Abstract
Background:
Helicobacter pylori (H. pylori) infection is related to peptic ulcer diseases and gastric cancer and eradication of H. pylori should be expected to decrease the risk of their development. Factors affecting H. pylori eradication are antibiotic resistance, CYP2C19 genotypes, drug regimen and patient compliance. Increment of omeprazole and amoxicillin dosage in clarithromycin-containing triple therapy regimen may overcome these problems and may be a better choice than the conventional clarithromycin-containing triple therapy regimen.
Objective:
To compare the eradication rates with modified triple therapy (MTT) and standard triple therapy (STT) as first-line treatment.
Materials and Methods:
The study was an open label, multicenter, randomized controlled trial. A total of 170 patients infected with H. pylori diagnosed by rapid urease test were randomly assigned into 2 groups. The first was treated with a 14-day MTT (20 mg omeprazole t.i.d., 500 mg amoxicillin t.i.d., and 500 mg clarithromycin b.i.d.) and the second with a 14-day STT (20 mg omeprazole b.i.d., 1000 mg amoxicillin b.i.d., and 500 mg clarithromycin b.i.d.). H. pylori eradication was evaluated by 14C-urea breath test. CYP2C19 genotypes, clarithromycin resistance, side effects and patient compliance were also recorded.
Results:
There were 85 patients in each group. The H. pylori eradication rate in the MTT group was 84.7% by ITT analysis and 91.1% by PP analysis, compared to the STT group values of 76.5% and 87.8% (p = 0.18 and 0.51), respectively. CYP2C19 genotypes and patient compliance were similar in both groups. Prevalence of clarithromycin resistance was 7.0%. Side effects were all mild with no significant differences between the twogroups.
Conclusions:
MTT is not superior to STT. From this study, MTT may not be recommended as the first-line treatment for H. pylori infection in Thailand because eradication rates proved to be less than 90% by ITT analysis.
Keywords: Modified high dose, omeprazole, amoxicillin, triple therapy, Helicobacter pylori eradication, Thailand
Introduction
H. pylori infection is related to peptic ulcer diseases and gastric cancer (Marshall et al, 1995; Correa et al, 1992). H. pylori infection is highly prevalent worldwide. More than half of the world’s population suffers from this in fection (Everhart, 2000). For example, the prevalence of H. pylori infection in the northeast region of Thailand is 67.1%. The premalignant histological change of gastric cancer (e.g. atrophic change and intestinal metaplasia) was also highly prevalent in this region with the rate of 59.5% (Atisook et al, 2003). According to previous studies, the best way to prevent gastric cancer development is to eradicate H. pylori infection (Ford et al, 2014; Fuccio et al, 2009).
The current rate of successful eradication with a clarithromycin-containing triple therapy regimen is lower than 80% in many Southeast Asian countries, including Thailand (Ang et al, 2015; Graham and Fischbach, 2010; Jianjaroonwong, 2013; Yoon et al, 2013). Factors affecting the eradication rate are antibiotic resistance of H. pylori, CYP2C19 genotypes in individual patients, drug regimen, and patient compliance. Clarithromycin and metronidazole resistance is increasing steadily and leads to triple therapy regimen ineffectiveness. Several studies in Thailand reported clarithromycin resistant rate from 3.7% to 29.2% and metronidazole resistant rate from 30.0% to 51.9% (Wongkusoltham et al, 2001; Mahachai et al, 2006; Tanuma et al, 2009; Vilaichone et al, 2011; Vilaichone et al, 2013). However, the most recent data from nationwide study in 2012 showed that clarithromycin resistant rate in northeast region of Thailand was only 2.1% (Vilaichone et al, 2013). Proton pump inhibitor (PPI) responsiveness depends on the expression of CYP2C19 enzyme in each individual (CYP2C19 genotypes). Tassaneeyakul et al predicted the majority of Thai northeast population (46.73%) as extensive metabolizer (EM) of CYP2C19. Based on Tassaneeyakul et al., (2002), EM patients have more rapid PPI clearance than the intermediate metabolizer (IM) and poor metabolizer (PM). Thus, acid inhibition is insufficient to accommodate antibiotic killing effect of H. pylori in the extensive metabolizers. The use of triple therapy regimen, including PPI, amoxicillin, and clarithromycin may be the first choice in the Northeast region, where the prevalence of clarithromycin resistance is low. Modification of triple therapy by increasing the dosage of omaeprazole may overcome the insufficient acid suppression for H pylori eradication among the extensive metabolizer patients.
Many studies have found that increasing the dose of omeprazole to 20 mg 3-4 times a day or increasing the frequency of amoxicillin to 3-4 times a day is more effective in the eradication of H. pylori with similar side effect as compared to standard dose of omeprazole or amoxicillin. No studies explored the combination of omeprazole and amoxicillin given three times a day together with clarithromycin which may be an alternative regimen to replace the conventional triple therapy regimen (Rokkas et al, 1995; Miehlke et al, 2003; Kim et al, 2008; Furuta et al, 2014). Omeprazole increment may help in cases whose CYP2C19 expression are extensive metabolizer. Amoxicillin administration using a regimen of three to four times daily is theoretically appropriate because its antibacterial effect depends on the time above the MIC and not the AUC or Cmax (Sugimoto et al, 2014). This study compared the modified triple therapy (omeprazole 20 mg tid, amoxicillin 500 mg tid, and clarithromycin 500 mg bid) withthe standard triple therapy (omeprazole 20 mg bid, amoxicillin 1,000 mg bid and clarithromycin 500 mg bid) in terms of eradication rate. The prevalence of each CYP2C19 genotypes and the prevalence of clarithromycin-resistant H.pylori strains, may in part explain the high or low eradication rate among the studied patients. The result of this study may help making decision whether MTT regimen can be recommended as the first choice for H pylori treatment among northeast Thai population.
Materials and Methods
Study design and participants
This was a prospective, open label, multicenter randomized controlled trial conducted in three hospitals in the northeast of Thailand (Srinagarind university hospital, Nongkhai, and Chaiyaphum provincial hospitals), from April to November 2015. Our protocol was approved by the office of the Khon Kaen university ethics committee in human research. The Thai Clinical Trials Registry (TCTR) registration identifier was TCTR20150910001. Adult patients (age ≥ 18 years old) with H. pylori infection who did not receive prior eradication therapy were eligible for the enrolment. The diagnosis of H. pylori infection was based on positivity to rapid urease test. Subjects with any one of the following criteria were excluded from the study: (1) history of gastric cancer or gastrectomy, (2) severe concurrent disease or malignancy, (3) pregnant or lactating women, (4) alcohol abuse or drug addiction, (5) previous allergic reaction to study drugs, and (6) use of PPI, bismuth containing drugs or antibiotics within 4 weeks.
Randomization and interventions
Eligible patients at each considered hospitals were randomized to receive either modified triple therapy (MTT; omeprazole 20 mg tid, amoxicillin 500 mg tid, and clarithromycin 500 mg bid) or standard triple therapy (STT; omeprazole 20 mg bid, amoxicillin 1000 mg bid and clarithromycin 500 mg bid) for 14 days. Randomization was performed in blocks of four by computer generation and the process was concealed to investigators until interventions were assigned. Signed informed consent was obtained to participate in this study. Patients were instructed to adhere to the drug regimen and were advised of the possible side effects. Eradication rate was assessed at 4 - 6 weeks after completion of therapy by performing 14C-urea breath test (UBT). Successful eradication was defined as negative UBT. Compliance and side effects were evaluated by self-reporting and direct interview at the end of the treatment. A good drug compliance was defined as drug consumption > 85% of the total dosage.
14C-Urea Breath Test
After six hours of fasting, one capsule of 14C-urea (Endo Supply Company Limited, Bangkok, Thailand) was administered orally. The breath sample was obtained 15 minutes later. The cutoff value was 45 (Negative result was defined as less than 45). Sensitivity and specificity of this test were over 95%.
Outcomes
The primary end point of the study was the eradication rate, which was assessed by intention-to-treat (ITT) and per-protocol (PP) analyses. All randomized patients were included in the ITT analysis. Patients who did not return for the follow up of 14C-UBT were considered treatment failures. Patients who failed to take at least 85 % of their prescribed drugs or who lost to follow up were excluded from the PP analysis.
The secondary end points were the prevalence of CYP2C19 genotypes in patients with H. pylori infection and the prevalence of clarithromycin-resistant H.pylori strains. Blood samples were collected from the patients before eradication therapy. The CYP2C19 genotyping for wild-type allele (*1) and three mutated alleles (*2, *3 and *17) were conducted by real-time PCR technique (Tassaneeyakul et al, 2002). The patients were categorized into three groups based on the CYP2C19 genotype, EM (*1/*1 or *1/*17), IM (*1/*2, *1/*3, *2/*17 or *3/*17), and PM (*2/*2, *2/*3, or *3/*3).
To evaluate clarithromycin resistance, because the A-to-G transition at position 2143 (A2143G) and 2142 (A2142G) in 23S rRNA were proposed as the major mechanism, two biopsy samples from gastric antrum and body were obtained for measurement of point mutations of 23S rRNA gene of H. pylori at the positions of 2142 and 2143 by PCR-RFLP (restriction fragment length polymorphism) technique. When 23S rRNA gene at the position of 2142 or 2143 was wild type, patients were diagnosed to be infected with clarithromycin-sensitive strains. When 23S rRNA gene was mutated (A2142G A/G, A2142G G/G, A2143G A/G or A2143G G/G), patients were diagnosed to be infected with clarithromycin-resistant strains (Versalovic et al, 1997; Wang and Taylor, 1998; Ménard et al, 2002).
Statistical analysis
For the sample size calculation, we hypothesized that there would be approximately 18% difference in the eradication rates between the two regimens. Knowing that the eradication rate of STT was approximately 72%, our sample size estimation was 85 for each group, given a power of 80% and a confidence level of 95%, assuming a 20% loss to follow up (Jianjaroonwong, 2013).
Statistical differences in eradication rates among the different regimen were assessed by chi-square test. The demographic data and frequencies of adverse reactions were compared using chi-square test or Fisher’s exact test, when appropriate. The p-values < 0.05 were considered to be statistically significant. The statistical analyses were performed using the Stata/SE 10.1.
Results
Baseline characteristics of patients
From April to November 2015, 186 patients with H. pylori infection from three hospitals were evaluated. Of these patients, 170 were enrolled and randomized to receive one of two regimens. Eighty-five patients were assigned to the MTT group and 85 patients to the STT group. The flow chart of patients included in the study is displayed in Figure 1. No differences were observed between two groups regarding baseline characteristics of patients (Table 1)
Figure 1.
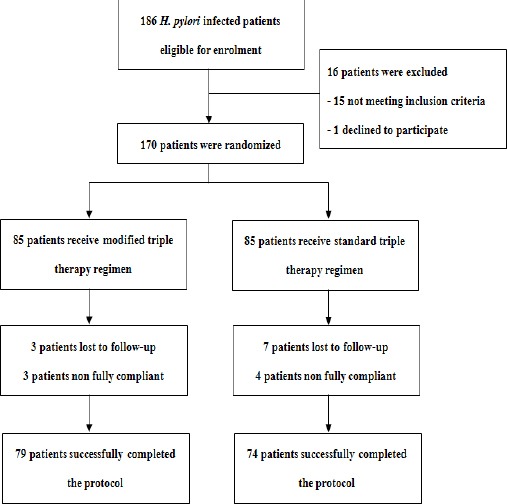
Flow Chart of Patients During the Study
Table 1.
Baseline Characteristics of Subjects in the Two Treatment Groups
| Modified triple therapy (n = 85) | Standard triple therapy (n = 85) | p-value | |
|---|---|---|---|
| Age, mean | 54.6 | 52.4 | 0.19 |
| Male gender, n (%) | 45 (52.9) | 40 (47.1) | 0.44 |
| BMI | 23.8 | 23.7 | 0.8 |
| Smoking > 5/day, n (%) | 5 (5.9) | 9 (10.6) | 0.26 |
| Alcohol consumption | 1 (1.2) | 1 (1.2) | 1.00 |
| > 1 day/week, n (%) | |||
| Underlying disease | |||
| Cardiovascular disease, n (%) | 3 (3.5) | 1 (1.2) | 0.62 |
| Diabetes mellitus, n (%) | 10 (11.8) | 11 (12.9) | 0.81 |
| Hypertension, n (%) | 16 (18.8) | 16 (18.8) | 1 |
| Dyslipidemia, n (%) | 11 (12.9) | 10 (11.8) | 0.82 |
| Cirrhosis, n (%) | 12 (14.1) | 18 (21.1) | 0.23 |
| Endoscopic findings | |||
| Chronic Gastritis without atrophy, n (%) | 41 (48.2) | 39 (45.9) | 0.76 |
| Atrophic gastritis, n (%) | 10 (11.9) | 14 (16.5) | 0.4 |
| Erosive gastritis, n (%) | 13 (15.3) | 9 (10.6) | 0.36 |
| Hemorrhagic gastritis, n (%) | 15 (17.7) | 16 (18.8) | 0.84 |
| Gastric ulcer, n (%) | 5 (5.9) | 8 (9.4) | 0.39 |
| Duodenal ulcer, n (%) | 4 (4.7) | 5 (5.9) | 1.00 |
Eradication rates of H. pylori infection
The eradication rates by PP and ITT analysis are shown in Table 2. In the MTT group, ITT and PP analyses of the eradication rates were 84.7% and 91.1%, respectively; whereas, the eradication rates in the STT group were 76.5% by ITT analysis and 87.8% by PP analysis. These eradication rates tended to be more successful in the MTT group than those in the STT group; however, it was not statistically significant.
Table 2.
Efficacy of Modified Triple Therapy and Standard Triple Therapy in the Study
| Modified triple therapy | Standard triple therapy | p- value | |
|---|---|---|---|
| Intention-to-treat | 72/85 (84.7%) | 65/85 (76.5%) | 0.18 |
| Per protocol | 72/79 (91.1%) | 65/74 (87.8%) | 0.5 |
Secondary outcomes
Eighty three patients in MTT group and 85 in STT group completed results of CYP2C19 genotypes. The prevalence of CYP2C19 genotypes were similar in each groups with no significant difference (p = 0.25), as shown in Table 3. Although this data suggested that the major benefit of the MTT may be in the subgroup of extensive metabolizers, the eradication rates classified by CYP2C19 genotypes did not differ significantly between the MTT and STT groups (Table 4).
Table 3.
Prevalence of CYP2C19 Genotypes
| Prevalence of | Modified triple therapy (n = 85) | Standard triple therapy (n = 85) | p-value | All subjects |
|---|---|---|---|---|
| Extensive metabolizer (EM), n (%) | 40 (48.2) | 34 (40.0) | 74 (44.1) | |
| Intermediate metabolizer (IM), n (%) | 38 (45.8) | 40 (47.1) | 0.25 | 78 (46.4) |
| Poor metabolizer (PM), n (%) | 5 (6.0) | 11 (12.9) | 16 (9.5) |
Table 4.
Outcome of Treatment Classified by CYP2C19 Genotypes
| Eradication rate of | Modified triple therapy (% eradication rate) | Standard triple therapy (% eradication rate) | p-value |
|---|---|---|---|
| Extensive metabolizer (RM) | |||
| ITT | 37/40 (92.5%) | 28/34 (82.4%) | 0.18 |
| PP | 37/40 (92.5%) | 28/32 (87.5%) | 0.48 |
| Intermediate metabolizer (IM) | |||
| ITT | 29/38 (76.3%) | 27/40 (67.5%) | 0.39 |
| PP | 29/35 (82.9%) | 27/36 (75.0%) | 0.42 |
| Poor metabolizer (PM) | |||
| ITT | 4/5 (80%) | 10/11 (90.9%) | 0.54 |
| PP | 4/5 (80%) | 10/10 (100.0%) | 0.14 |
23S rRNA gene of H.pylori could be amplified by PCR-RFLP technique in eighty six patient specimens from total of 170 specimens. In these 86 amplifiable specimens, 6 specimens (7.0%) had mutated 23S rRNA gene of H.pylori (1 with A2142G A/G, 3 with A2143G A/G and 2 with A2143G G/G) thus the owners were diagnosed to be infected with clarithromycin-resistant strains (Table 5).
Table 5.
Prevalence of Mutated 23S rRNA Gene of Clarithromycin-resistant H.pylori
| Mutation | Modified triple therapyn = 37(%) | Standard triple therapyn = 49 (%) | All subjects n = 86 |
|---|---|---|---|
| A2142G A/G, n (%) | - | 1 (2.0) | 1 (1.2) |
| A2143G A/G, n (%) | 1 (2.7) | 2 (4.1) | 3 (3.5) |
| A2143G G/G, n (%) | 1 (2.7) | 1 (2.0) | 2 (2.3) |
| All mutations, n (%) | 2 (5.4) | 4 (8.2) | 6 (7.0) |
Side effects and compliance with therapy
Side effects, including bitter taste, diarrhea, dizziness, headache, fatigue, and nausea were all mild and did not lead to significant difference between 2 groups as shown in Table 6. Bitter taste was the most commonly reported adverse effect in both groups (53.7% in the MTT group vs. 62.8% in the STT group; p = 0.24). Three patients in the MTT group and 7 patients in the STT group were lost to follow up with unknown reason. Drug compliance with the MTT regimen was 79/85 (92.9%) and 74/85 (87.1%) in the STT group indicating no significant difference (p = 0.20).
Table 6.
Side Effects of Modified Triple Therapy and Standard Triple Therapy
| Modified triple therapy (n = 75) | Standard triple therapy (n = 75) | p-value | |
|---|---|---|---|
| Bitter taste, n (%) | 44 (53.7) | 49 (62.8) | 0.24 |
| Diarrhea, n (%) | 0 (0) | 3 (3.9) | 0.11 |
| Dizziness, n (%) | 0 (0) | 4 (5.1) | 0.05 |
| Headache, n (%) | 3 (3.7) | 1 (1.3) | 0.62 |
| Nausea, n (%) | 4 (4.9) | 5 (6.5) | 0.74 |
| Skin rash, n (%) | 0 (0) | 0 (0) | - |
Discussion
Although the Maastricht IV consensus stated that clarithromycin-containing triple therapy can be recommended for first-line empirical treatment in the areas of low clarithromycin resistance rate (less than 15 - 20%) (Malfertheiner et al, 2012), the most recent data have shown that H. pylori eradication rate with triple therapy regimen, including PPI, amoxicillin, and clarithromycin in many regions has declined to 80% or below (Graham and Fischbach, 2010; Jianjaroonwong, 2013; Yoon et al, 2013). When the pattern of antibiotic resistance is unknown, only regimens that are expected to provide eradication rate at least 90% (by ITT analysis) should be prescribed as empiric therapy (Graham et al, 2014). Treatment success with eradication rate greater than 90% by intention-to-treat analysis has been defined as “good” and ≥ 95% as “excellent” outcome (Graham et al, 2007). Factors affecting the eradication rate are antibiotic resistance, CYP2C19 genotypes, drug regimen, and patient’s compliance. The regimen that can optimize these factors might be a candidate for ideal empiric therapy.
Modified triple therapy regimen is composed of clarithromycin plus three-times-daily dosing of omeprazole, which is aimed to overcome the effect of different CYP2C19 genotypes in patients, and three-times-daily dosing of amoxillin, which is more appropriate than twice-daily dose due to its pharmacokinetics. With respect to findings of current study, we found that modified triple therapy was not better than standard triple therapy (twice-daily dosing of omeprazole and amoxicillin plus clarithromycin) as we expected. The prevalence of CYP2C19 genotypes between these two groups of treatment were not different and were consistent previous study revealing that intermediate and extensive metabolizer were the most common genotypes in northeast of Thailand (Tassaneeyakul et al, 2002). When compared to STT group, H. pylori eradication rates in the MTT group seemed to be superior in the extensive and intermediate metabolizers; although, the magnitude of difference was not statistically significant. This result can be explained by the effect of multiple doses of omeprazole that decrease the influence of CYP2C19 genotype on gastric acid inhibition. Even that the eradication rate of MTT in extensive metabolizers achieved more than 90%, it was surprising that the eradication rate fall below 90 % in intermediate and poor metabolizers. Clarithromycin resistance may be the factor contributing to these low success rates and the three-times-daily dosing of omeprazole and amoxillin may not be adequate for patients infected with clarithromycin-resistant H. pylori. Although prevalence of clarithromycin-resistant H. pylori strains in our study was 7.0%, this result may underestimate the true prevalence because we could not amplified 23S rRNA gene of H. pylori in all patient specimens. There was also no difference regarding the safety profiles and patient compliance of these two regimens. According to our results, none of triple therapy regimens can be recommended as the first-line treatment for H. pylori infection because their eradication rates were estimated less than 90% by the ITT analysis.
The first limitation of our study was the fact that we could not evaluate the prevalence of clarithromycin resistance in all patients. For this reason, we cannot absolutely conclude that MTT and STT were not effective due to clarithromycin resistance or other factors. Second, we did not monitor intragastric pH in our subjects which can confirm whether three-times-daily dosing of omeprazole was sufficient to control gastric acid in extensive metabolizers. Another limitation is bias from open label study design, so we tried to reduce any potential bias by using randomization and objective measurement of primary outcome.
The modified triple therapy with three-times-daily dose of omeprazole and amoxicillin plus clarithromycin was not superior to standard triple therapy in terms of H. pylori eradication. Neither modified triple therapy nor standard triple therapy achieved acceptable eradication rates in this study; therefore, they are not recommended for the population of northeast Thailand. This low success rate may be due to more prevalence of clarithromycin resistance in our area than we expected or inadequacy of PPI and amoxicillin dosage. To explain this result, further studies are recommendedto explore the true prevalence of clarithromycin resistance in northeast region of Thailand. If the clarithromycin resistance rate in this region is more than 15 - 20%, clarithromycin-containing triple therapy should be absolutely abandoned, as suggested by Maastricht IV consensus (Malfertheiner et al, 2012). Moreover, if the clarithromycin resistance rate is still low, studies of triple therapy regimen with higher dose of omeprazole and amoxicillin (e.g. four-times-daily dosing omeprazole and amoxicillin) may be needed to test our hypothesis.
Acknowledgements
This study was partially supported by Research Fund at Faculty of Medicine, KhonKaen University, Thailand and the Gastroenterology Association of Thailand (GAT). We thank Siam Pharmaceutical Co., Ltd. (Thailand) for providing omeprazole, amoxicillin, and clarithromycin for the eradication regimens.
References
- Ang TL, Fock KM, Song M, et al. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J Gastroenterol Hepatol. 2015;30:1134–9. doi: 10.1111/jgh.12892. [DOI] [PubMed] [Google Scholar]
- Atisook K, Kachinthorn U, Luengrojanakul P, et al. Histology of gastritis and Helicobacter pylori infection in Thailand:a nationwide study of 3776 cases. Helicobacter. 2003;8:132–41. doi: 10.1046/j.1523-5378.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis:a multistep and multifactorial process. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–79. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals:systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis:can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–8. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- Furuta T, Sugimoto M, Yamade M, et al. Effect of dosing schemes of amoxicillin on eradication rates of Helicobacter pylori with amoxicillin-based triple therapy. J Clin Pharmacol. 2014;54:258–66. doi: 10.1002/jcph.195. [DOI] [PubMed] [Google Scholar]
- Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy:evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–86. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianjaroonwong V. Peptic ulcer healing and the efficacy of standard PPI-based triple therapy for Helicobacter pylori eradication at Nakhonpathom hospital. Reg Med J. 2013;32:81–95. [Google Scholar]
- Kim SY, Lee SW, Jung SW, et al. Comparative study of Helicobacter pylori eradication rates of twice versus four-times-daily amoxicillin administered with proton pump inhibitor and clarithromycin:a randomized study. Helicobacter. 2008;13:282–7. doi: 10.1111/j.1523-5378.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- Mahachai V, Thong-Ngam D, Noophun P, et al. Efficacy of clarithromycin-based triple therapy for treating Helicobacter pylori in Thai non-ulcer dyspeptic patients with clarithromycin-resistant strains. J Med Assoc Thai. 2006;89:74–8. [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O’Morain C, et al. Management of H. pylori infection – the maastricht IV/florence consensus report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- Marshall BJ. Helicobacter pylori in peptic ulcer:have Koch’s postulates been fulfilled? Ann Med. 1995;27:565–8. doi: 10.3109/07853899509002470. [DOI] [PubMed] [Google Scholar]
- Ménard A, Santos A, Mégraud F, et al. PCR-restriction fragment length polymorphism can also detect point mutation A2142C in the 23S rRNA gene, associated with Helicobacter pylori resistance to clarithromycin. Antimicrob Agents Chemother. 2002;46:1156–7. doi: 10.1128/AAC.46.4.1156-1157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehlke S, Kirsch C, Schneider-Brachert W, et al. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2003;8:310–9. doi: 10.1046/j.1523-5378.2003.00158.x. [DOI] [PubMed] [Google Scholar]
- Rokkas T, Mavrogeorgis A, Liatsos C, et al. Optimal dose of omeprazole in combination with amoxicillin in eradicating H. pylori and preventing relapses in duodenal ulcer patients. Hepatogastroenterology. 1995;42:842–6. [PubMed] [Google Scholar]
- Sugimoto M, Uotani T, Sahara S, et al. Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter. 2014;19:312–8. doi: 10.1111/hel.12128. [DOI] [PubMed] [Google Scholar]
- Tanuma M, Rimbara E, Noguchi N, et al. Analysis of clarithromycin resistance and CagA status in Helicobacter pylori by use of feces from children in Thailand. J Clin Microbiol. 2009;47:4144–5. doi: 10.1128/JCM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneeyakul W, Tawalee A, Tassaneeyakul W, et al. Analysis of the CYP2C19 polymorphism in a North-eastern Thai population. Pharmacogenetics. 2002;12:221–5. doi: 10.1097/00008571-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Vilaichone RK, Mahacahai V, Tumwasorn S, et al. CagA genotype and metronidazole resistant strain of Helicobacter pylori in functional dyspepsia in Thailand. J Gastroenterol Hepatol. 2011;26:46–8. doi: 10.1111/j.1440-1746.2011.06652.x. [DOI] [PubMed] [Google Scholar]
- Vilaichone RK, Gumnarai P, Ratanachu-Ek T, Mahachai V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagnost Microbiol Infect Dis. 2013;77:346–9. doi: 10.1016/j.diagmicrobio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Versalovic J, Osato MS, Spakovsky K, et al. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J Antimicrob Chemother. 1997;40:283–6. doi: 10.1093/jac/40.2.283. [DOI] [PubMed] [Google Scholar]
- Wang G, Taylor DE. Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob Agents Chemother. 1998;42:1952–8. doi: 10.1128/aac.42.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongkusoltham P, Vilaichone RK, Kullavanijaya P, et al. Eradication rates of Helicobacter pylori between metronidazole-sensitive and metronidazole-resistant strains with metronidazole containing regimen in Thai patients with peptic ulcer disease. J Med Assoc Thai. 2001;84:474–80. [PubMed] [Google Scholar]
- Yoon H, Lee DH, Kim N, et al. Meta-analysis:is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801–9. doi: 10.1111/jgh.12397. [DOI] [PubMed] [Google Scholar]


