Abstract
Objective:
The aim of this study is to determine the outcome of trastuzumab therapy for 6 or 12 months in patients with HER2 positive operable breast cancer who followed the national health insurance system in Indonesia.
Methods:
Data were extracted from medical records of Dr. M Djamil General Hospital Padang and Dharmais Cancer Hospital/ National Cancer Center Jakarta for post-operative breast HER-2 positive cancer patients treated with trastuzumab for 6 or 12 months who had been followed up for at least 5 years (January 1st in 2010 until December 31st in 2015). Disease free survival and overall survival rates and their relationship with trastuzumab duration was investigated using survival analysis (the Kaplan-Meier method and log rank test). Data were analyzed using the STATA program.
Results:
A total of 121 women fulfilled the criteria of the study, 80 who had received trastuzumab for 6 months and 41 patients who received 12 months’ therapy. Disease free survival was 54 months (95% CI 45-63) compared with 63 months (95% CI 54-72), respectively. The log-rank test p value was 0.048 so the 12 months’ treatment regime did result in a significantly lower probability of recurrence compared with the 6 months’ regime (HR = 2.6). Analysis of the overall survival rate revealed median survival of 57 months (95% CI 49-64) for 6 months’ therapy compared to 62 months (95% CI 53-72) for 12 months. However, the log-rank test p value of 0.073 indicated that the extra six months of therapy did not decrease the probability of death (HR = 2.4).
Conclusion:
Trastuzumab therapy for 12 months reduced the recurrence rate in post-operative breast HER-2 positive cancer patients but did not significantly reduce mortality.
Keywords: Breast cancer, HER2 positive, trastuzumab, survival
Introduction
Breast cancer is the most frequent cancer in women in the world. It is estimated that 1.4 million new cases of breast cancer occur and 450,000 women die each year. The incidence of breast cancer in developing countries continues to increase at least partially due to lifestyle changes (International Agency for Research on Cancer 2008). In Indonesia there are thought to be 40 cases per 100,000 women with a total of almost 40,000 new cases each year. The report from the National Hospital Data Base in 2007 showed breast cancer is the most commonly treated cancer in women and the leading cause of cancer death that most treated in women and also the most common cause of death from cancer in women who were treated in hospitals (Ministry of Health Republic of Indonesia, 2010).
Breast cancer is a heterogenic disease. Biomolecular changes in breast cancer involve the expression of genes and breast cancer is classified according to the four subtypes of genes expressed; luminal A, luminal B, HER2 and Basal Like. Each of these subtypes has different clinical features, response to therapy and prognosis. Globally 51-61% breast cancer patients have Luminal A subtype, 14-16% Luminal B, 15-20% HER2 and have 11-20% Basal like subtype (International Agency for Research on Cancer, 2008).
HER2 is one of 4 groups of receptor tyrosine kinases which mediate growth factors. HER2 protein overexpression is the result of amplification of the HER2 gene and occurs in 15-20% of all breast cancers. It is associated with aggressive tumor growth and consequent high rates of recurrence and mortality in patients (Slamon et al., 1987).
Trastuzumab is a monoclonal antibody that works by inhibiting the extracellular domain of HER2, which can improve survival and quality of life in patients with HER2-positive breast cancer. Since 2005, 12 months trastuzumab as adjuvant therapy is the standard treatment of patients with HER2 positive breast cancer internationally, based on the results of four major clinical trials from the last decade involving HER2-positive breast cancer; the HERA trial research, NSABP (B-31), NCCTG (N9831) and BCIRG 006. These trials demonstrated the use of trastuzumab would reduce recurrence and mortality by 24-30%. Nevertheless recurrence was recorded in nearly 39% of the cases, and about 35% of cases did not respond to trastuzumab (Perez et al., 2014). Since 2010, trastuzumab has been included in Indonesia national formularies as standard therapy in HER2 positive breast cancer for 8 cycles (6 months) financed by the national health insurance system (Indonesia’s Healthcare and Social Security Agency, 2010). To complete the course up to the full recommended 16 cycles (12 months) the breast cancer patients have to pay for the trastuzumab therapy themselves. Because not all patients are able to do this, less than one third of HER2-positive breast cancer patients get trastuzumab for the full 12 months. Therefore, this study aimed to evaluate the relative effectiveness of trastuzumab therapy for 6 months and 12 months in patients with HER2-positive operable breast cancer.
Materials and Methods
Study design and research sample
This study analyzed medical records of patients who belonged the national health insurance program with HER2-positive breast cancer. The study was conducted at the Dr M. Djamil General Hospital Padang and Dharmais Cancer Hospital / National Cancer Center in Jakarta. The population in this study are all HER-2 positive breast cancer patients who were given trastuzumab for 6 months or 12 months and were followed up for 5 years (1th January 2010 to 31th December 2015). Inclusion criteria for this study were women with breast cancer who received trastuzumab therapy for 6 or 12 months and were participants in the national health insurance scheme. Exclusion criteria were patients who did not complete the treatment, male breast cancer patients, patients with metastatic cancer and those who were unable to be followed up.
Operational definitions
The subjects of this study are operable breast cancer in women (stage I-III). The definition of Her2 positive breast cancer is Her2 positive with Immuno Histochemistry (IHC) score 3+. Disease free survival is determined from the length of time after completion of treatment until the onset of signs/ symptoms of cancer recurrence. Overall survival was calculated from diagnosis until the patient dies.
Research procedure
The study began by collecting data from medical records of patients with Her2 positive breast cancer who received trastuzumab therapy after surgery, with or without radiotherapy (Figure 1).
Figure 1.
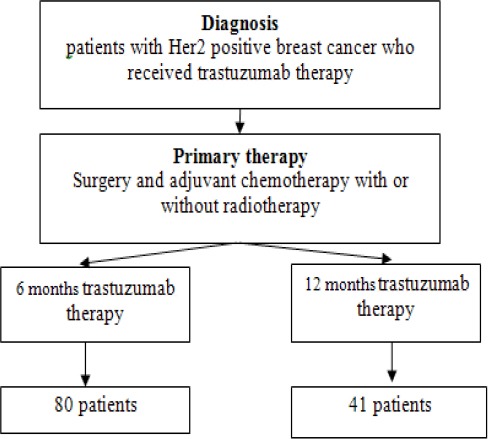
Research Procedure
Data collection technique
Data were collected from medical records at the Division of Surgical Oncology Dr M. Djamil General Hospital in Padang and in Dharmais Cancer Hospital/ National Center Cancer in Jakarta. This data was then coded and analyzed.
Data analysis
Univariate analysis of the data in this study was used to find the disease free and overall survival duration of the patients given trastuzumab therapy and bivariate analysis was conducted on these survival statistics using the Kaplan-Meier method and log rank test. Data were analyzed by using STATA program.
Results
Characteristics of research subjects
Research was conducted on data from January 1st, 2010 until December 31st 2015. Over this period 121 cases of HER2 positive breast cancer were identified 80 (66%) of whom received trastuzumab for 6 months and 41 (34%) who completed a full 12 months’ treatment. Data characteristics of the respondents are in Table 1.
Table 1.
Characteristics of Research Subjects
| Characteristics | Duration of Trastuzumab Therapy | p value | |||
|---|---|---|---|---|---|
| 6 months | 12 months | ||||
| f | % | f | % | ||
| Age | |||||
| < 35 years | 46 | 57.5 | 18 | 43.9 | 0.35 |
| 35-49 years | 33 | 41.3 | 22 | 53.7 | |
| 50-59 years | 1 | 1.3 | 1 | 2.4 | |
| Tumor Size (T) | |||||
| T1 | 3 | 3.8 | 4 | 9.8 | 0.405 |
| T2 | 31 | 38.8 | 18 | 43.9 | |
| T3 | 20 | 25.0 | 10 | 24.4 | |
| T4 | 26 | 32.5 | 9 | 22.0 | |
| Stage | |||||
| Stage I | 3 | 3.8 | 4 | 9.8 | 0.25 |
| Stage II | 36 | 45.0 | 21 | 51.2 | |
| Stage III | 41 | 51.3 | 16 | 39.0 | |
| Grade | |||||
| I | 13 | 16.3 | 3 | 7.3 | 0.077 |
| II | 53 | 66.3 | 24 | 58.5 | |
| III | 14 | 17.5 | 14 | 34.1 | |
| Nodal status | |||||
| Negative | 4 | 5.0 | 5 | 12.2 | 0.165 |
| Positive | 76 | 95.0 | 36 | 87.8 | |
| Histopathology | |||||
| Ductal carcinoma | 49 | 61.3 | 17 | 41.5 | 0.082 |
| Lobular carcinoma | 15 | 18.8 | 9 | 22.0 | |
| Other types | 16 | 20.0 | 15 | 36.6 | |
| Estrogen Receptor | |||||
| Negative | 60 | 75.0 | 25 | 61.0 | 0.165 |
| Positive | 20 | 25.0 | 16 | 39.0 | |
| Progesteron Receptor | |||||
| Negative | 58 | 72.5 | 30 | 73.2 | 1 |
| Positive | 22 | 27.5 | 11 | 26.8 | |
| KI67 | |||||
| < 14% | 20 | 25.0 | 5 | 12.2 | 0.159 |
| > 14% | 60 | 75.0 | 36 | 87.8 | |
| Status | |||||
| Life | 56 | 70.0 | 36 | 87.8 | 0.052 |
| Death | 24 | 30.0 | 5 | 12.2 | |
| Total | 80 | 100.0 | 41 | 100.0 | |
Table 1 shows most patients with Her2 positive breast cancer were less than 35 years old with only a fraction older than 50 years. Most of the patients had tumors > 5 cm so were in stage II and III. More than half of the cancers were grade II and III. 92,5% had positive nodal status. The most common histopathology was ductal carcinoma. A large proportion of patients had negative estrogen receptors; only 25% of patients in the 6 months therapy group were ER positive and 39% in the 12 month group. The expression of KI67 tended to be > 14%. Twenty four patients (30%) with 6 months of trastuzumab and 5 patients (12,2%) who completed 12 months of trastuzumab died before the five year follow up.
The effect of trastuzumab therapy on disease free survival and overall survival
The effect of trastuzumab therapy on disease free survival and overall survival is summarised in Table 2. Median follow up disease free survival for 6 months of therapy was 54 months (95% CI 45-63) compared to 63 months (95% CI 54-72) for 12 months. A log-rank test gave a p value of 0,048 meaning that the provision of a full 12 months of trastuzumab resulted in a statistically significantly lower probability of recurrence than only 6 months (HR = 2.6). The overall survival had a median of 57 months (95% CI 49-64) for 6 months of therapy compared to 62 months (95% CI 53-72) for 12 months trastuzumab therapy. The log-rank test p value was 0,073 meaning that the provision of longer trastuzumab therapy did not result in a statistically significant increase overall survival (HR = 2.4) (Figure 2).
Table 2.
The Effect of Trastuzumab Therapy with Disease Free Survival and Overall Survival
| Event | Censoring | Hazard Ratio | Median Follow Up | 95% CI | P value (Log-rank test) | ||
|---|---|---|---|---|---|---|---|
| Disease Free Survival | 6 months | 24 | 56 | 2.6 | 54 | 45-63 | 0.048 |
| 12 months | 5 | 36 | 63 | 54-72 | |||
| Overall Survival | 6 months | 24 | 56 | 2.4 | 57 | 49-64 | 0.073 |
| 12 months | 5 | 36 | 62 | 53-72 |
Figure 2.
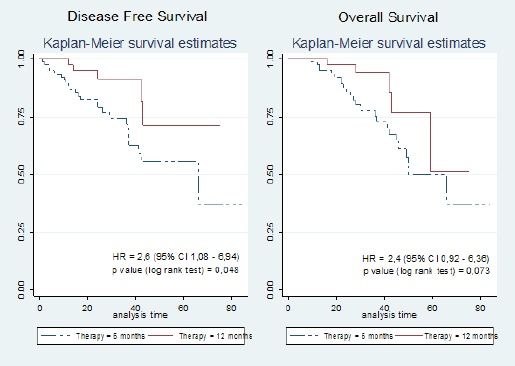
The Effect of Trastuzumab Therapy with Disease Free Survival and Overall Survival
Breast cancer recurrence and trastuzumab therapy duration
The Effect of trastuzumab therapy duration on recurrence is shown in Table 3. The 6 months to 12 months hazard ratio of local recurrence for trastuzumab therapy was 2.6. Patients given Trastuzumab therapy for 6 months experienced a local recurrence after a median 55 months (95% CI 44-66 months) compared to 61 months (95% CI 49-73 months) for patients who had the full 12 months’ treatment. For distant recurrence these values were 43 months (95% CI 29-57 months) and 46 months (95% CI 37-55 months) respectively. Local recurrence is related significantly at the 5% level to the duration of trastuzumab therapy (log-rank test p-value 0.049) but for distant recurrence does not (log-rank test p-value 0.074) (Figure 3).
Table 3.
The Effect of Trastuzumab Therapy with Recurrence
| Event | Censoring | Hazard Ratio | Median Follow Up | 95% CI | P value (Log-rank test) | ||
|---|---|---|---|---|---|---|---|
| Local recurrence | 6 months | 15 | 11 | 2.6 | 55 | 44-66 | 0.049 |
| 12 months | 4 | 28 | 61 | 49-73 | |||
| Distant metastasis | 6 months | 9 | 11 | 2.4 | 43 | 29-57 | 0.074 |
| 12 months | 1 | 8 | 46 | 37-55 |
Figure 3.
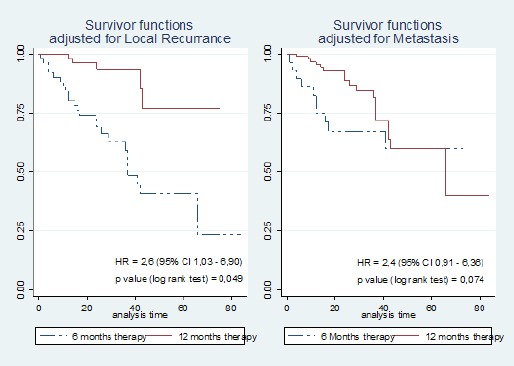
The Effect of Trastuzumab Therapy with Recurrence
Discussion
The formulary issued by national health insurance only covers 8 cycles (6 months) of trastuzumab even though current standard medical practice is 12 months. To complete the second 6 months of treatment a patient must pay themselves.
The four major clinical trials of trastuzumab (HERA trial, NSABP B-31, NCCTG N9831, BCIRG 006) included more than 13, 000 women with early stage HER2 positive breast cancer. All four indicate that the provision of trastuzumab resulted in recurrence within the first 3 years after diagnosis being halved (Baselga et al., 2006). In the HERA trial study 2 years of treatment was shown to not provide better outcomes than 1 year.
So the question arises whether the provision of trastuzumab in early stage HER2 positive breast cancer for only 6 months might not produce equally beneficial results to 12 months as was found by three other studies, including the PHARE study, which found no significant difference in recurrence between the provision of trastuzumab for 6 months compared to 12 months (Pivot et al. 2013).
Half the subjects in our study were less than 35 years old considerably younger than most of those in the clinical trials mentioned above where generally the ages were spread more evenly over 50 years (Baselga et al. 2006). For instance, in the HERA trial only 7.5% were less than 35 years old, 44% were 35-49, 32% 50-59 years and 16% over 60 (Piccart-Gebhart et al., 2005).
In this study, 53% of tumors sizes were T3, 40.5% were T4 or T2 and only 4.9% T1. This compares to a distribution sizes in the PHARE trial of 3307 HER2-positive breast cancer patients where 53.5% were still T1, 37.7%, T2 and only 7.3% T3 or T4. The HERA trial, NSABP (B-31), NCCTG (N9831) and BCIRG 006 also show similar distribution of tumor sizes to the PHARE Trial (Pivot et al. 2013). However, the distribution of tumor sizes in our study is similar to that found in studies in Malaysia and Thailand (Hisham et al., 2004).
The larger sizes of tumors could indicate that the subjects in our study, along with those in Malaysian and Thai Studies, had tumors with an aggressive phenotype or that breast cancers in these areas are less likely to be identified before they reach an locally advanced stage (stage IIIa and IIIb) (Pivot et al., 2013).
The histopathology of the cancers in this study showed mostly invasive ductal carcinoma (61.3%), ER positive (29.8%) and between grade II and III (86.7%). The HERA trial study found a much higher ER positive rate (63%). Several other Western trastuzumab studies also found ER positive rates above 50%. The low ER positive rate in patients in this study may be related to their younger age compared to patients from Western countries or a difference in lifestyle factors such as parity or obesity rates (Goldhirsch et al, 2013; Ejlertsen et al., 2014).
Analysis of 2010 and 2015 medical records found that the provision of trastuzumab for 6 months resulted in 24 cases of recurrence (30%) and in the 12-month group recurrence occurred in 5 cases (12%) (HR 2.6, p = 0.048). This demonstrates that the provision of trastuzumab for a full 12 months provides significantly better protection against recurrence of cancer.
This in in contrast to Mavroudis et al (2015) who compared 6 and 12 months trastuzumab therapy in conjunction with dose dense chemotherapy. After a median follow-up of 51 and 47 months respectively, 28 cases had relapsed (11.7%) for the 6-month group compared to 17 cases (7.1%) for the full year of treatment. However, this difference was not statistically significant. (p = 0.08) (Mavroudis et al., 2015). The PHARE Study also reported that the recurrence rate after a 3-5 year follow up period for 6 months’ therapy was 10.4% compared to 13% for 12 months (Pivot et al., 2013). Like the Mavroudis study this difference was not significant.
The HERA trial indicated that, after two years, there was a 46% reduction in the number of patients suffering from a recurrence in the group given trastuzumab for 12 months compared to patients who did not receive trastuzumab therapy (control) (Goldhirsch et al., 2013). However, most of the patients in this study were only stage I and II compared to the subjects in the present study who were generally diagnosed with more advanced cancers.
Approximately 35% of cases do not respond at all or were resistant to trastuzumab and further there is a secondary resistance in up to 70% of cases (Goldhirsch et al., 2013). Data from the present study showed median overall survival was not correlated significantly with the duration of trastuzumab therapy (p value 0.073). 24 deaths (29.6%) occurred in the 57 median month follow up period amongst patients who had 6 months therapy compared to only 5 (12.5%) who had 12 months – have you discussed the significance of this difference – survival at 5 years rather than median overall survival.
The HERA trial compared overall survival rates of 1 versus 2 years of trastuzumab treatment 1 vs 2 years and found no significant difference between the two groups (Piccart-Gebhart et al., 2005). Recent studies show trastuzumab reduces recurrence by 36-52% and a mortality by 33-37% when compared to a placebo but the optimal duration of trastuzumab as adjuvant therapy is still a matter of debate. There is a consensus that there is little advantage of 2 years over one year but it has been unclear whether a therapy duration of 12 months is better than 6 months. This study found that the provision of 12 months provided better rates of disease free survival, but not for overall survival. The fact that the patients in this study tended to be young and have cancer at a advanced local stage will have influenced these results.
The findings of the research which states that up to 12 months trastuzumab therapy is not effective in reducing mortality in patients with breast cancer. This illustrates that occurred ineffectiveness and inefficiency in drug treatment therapies. This differs from the study HERA trial, B-31 (NSABP), N9831 (NCCTG) and BCIRG006 caused by the sample in this study was not conducted selection of the samples tight, so it can be said to describe the actual conditions in the aftermath of therapy, however therapy 12 months reduced the rate of recurrence.
Cancer therapies are a very expensive component of the publicly funded Indonesia National Health Insurance formulary. Six months supply of trastuzumab therapy costs US$ 12,000 for 6 months. To extend the treatment to the full recommended 12 months costs US$ 20,000 more (Indonesia’s Healthcare and Social Security Agency 2010). However, this study shows that the longer course of therapy does reduce local recurrence significantly. But keep in mind is the level of effectiveness of the drug, so that the guarantees and government assistance be appropriate and targeted, this may be because in the era of Indonesia National Health Insurance, efforts should be made to improve health services and coverage thoroughly and can help many people. It actually should be evaluated and considered the government that the allocation and expenditure of the budget can be used for appropriate services. Then, medical personnel could focus on spending on public information and early detection breast screening program especially where there is a family history or presence of HER2 is know. If treatment is seen to be effective people will be more willing to see doctor sooner. The impact is higher cure rate and less expense for government.
Acknowledgements
The authors would like to thank Assoc. Dr. rer. nat Ichwan Sudji and Syifa’u Warahmah for collecting data. Fay Farley for translating.
References
- Baselga J, Perez EA, Pienkowski T. Adjuvant trastuzumab:A milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11:4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- Ejlertsen B, Ewertz M, Rasmussen BB. Divergent estrogen receptor positive and negative breast cancer trends and etiologic hertogeneity in Denmark. Int J Cancer. 2014;133:2201–6. doi: 10.1002/ijc.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA):an open-label, randomised controlled trial. Lancet. 2013;382:1021–8. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- Hisham AN, Yip CH. Overview of breast cancer in Malaysian women. Asian J Surg. 2004;27:130–3. doi: 10.1016/S1015-9584(09)60326-2. [DOI] [PubMed] [Google Scholar]
- Indonesia’s Healthcare and Social Security Agency. Jakarta: National Health Insurance of Indonesia; 2010. [Google Scholar]
- International Agency for Research on Cancer. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide. GLOBOCAN 2008; 2008. [Accessed March 7 2017]. Available at: http://globocan.iarc.fr . [Google Scholar]
- Mavroudis D, Saloustros E, Malamos N, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer:a multicenter randomized study by the Hellenic Oncology Research Group (HORG) Ann Oncol. 2015;26:1333–40. doi: 10.1093/annonc/mdv213. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Republic of Indonesia. Jakarta: Indonesia Basic Health Survey; 2010. [Google Scholar]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer:Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–52. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive positive early breast cancer (PHARE): a randomized phase 3 trial. Lancet Oncol. 2013;14:741–8. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer:correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]


