Abstract
Background:
Previously published data on any association of the fat mass and obesity-associated (FTO) gene with breast cancer risk remain inconclusive. Therefore, we conducted the present meta-analysis of links between breast cancer and the FTO rs9939609 polymorphism.
Methods:
We have conducted a systematic review of the English literature by searching PubMed, Google Scholar and ISI Web of Knowledge databases for studies on associations between the FTO rs9939609 polymorphism and breast cancer risk. Crude odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate the strength of the association using fixed- or random-effects model.
Results:
We included five studies with 1134 cases and 1453 controls. Overall, no significant association between the FTO rs9939609 polymorphism and risk of breast cancer was found. On subgroup analysis by ethnicity, there was still no significant association detected.
Conclusions:
To our knowledge, this is the first meta-analysis of the FTO rs9939609 polymorphism and risk of breast cancer. However, the present meta-analysis suggested that only there might be a significant association of the CXCL12 rs1801157 polymorphisms with breast cancer risk.
Keywords: Breast cancer, FTO rs9939609, polymorphism, risk, meta-analysis
Introduction
Breast cancer is the third most frequent cancer in the world, which is also the most common malignancy and cause of mortality among women worldwide (Garcia et al., 2007; Shiryazdi SM et al., 2015). In the US, one out of every eight women will be diagnosed with breast cancer in her lifetime (DeSantis et al., 2014; Yazdi et al., 2015). In 1994, breast cancer susceptibility gene 1 (BRCA1) was discovered as the first breast cancer susceptibility gene. A year later breast cancer susceptibility gene 2 (BRCA2) was identified (Forat-Yazdi et al., 2015; Shiryazdi SM et al., 2015). It is estimated that 30 % of hereditary breast cancer cases are due to mutations in one of these two genes (Forat-Yazdi et al., 2015; Neamatzadeh et al., 2015). However, other established environmental factors such as age, BMI, weight gain, reproductive history, menopausal status, and hormone therapy, etc., have identified to date (Stuckey 2011).
It is well known that obesity increases the risk of all-cause mortality in men and women worldwide (Aune et al 2016; Matthews et al., 2016). Obesity, a major epidemic problem, is an established risk factor and cause of poor prognosis for postmenopausal breast cancer worldwide (De Pergola et al., 2013). It is estimated that obesity increases the risks of postmenopausal breast cancer by 30-50% (La Vecchia, Negri et al. 1997). On the other hand, according to the World Health Organization (WHO), the worldwide incidence of obesity has increased over the past decades (Hurt et al., 2010). In a large size cohort of 16 years of follow-up among 900,000 free of cancer male and females, Calle et al. have investigated the effects of obesity on the occurrence of 57,000 cancer deaths. Their findings indicated that increased body weight was significantly associated with increased death rates for all cancers. In addition, they found that highest BMI doubled the death rate from breast cancer when compared with women in the lowest BMI (Calle et al. 2003). However, obesity in women is a controllable risk factor for different conditions such as type II diabetes mellitus, cardiovascular disease and several major cancers including breast and endometrial cancer (Rubenstein 2005; Pi-Sunyer 2009).
Like many other industrial world disease, obesity is a multifactorial disease that interplay between behavior, environment, and genetic factors (Shuldiner, 2008). To date, several studies have conducted to find variants in different genes that may contribute to obesity (Lyon et al., 2005; Herrera et al 2011). According to studies, obesity has a complicated association to both breast cancer risk and the clinical behavior of the established disease (Barone et al., 2016). Several genes associated with obesity have recently been identified by large scale genome-wide association studies, one being the FTO gene located on chromosome 16q12.2. Although the FTO gene physiology and function is still elusive (SanchezPulido and Andrade-Navarro 2007), recent studies have further shown the effects of the FTO gene rs9939609 polymorphism on the risk of BMI-related outcomes such as type 2 diabetes (T2D) (Steemburgo et al., 2013), endometrial (Lurie et al., 2011) and pancreatic cancer (Lin et al., 2013). To date, a few studies investigated the association of FTO gene common variant, rs9939609, with risk of breast cancer, however, the studies revealed conflicting results. Therefore, the purpose of our study is to investigate the weight gain related FTO gene rs9939609 polymorphism contribution to breast cancer patients.
Materials and Methods
Search Strategy
We have conducted a comprehensive literature research using PubMed, google scholar and Web of Science data bases up to 20 January 2017. The relevant publications were identified using the following keyword strings: “fat mass and obesity-associated gene”, “FTO”, “breast cancer”, “polymorphism”, “mutation”, “variant”, “gene”, “genotype”, “SNP”, and “allele”. Reference list from published original articles and previous reviews were scanned for more relevant studies not identified in the database search. The search was restricted to the English language and humans. In addition, we have identified additional studies by a hand search of references of the original publications on the topic.
Inclusion and Exclusion Criteria
We have considered the studies to the meta-analysis that met the following predetermined inclusion criteria: (1) studies investigating the between FTO rs9939609 polymorphism and breast cancer risk, (2) Studies with cohort and case–control design, (3) Studies provided sufficient data for estimating an odds ratio (OR) with a 95% confidence interval (95% CI) and (4) only conducted on the female breast cancer. The major exclusion criteria were as follow: (1) not conducted on human, (2) not breast cancer research, (3) investigated male breast cancer, (4) Only included cases, (5) duplicate of previous publications and (6) have not sufficient data for genotypes.
Data Extraction
We have extracted the following data about the eligible studies: first author name, year of publication, country of study, ethnicity of studied subjects, frequencies of genotypes in both case and control groups, and HWE. In this study the diverse ethnicity populations were categorized as Asian, Caucasian, African and Mixed. However, in the studies where the ethnicity of the case and controls was not clearly stated, we have inferred ethnicity on the basis of the largest ethnic group inhabiting the country of study. The data was extracted and confirmed by two authors; however, any disagreement was resolved by discussion among the three investigators.
Statistical Analysis
All analyses were performed with the comprehensive meta-analysis (CMA) V2.0 software (Biostat, USA). Two-sided P values < 0.05 were considered statistically significant. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the association of FTO rs9939609 polymorphism with breast cancer risk. The meta-analysis examined the association of FTO rs9939609 polymorphism for five genetic contrast including allele (A vs. T), heterozygote (AT vs. TT), homozygote (AA vs. TT), dominant (AA+AT vs. TT) and recessive (AA vs. AT+TT) contrasts.
The heterogeneity between studies was evaluated by applying a chi square-based Q-test and I2, which a lack of statistical heterogeneity (p>0.05 or I2>25%) suggesting the variability in effect sizes is larger than that expected from chance alone (Higgins et al., 2003). Moreover, a random effects model using the DerSimonian was utilized to calculate the OR and 95% CI for comparisons with moderate to high heterogeneity (P-value > 0.1 and I2 > 25%) (DerSimonian et al., 1986). Otherwise, a fixed-effects model using the Mantel–Haenszel method was used. We have performed the sensitivity analysis to assess the influence of each individual study by sequential omission of individual studies for various genetic models in the overall population and also for subgroup analysis by ethnicity. Then, publication bias was estimated graphically by Begg’s funnel plot test and statistically Egger’s linear regression test (Egger et al., 1997); P<0.05 indicated that the result was statistically significant.
Results
Characteristics of Eligible Studies
The characteristics of the included studies were summarized in Table 1. We have identified a total of 16 publication after an initial search. Of these studies, the first screening 11 studies were excluded based on established criteria, which of these excluded studies, 6 articles were redundant studies, 1 was review and 4 were not involved with the FTO rs9939609 polymorphism with breast cancer. Here, we have analyzed five studies with a total of 1134 cases and 1453 controls for the FTO rs9939609 polymorphism (Kaklamani et al. 2011; Kusinska et al. 2012; da Cunha et al. 2013; Mojaver et al. 2015; Zeng et al. 2015). The included studies were published between 2011 and 2015. There were 3 studies of Caucasian descendants (USA, Poland, and Brazil) and 2 studies of Asian descendants communities (Iran and China). Genotype distributions in the controls of all studies were in agreement with HWE (p < 0.05).
Table 1.
Characteristics of Studies Included In the Meta-Analysis of FTO Rs9939609 Polymorphism and Breast Cancer
| First author | Country (Ethnicity) | Case/Control | Cases | Controls | HWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Allele | Genotype | Allele | ||||||||||
| TT | AT | AA | T | A | TT | AT | AA | T | A | ||||
| Kaklamani et al. 2011 | USA (Caucasian) | 302/349 | 49 | 124 | 129 | 222 | 382 | 52 | 161 | 136 | 265 | 433 | 0.7 |
| Kusinska et al. 2012 | Poland (Caucasian) | 134/357 | 40 | 65 | 29 | 145 | 123 | 110 | 180 | 67 | 400 | 314 | 0.66 |
| da Cunha et al. 2013 | Brazil (Caucasian) | 100/148 | 37 | 50 | 13 | 124 | 76 | 48 | 78 | 22 | 174 | 122 | 0.286 |
| Mojaver et al. 2015 | Iran (Asian) | 62/62 | 26 | 25 | 11 | 77 | 47 | 20 | 32 | 10 | 72 | 52 | 0.637 |
| Zeng et al. 2015 | China (Asian) | 536/537 | 389 | 130 | 17 | 908 | 164 | 408 | 119 | 10 | 935 | 139 | 0.7 |
Meta-Analysis
Overall Findings
After pooling all of the selected studies into the meta-analysis, we not found a significant association between FTO rs9939609 polymorphism and risk of breast cancer under all 5 genetics contrasts (Allele: A vs. T, OR= 1.062, 95% CI: 0.934-1.208, p= 0.360, Figure 1A; heterozygote: AT vs. TT: OR= 0.972, 95% CI: 0.801-1.180, p= 0.775; homozygote : AA vs. TT, OR= 1.081, 95% CI: 0.809-1.444, p= 0.597; dominant: AA+AT vs. TT, OR= 1,019, 95% CI: 0.848-1.225, p= 0.838, Figure 1B; and recessive: AA vs. AT+TT, OR= 1.174, 95% CI: 0.934-1.478, p= 0.170; Table 2).
Figure 1.
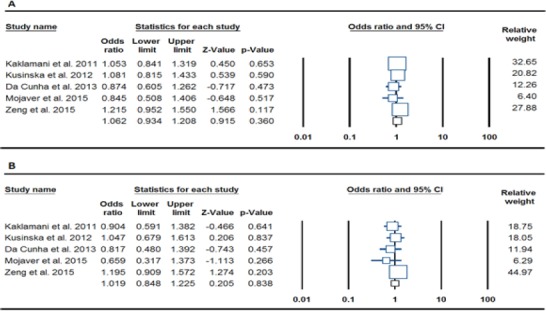
Forest Plot for the Association of FTO rs9939609 Polymorphism with Breast Cancer Risk. A: allelic contrast (A vs. T), B: dominant contrast (AA+AT vs. TT)
Table 2.
Meta-Analysis of the Association of FTO rs9939609 Polymorphism and Breast Cancer
| Genetic model | Type of model | Heterogeneity | Odds ratio | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| I2(%) | PH | OR | 95% CI | POR | PBeggs | PEggers | |||
| Overall | |||||||||
| A vs. T | Fixed | 0 | 0.551 | 1.062 | 0.934-1.208 | 0.36 | 0.462 | 0.126 | |
| AT vs. TT | Fixed | 0 | 0.463 | 0.972 | 0.801-1.180 | 0.775 | 0.22 | 0.015 | |
| AA vs. TT | Fixed | 0 | 0.62 | 1.081 | 0.809-1.444 | 0.597 | 0.806 | 0.956 | |
| AA+AT vs. TT | Fixed | 0 | 0.458 | 1,019 | 0.848-1.225 | 0.838 | 0.086 | 0.009 | |
| AA vs. AT+TT | Fixed | 0 | 0.803 | 1.174 | 0.934-1.478 | 0.17 | 1 | 0.939 | |
| Caucasian | |||||||||
| A vs. T | Fixed | 0 | 0.634 | 1.025 | 0.875-1.202 | 0.756 | 1 | 0.405 | |
| AT vs. TT | Fixed | 0 | 0.816 | 0.882 | 0.667-1.167 | 0.38 | 1 | 0.783 | |
| AA vs. TT | Fixed | 0 | 0.681 | 1.018 | 0.735-1.411 | 0.915 | 1 | 0.599 | |
| AA+AT vs. TT | Fixed | 0 | 0.768 | 0.931 | 0.715-1.212 | 0.595 | 1 | 0.501 | |
| AA vs. AT+TT | Fixed | 0 | 0.727 | 1.134 | 0.885-1.454 | 0.321 | 0.296 | 0.423 | |
| Asian | |||||||||
| A vs. T | Fixed | 37.1 | 0.207 | 1.135 | 0.911-1.414 | 0.258 | NA | NA | |
| AT vs. TT | Fixed | 56.57 | 0.129 | 1.063 | 0.813-1.389 | 0.657 | NA | NA | |
| AA vs. TT | Fixed | 20.22 | 0.263 | 1.354 | 0.721-2.541 | 0.346 | NA | NA | |
| AA+AT vs. TT | Fixed | 54.88 | 0.137 | 1.111 | 0.859-1.437 | 0.421 | NA | NA | |
| AA vs. AT+TT | Fixed | 0 | 0.491 | 1.444 | 0.788-2.643 | 0.234 | NA | NA | |
Subgroup Analysis
Subgroup analysis stratified by ethnicity also not suggested a significant association between FTO rs9939609 polymorphism and risk of breast cancer in Caucasian (Allele: A vs. T, OR= 1.025, 95% CI: 0.875-1.202, p= 0.756; heterozygote: AT vs. TT: OR= 0.882, 95% CI 0.667-1.167, p= 0.380; homozygote : AA vs. TT, OR= 1.018, 95% CI: 0.735-1.411, p= 0.915; dominant: AA+AT vs. TT, OR= 0.931, 95% CI: 0.715-1.212, p= 0.595 and recessive: AA vs. AT+TT, OR= 1.134, 95% CI: 0.885-1.454, p= 0.321) and Asian (Allele: A vs. T, OR= 1.135, 95% CI: 0.911-1.414, p= 0.258; heterozygote: AT vs. TT: OR= 1.063, 95% CI: 0.813-1.389, p= 0.657; homozygote : AA vs. TT, OR= 1.354, 95% CI: 0.721-2.541, p= 0.346; dominant: AA+AT vs. TT, OR= 1,111, 95% CI: 0.859-1.437, p= 0.421 and recessive: AA vs. AT+TT, OR= 1.444, 95% CI: 0.788-2.643, p= 0.234; Table 2).
Sensitivity Analysis
We have conducted the sensitivity analysis to evaluation the stability of this meta-analysis results through removing each study sequentially. However, no obvious changes were found in the results, which confirmed our results were stable under the five genetic contrasts for FTO rs9939609 polymorphism.
Publication Bias
Egger’s test and Begg’s funnel plot were used to evaluate publication bias quantitatively and qualitatively respectively. Slight asymmetry was found in heterozygote and dominant plots for FTO rs9939609 polymorphism association (Figure). The Egger’s test also showed obvious publication bias under heterozygote (PEggers=0.015) and dominant (PEggers=0.009) contrasts (Table 2, Figure 2A, 2B). Moreover, the examination of publication bias after stratified by ethnicity for Asians was not conducted because only two studies were included.
Figure 2.
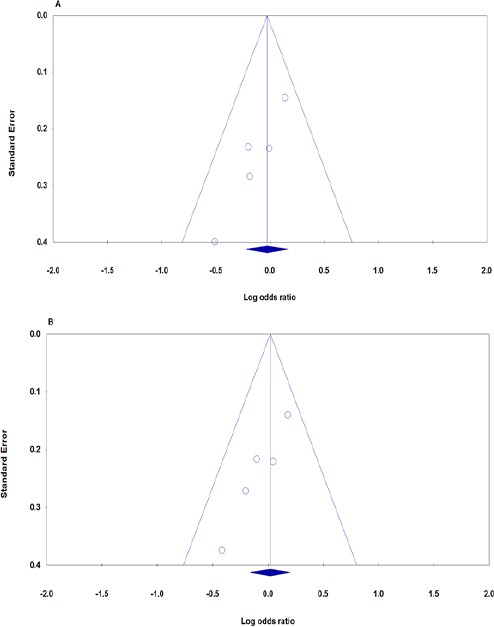
Begg’s Funnel Plots of FTO rs9939609 Polymorphism Association with Breast Cancer Risk for Publication Bias Test. A: Heterozygote contrast (AT vs. TT), B: Dominant contrast (AA+AT vs. TT). Each point represents a separate study for the indicated association.
Discussion
The FTO is a large gene with nine exons spanning more than 400 kb on chromosome 16 in humans (Mojaver et al., 2015; Huang et al., 2016). FTO gene also known as alpha-ketoglutarate-dependent dioxygenase FTO is a nuclear protein of the AlkB related non-haem iron and 2-oxoglutarate-dependent oxygenase superfamily which is the first mRNA demethylase that has been identified (Fedeles et al., 2015; Celis-Morales et al., 2016). However, the FTO gene exact physiological function of this gene is not known, the studies indicate a role in nervous and cardiovascular systems and a strong association with body mass index, obesity, and T2D (Fawcett et al., 2010; Manco et al., 2012). To date, several polymorphisms have been identified in the FTO gene such as rs9939609, rs17817449, rs8050136, rs1477196, rs6499640, rs16953002, rs11075995 and rs1121980, which associated with the risk of developing cancer (Hernández-Caballero et al., 2015). All the SNPs identified so far are located in the first and largest intron of the gene, a region where the sequence is strongly conserved across species (Loos et al. 2014). Of these SNPs, rs9939609 polymorphism, which is caused by A to T transition in the intron region of the FTO gene, is the most commonly studied polymorphism due to its various biology (Feng et al., 2014). On the other hand, it seems FTO rs9939609 may not be the true causal variant. Recent genome-wide association studies have showed that FTO rs9939609 was marginally associated with increased weight, raised BMI and several related traits (Wojciechowski et al., 2012). However, other studies suggested that this polymorphism may be involved in the development of other conditions such as T2D, metabolic syndrome (MetS) (Zhou et al., 2012), pancreatic cancer (Li et al., 2012), polycystic ovary syndrome (Li et al., 2013), major depressive disorder (Yao et al., 2016), etc.
The identification of the FTO gene and its potential role in obesity has provoked a series of studies to finds its association and also obesity with different malignancies, especially breast cancer, a multi-factorial and multi genic health problem. Recent studies have shown that FTO expression may have a vital role in the development of breast cancer (Tan et al., 2015). To date, a few studies investigated the association of FTO gene common variant, rs9939609, with risk of breast cancer, however, the studies revealed conflicting results. In this meta-analysis, we have surveyed the association of FTO rs9939609 polymorphism with breast cancer in the five studies with a total of 1,134 cases and 1,453 controls. However, there was no a significant association between this polymorphism and risk of breast cancer. Similarly, Mojaver et al. have reported that rs9939609 and rs1477196 polymorphisms not confer with risk of breast cancer in the studied Iranian population (Mojaver et al. 2014). In the other in a case control study of 354 cases and 364 controls at Northwestern University, USA, Kaklamani et al., have examined the role of 4 different FTO SNPs including rs7206790, rs8047395, rs9939609 and rs1477196 in development of breast cancer. They have found that all those SNPs were significantly associated with breast cancer risk (Kaklamani et al., 2011). This inconsistence was not reasonable among our results, Mojaver et al., and Kaklamani et al., findings, because our meta-analysis like to the Kaklamani et al., study was focused on Caucasians (Kaklamani et al. 2011). Similarly, Huang et al. in a meta-analysis of 15 studies with 17,403 cases and 34,465 controls have reported a significant association of FTO rs9939609 polymorphism with endometrial cancer and pancreatic cancer, but not breast cancer. In addition, they found that FTO rs9939609 polymorphism was associated with cancer risk in the Asians (OR = 1.15, 95% CI = 1.01–1.32) (Huang et al., 2016). However, Delahanty et al. in a case control study comprised 832 endometrial cancer cases and 2,049 controls have not reported such association (Delahanty et al., 2011). Huang et al. suggested that this inconsistence between their findings and Delahanty et al. may be due to the ethnicity backgrounds (Huang et al., 2016). Because the subgroup of endometrial cancer in Huang et al. meta-analysis was focused on Caucasians while Delahanty et al.’s study was based on Chinese patients (Delahanty et al., 2011). Although, we thought Huang et al. suggestion was wrong due to our inconsistence with previous two case controls study findings in the Iran and USA (Huang et al., 2016).
This is the first meta-analysis that particularly estimated the associations between two FTO rs9939609 polymorphism and risk of breast cancer. However, some limitations should be taken into consideration when explaining the results as follow: Firstly, most of the studies included in the meta-analysis were performed in the Caucasian population, only two publications were from Asians and there was no relevant study from Africans. Thus, to obtain more precise contribution of FTO rs9939609 polymorphism on breast cancer risk, additional studies with larger sample size and involving different ethnicities especially Asians and African are crucial. Second, because only published papers and written in English language were included to the meta-analysis, publication bias may have occurred, even though it was not found by making use of statistical tests. Third, in this meta-analysis the overall outcomes were based on individual unadjusted ORs without adjustment for other risk factors such as age, weight, menopausal status, post-menopausal weight, histological findings, and other controllable risk factors. Finally, it is well known both obesity and breast cancer are multifactorial and multigenic trait, therefore this meta-analysis could not address the gene-gene and gene-environmental interactions in the association between FTO rs9939609 polymorphism and breast cancer risk. Then, more studies with detailed information environmental factors exposure to assess the possible gene-gene and gene-environment interactions in the association between FTO rs9939609 polymorphism and risk of breast cancer are required.
Overall, there was no a significant association between FTO rs9939609 polymorphism and breast cancer risk was. As to subgroup stratified by ethnicity, there was no significant association in both Asians and Caucasians.
References
- Aune D, Sen A, Prasad M, et al. BMI and all-cause mortality:systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;4:353. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone I, Giordano C, Bonofiglio D, et al. Leptin, obesity and breast cancer:progress to understanding the molecular connections. Curr Opin Pharmacol. 2016;31:83–9. doi: 10.1016/j.coph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Celis-Morales C, Marsaux CF, Livingstone KM, et al. Physical activity attenuates the effect of the FTO genotype on obesity traits in European adults:The Food4Me study. Obesity (Silver Spring) 2016;24:962–9. doi: 10.1002/oby.21422. [DOI] [PubMed] [Google Scholar]
- da Cunha PA, de Carlos Back LK, Sereia AF, et al. Interaction between obesity-related genes, FTO and MC4R, associated to an increase of breast cancer risk. Mol Biol Rep. 2013;40:6657–64. doi: 10.1007/s11033-013-2780-3. [DOI] [PubMed] [Google Scholar]
- Delahanty RJ, Beeghly-Fadiel A, Xiang YB, et al. Association of obesity related genetic variants with endometrial cancer risk:a report from the Shanghai endometrial cancer genetics study. Am J Epidemiol. 2011;174:1115–26. doi: 10.1093/aje/kwr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis C, Ma J, Bryan L, et al. Breast cancer statistics 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- Fawcett KA, Barroso I. The genetics of obesity:FTO leads the way. Trends Genet. 2010;26:266–74. doi: 10.1016/j.tig.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wang F, Pan H, et al. Obesity-associated gene FTO rs9939609 polymorphism in relation to the risk of tuberculosis. BMC Infect Dis. 2014;7:592. doi: 10.1186/s12879-014-0592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedeles BI, Singh V, Delaney JC, et al. The AlkB family of Fe(II)/α-Ketoglutarate-dependent dioxygenases:Repairing nucleic acid alkylation damage and beyond. J Biol Chem. 2015;290:20734–42. doi: 10.1074/jbc.R115.656462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forat-Yazdi M, Neamatzadeh H, Sheikhha M, et al. BRCA1 and BRCA2 common mutations in iranian breast cancer patients, a meta-analysis. Asian Pac J Cancer Prev. 2015;16:1219–24. doi: 10.7314/apjcp.2015.16.3.1219. [DOI] [PubMed] [Google Scholar]
- Garcia M, Jemal A, Ward EM, et al. Global cancer facts and figures 2007. Atlanta, GA: American cancer sciety; 2007. pp. 250–3. [Google Scholar]
- Hernández-Caballero ME, Sierra-Ramírez JA. Single nucleotide polymorphisms of the FTO gene and cancer risk:an overview. Mol Biol Rep. 2015;42:699–704. doi: 10.1007/s11033-014-3817-y. [DOI] [PubMed] [Google Scholar]
- Herrera BM, Keildson S, Lindgrena CM. Genetics and epigenetics of obesity. Maturitas. 2011;69:41–9. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhao J, Yang M, et al. Association between FTO gene polymorphism (rs9939609 T/A) and cancer risk:a meta-analysis. Eur J Cancer Care (Engl) 2016 doi: 10.1111/ecc.12464. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hurt RT, Kulisek C, Buchanan LA, et al. The obesity epidemic:challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (NY) 2010;6:780–92. [PMC free article] [PubMed] [Google Scholar]
- Kaklamani V, Yi N, Sadim M, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12:52. doi: 10.1186/1471-2350-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusinska R, Górniak P, Pastorczak A, et al. Influence of genomic variation in FTO at 16q12.2, MC4R at 18q22 and NRXN3 at 14q31 genes on breast cancer risk. Mol Biol Rep. 2012;39:2915–9. doi: 10.1007/s11033-011-1053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chen Q, Wang L, et al. Association between FTO gene polymorphism and cancer risk:evidence from 16,277 cases and 31,153 controls. Tumour Biol. 2012;33:1237–43. doi: 10.1007/s13277-012-0372-9. [DOI] [PubMed] [Google Scholar]
- Li T, Wu K, You L, et al. Common variant rs9939609 in gene FTO confers risk to polycystic ovary syndrome. PLoS One. 2013;8:e66250. doi: 10.1371/journal.pone.0066250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ueda J, Yagyu K, et al. Association between variations in the fat mass and obesity-associated gene and pancreatic cancer risk:a case-control study in Japan. BMC Cancer. 2013;13:337. doi: 10.1186/1471-2407-13-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RJ, Yeo GS. The bigger picture of FTO:the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie G, Gaudet MM, Spurdle AB, et al. The obesity-associated polymorphisms FTO rs9939609 and MC4R rs17782313 and endometrial cancer risk in non-Hispanic white women. PLoS One. 2011;6:e16756. doi: 10.1371/journal.pone.0016756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon HN, Hirschhorn JN. Genetics of common forms of obesity:a brief overview. Am J Clin Nutr. 2005;82:215–17. doi: 10.1093/ajcn/82.1.215S. [DOI] [PubMed] [Google Scholar]
- Matthews SB, Thompson HJ. The obesity-breast cancer conundrum:An analysis of the issues. Int J Mol Sci. 2016;17:989. doi: 10.3390/ijms17060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco M, Dallapiccola B. Genetics of pediatric obesity. Pediatrics. 2012;130:123–33. doi: 10.1542/peds.2011-2717. [DOI] [PubMed] [Google Scholar]
- Mojaver M, Mokarian F, Kazemi M, et al. Specific TaqMan allelic discrimination assay for rs1477196 and rs9939609 single nucleotide polymorphisms of FTO gene demonstrated that there is no association between these SNPs and risk of breast cancer in Iranian women. Adv Biomed Res. 2015;4:136. doi: 10.4103/2277-9175.161532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neamatzadeh H, Shiryazdi SM, Kalantar SM. BRCA1 and BRCA2 mutations in Iranian breast cancer patients, a systematic review. J Res Med Sci. 2015;20:284–93. [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein AH. Obesity: A modern epidemic. Trans Am Clin Climatol Assoc. 2005;116:103–113. [PMC free article] [PubMed] [Google Scholar]
- Shiryazdi SM, Kargar S, Nasaj HT, et al. The accuracy of Breastlight in detection of breast lesions. Indian J Cancer. 2015;52:513. doi: 10.4103/0019-509X.178389. [DOI] [PubMed] [Google Scholar]
- Shiryazdi SM, Kargar S, Taheri-Nasaj H, et al. Breast light apparatus performance in detection of breast masses depends on mass size. Asian Pac J Cancer Prev. 2015;16:1181–4. doi: 10.7314/apjcp.2015.16.3.1181. [DOI] [PubMed] [Google Scholar]
- Shuldiner AR. Obesity genes and gene-environment-behavior interactions:recommendations for a way forward. Obesity (Silver Spring) 2008;16:79–81. doi: 10.1038/oby.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steemburgo T, Azevedo MJ, Gross JL, et al. The rs9939609 polymorphism in the FTO gene is associated with fat and fiber intakes in patients with type 2 diabetes. J Nutrigenet Nutrigenomics. 2013;6:97–106. doi: 10.1159/000350741. [DOI] [PubMed] [Google Scholar]
- Stuckey A. Breast cancer: epidemiology and risk factors. Clin Obstet Gynecol. 2011;54:96–102. doi: 10.1097/GRF.0b013e3182080056. [DOI] [PubMed] [Google Scholar]
- Tan A, Dang Y, Chen G, et al. Overexpression of the fat mass and obesity associated gene (FTO) in breast cancer and its clinical implications. Int J Clin Exp Pathol. 2015;8:13405–10. [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski P, Lipowska A, Rys P, et al. Impact of FTO genotypes on BMI and weight in polycystic ovary syndrome:a systematic review and meta-analysis. Diabetologia. 2012;55:2636–45. doi: 10.1007/s00125-012-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Wen Y, Du T, et al. Meta-analysis indicates that SNP rs9939609 within FTO is not associated with major depressive disorder (MDD) in Asian population. J Affect Disord. 2016;193:27–30. doi: 10.1016/j.jad.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Yazdi MF, Rafieian S, Gholi-Nataj M, et al. CYP2D6 Genotype and Risk of Recurrence in Tamoxifen Treated Breast Cancer Patients. Asian Pac Cancer Prev. 2015;16:6783–7. doi: 10.7314/apjcp.2015.16.15.6783. [DOI] [PubMed] [Google Scholar]
- Zeng X, Ban Z, Cao J, et al. Association of FTO mutations with risk and survival of breast cancer in a Chinese population. Dis Markers. 2015;2015:101032. doi: 10.1155/2015/101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Liu H, Zhou M, et al. Common variant (rs9939609) in the FTO gene is associated with metabolic syndrome. Mol Biol Rep. 2012;39:6555–61. doi: 10.1007/s11033-012-1484-4. [DOI] [PubMed] [Google Scholar]


