work could examine the extent to which different measures of subjective status during development affect cognition and correlated neural activity.
Finally, there is reason to believe that social status is perceived differently among children with certain developmental disorders. Children with autism spend less time with peers, have fewer friends, and are less likely to be socially accepted (Chamberlain, Kasari, & Rotheram-Fuller, 2007). They are also immune to the “audience effect” and appear to care little about reputation management (Chevallier et al., 2014). Future research should address whether individuals with autism lack the ability to perceive or interpret status cues, and whether they experience different consequences of social status as a result. Perhaps teaching children who cannot understand social organization how to recognize well-known status cues can help them to improve their social interactions.
To conclude, social hierarchies are an important and unavoidable aspect of social organization, and relative status within a group influences individual members' cognitions and behaviors. Given the dynamic and relational nature of status hierarchy, status-related cognitions and behaviors are critical for individual adaptation and group functioning. Recent evidence from neuroimaging suggests an underlying neural basis for status processing and developmental changes in both the nature of social hierarchies and social brain regions, but further research is necessary to fully understand the mechanisms of status processing, particularly during critical developmental periods such as adolescence.
Acknowledgments
This work was supported by a dissertation completion grant to J. Koski and a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- Abramovitch R, Grusec JE. Peer imitation in a natural setting. Child Development. 1978:60–65. [Google Scholar]
- Allen JP, Chango J, Szwedo D, Schad M, Marston E. Predictors of susceptibility to peer influence regarding substance use in adolescence. Child Development. 2012;83(1):337–350. doi: 10.1111/j.1467-8624.2011.01682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Porter MR, Mcfarland FC, Marsh P, Mcelhaney KB. The two faces of adolescents' success with peers: Adolescent popularity, social adaptation, and deviant behavior. Deviant Behavior. 2005;76(3):747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Beer JS, Spataro SE, Chatman JA. Knowing your place: Self-perceptions of status in face-to-face groups. Journal of Personality and Social Psychology. 2006;91(6):1094–1110. doi: 10.1037/0022-3514.91.6.1094. [DOI] [PubMed] [Google Scholar]
- Anderson C, Berdahl JL. The experience of power: Examining the effects of power on approach and inhibition tendencies. Journal of Personality and Social Psychology. 2002;83(6):1362–1377. doi: 10.1037//0022-3514.83.6.1362. [DOI] [PubMed] [Google Scholar]
- Anderson C, John OP, Keltner D, Kring AM. Who attains social status? Effects of personality and physical attractiveness in social groups. Journal of Personality. 2001;81(I):116–132. doi: 10.1037//0022-3514.81.1.116. [DOI] [PubMed] [Google Scholar]
- Anderson C, Kilduff GJ. The pursuit of status in social groups. Psychological Science. 2009;18(5):295–299. [Google Scholar]
- Ansari D, Dhital B. Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. Cognitive Neuroscience, Journal of. 2006;18(11):1820–1828. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Babad E. On the conception and measurement of popularity: More facts and some straight conclusions. Social Psychology of Education. 2001;5(1):3–29. [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behavioral Ecology and Sociobiology. 2006;59(4):469–479. [Google Scholar]
- Berger J, Rosenholtz SJ, Zelditch M. Status organizing processes. Annual Review of Sociology. 1980;6:479–508. [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science. 2011:1–14. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowska B, Pawlowski B. Female voice frequency in the context of dominance and attractiveness perception. Animal Behaviour. 2011;82(1):55–59. [Google Scholar]
- Brody LR. The socialization of gender differences in emotional expression: Display rules, infant temperament, and differentiation. In: Fischer AH, editor. Gender and emotion: Social psychological perspectives. New York, NY: Cambridge University Press; 2000. pp. 24–47. [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British journal of psychology. 1986;77(3):305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD. The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience. 2007;2(3-4):238–253. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. Neuroscience and Biobehavioral Reviews. 8. Vol. 35. Elsevier Ltd; 2011. The social brain in adolescence : Evidence from functional magnetic resonance imaging and behavioural studies; pp. 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buunk AP, Park JH, Zurriaga R, Klavina L, Massar K. Height predicts jealousy differently for men and women. Evolution and Human Behavior. 2008;29:133–139. doi: 10.1016/j.evolhumbehav.2007.11.006. [DOI] [Google Scholar]
- Cairns RB. Sociometry, psychometry, and social structure: A commentary on six recent studies of popular, rejected, and neglected children. Merrill-Palmer Quarterly (1982-) 1983:429–438. [Google Scholar]
- Chamberlain B, Kasari C, Rotheram-Fuller E. Involvement or isolation? The social networks of children with autism in regular classrooms. Journal of autism and developmental disorders. 2007;37(2):230–242. doi: 10.1007/s10803-006-0164-4. [DOI] [PubMed] [Google Scholar]
- Chance MR. Attention structure as the basis of primate rank orders. Man. 1967;2(4):503–518. [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) PNAS. 2012;109(3) doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth WR. Co-operation and competition: Contributions to an evolutionary and developmental model. International Journal of Behavioral Development. 1996;19(1):25–38. [Google Scholar]
- Chase ID. Social process and hierarchy formation in small groups : A comparative perspective. American Sociological Review. 1980;45(6):905–924. [Google Scholar]
- Chase ID. Dynamics of hierarchy formation: The sequential development of dominance relationships. Behaviour. 1982;80(3/4):218–240. [Google Scholar]
- Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. Individual differences versus social dynamics in the formation of animal dominance hierarchies. PNAS. 2002;99(8):5744–5749. doi: 10.1073/pnas.082104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafeddine R, Mercier H, Clément F, Kaufmann L, Berchtold A, Reboul A, Van der Henst JB. How Preschoolers Use Cues of Dominance to Make Sense of Their Social Environment. Journal of Cognition and Development 2014 [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon metaphysics: The evolution of a social mind. Chicago, IL: The University of Chicago Press; 2008. [Google Scholar]
- Cheng JT, Tracy JL, Foulsham T, Kingstone A, Henrich J. Two ways to the top: Evidence that dominance and prestige are distinct yet viable avenues to social rank and influence. Journal of Personality and Social Psychology. 2013;104(1):103–125. doi: 10.1037/a0030398. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Parish-Morris J, Tonge N, Le L, Miller J, Schultz RT. Susceptibility to the audience effect explains performance gap between children with and without autism in a theory of mind task. Journal of Experimental Psychology: General. 2014 Jan 6; doi: 10.1037/a0035483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Adams RB, Peter UT, Lowenthal WT, Richeson JA, Ambady N. Knowing who's boss: fMRI and ERP investigations of social dominance perception. Group Processes & Intergroup Relations. 2008;11(2):201–214. doi: 10.1177/1368430207088038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Bordeaux AR, Ambady Nalini. Mental representations of social status. Cognition. 2004;93:49–57. doi: 10.1016/j.cognition.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Li Z, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Cillessen AHN, Mayeux L. From censure to reinforcement: Developmental changes in the association between aggression and social status. 2004;75(1):147–163. doi: 10.1111/j.1467-8624.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- Cillessen AH, Marks PE. Conceptualizing and measuring popularity. In: Cillessen AHN, Schwartz D, Mayeaux L, editors. Popularity in the peer system. New York: The Guilford Press; 2011. pp. 25–56. [Google Scholar]
- Cillessen AHN, Rose AJ. Understanding popularity in the peer system. Current Directions in Psychological Science. 2005;14:102–105. doi: 10.1111/j.0963-7214.2005.00343.x. [DOI] [Google Scholar]
- Cloutier J, Ambady N, Meagher T, Gabrieli JDE. The neural substrates of person perception: Spontaneous use of financial and moral status knowledge. Neuropsychologia. 2012;50(9):2371–2376. doi: 10.1016/j.neuropsychologia.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Gyurovski I. Intraparietal sulcus activity during explicit self-referential social status judgments about others. International Journal of Psychological Research. 2013;6(1):68–79. [Google Scholar]
- Cloutier J, Gyurovski I. Ventral medial prefrontal cortex and person evaluation: Forming impressions of others varying in financial and moral status. NeuroImage. 2014;100:535–543. doi: 10.1016/j.neuroimage.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA, Coppotelli H. Dimensions and types of social status: A cross-age perspective. Developmental Psychology. 1982;18(4):557–570. [Google Scholar]
- Coie JD, Dodge KA, Kupersmidt JB. Peer group behavior and social status. In: Asher SR, Coie JD, editors. Peer Rejection in Childhood. New York, NY: Cambridge University Press; 1990. pp. 17–59. [Google Scholar]
- Collins J, Olson IR. Beyond the FFA: The role of the ventral anterior temporal lobes in face processing. 2014 doi: 10.1016/j.neuropsychologia.2014.06.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick NR, Casas JF, Mosher M. Relational and overt aggression in preschool. Developmental psychology. 1997;33(4):579. doi: 10.1037//0012-1649.33.4.579. [DOI] [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Relational aggression, gender, and social-psychological adjustment. Child development. 1995;66(3):710–722. doi: 10.1111/j.1467-8624.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Dalmaso M, Pavan G, Castelli L, Galfano G. Social status gates social attention in humans. Biology. 2012;8(3):450–452. doi: 10.1098/rsbl.2011.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- De Bruyn EH, Cillessen AH. Popularity in early adolescence: Prosocial and antisocial subtypes. Journal of Adolescent Research. 2006;21(6):607–627. [Google Scholar]
- Delgado MR. Reward-Related Responses in the Human Striatum. Annals of the New York Academy of Sciences. 2007;1104(1):70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Dominance rank, copulatory behavior, and differential reproduction. The Quarterly Review of Biology. 1982;57(2):135–159. doi: 10.1086/412672. [DOI] [PubMed] [Google Scholar]
- Dodge KA. Behavioral Antecedents of Peer Social Status, Child Development. 1983;54(6):1386–1399. [Google Scholar]
- Dovidio JF, Ellyson SL. Decoding visual dominance: Attributions of power based on relative percentages of looking while speaking and looking while listening. Social Psychology. 1982;45(2):106–113. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Exclusion does rejection hurt ? An fMRI study of social exclusion. Science. 2007;302(290):2003. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ellis WE, Zarbatany L. Peer group status as a moderator of group influence on children's deviant, aggressive, and prosocial behavior. Child Development. 2007;78(4):1240–1254. doi: 10.1111/j.1467-8624.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Ellyson SL, Dovidio JF, Fehr BJ. In Gender and nonverbal behavior. Springer; New York: 1981. Visual behavior and dominance in women and men; pp. 63–79. [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. American Journal of Primatology. 2004;17(July):1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Farmer TW, Estell DB, Bishop JL, O'Neal KK, Cairns BD. Rejected bullies or popular leaders? The social relations of aggressive subtypes of rural african american early adolescents. Developmental psychology. 2003;39(6):992. doi: 10.1037/0012-1649.39.6.992. [DOI] [PubMed] [Google Scholar]
- Farrow TFD, Jones SC, Kaylor-Hughes CJ, Wilkinson ID, Woodruff PWR, Hunter MD, et al. Higher or lower? The functional anatomy of perceievd allocentric social hierarchies. Neuroimage. 2011;57(4):1552–1560. doi: 10.1016/j.neuroimage.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Controlling other people: The impact of power on sterotyping. American Psychologist. 1993;48(6):621–628. doi: 10.1037//0003-066x.48.6.621. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Interpersonal stratification: Status, power, and subordination. Handbook of Social Psychology. 2010:941–982. [Google Scholar]
- Fiske ST, Cuddy AJ, Glick P, Xu J. A model of (often mixed) stereotype content: competence and warmth respectively follow from perceived status and competition. Journal of personality and social psychology. 2002;82(6):878. [PubMed] [Google Scholar]
- Foulsham T, Cheng JT, Tracy JL, Henrich J, Kingstone A. Cognition. 3. Vol. 117. Elsevier B.V; 2010. Gaze allocation in a dynamic situation : Effects of social status and speaking; pp. 319–331. [DOI] [PubMed] [Google Scholar]
- Fragale AR, Overbeck JR, Neale MA. Journal of Experimental Social Psychology. 4. Vol. 47. Elsevier Inc; 2011. Resources versus respect : Social judgments based on targets' power and status positions; pp. 767–775. [DOI] [Google Scholar]
- Galinsky AD, Magee JC, Inesi ME, Gruenfeld DH. Power and perspectives not taken. Psychological Science. 2006;17(12):1068–1074. doi: 10.1111/j.1467-9280.2006.01824.x. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MD, Gareis KC. The influence of status on decisions to help. Psychology. 1993;133(1):23–31. [Google Scholar]
- Gould RV. The origins of status hierarchies: A formal theory and empirical test. American journal of sociology. 2002;107(5):1143–1178. [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social cognitive and affective neuroscience. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy N, Chou EY, Galinsky AD. A functional model of hierarchy: Why, how, and when vertical differentiationenhances group performance. Psychology Review. 2011;1(1):32–52. doi: 10.1177/2041386610380991. [DOI] [Google Scholar]
- Hall JA, Coats EJ, Lebeau LS. Nonverbal behavior and the vertical dimension of social relations: A meta-analysis. Psychological Bulletin. 2005;131(6):898–924. doi: 10.1037/0033-2909.131.6.898. [DOI] [PubMed] [Google Scholar]
- Harper B. Beauty, stature and the labour market: A British cohort study. Oxford Bulletin of Economics and Statistics. 2000;62(s1):771–800. [Google Scholar]
- Hawley PH. The ontogenesis of social dominance: A strategy-based evolutionary perspective. Developmental Review. 1999;132:97–132. [Google Scholar]
- Henrich J, Gil-White FJ. The evolution of prestige: Freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evolution and human behavior. 2001;22(3):165–196. doi: 10.1016/s1090-5138(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Henrich J, Mcelreath R. The evolution of cultural evolution. Evolutionary Anthropology. 2003;12:123–135. doi: 10.1002/evan.10110. [DOI] [Google Scholar]
- Hess U, Adams RB, Kleck RE. Who may frown and who should smile? Dominance, affiliation, and the display of happiness and anger. Psychology. 2005;19(4):515–536. doi: 10.1080/02699930441000364. [DOI] [Google Scholar]
- Hogg MA. A social identity theory of leadership. Personality and Social Psychology Review. 2001;5(3):184–200. [Google Scholar]
- Hymel S, Closson LM, Caravita S, Vaillancourt T. Social status among peers: From sociometric attraction to peer acceptance to perceived popularity. Second. The Wiley-Blackwell Handbook of Childhood Social Development; 2011. pp. 375–392. [Google Scholar]
- Jones BC, Debruine LM, Main JC, Little AC, Welling LLM, R D, et al. Facial cues of dominance modulate the short-term gaze-cuing effect in human observers. Proceedings of the Royal Society B. 2010a;277:617–624. doi: 10.1098/rspb.2009.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Feinberg DR, DeBruine LM, Little AC, Vukovic J. A domain-specific opposite-sex bias in human preferences for manipulated voice pitch. Animal Behaviour. 2010b;79(1):57–62. [Google Scholar]
- Kalma AP, Visser L, Peeters A. Sociable and aggressive dominance: Personality difference in leadership style? Leadership. 1993;4(1):45–64. [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26:431–443. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Karafin MS, Tranel D, Adolphs R. Dominance attributions following damage to the ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2004;10:1796–1804. doi: 10.1162/0898929042947856. [DOI] [PubMed] [Google Scholar]
- Keating CF, Mazur A, Segall MH. A cross-cultural exploration of physiognomic traits of dominance and happiness. Ethology and Sociobiology. 1981;2(1):41–48. [Google Scholar]
- Keating CF, Doyle J. The faces of desirable mates and dates contain mixed social status cues. Journal of Experimental Social Psychology. 2002;38(4):414–424. [Google Scholar]
- Kishida KT, Yang D, Quartz KH, Quartz SR. Implicit signals in small group settings and their impact on the expression of cognitive capacity and associated brain responses. Phil Trans R Soc B. 2012;367:704–716. doi: 10.1098/rstb.2011.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Platt ML. Current Biology. 20. Vol. 19. Elsevier; 2009. Social attention and the brain minireview; pp. R958–R962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski J, Smith AR, Chein J, Steinberg L, Olson IR. The Influence of Social Status on Peer Influence and Risky Decisions. Poster presented at the 2014 Society for Research on adolescence 2014 March. [Google Scholar]
- Kraus MW, Keltner D. Signs of socioeconomic status: A thin-slicing approach. Psychological Science. 2009;20:99–106. doi: 10.1111/j.1467-9280.2008.02251.x. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Magoon NS, Hull JG, Heatherton TF. Judging a book by its cover: The differential impact of attractiveness on predicting one's acceptance to high- or low-status social groups. 2011;41(10):2538–2550. [Google Scholar]
- Kumaran D, Melo HL, Duzel E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron. 2012;76(3):653–666. doi: 10.1016/j.neuron.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFontana KM, Cillessen AH. Developmental changes in the priority of perceived status in childhood and adolescence. Social Development. 2010;19(1):130–147. [Google Scholar]
- La Freniere P, Chariesworth WR. Dominance, attention, and affiliation in a preschool group: A nine-month longit udinal study. Ethology and Sociobiology. 1983;4:55–67. [Google Scholar]
- Lansu TA, Cillessen AH. Peer status in emerging adulthood: Associations of popularity and preference with social roles and behavior. Journal of Adolescent Research. 2011 0743558411402341. [Google Scholar]
- Lansu TA, Cillessen AH, Karremans JC. Adolescents' Selective Visual Attention for High-Status Peers: The Role of Perceiver Status and Gender. Child development. 2014;85(2):421–428. doi: 10.1111/cdev.12139. [DOI] [PubMed] [Google Scholar]
- Lease AM, Musgrove KT, Axelrod JL. Dimensions of social status in preadolescent peer groups: Likability, perceived popularity, and social dominance. Social Development. 2002;11(4):508–533. [Google Scholar]
- Lount RB, Pettit NC. The social context of trust: The role of status. Organizational Behavior and Human Decision Processes. 2012;117(1):15–23. doi: 10.1016/j.obhdp.2011.07.005. [DOI] [Google Scholar]
- Lundborg P, Nystedt P, Rooth DO. Height and earnings: The role of cognitive and noncognitive skills. Journal of Human Resources. 2014;49(1):141–166. [Google Scholar]
- Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF. Subjective socioeconomic status predicts human ventral striatal responses to social status information. Current Biology. 2011;21(9):794–797. doi: 10.1016/j.cub.2011.03.050. [DOI] [PubMed] [Google Scholar]
- Magee JC, Galinsky AD. Social hierarchy: The self-reinforcing nature of power and status. The Academy of Management Annals. 2008;2(1):351–398. [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Markey PM, Funder DC, Ozer DJ. Complementarity of interpersonal behaviors in dyadic interactions. Personality and Social Psychology Bulletin. 2003;29:1082–1090. doi: 10.1177/0146167203253474. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR. Dominance and submission: The ventrolateral prefrontal cortex and responses to status cues. 2009;21(4):713–724. doi: 10.1162/jocn.2009.21052.Dominance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaro O, Csibra G. Representation of stable social dominance relations by human infants. PNAS. 2012;109(18):6862–6867. doi: 10.1073/pnas.1113194109. doi: 10.1073/pnas.1113194109/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1113194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M, Magee JC, Fiske ST. Neural substrates of social status inference: Roles of medial prefrontal cortex and superior temporal sulcus. Journal of Cognitive Neuroscience. 2014;26(5):1131–1140. doi: 10.1162/jocn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, Mcnealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence : understanding the distress of peer rejection. 2009:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. SCAN. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A. A biosocial model of status in face-to-face primate groups. Social Forces. 1985;64(2):377–402. [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- McNelis NL, Boatright-Horowitz SL. Social monitoring in a primate group: the relationship between visual attention and hierarchical ranks. Animal Cognition. 1998;1:65–69. [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moors A, Houwer JD. Automatic processing of dominance and submissiveness. Experimental Psychology. 2005;52(4):296–302. doi: 10.1027/1618-3169.52.4.296. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JAYR, Nader MA, et al. Predictors of social status in Cynomolgus monkeys (Macaca fascicularis) after group formation. American Journal of Primatology. 2000;131(April 1999):115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mueller U, Mazur A. Facial dominance of West Point cadets as a predictor of later military rank. Social forces. 1996;74(3):823–850. [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, et al. NeuroImage. Vol. 60. Elsevier B.V; 2012. Social status modulates neural activity in the mentalizing network; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-fisher NE. Hierarchy and social status in Budongo chimpanzees. Primates. 2004;45:81–87. doi: 10.1007/s10329-003-0064-6. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Mars RB, Neubert FX, Reilly JXO, Andersson JL, et al. A Neural Circuit Covarying with Social Hierarchy in Macaques. PLoS Biology. 2014;12(9) doi: 10.1371/journal.pbio.1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Parkhurst JT, Hopmeyer A. Sociometric popularity and peer-perceived popularity two distinct dimensions of peer status. The Journal of Early Adolescence. 1998;18(2):125–144. [Google Scholar]
- Paxton R, Basile BM, Hampton RR. Rhesus monkeys (Macaca mulatta) rapidly learn to select dominant individuals in videos of artificial social interactions between unfamiliar conspecifics. 2011;124(4):395–401. doi: 10.1037/a0019751.Rhesus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini AD, Bartini M. Dominance in early adolescent boys: Affiliative and aggressive dimensions and possible functions. Merrill-Palmer Quartlerly. 2001;47(1):142–163. doi: 10.1353/mpq.2001.0004. [DOI] [Google Scholar]
- Pereira ME. Development and social dominance among group-living primates. Development. 1995;175:143–175. doi: 10.1002/ajp.1350370207. [DOI] [PubMed] [Google Scholar]
- Pettit GS, Bakshi Dodge, K A, Coie JD. The emergence of social dominance in young boys' play groups: Developmental differences and behavioral correlates. Developmental Psychology. 1990;26(6):1017–1025. [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piff PK, Stancato DM, Côté S, Mendoza-denton R, Keltner D. Higher social class predicts increased unethical behavior. PNAS. 2012;109(11):4086–4091. doi: 10.1073/pnas.1118373109. doi: 10.1073/pnas.1118373109/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1118373109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, Heatherton TF. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Analysis. 2011:1–7. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist MC, Watkins CD, Smith FG, Debruine LM, Jones BC. Facial masculinity is a cue to women's dominance. Personality and Individual Differences. 2011;50:1089–1093. doi: 10.1016/j.paid.2011.01.032. [DOI] [Google Scholar]
- Raleigh MJ, Mcguire MT, Brammer GL, B D, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Animals. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Ratcliff NJ, Hugenberg K, Shriver ER, Bernstein MJ. The allure of status: High-status targets are privilege din face processing and memory. Personality and Social Psychology Bulletin. 2011;37(8):1003–1015. doi: 10.1177/0146167211407210. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Hickford C, Jeffery L. Sex-typicality and attractiveness: Are supermale and superfemale faces super-attractive? British Journal of Psychology. 2000;91(1):125–140. doi: 10.1348/000712600161718. [DOI] [PubMed] [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rose RM, Berstein IS, Gordon TP. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psychosomatic Medicine. 1975;37(1):50–61. doi: 10.1097/00006842-197501000-00006. [DOI] [PubMed] [Google Scholar]
- Sadler P, Woody E. Is who you are who you're talking to? Interpersonal style and complementarity in mixed-sex interactions. Journal of Personality and Social Psychology. 2003;84(1):80–96. doi: 10.1037/0022-3514.84.1.80. [DOI] [PubMed] [Google Scholar]
- Salmivalli C, Lagerspetz K, Osterman Karin, Bjorkvist K, Kaukiainen A. Bullying as a group process: Participant roles and their relations to social status within the group. 1996;22:1–15. [Google Scholar]
- Sandstrom MJ, Cillessen AH. Life after high school: Adjustment of popular teens in emerging adulthood. Merrill-Palmer Quarterly. 2010;56(4):474–499. [Google Scholar]
- Santos GS, Nagasaka Y, Fujii N, Nakahara H. Encoding of social state information by neuronal activities in the macaque caudate nucleus. Computational Intelligence. 2012;7(1):42–48. doi: 10.1080/17470919.2011.578465. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Annual Review of Anthropology. 2004;33(2004):393–418. doi: 10.1146/annurev.anthro.33.070203.144000. [DOI] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;648(2005):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Savin-Williams RC. Dominance hierarchies in groups of early adolescents. 1979;50(4):923–935. [Google Scholar]
- Schaal B, Tremblay RE, Soussignan R, Susman EJ. Male testosterone linked to high social dominance but low physical aggression in early adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(10):1322–1330. doi: 10.1097/00004583-199610000-00019. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. NeuroImage. 3. Vol. 57. Elsevier Inc; 2011. NeuroImage Developmental influences on the neural bases of responses to social rejection : Implications of social neuroscience for education; pp. 686–694. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Brain and Cognition. 1. Vol. 72. Elsevier Inc; 2010. Social brain development and the affective consequences of ostracism in adolescence; pp. 134–145. [DOI] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Social cognitive and affective neuroscience. 2012;7(1):93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RE, Schaal B, Boulerice B, Arseneault L, Soussignan R, Perusse D. Biosocial bases of violence. Springer US; 1997. Male physical aggression, social dominance and testosterone levels at puberty; pp. 271–291. [Google Scholar]
- Schwartz B, Tesser A, Powell E, Schwartz B, Tesser A. Behavior dominance cues in nonverbal behavior. Social Psychology Quarterly. 1982;45(2):114–120. [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16(4):119–120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Shoulberg EK, Sijtsema JJ, Murray-Close D. The association between valuing popularity and relational aggression: The moderating effects of actual popularity and physiological reactivity to exclusion. Journal of experimental child psychology. 2011;110(1):20–37. doi: 10.1016/j.jecp.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Sidanius J, Pratto F. Social Dominance: An Intergroup Theory of Social Hierarchy and Oppression System. Cambridge University Press; 1999. [Google Scholar]
- Smith PK, Jostmann NB, Galinsky AD, Dijk WWV. Lacking power impairs executive functions. Psychological Science. 2008;19(5):441–447. doi: 10.1111/j.1467-9280.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- Strayer FF, Strayer J. An ethological analysis of social agonism and dominance relations among preschool children. Child Development. 1976;47(4):980–989. [Google Scholar]
- Strayer FF, Trudel M. Developmental changes in the nature and function of social dominance among young children. Ethology and Sociobiology. 1984;295:279–295. [Google Scholar]
- Stulp G, Buunk AP, Verhulst S, Pollet TV. Tall claims? Sense and nonsense about the importance of height of US presidents. The Leadership Quarterly. 2012:1–13. doi: 10.1016/j.leaqua.2012.09.002. [DOI] [Google Scholar]
- Terburg D, van Honk J. Approach–Avoidance versus dominance–submissiveness: A multilevel neural framework on how testosterone promotes social status. Emotion review. 2013;5(3):296–302. doi: 10.1177/1754073913477510. [DOI] [Google Scholar]
- Thomsen L, Frankenhuis WE, Ingold-Smith Mc, Carey S. Big and mighty: Preverbal infants mentally represent social dominance. Science. 2011;331:477–480. doi: 10.1126/science.1199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedens LZ, Fragale AR. Power moves: Complementarity in dominant and submissive Nnonverbal behavior. Journal of Personality and Social Psychology. 2003;84(3):558–568. doi: 10.1037/0022-3514.84.3.558. [DOI] [PubMed] [Google Scholar]
- Timbie C, Barbas H. Specialized Pathways from the Primate Amygdala to Posterior Orbitofrontal Cortex. The Journal of Neuroscience. 2014;34(24):8106–8118. doi: 10.1523/JNEUROSCI.5014-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JM. Sexuality and partner selection: Sex differences among college students. Ethology and Sociobiology. 1993;14(5):305–329. [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences. 2008;105(49):19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt T, Hymel S, McDiugall P. Bullying is power: Implication for school-based intervention strategies. Journal Of Applied School Psychology. 2003;19(2):157–176. doi: 10.1300/J008v19n02. [DOI] [Google Scholar]
- Vaughn BE, Waters E. Attention structure, sociometric status, and dominance: Interrelations, behavioral correlates, and relationships to social competence. Developmental Psychology. 1981;17(3):275–288. [Google Scholar]
- Veenstra R, Lindenberg S, Zijlstra BJH, De Winter AF, Verhulst FC, Ormel J. The dyadic nature of bullying and victimization: Testing a dual-perspective theory. Child Development. 2007;78:1843–1854. doi: 10.1111/j.1467-8624.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: A meta-analysis and empirical study. Frontiers in Human Neuroscience. 2013;7:17. doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Rueden C, Gurven M, Kaplan H. The multiple dimensions of male social status in an Amazonian society. Evolution and Human Behavior. 2008;29(6):402–415. doi: 10.1016/j.evolhumbehav.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM. The integration of dominance and social bonding in primates. The Quarterly Review of Biology. 1986;61(4):459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. Downloaded from http://scan.oxfordjournals.org/ at Temple University on December 15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CD, Jones BC, Debruine LM. Personality and Individual Differences. 8. Vol. 49. Elsevier Ltd; 2010. Individual differences in dominance perception: Dominant men are less sensitive to facial cues of male dominance; pp. 967–971. [DOI] [Google Scholar]
- Weisfeld GE, Beresford JM. Erectness of posture as indicator of dominance or success in humans. 1982;6(2):113–131. [Google Scholar]
- Witvliet M, Olthof T, Hoeksma JB, Goossens FA, Smits MS, Koot HM. Peer group affiliation of children: The role of perceived popularity, likeability, and behavioral similarity in bullying. Social Development. 2010;19(2):285–303. [Google Scholar]
- Wolff SE, Puts DA. Vocal masculinity is a robust dominance signal in men. Behavioral Ecology and Sociobiology. 2010;64(10):1673–1683. [Google Scholar]
- Xie H, Li Y, Boucher SM, Hutchins BC, Cairns BD. What makes a girl (or a boy) popular (or unpopular)? African American children's perceptions and developmental differences. Developmental Psychology. 2006;42(4):599–612. doi: 10.1037/0012-1649.42.4.599. [DOI] [PubMed] [Google Scholar]
- Zettergren P, Bergman LR, Wångby M. Girls' stable peer status and their adulthood adjustment: A longitudinal study from age 10 to age 43. International Journal of Behavioral Development. 2006;30(4):315–325. [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL. Know your place: Neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitek EM, Tiedens LZ. The fluency of social hierarchy: The ease with which hierarchical relationships are seen, remembered, learned, and liked. Journal of Personality and Social Psychology. 2012;102(1):98–115. doi: 10.1037/a0025345. [DOI] [PubMed] [Google Scholar]
- Zumpe D, Michael RP. Dominance index: A simple measure of relative dominance status in primates. American Journal of Primatology. 1986;300:291–300. doi: 10.1002/ajp.1350100402. [DOI] [PubMed] [Google Scholar]


