Abstract
Objectives:
To evaluate the tolerance and effectiveness of stereotactic ablative radiotherapy (SABR) applied in the treatment of low and intermediate risk (LR & IR) prostate cancer patients (PCP) and provide an evaluation of the level of risk group impact on tr results. In addition, androgen deprivation therapy (ADT) usage and prostatic specific antigen (PSA) decline after SABR were assessed.
Material and Methods:
A total of 400 PCP (213 LR and 187 IR, including T2c) were irradiated with a CyberKnife using fd 7.25 Gy to TD 36.25 Gy. At the start of treatment, 60.3% of patients were undergoing ADT and this gradually decreased to 0% after 38 months. Follow-up was for a median of 15.0 months. Patients were monitored on SABR completion and 1, 4, 8 months later and then subsequently every 6 months. GI (Gastro-Intestinal) and GU (Genito-Urinary) acute and late adverse effects, PSA and ADT usage were evaluated.
Results:
Failure was noted in 9 patients (2.25%) (5 in LR and 4 in IR groups) - 4 relapses and 5 nodal metastases. No G3/4 late adverse effects (EORTC/RTOG) were observed. Some 0.5% of G3 GU and 0.3% of G3 GI acute reactions were noted respectively on the SABR completion day and one month later. The median of PSA declined 1.5 ng/ml during the first month and 0.6 ng/ml during the next three months. No impact of risk groups on treatment results was found. An impact of ADT on PSA decline was only confirmed for time point interactions.
Conclusions:
SABR for LR and IR PCP is a safe and effective treatment. The inclusion of T2c patients and the low percentage of IR patient failure permit us the assumption that this procedure could be utilized in the treatment of more advanced cases. The results do not allow clear definition of the impact of ADT on radioablation results in LR and IR+ T2c cases.
Keywords: SABR, SBRT, CyberKnife, prostate cancer radioablation, prostate radiotherapy
Introduction
The high incidence of prostate cancer (PC) in developed countries justifies the increasing interest in new radiotherapy technologies. There is consensus that radical treatment of PCP can be based on surgery or radiotherapy. The radiation treatment can be performed as brachy or radiotherapy (RT). Brachytherapy, irresepective of its form, is a relatively short treatment, but the vast majority of radiotherapy schedules are based on a lengthy treatment plan (8-9 weeks); often unacceptable for patients. Hence numerous attempts at hypofractionation introduction (Incrocci et al., 2016; Lee et al., 2016). There is an additional radiobiological argument for this; the α/β ratio for PC is considered low – 1.5 Gy, making it sensitive to large fractions (Fowler, 2005; Fowler et al., 2013). However, one major problem persists: the risk of late effects. There is also a connection with the irradiated volume and margins used. Due to prostate mobility with bladder and rectum filling, and in order to hit the target, we must enlarge the margins or track the prostate. The latter option is used in the CyberKnife system, enabling margin shrinkage while decreasing the likelihood of severe adverse effects.
Objectives
The main aim of this study was to evaluate the tolerance and effectiveness of SABR on PCP.
The secondary aims were to provide an evaluation of the level of risk group impact on the treatment results and an assessment of ADT usage on PSA decline after SABR.
Materials and Methods
Material
213 LR and 187 IR (including T2c) consecutive PCP, age range 53-83 (mean and median 69) treated between June 2011 and November 2015 according to routine clinical protocol in one RT center. The LR group included T2a or lower stage patients with PSA ≤ 10 ng/ml and Gleason score ≤ 6. The IR group comprised T2b-2 cpatients with PSA≤ 20 ng/ml and Gleason score 7 (3+4 only). Maximum prostate dimension 50 mm.
Prior to SABR, patients were informed of their options by urologists and given an informed choice.
Detailed data concerning PSA concentration, Gleason score, T stage, risk group and ADT usage of irradiated patients are presented in Table 1. Diagnosis was formed on the basis of the core biopsy performed transrectally (eight cores - four from each lobe). From one core, six slices were examined. In ambiguous cases AMCR and P63 expressions were checked. PC was identified if AMCR was positive and P63 negative. As we could not verify some cores from remote urological ambulatories; in 31 cases the Gleason score was below 5.
Table 1.
Patient’s Characteristics
| PSA concentration | PSA ≤ 10 ng/ml | 10 ng/ml> PSA <20 ng/ml | ||||
|---|---|---|---|---|---|---|
| 302 | 98 | |||||
| Gleason score | 2 | 3 | 4 | 5 | 6 | 7 |
| 4 | 2 | 25 | 84 | 223 | 62 | |
| TNM stage | T1c | T2a | T2b | T2c | ||
| 218 | 107 | 49 | 26 | |||
| Risk group | LR | LR | ||||
| ADT | (-) | (+) | (-) | (+) | ||
| (before SABR) | 95 | 119 | 72 | 114 | ||
PSA, Prostate Specific Antigen; TNM, Tumor, Nodes, Metastases; ADT, Androgen Deprivation Therapy; SABR, Stereotactic Ablative Radiotherapy
The TNM stage was evaluated based on MRI (if contraindicated, CT was performed), abdomen USG, chest X-ray and bone scintigraphy. If scintigraphy was unclear, a fluorocholine or sodium fluoride PET was undertaken.
Means of prostate dimensions (X-Y-Z) - 42.9 x 37.3 x 40.6 mm.
117 patients were comorbidities-free. 254 suffered from cardiovascular diseases, 45 diabetes, 24 pulmonary diseases, 22 arthritis, 9 GI diseases, 8 other neoplasms, 4 renal failure, 2 anemia, 2 Parkinson’s disease, 1 hyperthyroidism and 1 syringomielia.
122 patients had no urinary symptoms. 229 nycturia, 110 polyuria, 54 difficult urination, 14 dysuria, 2 rectal bleeding and 1 hematuria.
According to our protocols, ADT is not a standard treatment for LR and IR PCP, although urologists use it. Thus, at SABR initiation 60.3% of patients took ADT and PSA varying from 0.008 to 20.4 ng/ml (median 2.3). 159 patients used a combination of LHRH analogs and flutamide, 42 LHRH analogs alone, 18 flutamide, 1 bicalutamide and in 12 cases no information concerning ADT was available. ADT duration before SABR ranged from 0.5 to 48 months (median 2.3). During FU we tried to convince urologists to cease ADT.
FU varied from 1 to 53.9 months (mean 16.9, median 15.0) (time after failure was not included in the FU).
The last update was completed 12th May 2016.
Method
Patients were irradiated using the CyberKnife system, comprising a 6MV linear accelerator installed on a robotic arm with six degrees of freedom. The system is connected to a robotic couch (six degrees of freedom) and a tracking system allowing correction of the patient position and beams inlet. For this purpose, three markers (Gold Anchors, 2-cm-long golden wires, 0.3 mm in diameter, incised every 2 mm) were implanted transrectally, under USG control, in a triangular-like configuration. During implantation it bends itself to prevent migration. From one week after implantation, treatment planning procedures were initiated. An individual vacuum system was prepared followed by CT and MRI scans.
The treatment plan was prepared on the basis of CT-MRI fusion using the Multiplan system. 180 to 250 non-isocentric beams were used and the time of fraction delivery varied from 40 to 65 min.
The prostate position was checked every 5 to 150 seconds (depending on stability).
Patients were irradiated on alternate days (9 days) with fd of 7.25 Gy to the TD 36.25 Gy delivered to the Planning Target Volume (PTV) comprising Clinical Target Volume (CTV) (prostate + proximal 1 cm of seminal vesicles) and 3 mm of margin in posterior and 5 mm in other directions. The organs at risk were: the rectum, bladder, penile bulb and femoral heads.
Patients were monitored on the day of SABR completion and subsequently 1, 4, 8 months thereafter and then every 6 months. GI and GU acute (till the 4th month) and thenceforth, late adverse effects using the EORTC/RTOG grading system were evaluated. Moreover, additional urological symptoms, PSA and ADT usage were monitored. If a treatment failure occurred, a salvage treatment was undertaken.
Biochemical failures (BF) were evaluated using the Phoenix criterion.
Statistical analysis
Cox analysis was performed to check the impact of various factors on nodal dissemination as well as relapse risk.
Dependencies between symptoms and potentially influencing factors were analyzed using logistic hierarchical regression.
Linear hierarchical regression was utilized to evaluate the impact of treatment-dependent factors for the intensity of adverse effects and PSA.
A random effects ANOVA was applied to estimate the effects of the risk groups and ADT in time points on the PSA concentration.
All analyses took place within 26 months of FU; subsequent data were too limited to obtain reliable results.
Results
Detailed results concerning ADT usage, adverse effects and PSA are presented in Table 2.
Table 2.
The Percentage of Evaluated Patients without ADT, GI and GU Adverse Effects and PSA Concentration of Evaluated Patients During FU
| RT end | 1 month | 4 months | 8 months | 14 months | 20 months | 26 months | 32 months | 38 months | |
|---|---|---|---|---|---|---|---|---|---|
| N of observed patients | 400 | 250 | 343 | 303 | 242 | 143 | 87 | 40 | 14 |
| No ADT [%] | 41.8 | 61.5 | 56.3 | 75.7 | 82.2 | 86.1 | 97.7 | 95 | 100 |
| GI 0 [%] | 90.7 | 89.6 | 94.4 | 95 | 97.5 | 93.7 | 96.5 | 100 | 92.9 |
| GI 1 [%] | 8.8 | 8.4 | 4.7 | 4.3 | 2.1 | 5.6 | 2.3 | - | 7.1 |
| GI 2 [%] | 0.5 | 1.6 | 0.6 | 0.7 | 0.4 | 0.7 | 1.2 | - | - |
| GI 3 [%] | - | 0.4 | 0.3 | - | - | - | - | - | - |
| GU 0 [%] | 77.5 | 70.1 | 90.1 | 95.7 | 91.3 | 96.5 | 95.3 | 92.5 | 100 |
| GU 1 [%] | 16 | 25.5 | 7 | 3.6 | 7.1 | 2.1 | 2.3 | 5 | - |
| GU 2 [%] | 6 | 4 | 2.9 | 0.7 | 1.6 | 1.4 | 2.4 | 2.5 | - |
| GU 3 [%] | 0.5 | 0.4 | - | - | - | - | - | - | - |
| PSA mean | 3.8 | 1.8 | 0.9 | 0.7 | 0.7 | 0.5 | 0.5 | 0.4 | 0.2 |
| PSA median | 2.3 | 0.8 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 |
ADT, Androgen Deprivation Therapy; GI, Gastro-Intestinal; GU, Genito-Urinary; PSA, Prostate Specific Antigen; FU, Follow-Up
During FU 9 patients (2.25%) failed: 4 relapses and 5 nodal dissemination, as specified:
Relapses
- Patient 1 – BF after 18 months; the biopsy, relapse confirmation and salvage BT. Now disease-free (DF).
- Patient 2 – BF after 32 months; urologist started ADT, patient lost from FU.
- Patient 3 – BF after 32 months, a biopsy proposed, patient disagreement (poor PS). ADT introduced.
- Patient 4 - BF after 14 months; urologist started ADT, patient lost from FU.
Nodal disseminations
- Patient 5 – BF after 26 months, choline-PET performed - solitary node (illiac) metastasis. ADT was initiated by the brachytherapist and CK based SABR was undertaken. Now DF.
- Patient 6 - BF after 26 months; metastasis in illiac node found (choline-PET) and CK based SABR was undertaken. Now DF.
- Patient 7 – BF after 14 months; metastasis in illiac node found (choline-PET). Urologist started ADT, patient lost from FU.
- Patient 8 – BF after 14 months, metastasis in illiac node found (choline-PET). Next, CK based SABR was done. Now DF.
- Patient 9 – BF after 14 months; illiac node metastasis found (choline-PET). Next, CK based SABR was done; now DF.
Among the patients with failures, five were LR and four IR. The median of the Gleason score was 6.0; the median of PSA 8.6. Seven patients had T1c, one T2a and one T2b stage. Three had ADT at the SABR start (two nodal disseminations and one local relapse).
Acute and late GI and GU adverse effects were low and their percentages during FU are presented in Table 2.
The PSA decline in the whole group of patients is shown in Figure 1. It was most rapid during the first month (median of fall 1.5 ng/ml). During the next three months, PSA decline was 0.6 ng/ml (median). PSA decreased at a slower pace – 0.1 ng/ml (median) in the following 28 months. The courses of means and medians of PSA in subgroups of patients with and without ADT (at the SABR start) are presented on Figures 2 and 3. The PSA decline is faster (equally for the mean as the median) in the subgroup without ADT (patients using ADT had lower PSA at the beginning) and from the 20th month of FU values in both subgroups are highly similar. Courses of PSA during FU for LR and IR patients with and without ADT are shown in Figure 4. Furthermore, we can notice the impact of ADT (mainly in IR patients group) until the 20th month of FU only.
Figure 1.
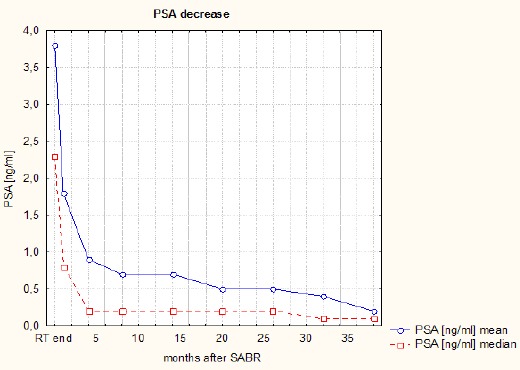
The Course of PSA Concentration During Follow-Up
Figure 2.
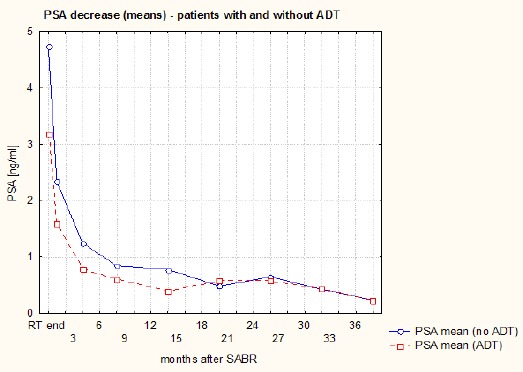
The Course of PSA Means During Follow-Up (Patients with and without ADT)
Figure 3.
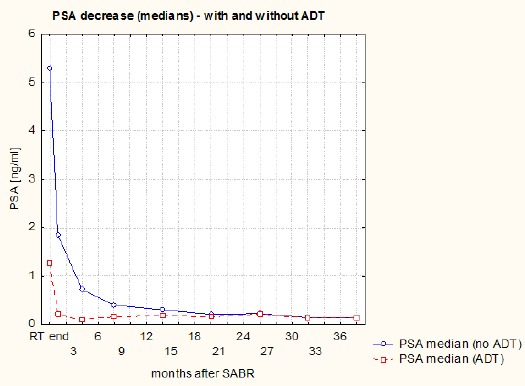
The Course of PSA Medians During Follow-Up (Patients with and without ADT)
Figure 4.
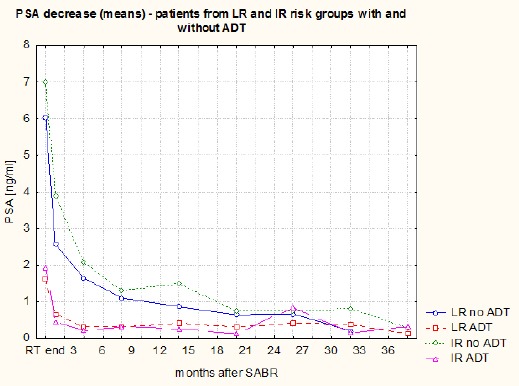
The Course of PSA Means During Follow-Up (LR and IR Patients with and without ADT)
Statistical analysis - results
The Cox analysis showed that the risk of nodal dissemination and relapse is reduced in patients using ADT (p=0.018 and p=0.046 respectively). Moreover, this analysis revealed risk reduction failure in correlation with the patient’s age (p=0.029); each additional year resulted in a 10% risk decrease; if the difference was ten years, then the risk decreased over (1-0.910)*100%=65%.
Positive dependencies between the presence of nycturia and the patient’s age (p=0.0055) and tumor stage (TNM) (p=0.0281) and between the presence of dysuria and TNM (p=0.0089) and ADT usage (p=0.0008) were clearly identified (logistic regression). This analysis showed inverse dependency between the presence of both the aforementioned symptoms and FU (p=0.000).
Linear regression showed an impact of diabetes on GI reaction (p=0.0411) – it increased GI acute and late adverse effects. Inverse dependencies between the acute GU reaction and Gleason score (p<0.026) and ADT usage (p=0.008) were identified.
Linear regression revealed an inverse dependency between PSA and FU, age, ADT usage and the presence of comorbidities (p=0.0000, p=0.0.0014, p=0.0000 and p=0.0064 respectively). A positive dependency was found for PSA and TNM (p=0.045).
A random effects ANOVA presented a difference in means of the PSA between the time points (p<0.001), however, no statistical difference was observed between the risk groups and the risk groups and time points interaction. No difference was found for ADT usage but statistical difference was registered for groups with and without ADT and time points interaction (p<0.001). The same results were observed for ADT impact evaluated separately in both risk groups (p<0.001 for both).
Ten patients developed second malignancies during FU: 2 bladder, 2 colon, 2 lung and one stomach cancer, lymphoma, multiple myeloma and brain tumor.
Discussion
Failures
The failure ratio is unexpectedly low (2.25%) and not connected with TNM (none of the failures in T2c subgroup) nor with the risk group (5 failures in LR and 4 in IR group). It suggests that there is no relevant impact of the risk group (taking into account LR, IR and T2c) on SABR treatment results. Additionally, we did not find statistical impact of the risk group on PSA values, even with time interaction. The low failure ratio is probably connected to a short FU (median 15 months). It could be supported by our previous results based on 200 patients with shorter FU (only 0.5% of failures) (Miszczyk et al., 2015). Other authors reported a larger failure ratio during longer FU: 9.8% (FU median 36 months) (Fan et al., 2015), 6.1% (FU median 30 months) (Jeong et al., 2015), 10.3% (FU median 63 months) (Lee et al., 2014), 4.4% and 10.4% for low and intermediate risk patients respectively (FU median 72 months) (Katz et al., 2014) and 4.5% (FU median 36 months) (King et al., 2013). An interpretation of these reports is difficult because of the low patient number i.e. Fan (2015), Jeong (2015) and Lee (2014) reported 31, 39 and 45 patients respectively; Katz (2014) and King (2013) had larger groups – 477 and 1100 (a multicentric study). Large groups were also analyzed by Freeman (2014) - a multi-institutional study comprising 45 radiotherapy centers and 2000 men (8% of failures at 2 years FU), by Bernetich (2014) – 0%, 8.3%, 4.8%, 10% and 13.3% of failures (5-years actuarial analysis) for very low, low, intermediate, high and very high risk patients respectively (142 men) and by Tan (2014) (a review of 14 CK studies – 1472 patients) – the failures rate varied from 0 to 19%. An exception is the Kim (2016) study, illustrating a lack of failures (33 patients). In all cited studies, the fractionation was the same (5 fractions) and the TD were highly similar: 35 Gy (Bernetich et al., 2014; Katz et al., 2014; Tan et al., 2014), 36 Gy (Lee et al., 2014), 36.25 Gy (the vast majority of patients) (King et al., 2013; Bernetich et al., 2014; Katz et al., 2014; Tan et al., 2014; Miszczyk et al., 2015; Kim et al., 2016) and 37.5 Gy (Fan et al., 2015; Jeong et al., 2015).
One possible explanation for failure disproportion in risk groups: 5 in LR vs 4 in IR could be the inconsistency in patient numbers [213 LR and 187 IR]). A similar phenomenon is described by Bernetich (2014) - 8.3% vs 4.8% of failures in LR and IR group respectively. On the other hand, Katz (2014) reported a larger failure number in IR than in LR group (14 vs 11) (LR group was more numerous – 324 vs 153).
Adverse effects
The intensity and frequency of acute as well as late GI and GU adverse effects were low. None of the patients had rectal bleeding. Admittedly, Joh (2014) reported 1.5% of rectal bleeding (269 men) during a 2-year observation, but the percentage was minimal. Also, Davis (2015) described low toxicity in the group of 437 patients. No grade 3 GI or GU acute and late toxicity was reported (in our material no G3 late toxicity was found, but we noted 0.4% of G3 acute GI and 0.5% of G3 acute GU toxicity). These authors (Davis et al., 2015) reported 3% G1 and 2% G2 late proctitis in comparison to 5.6% and 1.2% observed by us. Similar results were reported (515 patients) by Katz (2014) – no G3 and 4 acute toxicity and 1.7% of G3 GU late toxicity; acute G2 GI and GU adverse effects were observed in less than 5% of patients and G2 late GI and GU toxicity in 4% and 9.1% respectively. Good SABR tolerance and a low percentage (1.5%) of late urinary retention needing catheterization or TURP was also described by Arscott (2014) (269 men). Janowski (2014) described 3.5% of late GU G3 toxicity (no GI G3) in the group of 57 men with a large prostate (>50 ccm). More frequent and intense adverse GU effects were reported by Woo (2015). All aforementioned results regarded similar fractionation (35-36.25 Gy in 5 fractions), but even, after more intense SABR (38 Gy in 4 fractions) (Pontoriero et al., 2016) no G3 toxicity was reported.
Urinary incontinence after SABR, described by Chen (2014) - 5.7% leaking, more than once daily, was not observed in our material.
PSA decline
The decline of PSA concentration observed was similar to other reports. We can divide FU into three periods: the first month – rapid PSA fall, the next three months, slower but still intense PSA decrease and the rest of FU – with a slow PSA decline. Similar observations were reported by Kim (2015) – a rapid initial PSA decline followed by a slow decrease and Park (2015) – rapid decline during the first month and a slow decline within the next 2 years. Despite very fast PSA decline in first 4 months after the radioablation, Lee (2016) revealed that this decline is slower than after conventional irradiation (0.43 vs 0.53 ng/ml/month during the first year after RT). On the other hand Kim (2016) reported even larger PSA decline in the group of intermediate and high risk patients after combination of whole pelvis irradiation and SABR boost (0.61 ng/ml/month during the first year after RT).
Figures 2 and 3 illustrate that ADT has no real effect in terms of the velocity of PSA decline (in the subgroup with ADT, PSA decline is even slower) and from the 20th month PSA in both subgroups is similar. A random effects ANOVA revealed lack of significant differences between PSA during FU in subgroups with and without ADT, but significant difference for ADT and time interaction. The same results for ADT usage was found in LR and IR subgroups. In analyzing this, we should consider that the percentage of patients using ADT decreases over time.
The most unexpected result is the lack of a clear risk group impact on PSA decline.
Statistical analysis - results discussion
The observed dependency between failure risk and ADT usage and patient age seems obvious. Taking into account the character of SABR, we can compare it to brachytherapy. In some studies, the lack of ADT impact on biochemical outcome in LR and IR risk groups was reported (Merrick et al., 2005); in another, ADT deteriorated treatment results (among men of African descent) (Kovtun et al., 2016). Age impact is unsurprising as prostate cancer among younger patients is usually more aggressive (Ruska et al., 1999; Kanto et al., 2002).
The positive dependency between nycturia and the patient age and TNM is also clear; the probability of this symptom increases with age and tumor stage. A similar justification works for dysuria and TNM and ADT usage; the risk of dysuria is higher for larger T stage and, on the other hand, T stage is strictly interlinked with the probability of ADT usage. All patients were treated due to PC, therefore during FU the frequency of both symptoms decreased.
An impact of diabetes on adverse acute GI effects was previously described by us on the basis of a smaller study (Glowacki et al., 2015). We cannot find an explanation for acute GU decline with Gleason increase and its dependency on ADT usage.
A clear explanation for inverse dependencies among PSA, FU and ADT usage is as follows: longer FU after the treatment leads to better results, and PSA decline; the impact of ADT is commonly known. In contrast, the explanation for PSA and comorbidities connection is less obvious. The positive diabetes impact on PSA decline could be explained by metformin use. Such an effect is widely described in the literature (Gillessen et al., 2016; Jayalath et al., 2016). Unfortunately, we do not have data on metformin use at present.
Study limitations
Reading this text, we should be aware of four limitations of this study. Two of them concern patient recruitment, one treatment and one Follow-Up.
The first two limitations consider patient enrollment. Choline and/or PSMA-PET was not an obligatory examination before the SABR. Due to financial reasons, we undergo this examination for HR patients only; but this could, potentially, exclude patients with solitary, early metastases.
On the other hand, the 50 mm limit of maximal prostate dimension could impact the final results contrarily – there is some possibility that the cancer mass in a larger prostatic gland is bigger, which could influence indirectly treatment results (this 50 mm limit could improve them).
One of the weaknesses is connected to the treatment itself – not meeting ADT usage criteria (an additional issue was the different kinds of anti-hormonal drugs used). As aforementioned, we as radiation oncologists have a clear protocol of ADT usage and we had a limited impact on this phenomenon, but such a situation could lead to a change in the treatment results. On the other hand, it permitted ADT impact analysis.
The main weak-point is the short Follow-Up (mean 16.9, median 15.0 months). As mentioned in the first part of the Discussion, it probably resulted in a very low failure percentage, enabling sustained analysis of their causes.
The results obtained and the performed discussion enable us to conclude that stereotactic ablative radiotherapy of low and intermediate risk prostate cancer patients is a safe, well-tolerated and effective treatment modality (2.25% of failures).
The inclusion of T2c patients to the treatment group and the low percentage of failures in the intermediate risk group, as well as a lack of risk group impact on PSA decline, allow us to form the assumption that such a treatment could even be used in more advanced cases.
The results do not enable us to define clearly the impact of ADT on radioablation results of LR and IR+ T2c prostate cancer patients.
Conflict of interest statement
The main author has lectured on this topic during Accuray meetings.
References
- Arscott WT, Chen LN, Wilson N, et al. Obstructive voiding symptoms following stereotactic body radiation therapy for prostate cancer. Radiat Oncol. 2014;9:163. doi: 10.1186/1748-717X-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernetich M, Oliai C, Lanciano R, et al. SBRT for the primary treatment of localized prostate cancer:The effect of Gleason score, dose and heterogeneity of intermediate risk on outcome utilizing 2. 2014 NCCN risk stratification guidelines. Front Oncol. 2014;4:312. doi: 10.3389/fonc.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LN, Suy S, Wang H, et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol. 2014;9:148. doi: 10.1186/1748-717X-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Sharma S, Shumway R, et al. Stereotactic body radiotherapy for clinically localized prostate cancer:Toxicity and biochemical disease-free outcomes from a multi-institutional patient pegistry. Cureus. 2015;7:395. doi: 10.7759/cureus.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Chao HL, Huang WY, et al. Stereotactic ablative radiotherapy with CyberKnife in the treatment of locally advanced prostate cancer:preliminary results. Tumori. 2015;101:684–91. doi: 10.5301/tj.5000355. [DOI] [PubMed] [Google Scholar]
- Fowler JF. The radiobiology of prostate cancer, including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–76. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- Fowler JF, Toma-Dasu I, Dasu A. Is the α/βratio for prostate tumours really low and does it vary with the level of risk at diagnosis? Anticancer Res. 2013;33:1009–11. [PubMed] [Google Scholar]
- Freeman D, Dickerson G, Perman M. Multi-institutional registry for prostate cancer radiosurgery:a prospective observational clinical trial. Front Oncol. 2014;4:369. doi: 10.3389/fonc.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillessen S, Gilson C, James N, et al. STAMPEDE trial management group. Repurposing metformin as therapy for prostate cancer within the STAMPEDE trial platform. Eur Urol. 2016;70:906–8. doi: 10.1016/j.eururo.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Glowacki G, Majewski W, Wojcieszek P, et al. Acute toxicity of robotic ultrahypofractionated radiotherapy CyberKnifeTM in prostate cancer patients. Neoplasma. 2015;62:674–82. doi: 10.4149/neo_2015_081. [DOI] [PubMed] [Google Scholar]
- Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO):final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061–9. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- Janowski E, Chen LN, Kim JS, et al. Stereotactic body radiation therapy (SBRT) for prostate cancer in men with large prostates (≥50 cm(3)) Radiat Oncol. 2014;9:241. doi: 10.1186/s13014-014-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayalath VH, Ireland C, Fleshner NE, et al. The relationship between metformin and serum prostate-specific antigen levels. Prostate. 2016;76:1445–53. doi: 10.1002/pros.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong BK, Jeong H, Ha IB, et al. Stereotactic body radiation therapy for low- to intermediate-risk prostate adenocarcinoma. J Korean Med Sci. 2015;30:710–5. doi: 10.3346/jkms.2015.30.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh DY, Chen LN, Porter G, et al. Proctitis following stereotactic body radiation therapy for prostate cancer. Radiat Oncol. 2014;9:277. doi: 10.1186/s13014-014-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanto S, Ohyama C, Okada Y, et al. Clinical features of prostate cancer patients younger than 50 years:report of seven cases. Int J Urol. 2002;9:91–4. doi: 10.1046/j.1442-2042.2002.00427.x. [DOI] [PubMed] [Google Scholar]
- Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol. 2014;4:240. doi: 10.3389/fonc.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol. 2014;4:301. doi: 10.3389/fonc.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Phak JH, Kim WC. Prostate-specific antigen kinetics after stereotactic body radiotherapy as monotherapy or boost after whole pelvic radiotherapy for localized prostate cancer. Prostate Int. 2015;3:118–22. doi: 10.1016/j.prnil.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Phak JH, Kim WC. Hypofractionated stereotactic body radiotherapy in low- and intermediate-risk prostate carcinoma. Radiat Oncol J. 2016 doi: 10.3857/roj.2015.01571. doi:10.3857/roj.2015.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer:pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–21. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Phak JH, Kim WC. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy boost and whole pelvic radiotherapy for intermediate- and high-risk prostate cancer. Asia Pac J Clin Oncol. 2016 doi: 10.1111/ajco.12472. doi:10.1111/ajco.12472. [DOI] [PubMed] [Google Scholar]
- Kovtun KA, Chen MH, Braccioforte MH, et al. Race and mortality risk after radiation therapy in men treated with or without androgen-suppression therapy for favorable-risk prostate cancer. Cancer. 2016;22:3608–14. doi: 10.1002/cncr.30224. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim HJ, Kim WC. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy versus conventionally fractionated external beam radiotherapy for low- and intermediate-risk prostate cancer. Asia Pac J Clin Oncol. 2016;12:388–95. doi: 10.1111/ajco.12566. [DOI] [PubMed] [Google Scholar]
- Lee SW, Jang HS, Lee JH, et al. Stereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity:a 5-year follow-up of an investigational study. Medicine (Baltimore) 2014;93:e290. doi: 10.1097/MD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–32. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;61:32–43. doi: 10.1016/j.ijrobp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Miszczyk L, Napieralska A, Namysł-Kaletka A, et al. CyberKnife-based prostate cancer patient radioablation - early results of irradiation in 200 patients. Cent European J Urol. 2015;68:289–95. doi: 10.5173/ceju.2015.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Choi IY, Yoon SC, et al. Prostate-specific antigen kinetics after primary stereotactic body radiation therapy using CyberKnife for localized prostate cancer. Prostate Int. 2015;3:6–9. doi: 10.1016/j.prnil.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero A, Iatì G, Mondello S, et al. High-dose robotic stereotactic body radiotherapy in the treatment of patients with prostate cancer:Preliminary results in 26 patients. Technol Cancer Res Treat. 2016;15:179–85. doi: 10.1177/1533034614566994. [DOI] [PubMed] [Google Scholar]
- Ruska KM, Partin AW, Epstein JI, et al. Adenocarcinoma of the prostate in men younger than 40 years of age:diagnosis and treatment with emphasis on radical prostatectomy findings. Urology. 1999;53:1179–83. doi: 10.1016/s0090-4295(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Tan TJ, Siva S, Foroudi F, et al. Stereotactic body radiotherapy for primary prostate cancer:a systematic review. J Med Imaging Radiat Oncol. 2014;58:601–11. doi: 10.1111/1754-9485.12213. [DOI] [PubMed] [Google Scholar]
- Woo JA, Chen LN, Wang H, et al. Stereotactic body radiation therapy for prostate cancer:What is the appropriate patient-reported outcome for clinical trial design? Front Oncol. 2015;5:77. doi: 10.3389/fonc.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]


