Abstract
Background:
To enhance their own survival, tumor cells can manipulate their microenvironment through remodeling of the extra cellular matrix (ECM). The urokinase-type plasminogen activator (uPA) system catalyzes plasmin production which further mediates activation of matrix metalloproteinases (MMPs) and plays an important role in breast cancer invasion and metastasis through ECM remodeling. This provides a potential target for therapeutic intervention of breast cancer treatment. Enterolactone (EL) is derived from dietary flax lignans in the human body and is known to have anti-breast cancer activity. We here investigated molecular and cellular mechanisms of EL action on the uPA-plasmin-MMPs system.
Methods:
MTT and trypan blue dye exclusion assays, anchorage-dependent clonogenic assays and wound healing assays were carried out to study effects on cell proliferation and viability, clonogenicity and migration capacity, respectively. Real-time PCR was employed to study gene expression and gelatin zymography was used to assess MMP-2 and MMP-9 activities. All data were statistically analysed and presented as mean ± SEM values.
Results:
All the findings collectively demonstrated anticancer and antimetastatic potential of EL with antiproliferative, antimigratory and anticlonogenic cellular mechanisms. EL was found to exhibit multiple control of plasmin activation by down-regulating uPA expression and also up-regulating its natural inhibitor, PAI-1, at the mRNA level. Further, EL was found to down-regulate expression of MMP-2 and MMP-9 genes, and up-regulate TIMP-1 and TIMP-2; natural inhibitors of MMP-2 and MMP-9, respectively. This may be as a consequence of inhibition of plasmin activation, resulting in robust control over migration and invasion of breast cancer cells during metastasis.
Conclusions:
EL suppresses proliferation, migration and metastasis of MDA-MB-231 breast cancer cells by inhibiting induced ECM remodeling by the ‘uPA-plasmin-MMPs system’.
Keywords: Enterolactone, breast cancer, Urokinase-type plasminogen activator, matrix metalloproteinases
Introduction
The extracellular matrix (ECM) is a foremost component of the cellular microenvironment enclosing highly dynamic structure which is constantly undergoing a remodeling process. In addition to ECM’s structural role owing to its highly dynamic nature, its remodeling acts as an effective mechanism for regulating the most basic and diverse cellular behaviors such as cell proliferation, adhesion, migration, cell differentiation and cell death (Hynes, 2009) by pleiotropic ECM functions (Chaudhary et al., 2016). Dynamic remodeling of the ECM is also crucial for development, wound healing and normal organ homeostasis. An excessive or uncontrolled ECM remodeling contributes in the development of life-threatening pathological conditions such as fibrotic diseases and cancer (Cox and Erler, 2011). Tumor development is a complex, dynamic and progressive process in which the tumor microenvironment is mechanically and biologically active and continuously undergoes dynamic remodeling (Yu et al., 2011). To enhance their own survival, tumor cells can manipulate their microenvironment and conversely interact with an altered microenvironment to drive the malignancy (Bissell and Radisky, 2001). Thus, changes in the tumor microenvironment through remodeling of the ECM are important for metastatic dissemination.
The ECM is composed of a large collection of biochemically and structurally diverse components such as proteins (fibrillar collagen, elastin, fibronectin, laminin, and nidogen), proteoglycans, and glycoproteins which construct structurally both basement membrane (BM) and interstitial matrix (Daley et al., 2008; Vakonakis and Campbell, 2007). It has been known that compared to normal ECM, tumor-derived ECM is biochemically distinct and tumor stroma is typically stiffer than normal stroma (Butcher et al., 2009; Kass et al., 2007). Reportedly, in pre-malignant tissue increased matrix stiffness and ECM remodeling were observed and shown to contribute to malignant transformation in the breast (Levental et al., 2009). The effective strategies to remodel the ECM are degradation or removal of one or more of its protein components and modulation of the levels of ECM modifying enzymes and thereby altering the organization of ECM components assembled within the protein network (Lu et al., 2011). The extracellular proteinases such as matrix metalloproteinases (MMPs) (Kessenbrock et al., 2010), metzincin proteinases; a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) (Murphy, 2008) and serine proteases which include plasmin and cathepsin G (Green and Lund, 2005) play major role in ECM remodeling or degradation. The MMPs have been classified into four subgroups; collagenases (MMP-1, 8 and 13), stromelysins (MMP-3, 7, 10 and 11) gelatinases (MMP-2 and 9) and membrane type MMPs (MT1 to MT6 MMPs) (Kousidou et al., 2004). Collectively, MMPs are capable of degrading effectively all ECM components and BMs, thereby allowing cancer cells to invade and metastasize (Kessenbrock et al., 2010; Nabeshima et al., 2002). One of the serine proteases, plasmin also facilitates tumor cell motility by activating MMPs (MMP-1, MMP-3 and MMP-9), growth factors such as transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and by disrupting the BM and stromal barriers (Didiasova et al., 2014; Syrovets et al., 2012). Excessive plasmin production in tumors is frequent and is a direct consequence of increased expression and activity of plasminogen activators such as urokinase-type plasminogen activator (uPA) (Ranson, 2003). Briefly, uPA system catalyzes plasmin production which further mediates activation of MMPs and plays an important role in breast cancer invasion and metastasis (Didiasova et al., 2014; Shimizu et al., 2011). Thus, targeting these ECM-remodeling-enzymes (uPA-Plasmin-MMPs enzyme system) is now becoming an attractive and novel therapeutic strategy to prevent cancer progression and cancer metastasis (Cox and Erler, 2011).
Flaxseeds contain phenolic compounds called lignans of which secoisolariciresinol diglucoside (SDG) is a major lignan with proven health benefits such as cardio protective, antioxidant and anticancer effects (Alphonse and Aluko, 2015). In mammals, these plant lignans are metabolized by intestinal bacteria into mammalian lignans namely Enterodiol (ED) and Enterolactone (EL), also referred to as enterolignans (Fuentealba et al., 2014). Some previous studies have shown that flaxseed can inhibit the spontaneous metastasis of the estrogen receptor negative (ER−) human breast cancer MDA-MB-435 in nude mice (Chen et al., 2002; Dabrosin et al., 2002) and the experimental lung metastasis of murine melanoma B16BL6 cells in mice (Yan et al., 1998). Lignans and tamoxifen (TAM), alone or in combination can inhibit the steps involved in the metastasis cascade (Jianmin Chen and Thompson, 2003). Although taken together these findings suggest that certain flaxseed components, particularly lignans have the potential to inhibit metastasis by blocking or interfering the metastatic cascade, the role of EL in these pathways that are involved in breast cancer metastasis have not been explored. In the present study, we investigated the potential of EL to inhibit the ECM remodeling through inhibition of uPA-plasmin-MMPs enzyme system in metastatic breast cancer cells; MDA-MB-231. This is in continuation with our preliminary study where in, we investigated the possibility of EL acting as antimetastatic agent. The effects of EL on MMP-2 and MMP-9 gene expressions by reverse transcriptase PCR and its effects on migratory abilities in MDA-MB-231 cells observed were suggestive and qualitative (Mali et al., 2012). This needed further validation by quantitative and statistical verification. For this, we have now carried out the investigations of EL on MMP-2 and MMP-9 genes by quantitative real time PCR (qPCR) and EL’s effects on migratory abilities of MDA-MB-231 cells by a computational tool; TScratch software. Now we not only confirm our previous observations but also demonstrate that the mechanism of antimetastatic action of EL is through inhibition of uPA-plasmin-MMPs enzyme system.
Materials and Methods
Reagents and materials
EL, Tamoxifen (TAM) and Paclitaxel (PAC), Dulbecco’s phosphate-buffered saline (DPBS), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), Dimethyl Sulfoxide (DMSO), Trypan Blue and Antibiotic Antimycotic Solution containing 10,000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B per ml were purchased from Sigma-Aldrich (St. Louis, MO, USA). Leibovitz’s L-15 Medium, Trypsin-EDTA (0.25%), Fetal Bovine Serum (FBS) were procured from Gibco (BRL, CA, USA). Real-time PCR kits and reagents; KiCqStart™ SYBR® Green qPCR ReadyMix™ with ROX™, ReadyScript™ cDNA Synthesis Mix, TRIzol reagent, Chloroform, Isopropanol and DEPC treated water were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other common reagents were procured from HiMedia Laboratories Pvt. Ltd. (Mumbai, India).
Cell culture and treatment
Breast cancer cell line MDA-MB-231 was obtained from National Centre for Cell Science (NCCS), Pune, India. The cells were cultured as monolayers in Leibovitz’s L-15 medium supplemented with L-glutamine and sodium pyruvate, antibiotic antimycotic solution and 10% FBS in a humidified atmosphere at 37°C without CO2; in exchange with air. Stock solution of 60 mM EL was prepared by dissolving it in an absolute ethanol and stored at 4°C. Working solutions of EL were then made by serial dilutions of the stock solution with cell culture medium and ethanol was employed as a negative control at the final concentration not exceeding 0.2% (v/v) in cell culture experiments. Similarly, stock solutions of TAM and PAC (20 mM) were prepared in DMSO and same was used as a negative control at the final concentration less than 0.5 % (v/v) in cell culture experiments.
MTT assay
The effects of cytotoxicity of EL and TAM on cell proliferation and cell survival were studied by MTT assay as previously described (Mellatyar et al., 2014) with some modification. For the assay, 10 x 103 cells/well were seeded in 96 well plates and cultured overnight in L-15 medium containing 10% FBS. After 24 hrs, the cells were incubated with the fresh medium containing 8 different concentrations of EL (0-200 μM) and TAM (0-50 μM). The plates were further incubated for 24, 48 and 72 hours. After treatment period, the MTT solution (1 mg/ml) was added as 100μl per well and incubated further for 4 hrs at 37°C in dark. After confirming the formation of the purple coloured formazan crystals, MTT solution was removed and 100 μl of DMSO was added in each well to dissolve formazan and plates were incubated for 1 to 2 hours at 37°C. The intensity of colored formazan derivative was determined by measuring optical density (OD) with the iMark™ microplate reader (BIO-RAD, California, USA) at 570 nm test wavelength and 630 nm reference wavelength (OD 570–630 nm). A value of 100% viability/0% toxicity was assigned to untreated control cultures, and the concentration of drug that reduced the number of viable cells to 50% after 24, 48 and 72 hours of exposure (IC50) was derived by an interpolate logarithmic concentration curve. Results were derived from at least three independent sets of triplicate experiments.
Cell viability assay
Effects of EL and TAM on cell viability were determined by trypan blue dye exclusion assay (Strober, 2001). The MDA-MB-231 cells were seeded in triplicates at a density 5×104 cells/well in 24-well plates under standard culture conditions. After 24 h, the cells were treated with 25, 50, 75 μM doses of EL and ethanol alone as a negative control. After 24, 48 or 72 h of treatment, both floating and adhered cells were collected and resuspended in the medium. The cell suspensions were mixed with trypan blue dye and both viable as well as nonviable cells were counted by hemocytometer. The % cell viability of each EL dose was determined as a cell growth % of control.
Anchorage-dependent clonogenic assay
In vitro colony forming potential of MDA-MB-231 cells was assessed by anchorage-dependent clonogenic assay with some modification, as described previously (Elangovan et al., 2008). The MDA-MB-231 cells were seeded in 6-well tissue culture plates at a density of 800 cells per well in 2 ml of complete medium. After 24 hours of incubation, the cells were treated with 25, 50, 75 μM doses of EL, 12.5 and 25 nM doses of PAC and ethanol as negative control followed by an additional 8-days incubation to allow colonies to form. The colonies were fixed by 4% paraformaldehyde solution and imaged by inverted microscope (Magnus-INVI, New Delhi, India). Further colonies were stained with 0.1 % crystal violet followed by washing with PBS to remove excessive dye. Quantitative changes in clonogenicity were determined by extracting the colonies with 10% acetic acid and measuring the absorbance of the extracted dye at 595 nm.
Cell migration assay
The effects of EL on migration potential of breast cancer MDA-MB-231cells were analyzed by cell migration assay (Justus et al., 2014). MDA-MB-231cells were seeded at a seeding density of 0.3 x 106 cells per well in 6-well plates and incubated overnight at 37°C in 5% CO2 incubator. An artificial wound was made with a sterile tip. Detached cells were removed by discarding existing media and replacing with fresh media containing different concentrations of EL; 25, 50, 75 μM and ethanol alone as negative control. The speed of wound closure and cell migration was quantified by taking snapshot pictures with an inverted microscope (Magnus-INVI, New Delhi, India) at 24, 48 and 72 hours time intervals. To determine statistical significance of the data, we carried out automated analysis of monolayer wound healing assay by analyzing the images in the computational tool; TScratch software, as described elsewhere (Gebäck et al., 2009).
Real-time qPCR
Total RNA was isolated by TRIzol reagent (Invitrogen, California, USA) after treatment with 25, 50, 75 μM of EL and 0.5 μM of TAM; from three independent experiments. The purity and concentration of the isolated RNA were measured using BioPhometer® plus (Eppendorf, Hamburg, Germany). The first strand cDNA synthesis was performed by ReadyScript™ cDNA Synthesis Mix (Sigma-Aldrich, St. Louis, USA) according to the manufacturer’s protocol. The human oligonucleotide primers were synthesized from Sigma-Aldrich, St. Louis, USA for multiple genes under investigation as illustrated in Table 1. Afterwards, real-time PCR was carried out using the KiCqStart™ SYBR® Green qPCR ReadyMix™ with ROX™ (Sigma-Aldrich, St. Louis, USA) as per the manufacturer’s protocol on StepOne™ real time PCR system (Applied Biosystems, California, USA). Expression of each gene was assayed using three replicates for each primer along with GAPDH primer as an internal standard and negative control lacking the cDNA template. The analysis of real-time PCR quantitative gene expression data was carried out by relative quantification method and changes in gene expressions were presented as comparative CT method (2-∆∆CT method), described earlier (Schmittgen and Livak, 2008).
Table 1.
List of Oligonucleotide Primers Synthesized for Real Time PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| uPA | GAAAACCTCATCCTACACAAG | ATTCTCTTTTCCAAAGCCAG |
| PAI-1 | ATCCACAGCTGTCATAGTC | CACTTGGCCCATGAAAAG |
| MMP-2 | GTGATCTTGACCAGAATACC | GCCAATGATCCTGTATGTG |
| MMP-9 | AAGGATGGGAAGTACTGG | GCCCAGAGAAGAAGAAAAG |
| TIMP-1 | CACCTTATACCAGCGTTATG | TTTCCAGCAATGAGAAACTC |
| TIMP-2 | GGCCTGAGAAGGATATAGAG | CTTTCCTGCAATGAGATATTCC |
Gelatin Zymography
The activities of the gelatinases, MMP-2 and MMP-9 in the conditioned medium of the MDA-MB-231 cells were determined by gelatin Zymography as described earlier (Frankowski et al., 2012). MDA-MB-231 cells were seeded at a density of 0.4 x 106 cells/well in 6 well plates containing complete medium and allowed to adhere overnight at 37°C in 5% CO2 incubator. Next day, cells were washed three times with 1X PBS and serum starved for an additional 24 hours by culturing them into the serum free medium. Following serum starvation the cells were treated with 25, 50 and 75 µM concentrations of EL and 0.5 µM of TAM as a positive control. After 24 hours of treatment, the conditioned media for the each designated treatment were collected and centrifuged at 400g for 5min at 4°C to remove cellular debris. Further the conditioned media from untreated (control) cells and treated cells were collected and concentrated in Amicon® Ultra-4 Centrifugal Filter Units (Millipore, MA, US). The samples containing 20µg of total proteins were mixed with 5X sample buffer (0.313 M Tris-HCl; pH 6.8, 10% SDS, 50% glycerol and 0.05% bromophenol blue) and subjected to electrophoresis under non-reducing condition on to the 8% SDS-polyacrylamide gel containing gelatin (10mg/ml). Following electrophoresis, gel was removed and washed 3 times with 60 ml gel washing buffer (2.5% Triton X-100) and incubated in development buffer (50mM Tris-HCl; pH 7.4, 10 mM CaCl2, 0.02% NaN3) for 42h. The gel was further stained with staining solution (0.125% coomassie brilliant blue R-250, 1% acetic acid, 45% ethanol) for 1h and destained in destaining solution-I (25% ethanol, 10% acetic acid) for 15-30 min and destaining solution-II (5% ethanol, 7.5% acetic acid). Gelatinolytic activity was detected as unstained bands against blue background by Gel Doc™ XR+ Gel Documentation System (Bio-Rad, California, USA). The quantification of bands was carried out by densitometric analysis with NIH ImageJ 1.50i software.
Data analyses
All the experiments were performed in triplicates and repeated thrice. The data were presented as the mean ± SEM. Statistical analysis was performed with the GraphPad Prism 5 program and differences between the groups were examined for statistical significance using one-way analysis of variance (1 way ANOVA) followed by Tukey’s multiple comparison test and independent samples t-test. The p < 0.05 was considered as statistically significant.
Results
EL exhibited anticancer and antiproliferative effects on metastatic MDA-MB-231 breast cancer cells
The effects of EL and TAM on cell viability, cell survival and cell proliferation were assessed in estrogen receptor negative (ER-negative) breast cancer cells MDA-MB-231 by MTT assay at 24, 48 and 72 hours. The results were derived by interpolate logarithmic concentration curves in terms of mean % cytotoxicity (Figure 1A and B) and represented as IC50 values of Both EL and TAM (Table 2). These results show that both EL and TAM induced dose dependent reductions in cell viability and cell survival indicating the anticancer and cytotoxic/antiproliferative potential of EL on metastatic MDA-MB-231 breast cancer cells.
Figure 1.
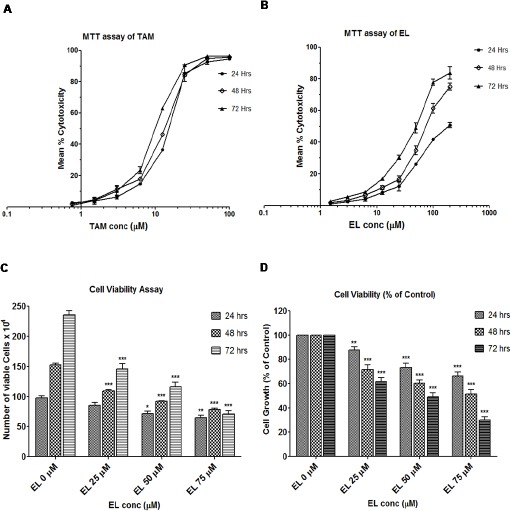
Effects of EL and TAM on Viability and Proliferation of Metastatic MDA-MB-231 Breast Cancer Cells. (A and B) Effects of TAM and EL on viability; determined in terms of mean % cytotoxicity by MTT assay. (C) Effect of EL on proliferation and growth kinetics in a dose and time dependent manner. (D) Effect of EL on relative proliferation % of control in a dose and time dependent manner. Data are means ± SEM, (n = 3). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs DMSO or ethanol control.
Table 2.
IC50 Values of EL and TAM at 24, 48 and 72 Hours
| Exposure Time in hours | IC50 values ± SEM | IC50 values ± SEM |
|---|---|---|
| TAM | EL | |
| 24 hours | 16.07 ± 0.15 μM | 182.67 ± 2.16 μM |
| 48 hours | 14.33 ± 0.08 μM | 73.00 ± 2.83 μM |
| 72 hours | 9.83 ± 0.15 μM | 54.33 ± 1.47 μM |
To reconfirm the antiproliferative potential of EL, further we carried out cell viability assay by trypan blue dye exclusion method. This assay enabled us to estimate the effect of EL on both the rate of proliferation and the % of viable cells. The Figure 1C depicts that there was a dose as well as time-dependent reduction in the growth kinetics i.e. in rate of cell proliferation (counted as total number of viable cells) in EL treated cells compared to untreated control cells. Moreover at 24 hours of treatment, 25μM EL showed non-significant decrease, 50μM EL showed significant decrease (p ≤ 0.05) and 75μM EL showed very significant decrease (p ≤ 0.01) in growth compared to that observed in the untreated control cells. While at 48 and 72 hours of treatments all 25, 50 and 75 μM EL doses showed extremely significant decrease in growth (p ≤ 0.001 for 24, 48 and 72 h) compared to untreated cells. Moreover, Figure 1D revealed that there was also significant decrease in the relative proliferation (cell growth percentage of control) in a dose dependent manner after EL treatment. About ~ 2 fold and ~ 3.3 fold extremely significant decrease was observed at 50 μM (p ≤ 0.001) and 75 μM (p ≤ 0.001) concentrations of EL treatments for 72 hours, respectively. These findings collectively suggest that ER-negative metastatic MDA-MB-231 breast cancer cells are susceptible to the cytotoxic and antiproliferative effects of both TAM and EL in a time as well as dose dependent manner, irrespective of their ER binding affinity.
EL inhibited in-vitro colony formation potential of metastatic MDA-MB-231 breast cancer cells
Changes found in the growth kinetics were further confirmed by carrying out anchorage-dependent clonogenic assay or colony formation assay. Through this assay, the effects of different concentrations of EL on the ability of individual cells in the population to undergo unlimited cell divisions were assessed against ethanol as a negative control. Since prevention of cell division is a key mechanism of the drug PAC, we used it as a positive control in this experiment. Quantitative changes in clonogenicity were determined by extracting the colonies with 10% acetic acid and measuring the absorbance of the extracted dye at 595 nm. As shown in Figure 2A, the EL induced a dose dependent significant decrease in colony formation visualized as reduced number of colonies by crystal violet staining. The morphological changes after EL treatment such as size of the colonies, number of cells per colony were observed under invert phase microscope. It was observed that at 50 and 75 μM concentrations of EL, colonies were smaller in size with lesser number of cells per colony compared to untreated control cells, as shown in Figure 2B. Quantitative assessment showed that there was significant decrease (~1.6 fold) in colony formation at 25 μM (p ≤ 0.05), and extremely significant decrease (~ 2.5 and ~ 3.6 fold) in colony formation at 50 and 75 μM (p ≤ 0.001) concentrations of EL respectively (Figure 2C). On the other hand, PAC was found to exhibit comparatively very strong (~ 6.6 fold) and extremely significant inhibitory action (p ≤ 0.001) on colony forming ability of cells at 12.5 and 25 nanomolar (nM) concentrations.
Figure 2.
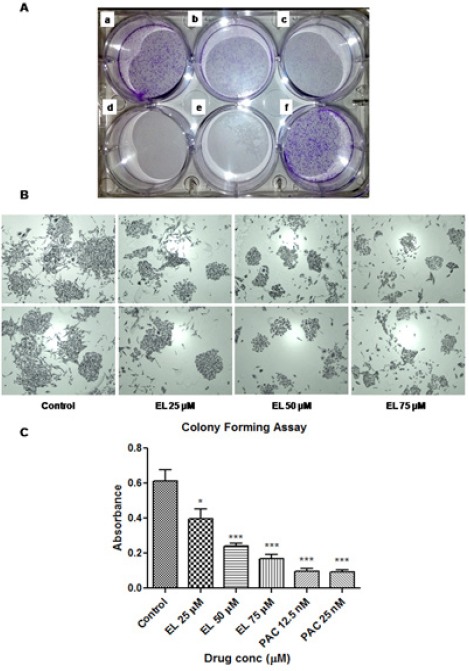
Effects of EL and PAC on Colony Formation of Metastatic MDA-MB-231 Breast Cancer cells. (A) Reduced number of colonies in treated cells at (a) EL 25 μM, (b) EL 50 μM, (c) EL75 μM, (d) PAC12.5 nM, (e) PAC 25 nM, compared to control (f), documented as a photograph after crystal violet staining. (B) Morphological changes such as decreased size of the colonies and reduced number of cells per colonies were observed under invert phase microscope. (C) Reductions in number of colonies were quantified by extracting the crystal violet stain with 10 % acetic acid and measuring the absorbance at 595 nm. Values are represented as mean ± SEM of three independent experiments (n =3) with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, indicating statistical significance.
EL inhibited migration of metastatic MDA-MB-231 breast cancer cells, in-vitro
To examine the effect of EL on cell migration of metastatic breast cancer cells, we performed wound healing assay on confluent monolayers of MDA-MB-231 cells. After making the wound with a pipette tip, the cells were cultured in presence or absence of different concentrations of the EL and imaged by inverted microscope (Magnus, INVI) for a period of 72 hours at an interval of 24 hours. It was observed that EL effectively inhibited the migration of cells in a dose dependent manner at all time points studied compared to the untreated control cells (Figure 3A and 3B). The control cells filled-up the wound gap completely after 48 hours whereas in cells treated with EL, particularly at 50 and 75 μM concentrations, the wound gap was not completely filled; even after 48 hours (Figure 3A). Further images were processed in the computational tool; TScratch software to quantify the open wound area as a ‘mean % open wound area’ and plotted as shown in Figure 3B. In untreated control cells there was about ~ 70%, 94% and 100% reduction in open wound area at 24, 48 and 72 hours of treatments respectively which indicates rapid migration of cells throughout the wound area. However, there was very significant decrease in cell migration at 25 μM of EL (p ≤ 0.01 for 24 and 48 hours) and extremely significant decrease in cell migration at 50 and 75 μM concentration of EL (p ≤ 0.001 for 24, 48 and 72 hours) compared to the untreated control cells, quantified as a dose and time dependent increase in mean % open wound area. Thus collectively. EL was conferred its potential to inhibit migration of metastatic MDA-MB-231 breast cancer cells in-vitro, in a dose and time dependent manner.
Figure 3.
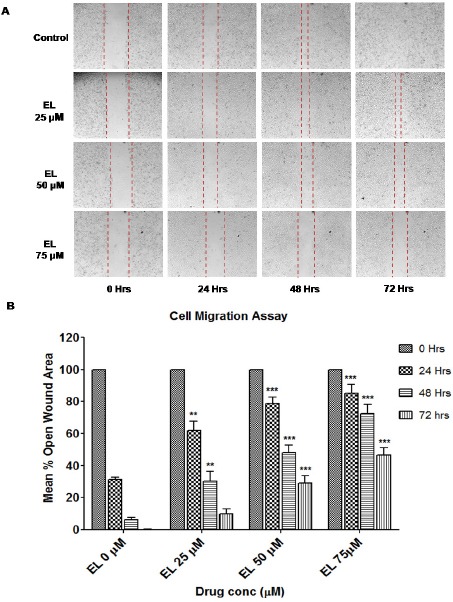
Effect of EL on the Migration of Metastatic MDA-MB-231 Breast Cancer Cells. (A) Decreased cell migration of MDA-MB-231 cells by EL in a dose and time dependent manner. The images were obtained by inverted microscope under 10X objective at 24, 48 and 72 hours of time intervals. (B) Quantification of a dose and time dependent decrease in cell migration induced by EL with the help of a computational tool; TScratch software. Analyzed data were represented graphically as mean % open wound area versus dose of EL at 24, 48 and 72 hours of time intervals
EL down-regulated uPA gene expression while up-regulated PAI-1 gene expression to inhibit plasmin activation
Further, to investigate the possible mechanisms of EL on ECM remodeling, owing to its ability to inhibit cell migration of metastatic breast cancer cells. We carried out investigations on the crucial uPA-Plasmin-MMPs enzyme system responsible for ECM remodeling during cancer metastasis. It was found that EL effectively reduced the mRNA levels of uPA in a time and dose dependent manner. For 24 hours of treatment, 25 μM of EL showed non-significant decrease while 50 and 75 μM of EL showed extremely significant decrease (p ≤ 0.001) in uPA gene expression. While for 48 hours of treatment, very significant decrease (p ≤ 0.01) with 25 μM of EL and extremely significant decrease (p ≤ 0.001) was observed with 50 and 75 μM of EL treatments (Figure 4A) in uPA gene expression. However, the expression of plasminogen activator inhibitor-1 (PAI-1) was increased by 2.1, 2.2 and 1.5 folds in response to treatment with 25 μM, 50 μM (p ≤ 0.001) and 75 (p ≤ 0.01) μM of EL respectively after 24 hours of incubation compared to the untreated cells. Similarly PAI-1 expression was increased by 2.3, 1.8 and 1.1 folds with 25 μM, 50 μM (p ≤ 0.001) and 75 μM of EL respectively after 48 hours of incubation (Figure 4B). These observations revealed that the changes in PAI-1 gene expression were not dose and time dependent. Maximum activity was observed with low concentrations; 25 and 50 μM of EL after 24 hours of incubation compared to the untreated cells, while 0.5 μM TAM was not found to be effective on both uPA and PAI-1 gene expressions in MDA-MB-231 cell lines.
Figure 4.
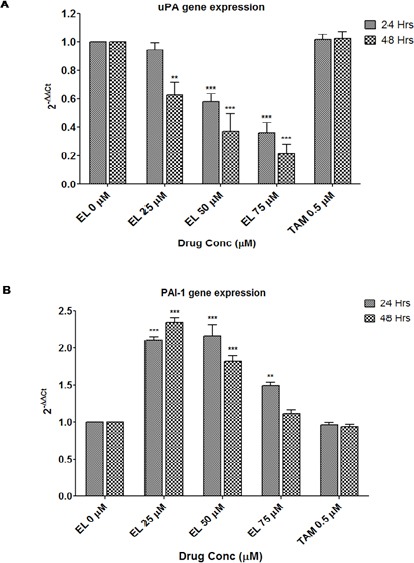
Effects of EL on Upa and PAI-1 Gene Expressions in MDA-MB-231 Cells. (A) Decreased gene expression of uPA in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 and 48 hours of incubation in a dose and time dependent manner. (B) Increased gene expression of PAI-1 in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 and 48 hours of incubation, compared to the untreated cells. Values are represented as mean ± SEM of three independent experiments (n =3) with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, indicating statistical significance.
EL down-regulated MMP-2 and MMP-9 gene expressions to inhibit ECM remodeling
Since uPA system catalyses the plasmin activation which further degrades BM and remodels ECM through MMPs activation, we investigated the concomitant effects of decreased uPA and increased PAI-1 gene expressions on MMPs gene expressions after the treatment with EL in MDA-MB-231 cells. It was found that EL inhibited the MMP-2 and MMP-9 gene expressions in a dose and time dependent manner in MDA-MB-231 cells. There was very significant decrease (~ 20% at 24 h and ~ 24% at 48h) with 25 µM of EL treatment (p ≤ 0.01) and extremely significant decrease in MMP-2 gene expression with 50 µM (~ 29% at 24h and ~ 32% at 48h) and 75 µM (~ 42% at 24h and ~ 49% at 48h) concentrations of EL treatments (p ≤ 0.001) compared to the untreated cells (Figure 5A). Similarly in the case of MMP-9 gene expression, there was significant decrease (~ 15% at 24h and ~ 19% at 48h) with 25 µM of EL treatment (p ≤ 0.01), very significant decrease (~ 24% at 24h) and extremely significant decrease (~ 31% at 48h) with 50 µM of EL treatment (p ≤ 0.05 for 24h and p ≤ 0.001 for 48h) and extremely significant decrease (~ 35% at 24h and 42% at 48h) with 75 µM of EL treatment (p ≤ 0.001) was observed compared to the untreated cells (Figure 5B). The positive control; 0.5 µM of TAM showed extremely significant increase in MMP-2 gene expression by 1.2 and 1.3 folds after 24h and 48h of treatments respectively (p ≤ 0.001). While in the case of MMP-9 gene expression, significant increase by 1.1 folds (p ≤ 0.05) and very significant increase by 1.2 folds (p ≤ 0.01) was observed after 24h and 48h of 0.5 µM of TAM treatments respectively, compared to the untreated cells.
Figure 5.

Effects of EL on MMP-2, MMP-9, TIMP-1 and TIMP-2 Gene Expressions in MDA-MB-231 Cells. (A) Decreased gene expression of MMP-2 in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 and 48 hours of incubation in a dose and time dependent manner. (B) Decreased gene expression of MMP-9 in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 and 48 hours of incubation in a dose and time dependent manner. (C) Increased gene expression of TIMP-1 in MDA-MB-231 cells with 25, 50 and 75 µM concentrations of EL after 24 and 48hours of treatment. (D) Increased gene expression of TIMP-1 in MDA-MB-231 cells with 25, 50 and 75 µM concentrations of EL after 24 and 48 hours of treatment, compared to the untreated cells as a negative control and 0.5 µM of TAM as a positive control. Values are represented as mean ± SEM of three independent experiments (n =3) with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, indicating statistical significance.
EL up-regulated TIMP-1 and TIMP-2 gene expressions (endogenous inhibitors of MMPs and ADAMTS)
In continuation, we also investigated the effects of EL on endogenous inhibitors of MMPs and ADAMTS in MDA-MB-231 metastatic breast cancer cells. As shown in Figure 5C, there was very significant increase by 1.8 and 2.4 folds after 24h and 48h of treatments with 25 µM of EL (p ≤ 0.01) and extremely significant increase with 50 µM of EL (~2.5 folds at 24h and ~3.2 folds at 48h) and 75 µM of EL (~3.2 folds at 24h and ~4.3 folds at 48h) treatments (p ≤ 0.001) in tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression. While in the case of tissue inhibitor of metalloproteinase-2 (TIMP-2) gene expression, 25 µM and 75 µM of EL treatments showed non-significant increase (except significant increase by 1.7 folds at 48h with 75 µM of EL, p ≤ 0.05) and 50 µM of EL showed extremely significant increase by 2.3 folds and 2.6 folds after 24h and 48h of treatments (p ≤ 0.001) compared to the untreated cells (Figure 5D). However the positive control TAM showed non-significant increase after 48h of treatments with 0.5µM concentration compared to the untreated cells.
EL reduces the proteolytic activities of gelatinases (MMP-2 and MMP-9) in MDA-MB-231 cells to inhibit ECM remodeling
After confirming the down-regulation of MMP-2 and MMP-9 by EL in MDA-MB-231 cells, we further assessed effects of EL on the potent gelatin-degrading activities of key MMPs, MMP-2 and MMP-9 involved in breast cancer metastasis through ECM remodeling. It was found that EL effectively reduced the proteolytic activities (gelatin degrading activities) of both gelatinases, MMP-2 and MMP-9 in a dose dependent manner in MDA-MB-231 cells. For 24 hours of treatment, EL showed significant decrease in the gelatinolytic activities of both MMP-2 and MMP-9, about 15% and 13% at 25μM (p ≤ 0.05), about 23% and 21% at 50 μM (p ≤ 0.01) and about 38% and 32% at 75 μM (p ≤ 0.001) of concentrations, respectively compared to the untreated cells. On the other hand, positive control TAM showed non-significant increase in the gelatinolytic activities of both MMP-2 and MMP-9, about 9% and 7% at 0.5 μM of concentration, respectively compared to the untreated cells.
Discussion
Breast cancer cell metastasis is one of the crucial events leading to treatment failure and death in breast cancer patients. This event is promoted by degradation of BM and ECM remodeling mainly by MMPs (Kousidou et al., 2004). It has long been known that tumor-derived ECM is different in its biochemical composition than that of normal ECM (Cox and Erler, 2011) owing to its ability to survive and drive the malignancy. It is now well accepted fact that an abnormal ECM influences cancer progression by directly promoting cellular transformation and metastasis (Lu et al., 2012). As mentioned earlier the extracellular proteinases; MMPs (Kessenbrock et al., 2010), metzincin proteinases; ADAMTS (Murphy, 2008) and serine proteases; plasmin and cathepsin G (Green and Lund, 2005) play major role as ECM modifying enzymes in ECM remodeling or degradation.
Collectively, MMPs not only target essentially all ECM components but also perform various pleiotropic functions other than digesting the ECM due to their extreme pleiotropic nature (Lu et al., 2011). Among these MMPs, MMP-2 and MMP-9 have capacity to remodel ECM through ECM and BM degradation along with induction of angiogenesis and thus they are related to tumor invasion and metastasis (Egeblad and Werb, 2002; Sternlicht and Werb, 2001). These MMPs are negatively regulated by the endogenous inhibitors of MMPs popularly known as tissue inhibitors of metalloproteinases (TIMPs) which also commonly expressed at tumor site (Deryugina and Quigley, 2006). Four members of TIMP family, TIMP-1, 2, 3 and 4 are reported to inhibit proteolytic activities of not only MMPs but also ADAMTS (Kessenbrock et al., 2010). Another ECM-remodeling-enzyme playing important role in the breast cancer invasion and metastasis is the plasmin or plasminogen activators (uPA system), belonging to the serine protease family (Deryugina and Quigley, 2012; Tang and Han, 2013) which actually mediate the activation of MMPs. The uPA system includes urokinase-type plasminogen activator (uPA), uPA receptor (uPAR) and plasminogen activator inhibitors (PAIs; PAI-1 and PAI-2). The uPA system catalyzes the inactive plasminogen to the active plasmin, which is capable of degrading the BM and ECM directly or indirectly by activating pro-matrix metalloproteinases (pro-MMPs) to MMPs which ultimately results in cancer metastasis (Tang and Han, 2013). In turn, an active plasmin catalyses the conversion of uPA precursor; pro-uPA to uPA and thus uPA and plasmin generate a positive feedback loop to activate each other (Andreasen et al., 2000). This plasminogen activation system can also be negatively regulated by PAI-1 and PAI-2 which inhibit uPA and by α2-antiplasmin which blocks plasmin activities (Green and Lund, 2005). Thus this uPA-Plasmin-MMPs enzyme system is playing important role in breast cancer metastasis through ECM remodeling and provides potential target for therapeutic intervention of breast cancer treatment (Figure 7).
Figure 6.
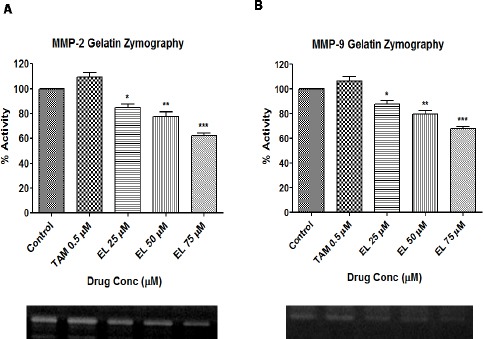
Effects of EL on the Gelatinolytic Activities of MMP-2 And MMP-9 assessed by Gelatin Zymography in MDA-MB-231 Cells. (A) Decreased gelatinolytic activity of MMP-2 in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 hours of incubation in a dose dependent manner. (B) Decreased gelatinolytic activity of MMP-9 in MDA-MB-231 cells with 25, 50 and 75 μM concentrations of EL after 24 hours of incubation in a dose dependent manner. Values are represented as mean ± SEM of three independent experiments (n =3) with *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, indicating statistical significance.
Figure 7.
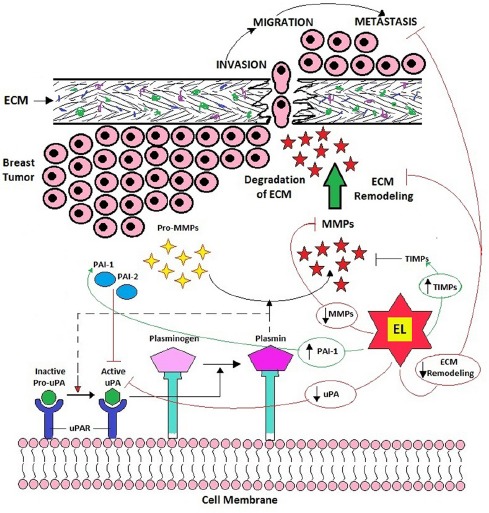
Role of uPA-Plasmin-MMPs Enzyme System in ECM Remodeling Leading to Breast Cancer Metastasis and the Potential Targets of EL to Inhibit ECM Remodeling to Arrest Breast Cancer Metastasis.
ED and EL; the mammalian lignans derived from the flax lignan are known for their anti-breast cancer activity (Flower et al., 2014). An antimetastatic potential of the flax lignan and its mammalian lignans ED and EL has been reported in several animal and human breast cancer xenografts studies (Chen et al., 2007, 2006, 2002; Dabrosin et al., 2002; Jungeström et al., 2007; Saarinen et al., 2006). Several in-vitro cell culture studies also explained some cellular and molecular mechanisms of the ED and EL in different breast cancer cell lines affecting various cellular behaviors such as adhesion, invasion and migration during metastasis (J Chen and Thompson, 2003; Magee et al., 2004; Wang et al., 2005; Wang, 2002; Welshons et al., 1987; Xiong et al., 2015). In order to confer the antimetastatic therapeutic potential to the ED and EL, detailed investigations regarding the cellular and molecular mechanistic endpoints of ED and EL in various cell-signaling pathways involved in breast cancer metastasis are needed to be carried out. In present study we investigated the inhibitory potential of EL on a crucial uPA-Plasmin-MMPs enzyme system carrying out ECM remodeling and metastasis in breast cancer.
In this study we found that EL induced a dose and time dependent reduction in cell viability and cell survival indicating the anticancer potential of EL on metastatic MDA-MB-231 breast cancer cells. Findings of both MTT assay and viability assay collectively suggest that ER-negative metastatic MDA-MB-231 breast cancer cells are susceptible to the cytotoxic and antiproliferative effects of both TAM and EL in a time as well as dose dependent manner, irrespective of their ER binding affinity. The anchorage dependent clonogenic assay also confirmed the potential of EL to affect the ability of individual MDA-MB-231 breast cancer cells in the population to undergo unlimited divisions i.e. clonogenicity. Different concentrations of EL have shown significant decrease in colony forming ability of the metastatic breast cancer cells; MDA-MB-231. We also analyzed effects of EL on migrating abilities of the MDA-MB-231 cells and results of migration assay have shown that EL significantly inhibited the migration of metastatic breast cancer cells, in-vitro in a dose and time dependent manner. Results of all these assays collectively conferred the anticancer and antimetastatic potential to the EL in MDA-MB-231 metastatic breast cancer cells with antiproliferative, antimigratory and anticlonogenic cellular mechanisms. All these cellular mechanisms of the EL suggest its possible potential to inhibit ECM remodeling in order to prevent adhesion, invasion and migration events in the metastatic cascade. So as to explore the underlying molecular mechanisms of EL on ECM remodeling and related cell signaling, we selected uPA-plasmin-MMPs system which supports protease activation, ECM remodeling and thus metastasis.
Taken together, our findings suggest that EL can inhibit activation of plasmin by down-regulating the uPA gene expression and up-regulating the PAI-1 gene expression since uPA converts inactive plasminogen to active plasmin which degrades BM and remodels ECM by consecutive activation of MMPs. It is noteworthy that EL not only down-regulates the uPA but also up-regulates the natural inhibitor of uPA, PAI-1 and thus exhibits strong control over plasmin activation. As discussed earlier, plasmin activates pro-MMPs to MMPs which degrade BM and facilitate invasion and migration of cancer cells. The EL was also found to down-regulate expression of MMP-2 and MMP-9 genes, and up-regulate TIMP-1 and TIMP-2 (natural inhibitors of MMP-2 and MMP-9 respectively), may be as a consequence of inhibition of plasmin activation which exhibits strong control over migration and invasion of breast cancer cells during metastasis. These findings suggest a strong evidence of an underlying molecular mechanism of EL to inhibit uPA-plasmin-MMPs system induced ECM remodeling and thereby arrest breast cancer metastasis (Figure 7). There could be several chemicals that could be synthesized to target different pathways in ECM remodeling to arrest metastasis, but EL that gets normally formed in human body with the consumption of flaxseeds or flax lignan may be an alternative to synthetic drugs. More research is needed to find its action on other pathways in ECM remodeling to establish the full potential of flax lignan.
Several studies support that ECM is an essential component of the both primary tumor niche and metastatic niche. Thus, to explore an abnormal ECM as an effective cancer therapeutic target will become an important area of future cancer research (Lu et al., 2012). Recent studies in cancer are aiming to target various factors that regulate ECM homeostasis so as to develop several novel therapeutics out of which some have been found to be promising, such as MMP inhibitors, collagen cross-linkers and anti uPA/uPAR drugs (Cox and Erler, 2011). Some promising data from animal models suggest that uPA, besides its biomarker role, may be a novel therapeutic target for the treatment of cancers including breast cancer (Carriero and Stoppelli, 2011; Duffy, 2004; Schmitt et al., 2011). Recently, a phase II clinical trial using the low-molecular-weight uPA inhibitor WX-671 reported activity in metastatic breast cancer (Duffy et al., 2014). Apart from its proteolytic activity, plasmin may also trigger intracellular signaling pathways of metastasis by activating growth factors such as TGF-β, bFGF and VEGF and thus affect cellular processes (Didiasova et al., 2014). Thus, uPA/uPAR may become crucial therapeutic target to inhibit plasmin activation and concurrent MMP mediated ECM remodeling leading to the cancer progression and metastasis in breast cancer. Here we have explored molecular and cellular mechanisms of EL on promising and novel therapeutic targets, uPA-plasmin-MMPs system and thereby ECM remodeling and demonstrated the antimetastatic breast cancer potential of EL. Furthermore, to our best knowledge, this is the first time to report the efficacy of EL on inhibition of ECM remodeling in breast cancer to arrest its metastasis. With these promising findings which project EL as a potential antimetastatic molecule capable of inhibiting MMPs, uPA activities and ECM remodeling in breast cancer, further studies on different receptor and intracellular signaling pathways taking place during breast cancer metastasis are warranted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- Alphonse P, Aluko R. A review on the anti-carcinogenic and anti-metastatic effects of flax seed lignan secolariciresinol diglucoside (SDG) Discov Phytomedicine. 2015;2:12–7. [Google Scholar]
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation:forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero MV, Stoppelli MP. The urokinase-type plasminogen activator and the generation of inhibitors of urokinase activity and signaling. Curr Pharm De. 2011;17:1944–61. doi: 10.2174/138161211796718143. [DOI] [PubMed] [Google Scholar]
- Chaudhary AK, Chaudhary S, Ghosh K, et al. Pleiotropic roles of metalloproteinases in hematological malignancies:an update. Asian Pacific J Cancer Prev. 2016;17:3043–51. [PubMed] [Google Scholar]
- Chen J, Power KA, Mann J, et al. Flaxseed alone or in combination with tamoxifen inhibits MCF-7 breast tumor growth in ovariectomized athymic mice with high circulating levels of estrogen. Exp Biol Med. 2007;232:1071–80. doi: 10.3181/0702-RM-36. [DOI] [PubMed] [Google Scholar]
- Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–92. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- Chen J, Thompson LU. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat. 2003;80:163–70. doi: 10.1023/A:1024513815374. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Thompson LU. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett. 2006;234:168–75. doi: 10.1016/j.canlet.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix:implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrosin C, Chen J, Wang L, et al. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;185:31–37. doi: 10.1016/s0304-3835(02)00239-2. [DOI] [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Cell surface remodeling by plasmin:A new function for an old enzyme. J Biomed Biotechnol. 2012;56:4259–80. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Didiasova M, Wujak L, Wygrecka M, et al. From plasminogen to plasmin:role of plasminogen receptors in human cancer. Int J Mol Sci. 2014;15:21229–52. doi: 10.3390/ijms151121229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MJ. The urokinase plasminogen activator system:role in malignancy. Curr Pharm Des. 2004;10:39–49. doi: 10.2174/1381612043453559. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, McGowan PM, Harbeck N, et al. uPA and PAI-1 as biomarkers in breast cancer:validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014;16:428. doi: 10.1186/s13058-014-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Elangovan S, Hsieh TC, Wu JM. Growth inhibition of human MDA-MB-231 breast cancer cells by?-tocotrienol is associated with loss of cyclin D1/CDK4 expression and accompanying changes in the state of phosphorylation of the retinoblastoma tumor suppressor gene product. Anticancer Res. 2008;28:2641–47. [PubMed] [Google Scholar]
- Flower G, Fritz H, Balneaves LG, et al. Flax and breast cancer:A systematic review. Integr Cancer Ther. 2014;13:181–92. doi: 10.1177/1534735413502076. [DOI] [PubMed] [Google Scholar]
- Frankowski H, Gu YH, Heo JH. Use of gel zymography to examine matrix metalloproteinase (Gelatinase) expression in brain tissue or in primary Glial cultures. Methods Mol Biol. 2012;814:221–33. doi: 10.1007/978-1-61779-452-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba C, Figuerola F, Estévez AM, et al. Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.). determined by single-batch in vitro simulation of the digestive process. J Sci Food Agric. 2014;94:1729–38. doi: 10.1002/jsfa.6482. [DOI] [PubMed] [Google Scholar]
- Gebäck T, Schulz MMP, Koumoutsakos P. TScratch:A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–74. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays. 2005;27:894–03. doi: 10.1002/bies.20281. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix:not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungeström MB, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–67. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M. In vitro cell migration and invasion assays. J Vis Exp. 2014;88:e51046. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L, Erler JT, Dembo M. Mammary epithelial cell:Influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–94. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases:regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousidou OC, Roussidis AE, Theocharis AD. Expression of MMPs and TIMPs genes in human breast cancer epithelial cells depends on cell culture conditions and is associated with their invasive potential. Anticancer Res. 2004;24:4025–30. [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–06. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:1–24. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix:A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–06. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee PJ, McGlynn H, Rowland IR. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004;208:35–41. doi: 10.1016/j.canlet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Mali AV, Wagh UV, Hegde MV. In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian J Cancer. 2012;49:181–7. doi: 10.4103/0019-509X.98948. [DOI] [PubMed] [Google Scholar]
- Mellatyar H, Akbarzadeh A, Rahmati M, et al. Comparison of inhibitory effect of 17-DMAG nanoparticles and free 17-DMAG in HSP90 gene expression in lung cancer. Asian Pacific J Cancer Prev. 2014;15:8693–98. doi: 10.7314/apjcp.2014.15.20.8693. [DOI] [PubMed] [Google Scholar]
- Murphy G. The ADAMs:signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–41. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Inoue T, Shimao Y, et al. Matrix metalloproteinases in tumor invasion:Role for cell migration. Pathol Int. 2002;52:255–64. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- Ranson M. Plasminogen binding and cancer:promises and pitfalls. Front Biosci. 2003;8:294–04. doi: 10.2741/1044. [DOI] [PubMed] [Google Scholar]
- Saarinen NM, Power K, Chen J, et al. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int J Cancer. 2006;119:925–31. doi: 10.1002/ijc.21898. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Harbeck N, Brünner N, et al. Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev Mol Diagn. 2011;11:617–34. doi: 10.1586/erm.11.47. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–08. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Cohen B, Goldvasser P, et al. Plasminogen activator uPA is a direct transcriptional target of the JAG1-notch receptor signaling pathway in breast cancer. Cancer Res. 2011;71:277–86. doi: 10.1158/0008-5472.CAN-10-2523. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–16. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. A3-B. (vol)(pages?) [DOI] [PubMed] [Google Scholar]
- Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–19. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother. 2013;67:179–82. doi: 10.1016/j.biopha.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Vakonakis I, Campbell ID. Extracellular matrix:from atomic resolution to ultrastructure. Curr Opin Cell Biol. 2007;19:578–83. doi: 10.1016/j.ceb.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components. Int J Cancer. 2005;116:793–8. doi: 10.1002/ijc.21067. [DOI] [PubMed] [Google Scholar]
- Wang LQ. Mammalian phytoestrogens:Enterodiol and enterolactone. J Chromatogr B. 2002;777:289–09. doi: 10.1016/s1570-0232(02)00281-7. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Murphy CS, Koch R, et al. Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res Treat. 1987;10:169–75. doi: 10.1007/BF01810580. [DOI] [PubMed] [Google Scholar]
- Xiong XY, Hu XJ, Li Y, et al. Inhibitory effects of enterolactone on growth and metastasis in human breast cancer. Nutr Cancer. 2015;67:1326–34. doi: 10.1080/01635581.2015.1082113. [DOI] [PubMed] [Google Scholar]
- Yan L, Yee JA, Li D, et al. Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer Lett. 1998;124:181–6. doi: 10.1016/s0304-3835(97)00470-9. [DOI] [PubMed] [Google Scholar]
- Yu H, Mouw JK, Weaver VM. Forcing form and function:Biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


