Abstract
Objective:
This study focused on recent changes in the incidence of colorectal cancer (CRC) in Khon Kaen, Thailand.
Methods:
Data for CRC over the period 1989 to 2012 from the population-based cancer registry of Khon Kaen province were employed. Age-standardized incidence rates (ASR) were calculated and classified into 4 age-groups for comparison. Joinpoint regression analysis was used to detect changes in trends among each line segment and an overall line was generated, whether increasing or decreasing, with annual percent change (APC) and average annual percent change (AAPC).
Results:
There were 3,364 CRC cases included in the analysis, 72.2% histological confirmed and 53.5% in men. Trends of ASRs generally demonstrated gradual increase over the period 1989 to 2012. For those aged under 45 or 50 years there was slight overall increase, with a somewhat zigzag pattern. From joinpoint analysis, the trends of all aged groups were found to be increasing among both men and women: aged 45 years and older group AAPC=3.40, 2.30 and 3.90, respectively); aged 50 years and older group AAPC=2.90, 2.20 and 3.40; aged under 45 years AAPC=6.30, 6.00 and 6.90; and aged under 50 years (AAPC=5.70, 3.20 and 5.70.
Conclusions:
ASRs for CRC have been gradually increasing in the northeast region of Thailand. Future studies should consider the subsite distribution.
Keywords: Colorectal cancer, incidence, joinpoint analysis, Thailand
Introduction
Colorectal cancer (CRC) is the third and fourth most common cancer in Thai men (age-standardized incidence rate (ASR)=14.4 per 100,000 population) and women (ASR=11.2 per 100,000 population), respectively(Pongnikorn et al., 2015). Khon Kaen is a province in the northeastern region of Thailand which was among those parts of the country affected by rapid socio-economic development in the late 1990s and early 2000s (Pongchaiyakul et al., 2005). Data from the population-based cancer registry of Khon Kaen have shown that the incidence of CRC had the same ranking as the whole country in both men (ASR=13.1 per 100,000 population) and women (ASR=9.0 per 100,000 population) (Pongnikorn et al., 2015). This rate may be low when compared with other parts of Thailand and some countries in Asia such as Japan, Singapore and Korea, but the trend during the last two decades has shown a slight overall increase in rates and noticeable increase in men (Suwanrungruang et al., 2006; Wirasorn et al., 2014).
CRC incidence and mortality are known to increase with age, and regular screening for early detection is beginning at age 50 years, while people who have high risk group, such as those with family history of CRC, should be screened at a younger age (Rahman et al., 2015). Recent incidence trends of young population in several studies have reported a rise in the incidence of CRC (Ahnen et al., 2014; Singh et al., 2014; Bailey et al., 2015), but incidence trends of this group have not been reported in Thailand. Monitoring disparities among long-term trends in incidence of some aged groups at a small-area geographic levels may help health officials and policy-makers in the determination of the local health plans, the prioritization of prevention activities, the allocation of health services, and in the evaluation of cancer control interventions and treatments (Jiang et al., 2007; Schootman et al., 2011).
The objectives of the present study were to describe the characteristics and changes in the incidence of CRC among four age-groups who lived in Khon Kaen province, Thailand, and whose diagnosis was recorded over the period 1989 to 2012 in the Khon Kaen population-based cancer registry. Since earlier work focused on age differences with cutoffs of either 45 or 50 years of age, both were here selected for comparison of older and younger patient groups.
Materials and Methods
Setting and Data Collection
The Khon Kaen population-based cancer registry in Thailand was started in January 1988 based in the Faculty of Medicine, Srinagarind Hospital of Khon Kaen University. Khon Kaen province is situated in the northeastern part of Thailand with a population of about 1.7 million. Data of the registry are collected from hospitals and health service centers by both passive and active methods (1 university hospital, 1 regional hospital, 1 military hospital, 8 private and 20 community hospitals, the Provincial Chief Medical Officer’s Office and Civil Registration Section of all districts), while duplication coded of all data are verified and checked by entry into the CANREGT software (Suwanrungruang et al., 2006). From this registry, all participants living in Khon Kaen Province and diagnosed as new cases with the C180-C209 coding based on the International Classification of Diseases for Oncology (ICDO) system during the period from January 1989 to December 2012 were selected.
Statistical Methods
We calculated ASRs of CRC for individual years (1989-2012) using the world standard population as a reference based on the direct method (Doll and Cook, 1967) and classified into 4 age-groups for comparison which consisted of (1) under 45 years, (2) 45years and older, (3) under 50 years, and (4) 50 years and older. Joinpoint regression analysis was employed to detect changes in trends among each line segment and overall lines whether increasing or decreasing with statistical significance using annual percent change (APC) and average annual percent change (AAPC) (Kim et al., 2000). The number of joinpoints started from 0 and was increased to test if the addition of joinpoints improved the fitness of model significantly (Lim et al., 2015). For such analysis, we used Joinpoint version 4.2.0.2 which is free software developed by the Statistical Methodology and Applications Branch, National Cancer Institute, USA (Institute, 2015).
Ethics Statement
This study was given ethical approval by the Khon Kaen University Ethics Committee for Human Research in Thailand. The reference number was HE581408.
Results
Characteristics of CRC
There were 3,364 cases of colorectal cancer diagnosed during January 1989 to December 2012, all being included in the analysis. Table 1 shows the distribution of all cases by sex, age at diagnosis, histology and staging. There were 53.5% men, 46.5% women, 72.2% with histological confirmed.
Table 1.
Demographic Data of Participants Including in the Analysis (n=3,364)
| Demographic data | Number | % |
|---|---|---|
| Sex | ||
| Men | 1,798 | 53.5 |
| Women | 1,566 | 46.5 |
| Age at diagnosis | ||
| < 50 years | 785 | 22.1 |
| 50 - 59 years | 813 | 23.7 |
| 60 - 69 years | 955 | 27.8 |
| 70 years and older | 905 | 26.4 |
| Mean(SD) =60.1(13.63) | ||
| Median =61, min =16, max =99 | ||
| Basis of diagnosis | ||
| Histological confirmed | 2,427 | 72.2 |
| Laboratory test confirmed | 17 | 0.5 |
| Endoscopy | 486 | 14.5 |
| Clinical only | 434 | 12.8 |
| Staging | ||
| I | 50 | 1.5 |
| II | 290 | 8.6 |
| III | 380 | 11.3 |
| IV | 851 | 25.3 |
| Unknown stage | 1,793 | 53.3 |
The trends of age-standardized incidence rate of CRC
The trends of age-standardized incidence rate of CRC between 1989 and 2012 in Khon Kaen of Thailand for aged 45 years and older, aged 50 years and older found that their trends in both sexes, men, and women were gradually increasing over time, while the trends of men were higher than women over the period 1989 to 2012, these results being shown in Figure 2 and Figure 4. Whereas the ASRs of CRC of aged under 45 years and aged under 50 years were lower than the two aged groups as mentioned above, but their trends were still a slight overall increase, and these trends were not different between men and women over the period 1989 to 2012. Surprisingly, their trends were either steeper in women than in men or steeper in men than in women like a zigzag pattern over the period 1989 to 2012, these results are shown in Figure 1 and Figure 3.
Figure 1.
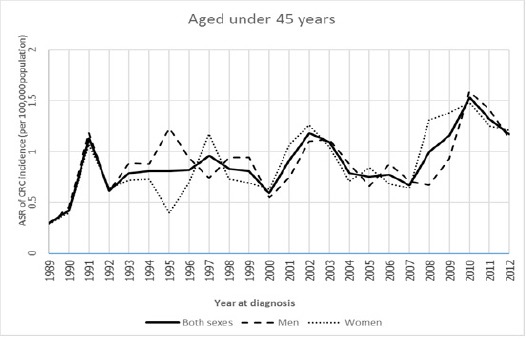
Age-Standardized Incidence Rate for Colorectal Cancer by Aged Under 45 Years among Both Sexes, Men, and Women in Khon Kaen, Thailand, 1989-2012.
Figure 2.
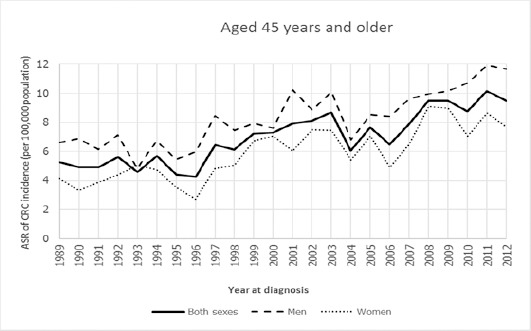
Age-Standardized Incidence Rate for Colorectal Cancer by Aged 45 Years and Older among Both Sexes, Men, and Women in Khon Kaen, Thailand, 1989-2012.
Figure 3.
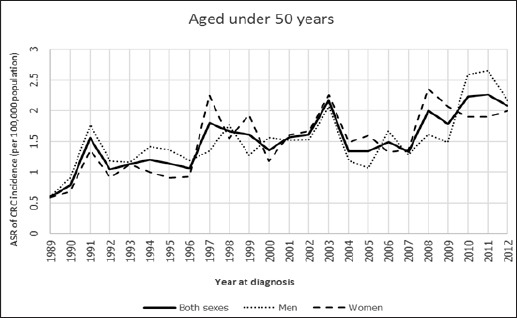
Age-Standardized Incidence Rate For Colorectal Cancer by Aged Under 50 Years Among Both Sexes, Men, and Women in Khon Kaen, Thailand, 1989-2012.
Figure 4.
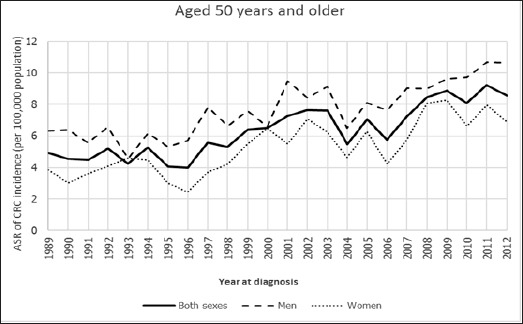
Age-Standardized Incidence Rate for Colorectal Cancer by Aged 50 Years and Older among both Sexes, Men, and Women in Khon Kaen, Thailand, 1989-2012.
Trends of age-standardized incidence rates of CRC by joinpoint analysis
According to joinpoint regression analysis, the trend of age-standardized CRC incidence rate of four aged groups were analyzed and compared for both sexes, men, and women.
The aged 45 years and older group
The trend of both sexes was increased significantly over the period 1989 to 2001 (APC=3.95) and the period 2001 to 2012 (APC=2.76), while the whole line was also increased significantly over the period 1989 to 2012 (AAPC=3.40). For men in this group, the trend was decreasing but not significant from 1989 to 1993 (APC=-2.04), and thereafter was increased significantly from 1993 to 2012 (APC=3.27), while the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=2.3). For women in this group, the trend was for very significant increase from 1989 to 2000 (APC=5.43), and thereafter was also increased but not significant from 2000 to 2012 (APC=2.48), while the whole line was still increased significantly over time from 1989 to 2012 (AAPC=3.90).
The aged 50 years and older group
The trend of both sexes was decreased to be not significant in the first period of 1989 to 1993 (APC=-0.01) and thereafter increased significantly in the period of 1993 to 2012 (APC=3.47), while the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=2.90). The trend for men was for decrease but not significant from 1989 to 1993 (APC=-2.07), and thereafter significant increase from 1993 to 2012 (APC=3.15), while the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=2.2). For women in this group, significant increase was noted from 1989 to 2009 (APC=4.05), and thereafter decrease withoutsignificance from 2009 to 2012 (APC=-0.82), while the whole line was still increased significantly over the period 1989 to 2012 (AAPC=3.40).
The aged under 45 years
The trend of both sexes wasfor increase but not significant from 1989 to 1991 (APC=64.19), and thereafter the trend was for further significant increase from 1991 to 2012 (APC=2.02), and the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=6.30). For men, the trends of both periods 1989 to 1991 and 1991 to 2012 were for increase withoutsignificance (APC=73.18 and APC=1.2, respectively), while the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=6.00). For women, the trend was for increase withoutsignificance from 1989-1991 (APC=60.48), and thereafter for gradual significant increase from 1991-2012 (APC=2.80), and the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=6.9).
The aged under 50 years
The trend of both sexes was for increase but not significant from 1989 to 1991 (APC=44.9), and thereafter for significant increase from 1991 to 2012 (APC=2.60), and the whole line was also increased over time but its trend was still not significant from 1989 to 2012 (AAPC=5.70). For men, the trends of both periods 1989 to 2007 and 2007 to 2012 were for non-significant increase (APC=1.30 and APC=10.40, respectively), while the whole line was also increased slightly to be significant from 1989 to 2012 (AAPC=3.20). For women, the trend was for significant increased from 1989-1997 (APC=10.30), and for further gradual significant increase from 1997-2012 (APC=1.40), and the whole line was increasingly higher to be significant over the period 1989 to 2012 (AAPC=5.70). These results are shown in Table 2.
Table 2.
The Trends of Age-Standardized Incidence Rate of Colorectal Cancer Incidence in Khon Kaen, Thailand by “Joinpoint” Analysis, 1988-2012
| Groups | Groups | Joinpoint analysis | ||||
|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | 1989-2012 | ||||
| Years | APC | Years | APC | AAPC | ||
| Aged 45 years and older | Both sexes | 1989-2001 | 3.95* | 2001-2012 | 2.76* | 3.40* |
| Men | 1989-1993 | -2.04 | 1993-2012 | 3.27* | 2.3 | |
| Women | 1989-2000 | 5.43* | 2000-2012 | 2.48 | 3.90* | |
| Aged 50 years and older | Both sexes | 1989-1993 | -0.01 | 1993-2012 | 3.47* | 2.9 |
| Men | 1989-1993 | -2.07 | 1993-2012 | 3.15* | 2.2 | |
| Women | 1989-2009 | 4.05* | 2009-2012 | -0.82 | 3.40* | |
| Aged under 45 years | Both sexes | 1989-1991 | 64.19 | 1991-2012 | 2.02* | 6.3 |
| Men | 1989-1991 | 73.18 | 1991-2012 | 1.2 | 6 | |
| Women | 1989-1991 | 60.48 | 1991-2012 | 2.80* | 6.9 | |
| Aged under 50 years | Both sexes | 1989-1991 | 44.9 | 1991-2012 | 2.60* | 5.7 |
| Men | 1989-2007 | 1.3 | 2007-2012 | 10.4 | 3.20* | |
| Women | 1989-1997 | 10.30* | 1997-2012 | 1.40* | 5.70* |
APC, annual percent change; AAPC, average annual percent change;
, APC or AAPC are significantly different from zero at alpha=0.05
Discussion
Based on our results, there are three main points for discussion. First, CRC incidence in Khon Kaen province has been gradually increasing over the period 1989 to 2012. Thus trends of ASRs of CRC among four aged groups increased all together, and joinpoint analysis demonstrated that AAPCs were relatively high among all aged groups examined, although for the significance of some periods and aged groups as indicated earlier was not attained. Our results are consistent with a previous study in Thailand, whereby the incidence of CRC was found to be increasing 10 years ago, especially in the capital, Bangkok, and other cities such as Chiang Mai, Khon Kaen, and Hat Yai (Sriplung et al., 2006). The increasing incidence of CRC in Thailand is probably due to changes in lifestyle and individual behavior risk factors (Khuhaprema and Srivatanakul, 2008) associated with economic development or westernization, with increase in sedentary habits and insufficient physical activity, smoking and heavy alcohol consumption, and unhealthy diets which include high amounts of red or processed meat and low amounts of fiber, fruit and vegetables (Giovannucci, 2002; Botteri et al., 2008; Khuhaprema et al., 2014).
Second, approximately 14% and 22% of all CRC cases occurred in persons aged under 45 and under50 years, respectively. These findings are higher than in a previous study in the United States which found 8% of cases involving persons younger than 50 years of age (Fairley et al., 2006). We found the CRC incidence in the young population (aged under 45 or under50 years) to have increased over the period 1989 to 2012. It is important to take into consideration differences between rectal and colon tumors in relation to age(Bailey et al., 2015). Thus our results may reflect differences in tumor subsite location but unfortunately the relevant data were not available for our study. A recent study found left-sided tumors (distal colon and rectum) to be increasingly observed in young adult patients (Siegel et al., 2009), whereas consumption of red and processed meat was shown to be associated with risk of both colon and rectal cancer (Larsson and Wolk, 2006). These studies could have relevance to our cases younger than 45 or 50 years of age in Thailand, younger people being more likely to replace Thai staples and side dishes by diets containing a higher proportion of fats and animal meat, and the prevalence of overweight and obesity among children and adolescents has increased dramatically during the past 20 years (Kosulwat, 2002). Similarly, some studies have been shown strong relationships between diabetes mellitus and increased risk of both colon and rectal cancer (Larsson et al., 2005; Larsson et al., 2005), a possibility which also needs to be clarified in Thailand.
Lastly, CRC incidence here tended to be higher in women than men over the period 1989 to 2012. Our results showed the AAPCs of all aged groups in women were higher than men, with statistical significance except in persons aged under 45 years. A previous study found women to have a higher risk of developing right-sided (proximal) colon cancer than men (Kim et al., 2015), while another indicated that possible sex differences in exposure to certain risk factors might modify risk for tumor development at certain sites, but supportive evidence remains limited (Murphy et al., 2011).
Trends of age-standardized incidence rate of CRC in Khon Kaen, Thailand including persons who aged 45 year and older, aged 50 years and older, aged under 45 years and aged under50 years have been gradually increasing. Future research should focus on causal factors related to these findings, with consideration of additional data on the subsite distribution of CRC in Thai populations, given the evidence of anatomic subsite, race and ethnicity relation with sex and age disparities (Brozek et al., 2009; Esteva et al., 2014; Yoo et al., 2016). In addition, molecular pathways of CRC and the roles of nuclear receptors and intestinal microbiota should be considered with regard to the current possible epidemiological transition in Asia (Bishehsari et al., 2014).
In our study, there were 72.2% of cases with histological confirmation. This figure is reliable since this is a study of the population-based cancer registry.
In conclusion; the incidences of colorectal cancer in Khon Kaen, Thailand have been gradually increasing. Future studies should also consider the subsite distribution.
Conflict of interest
No potential conflicts of interest were disclosed.
Acknowledgements
Thanks are due to Cancer Unit Staff, Faculty of Medicine, Khon Kaen University for the support at the first phase of data collection. Thanks are due to Dr. Malcolm A. Moore for his advice and assistance in writing this paper as part of KKU publication clinic.
References
- Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer:A call to action. Mayo Clinic Proceedings. 2014;89:216–24. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in age-related incidence of colon and rectal cancer in the United States 1975-2010. JAMA surgery. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishehsari F, Mahdavinia M, Vacca M, et al. Epidemiological transition of colorectal cancer in developing countries:Environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055–72. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer:A meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- Brozek W, Kriwanek S, Bonner E, et al. Mutual associations between malignancy, age, gender, and subsite incidence of colorectal cancer. Anticancer Res. 2009;29:3721–26. [PubMed] [Google Scholar]
- Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2:269–79. doi: 10.1002/ijc.2910020310. [DOI] [PubMed] [Google Scholar]
- Esteva M, Ruiz A, Ramos M, et al. Age differences in presentation, diagnosis pathway and management of colorectal cancer. Cancer Epidemiol. 2014;38:346–53. doi: 10.1016/j.canep.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in U.S. adults younger than 50 years of age 1998–2001. Cancer. 2006;107:1153–61. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–43. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- Institute NC. “Joinpoint regression program, Version 4.2.0.2.” retrieved september 09 2015. 2015 [Google Scholar]
- Jiang Z, Qiu Z, Hatcher J. Joinpoint trend analysis of cancer incidence and mortality using Alberta data. Canadian Partnership Against Cancer. 2007:5–50. [Google Scholar]
- Khuhaprema T, Sangrajrang S, Lalitwongsa S, et al. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open. 2014;4:1–11. doi: 10.1136/bmjopen-2013-003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuhaprema T, Srivatanakul P. Colon and rectum Cancer in Thailand:An Overview. Jpn J Clin Oncol. 2008;38:237–43. doi: 10.1093/jjco/hyn020. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kim S-E, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–75. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosulwat V. The nutrition and health transition in Thailand. Public Health Nutr. 2002;5:183–9. doi: 10.1079/PHN2001292. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28:1805–7. doi: 10.2337/diacare.28.7.1805. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Orsini N, Wolk A. Diabetes Mellitus and risk of colorectal cancer:A meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer:A meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- Lim D, Ha M, Song I. Trends in major cancer mortality in Korea 1983–2012, with a joinpoint analysis. Cancer Epidemiol. 2015;39:939–6. doi: 10.1016/j.canep.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. International journal of cancer. Int J Cancer. 2011;128:1668–75. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongchaiyakul C, Pongchaiyakul C, Pratipanawatr T. Prevalence of dyslipidemia in rural Thai adults:an epidemiologic study in Khon Kaen province. J Med Assoc Thai. 2005;88:1092–97. [PubMed] [Google Scholar]
- Pongnikorn D, Suwanrungruang K, Buasom R. 2010-2012, Cancer Registry Unit. VIII. National Cancer Institute Thailand; 2015. Cancer in Thailand; pp. 29–31. [Google Scholar]
- Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. 2015;4:1863–70. doi: 10.1002/cam4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schootman M, Lian M, Deshpande AD, et al. Temporal trends in geographic disparities in small-area-level colorectal cancer incidence and mortality in the United States. Cancer Causes Control. 2011;22:1173–81. doi: 10.1007/s10552-011-9796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Detect Prev Suppl. 2009;18:1695–98. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- Singh KE, Taylor TH, Pan C-JG, et al. Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol. 2014;3:176–184. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriplung H, Wiangnon S, Sontipong S, et al. Cancer incidence trends in Thailand 1989-2000. Asian Pacific J Cancer Prev. 2006;7:239–4. [PubMed] [Google Scholar]
- Suwanrungruang K, Wiangnon S, Sriamporn S, et al. Trends in incidences of stomach and colorectal cancer in Khon Kaen, Thailand 1985-2004. Asian Pacific J Cancer Prev. 2006;7:623–6. [PubMed] [Google Scholar]
- Wirasorn K, Suwanrungruag K, Wiangnon S, Punjaruk W. Numbers of new cases and trends of cancer 1993-2012: Srinagarind hospital based population, Khon Kaen, NorthEast Thailand. Asian Pac J Cancer Prev. 2014;15:8423–27. doi: 10.7314/apjcp.2014.15.19.8423. [DOI] [PubMed] [Google Scholar]
- Yoo W, De S, Wilkins T, et al. Age, race and regional disparities in colorectal cancer incidence rates in Georgia between 2000 and 2012. Ann Trop Med Public Health. 2016;3:1040. [PMC free article] [PubMed] [Google Scholar]


