Abstract
Hepatocellular carcinoma (HCC) is a cause of several deaths related to cancer worldwidely. In early stage, curative treatments such as surgical resection, liver transplant and local ablation can improve the patient ´s survival. However, the disease is detected in advanced stage; moreover some available therapies are restricted to palliative care and local treatment. Early detections of HCC and adequate therapy are crucial to increase survival as well as to improve the patient´s quality of life. Therefore, researchers have been investigating molecular biomarkers with high sensibility and reliability as Golgi 73 protein (GP73), Glypican-3 (GPC3), Osteopontin (OPN), microRNAs and others. MicroRNAs can regulate important pathways on carcinogenesis, as tumor angiogenesis and progression. So, they can be considered as possible markers of prognosis in HCC, and therapeutic target for this tumor type. In this review, we discuss the recent advances related to the cause (highlighting the main risk factors), treatment, biomarkers, clinic aspects, and outcome in hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma, causes, biomarkers, treatment, prognosis
Introduction
Hepatocellular carcinoma (HCC), the primary cancer of the liver, is derived from hepatocytes and occurs in more than approximately 80% of cases of liver cancer (Jemal, 2011).
The incidence of HCC is higher on mean ages from its diagnosis between 55 and 59 years in China, and 63-65 years in North America and Europe. Higher rates of HCC are observed in East and Southeast Asia, East and Western Africa (Jemal et al., 2011; El-Serag, 2012). The latest data estimated by GLOBOCAN showed about 782,000 new cases and 745,000 deaths of liver cancer in 2012 worldwide. Therefore, the World Health Organization (WHO) considers HCC as the second leading cause of cancer deaths (Ferlay et al., 2015).
HCC development results from the interaction between environmental and genetic factors. Liver cirrhosis, hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, excessive alcohol consumption, ingestion of aflatoxin B1, and nonalcoholic steatohepatitis (NASH) are important risk factors for HCC development (Pimenta et al., 2010; Gomes et al., 2013).
Life expectancy of patients with HCC depends on the stage of the cancer at diagnosis. In advanced stage, some months are expected, however, when the diagnosis is early and effective treatment performed, five- year survival rate can be accomplished (Forner et al., 2012). If the diagnosis is performed at early stage, its treatment is limited and effective; whereas, at advanced when traditional chemotherapy has no satisfactory effect, poor prognosis is expected (Liu et al., 2015). At early stage of HCC, curative treatments such as surgical resection, liver transplant and local ablation can improve the survival of the patients. Therefore, early detection and the adequate therapy are crucial to increase survival and improve the life quality of HCC patients. When classified as stage C (advanced stage) with the presence or absence of vascular invasion and preserved liver function, according to Barcelona Clinic Liver Cancer (BCLC) classification, the use of Sorafenib has been effective to improve these patients´ survival (Gomes et al., 2013; de Lope et al., 2012).
Alpha-fetoprotein (AFP) has been used as a biomarker in HCC diagnosis by serum. However, AFP is not a precise marker since it provides low sensibility and specificity (Morimoto et al., 2012; Lok et al., 2010). Therefore, a biomarker that presents higher diagnostic accuracy and high reliability are needed. Recent studies identified many tumor biomarkers in HCC as Golgi 73 protein (GP73), Glypican-3(GPC3), microRNAs and others (Ba et al., 2012; Feng et al., 2014; Bartel., 2004).
Current genetic research can contribute to the diagnosis, prognosis, and therapeutic in HCC as well as to provide insights on further steps of the molecular medicine applied to the cancer (Villanueva et al., 2010). In this context, the present review stands out the recent advances related to the causes, treatments, biomarkers, clinic aspects and outcome on hepatocellular carcinoma.
Causes/ Risk Fators
The HCC carcinogenesis is often associated with liver cirrhosis resultant from chronic liver diseases as chronic hepatitis, HBV or HCV infection, and autoimmune hepatitis. Other risk factors include excessive alcohol consumption, NASH, non-alcoholic fatty liver disease (NAFLD), exposure and ingestion of aflatoxin, diabetes mellitus, tobacco, and sporadically genetic diseases such as alpha-1 antitrypsin deficiency, hemochromatosis, tyrosinemia, porphyria and Wilson’s disease (Mittal and El-Serag, 2013; McGlynn and London, 2011; Pimenta and Massabki, 2010). Figure 1 represents the risk factors for the development of Hepatocellular Carcinoma.
Figure 1.
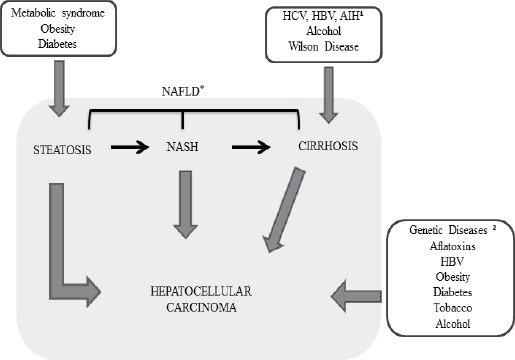
Risk Factors for the Development Stage of Hepatocellular Carcinoma. HCV (Hepatitis C virus), HBV (Hepatitis B virus), ¹AIH (Autoimmune Hepatitis), 2Genetic Diseases: Alpha-1-antitrypsin deficiency, Hemochromatosis, Porphyria and Tyrosinemia. *The Spectrum of NAFLD: Steatosis, NASH and Cirrhosis.
Yang and colleagues have studied other risk factors related to HCC development, as male gender, advanced age, obesity, and co-infection with VHB (Yang et al., 2010). A large population-based study with 11,801 male from Taiwan (followed up for 15 years) concluded that an increased risk for HCC development was influenced by three factors, in the following proportions: HBV (55.7%) and HCV (15.3%) infection, and independent alcohol consumed (2.1%). The study has observed an association between HBV and HCV infection and the increased risk of developing HCC (1.7%), and that the combination HBV infection with alcohol consumption increases the risk in 4,2% (Liao et al., 2012).
The presence of chronic HCV infection and excessive alcohol consumption can duplicate the risk of HCC when compared with the infection alone (Jelic et al., 2010). Women are more susceptible to liver lesion and cirrhosis development by alcohol ingestion compared to men, because the influence of hormones, such as estrogen. The liver is the organ responsible for metabolizing these hormones and the presence of alcohol in the liver results of an increase in oxidative stress and inflammation resulting from high levels of female hormones steroids (Guy and Peters, 2013; Eagon, 2010).
In the liver, the alcohol is metabolized to acetaldehyde, which is highly toxic to the hepatocytes and can result in the formation of adducts, which inactivate glutathione peroxidase system and cause mitochondrial damage. Deleterious effects of alcohol and its toxic metabolites include defect on synthesis and repair of DNA in the liver cells, and synthesis of reactive oxygen species (ROS), resulting in oxidative stress and up-regulation of pro-inflammatory signaling (Setshedi et al., 2010; Orman et al., 2013).
Some studies have shown that NAFLD is another risk factor for development of HCC. Currently, metabolic disorders have been a public health problem on a global scale, as diabetes mellitus, obesity, and metabolic syndrome. NAFLD is present in approximately 70% of the people with diabetes mellitus, and 90% of the people with obesity. Therefore, NAFLD is a potential risk factor for HCC (Gomes et al., 2013). Diabetic patients present a higher risk (2.5 fold) of developing HCC, independent of co-infection with HBV or HCV, and alcohol consumption (El-Serag, 2012). NAFLD can progress to NASH, cirrhosis, and HCC, due to the inflammation resulting from fat accumulation in the liver (Pocha et al., 2015; Starley et al., 2010; Michelotti et al., 2013). A study conducted in Europe suggested that tobacco consumption is a potential risk factor for the development of hepatocarcinogenesis. Tobacco was associated with liver disease and with an increased risk of mortality associated with alcohol consumption (Trichopoulos et al., 2011; Shih et al., 2012).
Another risk factor for hepatocarcinogenesis is the ingestion and exposure to fungal aflatoxins, particularly aflatoxin B1 caused by fungi (Aspergillus flavus and Aspergillus parasiticus), which often contaminate stored cereals, such as peanuts and wheat, in areas with limited resources. Mycotoxins bind to hepatocyte DNA, resulting in mutation of tumor suppressor genes or proto-oncogenes, particularly in the p53 gene, occurring in mitotically active cells, such as in livers with chronic HBV or cirrhotic (Pimenta et al., 2010; Yang et al., 2010).
Treatment
Actually, there are many curative and/or palliative treatments for HCC. The choice of the appropriate treatment should take into account the cancer stage, the expertise of the professionals, the available resources, age and comorbidities of the patient (Maida et al., 2014).
The BCLC classification considers the relevant parameters of HCC, and classifies the patients into very early, early, intermediate, advanced and terminal stage. Resection, ablation and transplantation are considered potentially curative options for patients in the early stages. Chemoembolization is indicated for intermediate stage, while patients in advanced stage can be treated with Sorafenib. Finally, patients in the terminal stage are submitted to palliative care for improve their quality of life (Yang et al., 2010; Forner et al., 2014; Maida et al.,2014; Mazzanti et al., 2016) (Figure 2).
Figure 2.
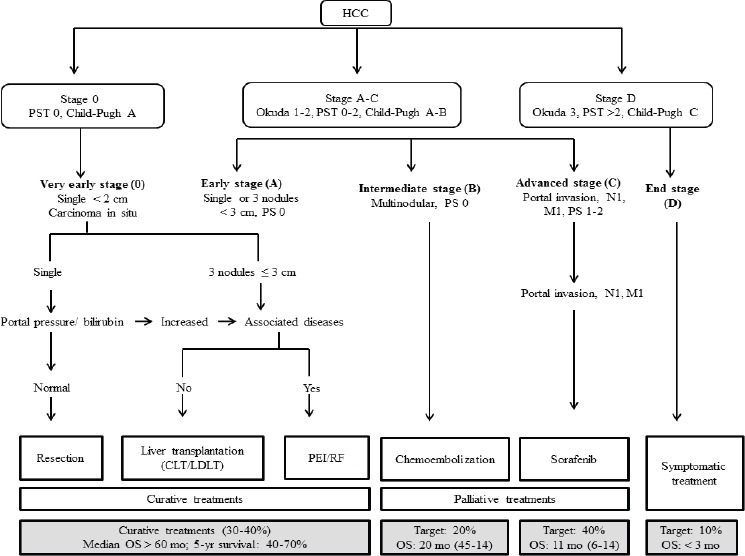
The Barcelona Clinic Liver Cancer Stanging and Treatment Allocation. Adapted from Bruix J. Sherman M. Management of Hepatocellular Carcinoma: an Update, 2010. CLT, Cadaveric Liver Transplantation; HCC, Hepatocellular Carcinoma; LDLT, Living Donor Liver Transplantation; M, Metastasis Classification; N, Node Classification; OS, Overall Survival; PEI, Percutaneous Ethanol Injection; PST, Performance Status Test; RF, Radiofrequency.
Surgical resection is the choice treatment for HCC patients at early stage without cirrhosis. In cirrhotic patients, surgical resection is indicated only in cases with preserved liver function, single nodule, and absence of portal hypertension. The five-year survival rate varies between 50 to 75% in patients undergoing resection, but the recurrence rate (tumor development and metastasis) can reach 50% (Bruix et al., 2011). Therefore, surgical resection is not recommended for HCC associated with vascular invasion or tumor metastasis (Maida et al., 2014).
A common therapy is the ablation, which involves the destruction of tumors <5 centimeters using radiofrequency applications. Ethanol and cryosurgery are alternatives for patients who cannot be subjected to resection or transplantation. The ablation causes necrosis in the tumor microcirculation and ischemia (Ryan et al., 2016). Studies of randomized controlled trials comparing surgical resection and radiofrequency ablation have reported that there were no significant differences in survival or recurrence rates, though the ablation was associated with lower rates of hospitalizations and complications related to the treatment (Kang et al., 2015).
In HCC patients with cirrhosis the transplantation is the indicated treatment, because the reduced recurrence rate and the increase of survival. However, due to the lack of organs available for transplant, patients who could present better results are prioritized for the liver transplant (Adam et al., 2012; Clavien et al., 2012). Because the limited availability of deceased donor liver, transplant from living donors has been a strategy to reduce the waiting time for the transplant and decrease the mortality rate. Transplant from living donors is the alternative treatment for patients with HCC and advanced cirrhosis. However, studies have shown that relapse rate is higher in transplant living donor (Akamatsu et al., 2014; Chen et al., 2015; Rahimi et al., 2015).
Arterial chemoembolization is defined as an intra-arterial infusion of a chemotherapeutic agent (doxorubicin or cisplatin) combined with embolization of tumor vascular supply, resulting in a cytotoxic and ischemic (hypoxia) effect (Waller et al., 2015). Hypoxia increases cell permeability, and the concentration of chemotherapeutic agent used. Chemotherapeutic agents are contraindicated for patients with impaired liver and portal vein function, encephalopathy and commitment of the biliary system. Studies have shown that the vascular obstruction induces the release of angiogenic factors, requiring the combination of Sorafenib and arterial chemoembolization in order to achieve greater therapeutic safety (Lencioni et al., 2012).
Currently, Sorafenib is an oral multikinase inhibitor with anti-angiogenic effect and antiproliferative and it is the choice treatment for advanced HCC patients with preserved liver function (Carr et al., 2010; Bruix et al., 2011). Meta-analysis and multicenter trials studies have shown the Sorafenib efficacy in prolonging survival and time to progression of the disease. However, common side effects such as dermatologic toxicity, diarrhea, fatigue, and increased incidence of hypertension were reasonably tolerated and provide conduits in clinical practice (Han et al., 2016). The treatment cost with Sorafenib is high and this medicament is not provided for use by Brazilian Public Heath (Carr et al., 2010).
Patients in the terminal stage are submitted to palliative care, aiming to improve the quality of life and increase the survival of patients. One method chosen by the healthcare team to the patient that is in this stage is brachytherapy, a less aggressive internal radiotherapy modality than the conventional one. It is an important role of the health team to monitor the patient in all aspects, physiological and psychological, once any alteration may be suggestive of metastasis (Kumar and Panda, 2014; Schlachterman et al., 2015).
The recent positive results of the therapy with multi-target blocking activity, for example, KRAS (Kirsten Rat Viral Sarcoma Oncogene Homolog) and VEGF (Vascular Endothelial Growth Factor) represent progress in the treatment of HCC patients, proving the better efficacy of molecular therapies in comparison to the conventional chemotherapy, strengthening the current clinical oncology (Villanueva et al., 2010; Baines et al., 2011).
A challenge in the search for new therapies is that the HCC is genetically heterogeneous. Therefore, VEGF is involved in several mechanisms responsible for tumor progression, invasion, and metastasis. The identification of new targets resulted from experimental studies could predict liver carcinogenesis, and describe new biomarkers, delineating efficient therapeutic strategies in order to reduce significantly the number of HCC related deaths (Schütte et al., 2015).
Biomarkers
Modern analytical techniques such as Next Generation Sequencing (NGS), mass spectrometry, proteomics and metabolomics can provide important information for medical oncology and contribute to the identification of novel molecular biomarkers for the diagnosis of HCC (Marquardt and Andersen, 2012). These biomarkers are identified by genomic platforms, and other genetic analysis resultant of blood, tissue, urine, feces and saliva, and could contribute for the development of individualized treatment according to the genetic composition and exposure to environmental risk factors. Biomarkers may be used for diagnostic, prognostic and for identification of the clinical staging of HCC (Carethers et al., 2015; Jameson et al., 2015).
Recent studies indicate promising molecular biomarkers, such as GPC3 (Glypican-3), OPN (Osteopontin), GP73 (Protein Golgi 73), VEGF gene (Vascular Endothelial Growth Factor), EGF gene (Epidermal Growth Factor), PDGF gene (Growth Factors Derived From Platelets) IGF gene (Growth Factor Similar To Insulin), mTOR (Protein In Mammalian Target Of Rapamycin), and microRNAs, are potential candidates to be clinically validated in the future (Mínguez et al., 2011; Schütte et al., 2015; Biselli-Chicote et al., 2012; Kedmi et al., 2015; Okada et al., 2015; Jung et al., 2015; Alqurashi et al., 2013). The most relevant genes, proteins, and their related pathways involved in HCC are summarized in the Table 1.
Table 1.
Biomarkers of HCC Pathways
| Function | Reference |
|---|---|
| Suppression/modulation of growth | Dargel et al., 2015; Haruyama et al., 2015, 2016 |
| Cytokine which upregulates expression of interferon gamma and interleukin-12 | Dong et al., 2016 |
| Membrane protein of the apparatus Golgi expressed in liver and biliary epithelial cells | Ba et al., 2012; Yang et al., 2015 |
| Repair pathways, DNA replication and transcription | Thurnherr et al., 2016 |
| Repair pathways, DNA replication and transcription | Thurnherr et al., 2016 |
| Repair pathways, DNA replication, transcription and autophagy mechanism | Thurnherr et al., 2016 |
| Metabolic pathways and the immune system | Thurnherr et al., 2016 |
| Metabolic pathways and the immune system | Thurnherr et al., 2016 |
| Metabolic pathways and the immune system | Thurnherr et al., 2016 |
| Vascularization | Fish et al., 2009; Fátima and Papa, 2010; Liu et al., 2016 |
| Angiogenesis | Fish et al., 2009; Fátima and Papa, 2010; Liu et al., 2016 |
| Suppressor migration and cell invasion | Fish et al., 2009; Fátima and Papa, 2010; Liu et al., 2016 |
| Cell proliferation and differentiation | Kedmi et al., 2015 |
| Angiogenesis | Shao et al., 2011; Okada et al., 2015 |
| Blockade of the cell cycle and apoptosis | Li et al., 2016 |
| Blockade of the cell cycle | Zhang et al, 2014 |
| Tumor suppressive | Zhang et al., 2014 |
| Angiogenesis | Moeini et al., 2012; Cheng et al., 2016 |
| Proliferation and cell migration | Kedmi et al., 2015 |
| Angiogenesis | Shao et al., 2011; Okada et al., 2015 |
| Cell development, homeostasis and aging | Su et al., 2010; Elmashad et al., 2015; Jung and Suh, 2015 |
| Cell growth, differentiation, proliferation and migration | Li et al., 2016; Buitrago-Molina and Vogel, 2012; Merkenschlager and Marcais, 2015. |
GPC3 is a member of the cell-surface proteoglycans family, which is anchored to the plasma membrane by a glycosyl-phosphatidylinositol bond, performing a role in the control of cell division and regulation. This protein can inhibit the activity of dipeptidyl-peptidase-4 (DPP4) inducing apoptosis in some cell types and it is overexpressed in HCC predicting the poor prognosis for the patients. Thus, studies suggest the GPC3 as a potential marker of malignancy with sensitivity (77%) and specificity (96%) in the detection of small dysplastic nodules (<2 cm). Currently, researchers have investigated new strategies for therapies using GPC3 in HCC (Feng et al., 2014).
OPN is a protein involved in the fusion of osteoclasts in the mineralized bone matrix. It is a cytokine which up regulates the expression of interferon-gamma and interleukin-12. Studies suggest that OPN can induce the transition mesenchymal epithelium (MET) of HCC cells, by means of an increase in the stability of vimentin protein, which promotes the maintenance of the cell structure, cytoplasmic integrity and stabilization of cytoskeletal interaction. Thus, understanding the mechanisms in which OPN participate, could result in new therapeutic possibilities in metastatic HCC (Dong et al., 2016; Wen et al., 2016).
In a study in Thailand, OPN serum levels were significantly higher in HCC patients compared to controls or patients with nonmalignant chronic liver disease. This result suggests that the dosage of OPN could be a possible diagnostic marker for HCC (Chimparlee et al., 2015). In order to assess the diagnostic and prognostic value of OPN serum levels, a meta-analysis including eight clinical trials (n = 1399) observed that the increase of OPN level was significantly associated with reduced overall survival and relapse-free survival. So, this protein could has a potential predictive significance to determine the HCC survival rate, despite of AFP (Alpha-fetoprotein) (Cheng et al., 2014).
GP73 is a membrane protein belonging to the Golgi complex expressed in the liver and biliary epithelial cells. GP73 expression is higher in patients with HCC, suggesting that this protein can be involved in an important mechanism of liver carcinogenesis (Ba et al., 2012).
In a multicenter study conducted in China and United States, the serum levels of GP73 and AFP were evaluated in 4217 patients and controls. The sensitivity and specificity of GP73 in HCC were 74.6% and 97.4%, respectively, compared to 58.2% and 85.3% for AFP, indicating that GP73 is a potential tumor marker with higher sensitivity and specificity. Corroborating other studies, GP73 levels were significantly higher in HCC patients compared to the healthy subjects, and serum levels decreased after surgical resection and increased with tumor recurrence. Thus, it can be a useful biomarker for the identification of HCC in high-risk populations (Mao et al., 2010).
In order to elucidate the mechanism of gene expression regulation related to the tumor formation, some studies have been investigated microRNAs (miRNAs). MiRNAs are small non-coding RNAs, compounds of ~ 21 nucleotides, regulators of gene expression and multiple cellular processes such as cell differentiation, maintenance of progenitor cells, and epithelial-mesenchymal transition. Changes in the regulation of miRNAs are a common feature of malignant cancers. The mechanisms involved in cancer development include gene amplification/deletion, chromosomal rearrangements, and epigenetic regulatory mechanisms, including DNA methylation, and histone modifications. MiRNAs can act as tumor suppressors (down-regulated) or oncogenes (up-regulated) depending on their target genes (Bartel, 2004).
Studies highlighted fundamental roles of miRNAs in the liver carcinogenesis, such as the modulation of the cell in the different phases as proliferation, maintenance, apoptosis, and metastasis. One of the main advantages of the use of miRNAs in therapies is the capacity of regulate multiple genes and signaling cascades, involved in the tumor growth. Thus, some miRNAs represent potential targets in the treatment of HCC (Gramantieri et al., 2008; Liu et al., 2014).
A retrospective study has found clinical parameters combined with miRNAs, it is possible to develop a score classifying low groups and high risk of recurrence and mortality. The study suggested that the use of a specific pattern of microRNAs expression in combination with tumor classification criteria could provide a more accurate estimate of the tumor recurrence. Thus, miRNAs can act as important biomarkers after the liver transplantation to assess postsurgical recurrence (Liese et al., 2016).
A recent study has evaluated in silico 829 miRNAs and their targets in HCC tissues and non-tumor tissues. It was observed that six of these miRNAs can regulate a significant quantity of the targets involved in the liver carcinogenesis. miR-26a, miR-122, and miR-130a were down-regulated in HCC and are involved in repair pathways, DNA replication, and transcription, while miR-21, miR-93, and miR-221, related to the metabolic pathways of carbohydrate, amino acids, lipids and the immune system, were up-regulated (Thurnherr et al., 2016).
A study in China has analyzed the expression of microRNAs in 96 tumor samples and non-tumor tissues of HCC patients, of which 88% were HBV infected. MiR-122 was down-expressed in HCC and would be responsible for the mitochondrial metabolic regulation. The absence of the miR-122 can be deleterious to the preservation of liver function, and can result in higher of mortality and morbidity rates in HCC patients (Burchard et al., 2010).
A recent study found that low expression of miR-122 in HCC cell lines results in cell proliferation, migration, and invasion, resulted of the activation of the epithelial-mesenchymal transition by Wnt-signaling pathway/β-catenin, which is involved in the control of cell proliferation and differentiation of the hepatocytes. The miR-122 is one of the most abundant and specific miRNA presented in the liver, and can be detected from embryogenesis to adulthood. Therefore it can be an important biomarker for liver diseases (Waisberg and Saba, 2015; Wang et al., 2016).
A retrospective study has evaluated the expression of miR-21 in 112 patients with HCC underwent surgical resection. It was concluded that the miR-21 expression was significantly up-regulated in tumor tissues compared to adjacent non-tumor tissues. Patients with high expression of miR-21 had lower survival rates compared to patients with low expression. Thus, it is suggested that high expression of miR-21 is associated with the tumor progression, and can be used as biomarker for HCC (Huang et al., 2015).
There are several signaling pathways that are implicated in the HCC pathogenesis, such as VEGF, EGF, PDGF, IGF, and mTOR. Activation of these pathways possibly the resistence apoptosis, and promote cell growth, angiogenesis, invasion, and metastasis (Cervello et al., 2012). VEGF is an important regulator of the liver angiogenesis because it is strongly involved in neovascularization and infiltration of cancer cells in the tumor capsule (Moon et al., 2003). Several studies have shown that VEGF overexpression is associated with proliferation, portal vein thrombosis, tumor aggressiveness, and poor prognosis in HCC patients (Moeini et al., 2012). The VEGF inhibition has been effective in many types of cancer. Angiogenesis blockage is a route for reduction in the tumor growth and an increase of the survival rates (Biselli-Chicote et al., 2012). Inhibition of angiogenesis can have a therapeutic potential for HCC. Actually, several anti-angiogenic agents are being evaluated in clinical trials in order to control the tumor growth (Moeini et al., 2012).
Some miRNAs are highly expressed in carotid artery of mice. It is proposed that these miRNAs belong to a specific class of miRNAs related to vascularization. The expression of miR-210 is induced by hypoxia and promotes the endothelial cell migration driven by VEGF, and formation of capillaries. MiR-126 induces angiogenesis in response to growth factors such as VEGF or fibroblast growth factor, repressing the negative regulators of signal transduction pathways. VEGF gene has been identified as a direct target of miR-101. This interaction results in the suppression of cell migration and invasion by the inhibition of VEGF. Therefore, miR-101 could be used for the development of therapies for HCC treatment (Fish et al., 2009; Fátima et al., 2010; Liu et al., 2016).
EGF leads to morphological and biochemical alterations in the target cells, resulting in proliferation and cell migration. It is known that miR-15b has the EGF gene as a target, and it is overexpressed in several tumor types, such as liver, colon, and cervical cancer, causing an increase in cell proliferation and differentiation. These alterations can be deleterious and promote the development and progression of cancer (Kedmi et al., 2015).
PDGF acts on transcriptional gene regulation by specific receptors, regulates the angiogenesis. A study has investigated the expression of miR-214 in the liver of transgenic mouse model expressing PDGF. Overexpression of PDGF was associated with hepatic fibrosis, steatosis, and HCC development in this study. MiR-214 appears to be involved in the development of liver fibrosis, by the modulation of EGFR (Epidermal growth factor receptor) and TGF-β (Transforming growth factor beta) signaling pathways. The antimiR-214 would be a possible therapeutic agent for the prevention of liver fibrosis and development of HCC (Okada et al., 2015).
The IGF is responsible for the regulation of several biological mechanisms, including cell development, homeostasis, and aging. The dysregulation of these pathways is related to metabolic disorders, cancer, and neurodegenerative diseases. IGF levels indicate the liver function and are inversely related to the severity of liver disease. A study in Egypt has examined the IGF serum levels in 89 patients with HCC. The patients were divided into three groups: 30 patients treated with Sorafenib, 30 patients who received best supportive care and 29 patients who performed arterial chemoembolization. It was found that the patients with controlled disease had significantly higher levels of IGF compared to the patients without disease control. Therefore, the dosage of IGF can predict the liver function and estimate the prognosis of HCC patients (Elmashad et al., 2015; Jung et al., 2015).
A study has shown that miR-145 could inhibit the expression of genes as IGF, blocking the cell cycle and apoptosis in HCC by modulation of the Wnt-/β-catenin (Law et al., 2012). Other miRNAs are associated with IGF expression, such as the miR-122. The reduced expression of miR-122 made the cells resistant to Sorafenib and induced apoptosis, it was found that IGF is a possible target of this microrna, being repressed to this miR-122. Therefore, miR-122 could act as potential predictive biomarker for therapeutic resistance in HCC (Xu et al., 2016). Another study concluded that down-expression of miR-122 in patients infected with HBV induces chronic inflammation in the liver, contributing to carcinogenesis. In this context, the increase of miR-122 expression could be used as a strategy for preventing the development of HCC in patients with Hepatitis B (Li et al., 2016).
The mTOR signaling pathway regulates important cellular aspects such as cell growth factors, oxidative stress, and cellular metabolism, which are closely related to cell growth, differentiation, proliferation, and migration (Buitrago-Molina et al., 2012). The activation of the mTOR pathway in HCC is associated with poor prognosis and increased tumor recurrence. Considering the role of miRNAs as modulators of several pathways related to the development and progression of the tumor, the increased levels of certain miRNAs could regulate mTOR pathways and control the progression of the disease (Alqurashi et al., 2013).
MiR-99 is considered a regulator of the mTOR pathway, and mTOR levels were inversely related to the expression of miR-99 in HCC tissue (Li et al., 2013). The high expression of miR-100 can also be involved in the inhibition of the mTOR pathway, resulting in autophagy and apoptosis of HCC cells (Ge et al., 2014). A study has shown that the low expression of miR-149 acts as tumor suppressor by modulating the mTOR pathway and reduces the tumorigenesis in HepG2 (Zhang et al., 2014).
A potential advantage of using miRNAs as biomarkers is that detected in various biological materials, such as tissues primary HCC, plasma, serum, urine, and saliva, providing a remarkable means for non-invasive early diagnosis of cancer. The analysis of the miRNAs expression has been done mainly by real time-PCR and micro array techniques. Recently, the introduction of new technologies, such as NGS, has facilitated the discovery of new miRNAs. However, the lack of standardization of the sample collection procedure can affect the results (Anwar et al., 2015).
MiRNAs have emerged as a new group of small RNAs that control gene expression at post-transcriptional level, at about 30% of human genes, modulating biological mechanisms such as inflammation, carcinogenesis, and fibrogenesis. The use of microRNAs, either alone or in combination with other biomarkers, can enable to categorize samples with respect to the liver function and evolution of HCC. However, many problems have been identified such as the lack of standardization in the protocols, which difficult the validation of the use of microRNAs in the diagnosis and therapy of cancer (Anwar et al., 2015; Hayes et al., 2016).
Clinical Issues, Healthcare Team and Palliative Care
The first symptoms suggestive of HCC are pain in the upper-right quadrant of the abdomen, the appearance of palpable masses, loss of appetite and weight loss, jaundice, ascites, edema of lower extremity, malaise, diarrhea, and fever. The growth of ascites gastrointestinal bleeding, splenomegaly, and encephalopathy can be directly related to impaired liver function (Pimenta et al., 2010; Gomes et al., 2013).
The improvement of the discovery of new therapies for cancer resulted in an increased life expectancy of the Brazilian population and increase of the demand for palliative care. However, the term palliative care still is associated many stigmas, as the dying process thus, requiring extensive reflection of the multidisciplinary team, orientation to the patients and their families (Abreu et al., 2013).
Regardless the choice treatment for HCC, it is necessary the knowledge of all the multidisciplinary healthcare team including doctors, nurses, pharmacists, dietitians, social workers and others. Therefore, each patient can be treated individualized according to the appropriate therapies based on clinical and scientific evidence from clinical trials. These professionals should evaluate the benefits and the disadvantage of the therapy for the quality of life, and give patient assistance throughout their biopsychosocial and health education. A planned course of palliative care provides the patient with counseling and management of the disorder, ensuring good intervention results. The deliveries of supportive care for patients with HCC are summarized in Table 2, including aspects such as analgesia, radiotherapy and nutrition (Sun et al., 2008; Gholz et al., 2010; Gish et al., 2012; Kumar and Panda, 2014).
Table 2.
Delivery of Supportive Care for HCC. Adapted from: Kumar M, Panda D, 2014. CBT, Cognitive Behavioral Therapy; NSAIDs, Nonsteroidal Anti-Inflamatorydrug; RT, Radiotherapy.
| Intervention | Options/ Types |
|---|---|
| Analgesia | NSAIDs |
| Selective COX-2 Inhibitors | |
| Opioids | |
| Acetaminophen | |
| Corticosteroids | |
| Morphine | |
| Fentanyl | |
| Radiotherapy | Palliative RT |
| Percutaneous cementoplasty | |
| Stereotaxic radiotherapy | |
| Nutrition | Dependent on nutritional status the patient (enteral or parenteral) |
| Anorexia–Cachexia | Megestrol acetate |
| Fatigue | Methylphenidat |
| Ascites | Diuretics |
| Nausea and vomiting | Opioid, metoclopramide, glucocorticoids, octreotide, ondansetron and scopolamine |
| Pruritus | Antihistamine, cholestyramine, rifampin and naltrexone |
| Constipation | Bulk-forming laxatives, osmotic laxatives, surfactants and stimulant laxatives |
| Psychosocial | Crisis intervention, |
| CBT, support groups, antidepressants, benzodiazepine and relaxing |
The HCC has a negative impact on patients, mainly on their quality of life, such as aspects related to their physical, mental, social, emotional and spiritual well-being. The healthcare team should be able to notice any changes in the patient, both physiological and psychological. Some individualized care should be taken into account for the improve of the quality of life of the patient (Fan et al., 2010).
Final Considerations
Actually, the HCC is one of the biggest challenges in cancer management in the clinical area, due to its different molecular pathways, causing agents, and late diagnosis. However, the risk factors for the development of carcinogenesis have been widely investigated and identified in the recent years by modern techniques as genomic sequencing, and can contribute for the discovery of new molecular biomarkers.
The improvement can also be associated to the discovery of new drugs, such as the Sorafenib, a multikinase inhibitor, from multi-center clinical studies, which have shown a possible cure to some advanced stage patients. However, the best way to significantly reduce the incidence and mortality rates related to the HCC remains in preventing HBV and HCV infection and reducing of alcohol consumption rates.
To achieve positive goals, effective health public policies coupled with the performance of health professionals in primary care should be implemented, conducting education and prevention of these diseases. So the HCC could leave the ranking of the most malignant tumors.
In conclusion, the emergence of the HCC is the result of a multifactorial process involving cirrhosis, HBV and HCV infection, alcohol consumption, presence of NAFLD or NASH, ingestion and exposure to fungal aflatoxins, tobacco, and genetic factors. Depending on the disease stage, there are several curative and/or palliative treatments, such as surgical resection, ablation, transplantation, chemoembolization, and Sorafenib, an oral multikinase inhibitor with anti-angiogenic and anti-proliferated effects, being widely used and bringing favorable results, with good cure rates and low relapse. However, all treatments can bring side effects that impair the quality of life of the patients.
Therefore, researchers have sought new molecular biomarkers with enhanced sensitivity and reliability, such as the GP73, GPC3, OPN, and microRNAs. The microRNAs can regulate different cellular pathways, which are closely linked to the tumor progression and angiogenesis, facilitating the diagnosis, prognosis, and therapy of HCC. A better understanding of these biomarkers facilitates the diagnosis of HCC to significantly benefit patients by the discovery of new drug target and subsequent increase in the cure rate. So, further studies are needed in order to fully understand the hepatic carcinogenesis.
References
- Abreu RM, Ferreira CS, Nasser PD, et al. Hepatocellular Carcinoma:The final moments of life. J Cancer Ther. 2013;4:377–83. [Google Scholar]
- Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European liver transplant registry (ELTR) J Hepatol. 2012;57:675–88. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Akamatsu N, Sugawara Y, Kokudo N. Living-donor vs deceased-donor liver transplantation for patients with hepatocellular carcinoma. World J Hepatol. 2014;6:626–31. doi: 10.4254/wjh.v6.i9.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqurashi N, Hashimi SM, Wei MQ. Chemical inhibitors and microRNAs (miRNA) targeting the mammalian target of rapamycin (mTOR) pathway:potential for novel anticancer therapeutics. Int J Mol Sci. 2013;14:3874–900. doi: 10.3390/ijms14023874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar SL, Lehmann U. MicroRNAs:Emerging novel clinical biomarkers for hepatocellular carcinomas. J Clin Med. 2015;4:1631–50. doi: 10.3390/jcm4081631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma:a review. Int J Clin Exp Pathol. 2012;5:874–81. [PMC free article] [PubMed] [Google Scholar]
- Baines AT, Xu D, Der CJ. Inhibition of Ras for cancer treatment:the search continues. Future Med Chem. 2011;3:1787–808. doi: 10.4155/fmc.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Biselli-Chicote PM, Oliveira AR, Pavarino EC, Goloni-Bertollo EM. VEGF gene alternative splicing:pro and anti-angiogenic isoforms in cancer. J Cancer Res Clin Oncol. 2012;138:363–70. doi: 10.1007/s00432-011-1073-2. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma:an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Molina LE, Vogel A. mTor as a potential target for the prevention and treatment of hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;12:1045–61. doi: 10.2174/156800912803988011. [DOI] [PubMed] [Google Scholar]
- Burchard J, Zhang C, Liu AM, et al. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol Syst Biol. 2010;6:1–12. doi: 10.1038/msb.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carethers JM, Braun J, Sands BE. Genetics, genetic testing, and biomarkers of digestive diseases. Gastroenterology. 2015;149:1131–3. doi: 10.1053/j.gastro.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1739–46. doi: 10.1111/j.1440-1746.2010.06404.x. [DOI] [PubMed] [Google Scholar]
- Cervello M, McCubrey JA, Cusimano A, et al. Targeted therapy for hepatocellular carcinoma:novel agents on the horizon. Oncotarget. 2012;3:236–60. doi: 10.18632/oncotarget.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LP, Li C, Wen TF, et al. Can living donor liver transplantation offer similar outcomes to deceased donor liver transplantation using expanded selection criteria for hepatocellular carcinoma? Pak J Med Sci. 2015;31:763–9. doi: 10.12669/pjms.314.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Wang W, Sun C, et al. Meta-analysis of the prognostic and diagnostic significance of serum/plasma osteopontin in hepatocellular carcinoma. J Clin Gastroenterol. 2014;48:806–14. doi: 10.1097/MCG.0000000000000018. [DOI] [PubMed] [Google Scholar]
- Chimparlee N, Chuaypen N, Khlaiphuengsin A, et al. Diagnostic and prognostic roles of serum osteopontin and osteopontin promoter polymorphisms in hepatitis B-related hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16:7211–7. doi: 10.7314/apjcp.2015.16.16.7211. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma:an international consensus conference report. Lancet Oncol. 2012;13:e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargel C, Stemberg B, Hasreiter J, et al. T Cells engineered to express a T-cell receptor specific for Glypican-3 to recognize and kill Hepatoma cells in vitro and in mice. Gastroenterol. 2015;149:1042–52. doi: 10.1053/j.gastro.2015.05.055. [DOI] [PubMed] [Google Scholar]
- De Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepato. 2012;56:75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- Dong Q, Zhu X, Dai C, et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7:12997–13012. doi: 10.18632/oncotarget.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagon P. Alcoholic liver injury:Influence of gender and hormones. World J Gastroenterol. 2010;16:1377–84. doi: 10.3748/wjg.v16.i11.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmashad N, Ibrahim WS, Mayah WW, et al. Predictive value of serum insulin-like growth factor-1 in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16:613–9. doi: 10.7314/apjcp.2015.16.2.613. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the liver. European organization for research and treatment of cancer. EASL-EORTC clinical practice guidelines:management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Fan SY, Eiser C, Ho MC. Health-related quality of life in patients with hepatocellular carcinoma:a systematic review. Clin Gastroenterol Hepatol. 2010;8:559–64. doi: 10.1016/j.cgh.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Fátima LA, Papa PC. Vascular endothelial growth factor (VEGF):transcriptional and postranscriptional regulation. Rev Biol. 2010;4:22–7. [Google Scholar]
- Feng M, Ho M. Glypican-3 antibodies:a new therapeutic target for liver cancer. FEBS Lett. 2014;588:377–82. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fish JE, Srivastava D. MicroRNAs:opening a new vein in angiogenesis research. Sci Signal. 2009;2:1–7. doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–35. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- Ge YY, Shi Q, Zheng ZY, et al. MicroRNA-100 promotes the autophagy of hepatocellular carcinoma cells by inhibiting the expression of mTOR and IGF-1R. Oncotarget. 2014;5:6218–28. doi: 10.18632/oncotarget.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholz R, Engstrom C. Managing Hepatocellular Carcinoma:The Nurse’s Role. Federal Practitioner Supplement. 2010;27:1–6. [Google Scholar]
- Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173–85. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- Gomes MA, Priolli DG, Tralhão JG, Botelho MF. Hepatocellular carcinoma:epidemiology, biology, diagnosis, and therapies. Rev Assoc Med Bras. 2013;59:514–24. doi: 10.1016/j.ramb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Gramantieri L, Fornari F, Callegari E, et al. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189–204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Peters M. Liver disease in women:The influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol. 2013;9:633–9. [PMC free article] [PubMed] [Google Scholar]
- Han K, Kim J, Ko G, Gwon D, Sung K. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis:A comprehensive review. World J Gastroenterol. 2016;22:407–16. doi: 10.3748/wjg.v22.i1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama Y, Yorita K, Yamaguchi T, et al. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer. 2015;137:1643–51. doi: 10.1002/ijc.29518. [DOI] [PubMed] [Google Scholar]
- Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2016;22:275–83. doi: 10.3748/wjg.v22.i1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CN, Chayama K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int J Mol Sci. 2016;17:280. doi: 10.3390/ijms17030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–92. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Yu W, Cui H, et al. Increased expression of miR-21 predicts poor prognosis in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:7234–8. [PMC free article] [PubMed] [Google Scholar]
- Jameson JL, Longo DL. Precision medicine-personalized, problematic, and promising. N Engl J Med. 2015;37:2229–34. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- Jelic S, Sotiropoulos GC. ESMO guidelines working group. Ann Oncol. 2010;21:59–64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center M, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Suh Y. Regulation of IGF -1 signaling by microRNAs. Front Genet. 2015;5:1–13. doi: 10.3389/fgene.2014.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015;4:176–87. doi: 10.1159/000367740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedmi M, Ben-Chetrit N, Körner C, et al. EGF induces microRNAs that target suppressors of cell migration:miR-15b targets MTSS1 in breast cancer. Sci Signal. 2015;8:1–12. doi: 10.1126/scisignal.2005866. [DOI] [PubMed] [Google Scholar]
- Kumar M, Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:130–9. doi: 10.1016/j.jceh.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PT, Ching AK, Chan AW, et al. MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis. 2012;33:1134–41. doi: 10.1093/carcin/bgs130. [DOI] [PubMed] [Google Scholar]
- Lencioni R. Chemoembolization in patients with hepatocellular carcinoma. Liver Cancer. 2012;1:41–50. doi: 10.1159/000339019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Deng M, Hu J, et al. Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR-122 levels. Oncotarget. 2016;7:17021–34. doi: 10.18632/oncotarget.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang J, Chen QD, et al. Insulin promotes glucose consumption via regulation of miR-99a/mTOR/PKM2 pathway. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SF, Yang HI, Lee MH, Chen CJ, Lee WC. Fifteen-year population attributable fractions and causal pies of risk factors for newly developed hepatocellular carcinomas in 11,801 men in Taiwan. PLoS One. 2012;7:1–7. doi: 10.1371/journal.pone.0034779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese J, Peveling-Oberhag J, Doering C, et al. A possible role of microRNAs as predictive markers for the recurrence of hepatocellular carcinoma after liver transplantation. Transpl Int. 2016;29:369–80. doi: 10.1111/tri.12733. [DOI] [PubMed] [Google Scholar]
- Liu M, Jiang L, Guan XY. The genetic and epigenetic alterations in human hepatocellular carcinoma:a recent update. Protein Cell. 2014;5:673–91. doi: 10.1007/s13238-014-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YR, Tang RX, Huang WT. Long noncoding RNAs in hepatocellular carcinoma:Novel insights into their mechanism. World J Hepatol. 2015;7:2781–91. doi: 10.4254/wjh.v7.i28.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang J, Mao Y, Zou B, Fan X. MicroRNA-101 suppresses migration and invasion via targeting vascular endothelial growth factor C in hepatocellular carcinoma cells. Oncol Lett. 2016;11:433–8. doi: 10.3892/ol.2015.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterol. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma:a review of literature. World J Gastroenterol. 2014;20:4141–50. doi: 10.3748/wjg.v20.i15.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–93. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- Marquardt J, Andersen J. Next-generation sequencing:application in liver cancer past, present and future? Biology. 2012;1:383–94. doi: 10.3390/biology1020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma:Where are we? World J Exp Med. 2016;6:21–36. doi: 10.5493/wjem.v6.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn K, London WT. The global epidemiology of hepatocellular carcinoma, present and future. Clin Liver Dis. 2011;15:1–22. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M, Marcais A. microRNAs calibrate T cell responses by regulating mTOR. Oncotarget. 2015;33:34059–60. doi: 10.18632/oncotarget.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–65. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- Mínguez B, Lachenmayer A. Diagnostic and prognostic molecular markers in hepatocellular carcinoma. Dis Markers. 2011;31:181–90. doi: 10.3233/DMA-2011-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma:consider the population. J Clin Gastroenterol. 2013;47:2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeini A, Cornella H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer. 2012;1:83–93. doi: 10.1159/000342405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2:a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16:552–7. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Numata K, Nozaki A, et al. Novel Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein:a biomarker of hepatocellular carcinoma recurrence in patients with low alpha-fetoprotein concentrations. Int J Clin Oncol. 2012;17:373–9. doi: 10.1007/s10147-011-0306-3. [DOI] [PubMed] [Google Scholar]
- Okada H, Honda M, Campbell JS, et al. Inhibition of microRNA-214 ameliorates hepatic fibrosis and tumor incidence in platelet-derived growth factor C transgenic mice. Cancer Sci. 2015;106:1143–52. doi: 10.1111/cas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman ES, Odena G, Bataller R. Alcoholic liver disease:pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28:77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta JR, Massabki OS. Hepatocellular carcinoma:a clinical outlook. Rev Bras Clin Med. 2010;8:59–67. [Google Scholar]
- Pocha C, Kolly P, Dufour JF. Nonalcoholic fatty liver disease-related hepatocellular carcinoma:A problem of growing magnitude. Semin Liver Dis. 2015;35:304–17. doi: 10.1055/s-0035-1562949. [DOI] [PubMed] [Google Scholar]
- Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma:outcomes and treatment options for recurrence. Ann Gastroenterol. 2015;28:323–30. [PMC free article] [PubMed] [Google Scholar]
- Ryan MJ, Willatt J, Majdalany BS, et al. Ablation techniques for primary and metastatic liver tumors. World J Hepatol. 2016;8:191–9. doi: 10.4254/wjh.v8.i3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachterman A, Craft WW, Jr, Hilgenfeldt E, et al. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol. 2015;21:8478–91. doi: 10.3748/wjg.v21.i28.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütte K, Schulz C, Link A, Malfertheiner P. Current biomarkers for hepatocellular carcinoma:Surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7:139–49. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178–85. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Hsu C, Huang C, et al. Use of plasma angiogenesis-related factors to investigate the association of interleukin 8 and interleukin 6 levels with efficacy of sorafenib-based antiangiogenic therapy in patients with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29:199–203. [Google Scholar]
- Shih W, Chang H, Liaw Y, et al. Influences of tobacco and alcohol use on hepatocellular carcinoma survival. Int J Cancer. 2012;131:2612–21. doi: 10.1002/ijc.27508. [DOI] [PubMed] [Google Scholar]
- Starley B, Calcagno C, Harrison S. Nonalcoholic fatty liver disease and hepatocellular carcinoma:A weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- Su W, Lee K, Yeh Y, et al. Association of circulating insulin-like growth factor 1 with hepatocellular carcinoma:one cross-sectional correlation study. J Clin Lab Anal. 2010;24:195–200. doi: 10.1002/jcla.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun VC, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs. 2008;2:759–66. doi: 10.1188/08.CJON.759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnherr T, Mah WC, Lei Z, et al. Differentially expressed miRNAs in hepatocellular carcinoma target genes in the genetic information processing and metabolism pathways. Sci Rep. 2016;6:20065. doi: 10.1038/srep20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulos D, Bamia C, Lagiou P, et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort:a nested case-control study. J Natl Cancer Inst. 2011;103:1686–95. doi: 10.1093/jnci/djr395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma:novel molecular approaches for diagnosis, prognosis and therapy. Annu Rev Med. 2010;61:317–28. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisberg J, Saba G. Wnt-/-β-catenin pathway signaling in human hepatocellular carcinoma. World J Hepatol. 2015;7:2631–35. doi: 10.4254/wjh.v7.i26.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma:A comprehensive review. World J Hepatol. 2015;7:2648–63. doi: 10.4254/wjh.v7.i26.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Wang Q, Shen D, et al. Downregulation of microRNA-122 promotes proliferation, migration, and invasion of human hepatocellular carcinoma cells by activating epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2035–47. doi: 10.2147/OTT.S92378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Jeong S, Xia Q, Kong X. Role of osteopontin in liver diseases. Int J Biol Sci. 2016;12:1121–8. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Huang J, Ma L, et al. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371:171–81. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Yang JD, Roberts LR. Hepatocellular carcinoma:A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo X, Xiong L, et al. Comprehensive analysis of microRNA-regulated protein interaction network reveals the tumor suppressive role of microRNA-149 in human hepatocellular carcinoma via targeting AKT-mTOR pathway. Mol Cancer. 2014;13:253. doi: 10.1186/1476-4598-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–39. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]


