Abstract
Lesion-deficit association studies are important as they can reveal brain regions essential for specific functions, but sometimes appear to yield conflicting results. We aimed to show how pitfalls of lesions studies can be avoided, and converging results obtained, illustrating from studies of the role of posterior superior temporal gyrus in auditory word comprehension. We review published lesion studies on auditory comprehension and present new data from both acute and chronic stroke that address weaknesses in some previous studies. Results demonstrate how convergence of positive results from diverse lesion studies provides strong evidence for the role of a particular region in a given behavior.
Keywords: aphasia, ischemic stroke, brain mapping, lesion studies
Introduction
A core aim of modern neuroscience is to identify the anatomical cortical networks underlying specific cognitive functions. It is widely agreed that while functional neuroimaging has provided substantial insights into networks engaged in particular cognitive tasks, these studies reveal areas where activity is correlated with task performance, but not necessarily critical for that task1, 2. Lesion-deficit association studies provide a complementary methodology, revealing areas essential for the task. The assumption is that if an individual has impairment of a particular function following brain damage, the lesioned region must have been necessary for that function. However, the opposite inference does not necessarily follow: if there is damage to a particular area, and the individual does not have impairment in a function, that area must not be necessary for that function. This latter assumption will be false when (1) other brain regions have assumed the function of the damaged area (e.g. in the course of recovery after stroke, or over time in the presence of slow-growing tumors3 or chronic brain disease4, or (2) damage to the critical area is insufficient to cause a detectable impairment due to residual function of surviving portions – the so-called partial-injury problem5.
Here we examine the strength of evidence, and conclusions that can be drawn, from lesion-deficit association studies, illustrating with studies of auditory word comprehension in stroke. We chose spoken word comprehension because this topic has recently engendered controversy. While many previous studies from autopsy6, direct cortical stimulation7 and stroke have indicated that superior temporal gyrus posterior to the temporal pole (hereafter pSTG) is necessary for auditory word comprehension, a recent study of primary progressive aphasia (PPA) shed doubt on this conclusion8. The investigators found that seven of 72 patients with PPA had peak cortical thinning in left temporoparietal cortex, but relatively intact word comprehension. Although at a group level, areas of cortical thinning associated with word comprehension rate included parts of pSTG, results were interpreted as evidence against classical thinking that “Wernicke’s area” (defined as pSTG, middle temporal gyrus, and inferior parietal cortex) is critical for auditory word comprehension.
This paper by Mesulam and colleagues illustrates that studies of neurodegenerative disorders may complement studies of patients with acute lesions and chronic lesions, to understand brain function. Their study emphasized the role of areas beyond the usual bounds of Wernickes area in word comprehension. While this information is important for identifying the entire network of brain regions that support word comprehension, consideration of focal lesion studies are also necessary to put these findings into context.
Here we focus on lesion studies that have evaluated areas critical for auditory word comprehension, and touch on areas critical for sentence comprehension. Both entail numerous cognitive functions (Table 1), and each function likely relies on a complex cortical-subcortical network. We cannot review all areas involved in all of these functions underlying comprehension. For example, a vast literature is devoted to the neural representation of semantics, showing broadly distributed areas including the bilateral anterior and inferior temporal cortex represent object semantics9–12. To elucidate our points we review studies of acute and chronic stroke patients that have evaluated the role of pSTG in auditory comprehension. We propose that lesion studies can use a variety of approaches to evaluating associations between deficits and tissue damage or dysfunction, but should rely on positive (rather than null) results. Furthermore, the strongest evidence is obtained when results from a variety of approaches converge on the same conclusions.
Table 1.
Cognitive Processes Underlying Word and Sentence Comprehension
| Spoken Word Comprehension |
| Early auditory processing (relying on the auditory pathway, from the auditory nerve synapses in the cochlear nuclei through the superior olivary nucleus in the medulla, lateral lemniscus, inferior colliculus, medial geniculate body, to Heschl’s gyrus in superior temporal gyrus) |
| Word recognition |
| Phonological processing |
| Access to word meaning |
| Distributed semantic representation (e.g. for an object, what it looks like may be represented by different regions from how it moves, what it smells like, what it sounds like, etc.) |
| Spoken Sentence Comprehension (many of these operate in parallel, rather than serially) |
| Early auditory processing (see above) |
| Word recognition |
| Distributed semantic representations of individual words |
| Combinatorial semantics |
| Syntactic parsing |
| Phonological short term memory |
| Semantic short term memory |
The Role of pSTG in Auditory Word Comprehension: Evidence from Chronic Stroke
An early lesion-deficit association study of 39 patients with left hemisphere ischemic stroke studied monthly for six months after stroke showed that lesions in left pSTG and infrasylvian SMG were strongly associated with poor auditory comprehension at one month through six months13. Furthermore, patients with initially intact auditory comprehension tended to have lesions that excluded these regions.
A study of 10 men with “Wernicke’s aphasia” showed a highly significant correlation between the extent of lesion in pSTG and severity of auditory comprehension deficit. There was not a significant correlation between total temporoparietal lesion size and severity of auditory comprehension deficit. Patients with damage to 50% or less of pSTG had good comprehension at six months after the onset of stroke, while those with damage to >50% of pSTG had poor auditory comprehension even one year post-stroke14.
In a meticulous study of 18 aphasic patients, Hart and Gordon15 identified patients with pure deficits in semantic comprehension at the single-word level, defined through detailed analyses that excluded possible confounding deficits (e.g. in auditory or visual perception or speech production). These pure semantic word comprehension deficits were found to be associated with infarct in pSTG and inferior parietal cortex. Patients without damage to this area did not have such deficits.
Furthermore, a study by Leff and colleagues16 of 210 right and left hemisphere chronic stroke patients (mean 35 months) who had high-resolution structural MRI and testing of language and auditory short-term memory found that grey matter density (a measure of structural integrity) of pSTG and superior temporal sulcus was associated with auditory comprehension as well as auditory short-term memory (measured with digit span), even after factoring out performance on other measures and lesion volume.
A recent study including 98 participants with chronic aphasia following left hemisphere stroke used support vector machine learning, a multivariate analysis approach, to distinguish between different aphasia types based solely on lesion location17. Results revealed that what distinguishes Wernicke’s aphasia from other aphasia types is that pSTG is damaged. In cases of aphasia where pSTG is spared, it is very unlikely that the patient would have Wernicke’s aphasia. Although this study did not focus specifically on single word comprehension, it also provides evidence that pSTG is important for auditory comprehension.
To specifically address whether damage to left pSTG is associated with impaired single word comprehension in chronic stroke, we conducted a VLSM study (N=138) where the dependent factor was error rate in one subtest of the Western Aphasia Battery-Revised18- auditory word recognition. This test requires matching a spoken word to one of six pictures or objects.
Inclusion criteria included > 1 year post onset of single left hemisphere ischemic stroke, right handed, at least 18 years old, native speaker of English, without previous neurological disease of the brain, or uncorrected visual or hearing loss. Mean age was 60.45 (SD=11.34); 38% were female.
We acquired MRI scans on a Siemens Trio 3T scanner equipped with a 12-element head coil within two days of language testing. We obtained T1-weighted MP-RAGE sequence with 1 mm isotropic voxels (TR = 2250 msec, TI = 925 msec, TE = 4.5 msec), a 256 × 256 matrix size, 192 slices and a 9-degree flip angle. In addition we acquired a T2-weighted image using a sampling perfection using a different flip angle evolution (3D-SPACE) sequence (TR = 2800 msec, TE of 402 msec, variable flip angle, 256 × 256 matrix size with 192 1 mm thick slices).
A trained neurologist manually drew lesions on the T2 weighted image and then coregistered the T2 image to the T1 image. We used these parameters to reslice the lesion into the native T1 space. Jagged edges associated with manual drawing on the resliced lesion maps were smoothed with a 3 mm full-width half maximum Gaussian kernel. Enantiomorphic normalization was accomplished using SPM12 and in-house Matlab scripts (for details of this process see19) VLSM was carried out in MRIcron (www.mccauslandcenter.sc.edu). The univariate VLSM analysis relied on permutation thresholding (4000 permutations; p<0.05), general linear regression, and only included regions where at least 10 patients had damage. Results revealed damage to pSTG and temporal pole (TP; Z>5.16; 55,119 voxels survived threshold) predicts poor performance on auditory word-picture/object matching (Figure 1, Panel A). As was the case in the Mesulam et al.8 study, single word comprehension scores were correlated with scores on the Pyramids and Palm Trees test (r=.58), a test that assesses amodal semantic processing and requires participants to match a target to one of two semantically related pictures20. To distinguish voxels critical for amodal semantic processing from those critical for auditory word comprehension, a multivariate analysis was conducted using the same parameters as before but now including Pyramids and Palm Trees test scores as a cofactor (N=99). Results from this multivariate analysis showed that damage to the middle pSTG (Z>4.87; 2,167 voxels survived threshold) is strongly associated with lower auditory comprehension scores (Figure 1, Panel B, left). Damage to the medial TP (Z>4.60; 99 voxels survived threshold) was related to lower scores on the Pyramids and Palm Trees test (Figure 1, Panel B, right). The analyses above were also run with lesion volume as a regressor but none yielded a statistically significant result. Our findings suggest damage to the left middle pSTG impairs single word auditory comprehension in chronic stroke; while damage to TP disrupts amodal semantic processing9,10.
Figure 1.
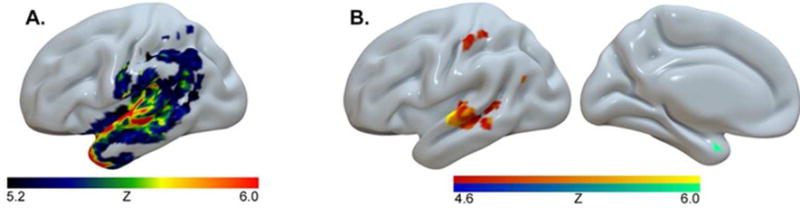
Panel A. Lesions associated with auditory word recognition and object semantics. Damage to pSTG and temporal pole (TP) is associated with poor performance on the Auditory Word Recognition sub-test of the Western Aphasia Battery.
Panel B, left. A multivariate analysis revealed the lesion location that most accurately predicts WAB Auditory Word Recognition scores, with Pyramids and Palm Trees scores included as a cofactor, is in left pSTG and superior temporal sulcus (STS).
Panel B, right. The same analysis revealed damage to the medial portion of left temporal pole best predicts scores on the Pyramids and Palm Trees test, when WAB Auditory Word Recognition scores are included as cofactor.
The Role of pSTG in Auditory Word Comprehension: Evidence from Acute Stroke
Ischemic stroke results in relatively focal lesions in premorbidly normal individuals, making it an excellent model for evaluating lesion-deficit associations. Nonetheless, one caveat is that there is often substantial recovery and structure/function reorganization after stroke. This limitation can be avoided by studying patients acutely after stroke. However, in acute stroke, deficits are often due to areas of hypoperfusion beyond the infarct as well as the infarct itself21,22. Therefore, the entire region of dysfunctional tissue must be measured to determine the area responsible for acute deficits23.
Studies of patients with acute ischemic stroke that have evaluated areas of hypoperfusion and infarct provide evidence that left pSTG is critical for word comprehension by demonstrating: (1) acute dysfunction of pSTG is statistically associated with impairment in auditory word comprehension; (2) reperfusion of pSTG results in restored auditory word comprehension; and (3) severity of hypoperfusion in pSTG is correlated with severity of impairment in word comprehension. These studies are briefly reviewed below.
Abrupt dysfunction of pSTG is statistically associated with impairment in auditory word comprehension
Several previous studies, using various statistical tests, have shown a reliable association between acute tissue dysfunction in left pSTG and impairment in auditory word comprehension. Studies from our laboratory employed a spoken word/picture verification test in which a picture of a common object is presented once with a target (e.g., a picture of a horse is presented with the question, “Is this a horse?”), once with a semantic foil (e.g. cow for horse), and once with a phonological foil (e.g. nurse for horse) (17 items, 51 trials). Error rate was evaluated as a continuous variable or defined as impaired if > 2 SD below the mean for normal age-matched controls (> 10% errors)24.
In each of these studies, areas of dysfunctional brain tissue, defined by Brodmann areas (BA)25, clusters of voxels, or grey and white matter parcels on the JHU-MNI Brain Parcellation Map (cmrm.med.jhmi.edu), were defined as areas of ischemia identified on diffusion weighted imaging (DWI) and/or perfusion-weighted imaging (PWI). That is, if ROI was acutely infarcted on DWI or significantly hypoperfused on PWI (>4 sec delay in time to peak (TTP) arrival of contrast, it was considered dysfunctional26.
An initial study evaluated BAs in “language cortex” where hypoperfusion/infarct was associated with impaired auditory word comprehension, both as dichotomous variables in 66 patients within 24 hours of acute ischemic stroke. The following BAs were evaluated by technicians masked to the language testing: BA 6, 9, 21, 22, 37, 38, 39, 40, 44, 45 (in left frontal, temporal, and parietal cortex), using a published atlas25. In this atlas, BA 22 is the segment of STG that is posterior to the temporal pole, excluding Heschl’s gyrus (pSTG as we have defined it). The strongest association was between BA 22 (pSTG) and impaired auditory word comprehension: X2 (df1) = 47.2; p < 0.00000127.
The above studies, however, only evaluated associations between BAs (cortical regions) and comprehension. We recently carried out a VSLM study evaluating the association between severity of auditory word comprehension (measured with the word/picture verification test described above) and percentage of voxels with dysfunction (infarct and/or hypoperfusion on DWI or TTP as a dichotomous variable) in both gray and white matter regions on the JHU-MNI atlas.
Participants were 169 patients who met the following inclusion criteria: <48 hours from onset of first ever left hemisphere ischemic stroke, right handed, at least 18 years old, native speaker of English, without reduced level of consciousness or sedation, previous neurological disease of the brain, or uncorrected visual or hearing loss. Mean age was 60.1 (SD 14.5) years. Mean education was 12.2 (SD 3.4) years; 49% were female. A subset of the 23 of the 66 patients in the previously published paper was also included in this study of 169 patients. We did not include patients who did not have available B0 maps needed for normalization.
MRI scans obtained within 48 hours of stroke onset were first registered to the MNI atlas using Matlab. Using MRIcron, regions of infarct were drawn on DWI trace images and hypoperfusion was identified on PWI images (TTP maps, co-registered to DWI) by a physician masked to behavioral tests. A single area of “dysfunctional tissue” – areas of abnormality on DWI and/or TTP – was drawn for each patient. Images were then normalized to standard space using SPM12. Next, we computed the normalization transforms for the DWI B=0 image to a template based on age-matched controls28. Finally, these normalization transforms were applied to the lesion maps. Note this method leverages the fact that lesions clearly visible on the TTP and DWI Trace images are typically not yet apparent on the DWI B=0 image, encouraging a normalization which is not disrupted by the unusual appearance of the injury. Once the lesion maps were normalized to standard space, the percentage of voxels in each parcel of the JHU-MNI parcellation map that were dysfunctional (infarcted/hypoperfused) was determined. We evaluated associations between error rate on word-picture verification and dysfunctional tissue using GLM (pooled-variance t-test, linear regression) for gray and white matter regions/voxels on the JHU-MNI atlas where at least 10 patients had lesions. We corrected for multiple comparisons using permutation thresholding (5000 permutations) and corrected for total lesion volume. Figure 2 shows the distribution of all lesions of the patients.
Figure 2.
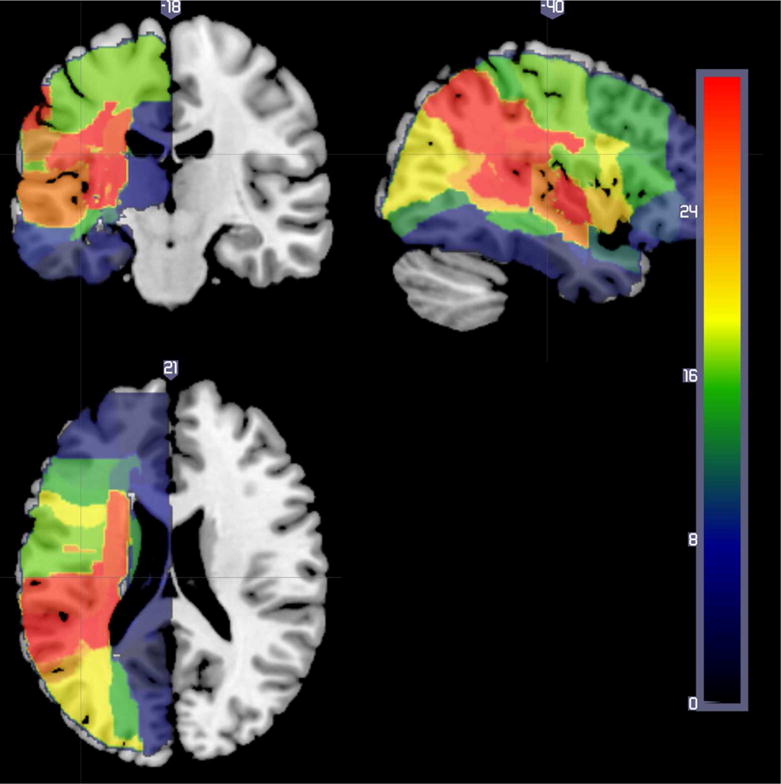
Lesion overlap of all patients in VSLM study of acute patients. The color bar indicates the number of participants with tissue dysfunction in each voxel.
Only one region (left pSTG) had a significant negative Z score (indicating that more voxels with tissue dysfunction was associated with low accuracy; Z= −3.66). Another three regions (superior frontal gyrus, superior parietal gyrus, precuneas left) had Z scores ranging from 2.56 to 2.77, indicating that more voxels with tissue dysfunction in these areas was significantly associated with relatively high performance on word comprehension (Figure 3). In this analysis, neither damage to white matter regions nor damage to temporal pole were significantly associated with severity of auditory word comprehension deficit, although there may have been inadequate power to evaluate the association with temporal pole (particularly after controlling for lesion volume).
Figure 3.
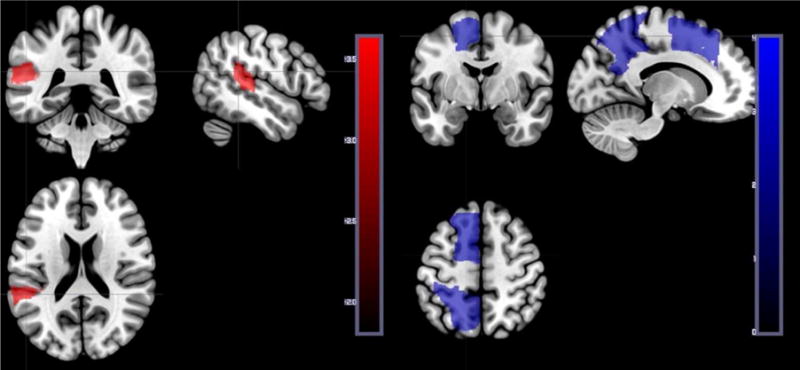
Left panel. Voxels where hypoperfusion and/or infarct was significantly associated with lower accuracy on auditory word-picture verification (in posterior pSTG).
Right panel. Voxels where hypoperfusion and/or infarct was significantly associated with higher accuracy on auditory word-picture verification (i.e. where dysfunction in the area is associated relatively better performance). These results can be accounted for by the fact that all participants had stroke, and those with anterior cerebral artery stroke, superior division MCA stroke, or posterior cerebral artery stroke tended to have spared auditory word comprehension.
One could argue that using permutation thresholding for all regions of interest is necessarily conservative, and therefore while Figure 3 provides strong evidence that pSTG is involved in comprehension, our null result regarding the role of the temporal pole (TP) is necessarily weak. As an extension of this viewpoint, one could argue that perhaps both pSTG and TP show similar trends but due to random noise only the pSTG survives our stringent statistical threshold. To address these concerns we conducted a 10,000 permutation Freedman-Lane analysis29, where proportional injury to pSTG, TP, and total lesion volume were included as predictors. In this analysis, the TP was not a reliable predictor when we included the pSTG and lesion volume as nuisance regressors (t(157)=0.0884, one-tailed p=0.46), while the pSTG was a significant predictor (t(157)=4.52 one-tailed p<0.00001) after removing variability described by lesion volume and proportional TP injury.
Restoration of the function of pSTG results in restored auditory word comprehension
Stronger evidence that an area is essential for a task is obtained if the task is impaired when the area is dysfunctional and the task is restored when the brain area becomes functional again. Thus, in an earlier study30 we examined BAs where hypoperfusion was associated with impaired auditory word comprehension and reperfusion was associated with restored auditory word comprehension. Participants were 90 patients with acute left hemisphere stroke, initially studied at a mean of 10.3 ± 8.4 hours post symptom onset and again within 24 hours after intervention to restore blood flood (or at 3–5 days post stroke onset if there was no intervention). At both the initial and second time point, participants underwent MRI with DWI and PWI and had language testing, including the word-picture verification test, using two forms of the test matched in difficulty. We identified associations between recovery of word comprehension and reperfusion of BAs using chi square tests (with Bonferroni correction for multiple comparisons). The only area where there was a significant association between reperfusion and recovery of word comprehension was in BA 22 (pSTG): X2 (df1) = 18; p<0.0000130. For example, one patient showed improvement from 47% correct on Day 1 to 94% correct on Day 3 on the word-picture verification test described above when blood flow was restored to the entire left pSTG by Day 3. (Figure 4). In this case, reperfusion was brought about by induced blood pressure elevation31.
Figure 4.
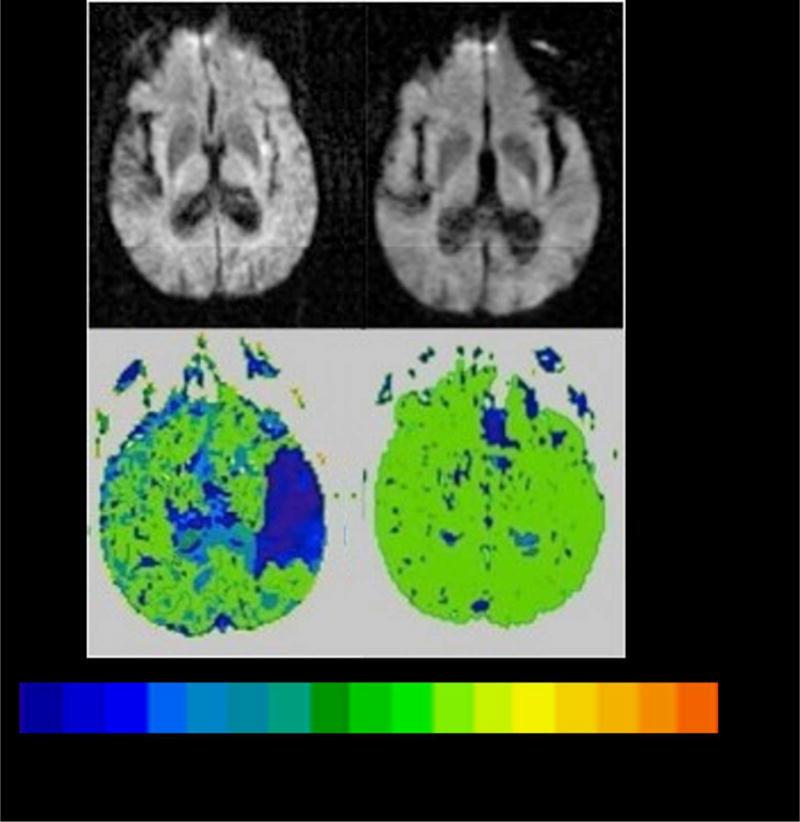
Scans showing reperfusion of left pSTG associated with auditory word comprehension,
Top Panel. DWI on Day 1(left) and Day 3 (right) showing tiny area of infarct in left insular cortex.
Lower Panel. PWI on Day 1 (left) and Day 3 (right) showing severe hypoperfusion of left superior pSTG on Day 1 and reperfusion of left superior pSTG on Day 3, when auditory word comprehension had recovered. The color bar shows the number of seconds of delay in time to peak arrival of contrast, compared to normally perfused tissue (0 seconds).
Severity of dysfunction in pSTG is correlated with severity of impairment in word comprehension
In a previous study of 50 patients within 24 hours post-onset of acute left hemisphere ischemic stroke, severity of auditory word comprehension deficit was measured by error rate in word-picture verification32. Severity of tissue dysfunction was measured by number of seconds of delay in TTP arrival of contrast to each ROI. The strongest correlation was found between severity of word comprehension deficit and severity of hypoperfusion of left BA 22 (r = 0.84; p<<0.000001). (Figure 5). Hypoperfusion of left BA 22 (pSTG) was not significantly associated with impairment of other lexical or sublexical language functions.
Figure 5.
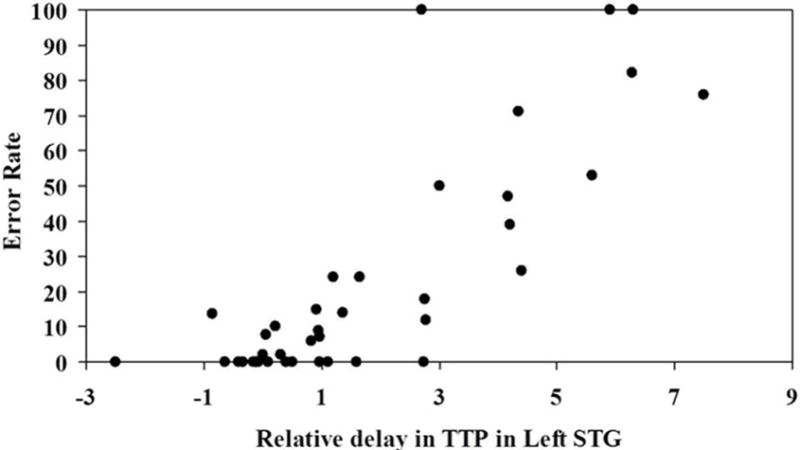
Association between severity of hypoperfusion in left pSTG and severity of deficit (error rate) in auditory word comprehension.
A subsequent study of 116 acute ischemic left hemisphere stroke patients showed that two discriminant functions based on severity of hypoperfusion of six brain regions (BA 21, 22, 37, 39, 40, 44) discriminated between no deficit, word comprehension deficit, and deficits in word production (lexical retrieval or motor speech) (Wilkes Lambda=0.238; Х2 = 142; df14, p < 0.0001)33. These two discriminant functions correctly classified 80% of patients (77.1% in cross-validation). Function 1 was highest (most abnormal) in patients with word comprehension deficits and correlated most strongly with severity of hypoperfusion in left BA 22 or pSTG (r=0.965).
A final published study, of 156 acute left hemisphere ischemic stroke patients, showed that severity of dysfunction in left pSTG was associated with severity of impaired auditory word comprehension, independently of the severity of hypoperfusion of other regions. In multivariable regression, the dependent variable was severity of word comprehension impairment measured with word-picture verification and the independent variables were severity of hypoperfusion in each BA of language cortex. The only area where hypoperfusion was independently associated with error rate in auditory word comprehension was BA 22 (pSTG) (p<0.00001; r2= 0.51)34.
Discussion
Here we illustrated how converging evidence from acute and chronic stroke, focusing on positive results, can provide strong evidence that a particular area is essential for a given function. Most of the studies we reviewed converge in support of the hypothesis that left posterior pSTG (often considered at least part of “Wernicke’s area”), is normally needed for auditory word comprehension. This type of evidence is consistent with, but extends, findings from functional imaging studies that left pSTG is engaged in auditory word comprehension35, 36. Our review shows that auditory word comprehension tends to fail if this region abruptly becomes dysfunctional. In some cases the brain can eventually adapt to damage to this area; other areas may assume its function over time37. On the other hand, Von Monakow in 1914 rejected the concept that other areas of the brain are recruiting to assume the function of the damaged tissue38,39. He attributed recovery to the resolution of diaschisis. That is, he argued that a small lesion to one component of a large network causes impaired function of a widely distributed network of brain regions. The uninjured part of this network is anatomically intact but functionally inhibited. Recovery occurs when this inhibition slowly resolves. Von Monakow argued that localization of symptoms and localization of function are distinct. Thus, pSTG might be one component of a widely distributed network that supports the function spoken word comprehension, and damage to it causes the symptoms of impaired word comprehension; but recovery occurs when inhibition of the remaining network resolves. Nevertheless, those who do not recover spoken comprehension predictably have lesions in left posterior pSTG and sulcus (see data presented above from chronic stroke), indicating that it is likely to be an essential node in the network.
Compatible with the notion that a broadly distributed network supports word comprehension, a few studies support the hypothesis that left TP is critical to auditory word comprehension8. However, it is likely that TP and pSTG have different roles in auditory word comprehension. Left pSTG seems to be more critical for early processing, in access to phonology and meaning40; whereas temporal poles seem to be among the areas critical for amodal representation of semantics9,10. Our study of chronic patients showed that performance on auditory word comprehension was associated with damage to pSTG and STS when Pyramids and Palm Trees scores were included as a cofactor, while performance on Pyramids and Palms Trees was associated with damage to left TP when performance on word comprehension was factored out.
One approach to identifying the broader network that supports a function like word comprehension, using data from brain-damaged patients, has been described by Boes and colleagues41. This approach involves registering the three-dimensional brain lesions to a common reference brain; identifying the functional connectivity of the lesioned area with the rest of the brain using connectome data from healthy controls; and overlapping the networks associated with the lesioned area. This approach has not been taken yet to identify networks that support word comprehension, but represents an important path for future research.
Our definition of “Wernicke’s area” is broader than in some articles, as we included all of the left STG posterior to the temporal pole (rather than just the segment posterior to Heschl’s gyrus), but narrower than other papers that have also included inferior parietal cortex and middle temporal gyrus8. Localization and extent of this area has been controversial8. In some of the studies we have reported, the area where damage was most associated with word comprehension was in the segment of pSTG (BA 22) anterior to Heschl’s gyrus (see Figure 1b, in chronic stroke), the area where a meta-analysis of fMRI studies found activation to be associated with integration of phonemes into words42. In other studies, the area where damage was most strongly associated with impaired word comprehension was posterior to Heschl’s gyrus (e.g. Figure 3, in acute stroke). In some of our studies that used a ROI approach, based on BAs, we did not distinguish these two areas, but found dysfunction in BA 22 was associated with impaired word comprehension (e.g. Figure 5). It seems likely that different parts of pSTG may have slightly different roles because of their connections with other areas. The task employed (e.g. a forced choice word-picture/object matching task in the chronic stroke patients; Figure 1b) might rely on somewhat different cognitive processes and connections than the word-picture verification task with both semantic and phonological foils used in the acute patients (Figure 3). Alternatively, the more posterior segment of pSTG in Figure 3 may be associated with acute auditory word comprehension deficits, while the more anterior area in pSTG may be critical for recovery of word comprehension deficits.
How can we account for data offered against the role of pSTG in word comprehension, such as PPA patients with atrophy in pSTG (and/or posterior middle temporal cortex and inferior parietal lobule) but without substantial word comprehension deficits?8 It should be noted that other studies of areas of atrophy in PPA that are associated with word comprehension deficits have led to different results, although atrophy was evaluated using different methods43,44. Moreover, none of the PPA patients in the Mesulam et al.8 study had complete destruction of pSTG. Rather, they each had cortical thinning (relative to normal controls) in parts of left temporoparietal cortex; it is quite possible that the remaining neural tissue in left pSTG was sufficient to support word comprehension. They also had a slowly progressive disorder, and many had language therapy. Other areas of brain may have assumed functions of some atrophied areas. These issues illustrate the barriers in interpreting null results.
The studies we reviewed used a variety of statistical approaches to lesion-deficit analysis. Some used a region of interest approach and others used a voxel-based approach. Some evaluated auditory comprehension as a continuous variable, some as a dichotomous variable (impaired versus intact). Likewise, some studies evaluated structural damage or tissue dysfunction as a continuous variable, others evaluated structural damage or tissue dysfunction as a dichotomous variable (impaired versus intact), and still others evaluated the number of damaged voxels in regions of interest as continuous variables. Importantly, the studies converged on the same result – that posterior pSTG and sulcus are critical for auditory word (and sentence) comprehension. These studies illustrate that there is no single, correct method of lesion-deficit analysis45. Similarly, studies we reviewed used a variety of statistical approaches, including multivariable regression and discriminant function analyses. Bayesian approaches to identifying networks of regions critical to language functions have also been applied successfully.46
Here we have not considered how functional imaging studies can be used to complement lesion studies in identifying networks essential for language studies47. Recent studies combining resting state functional connectivity MRI with lesion overlap within specific regions of interest have shown that the effect of the structural lesion extends beyond the area of damage, within the bounds of the existing network connections41. Likewise, we have not discussed how task-related functional imaging in stroke patients can provide useful predictive information beyond information provided by the lesion. For example, using a multivariate machine learning technique, data from a functional imaging study of auditory language comprehension at 2 weeks post-stroke, along with age and language scores, accurately predicted language outcome at 6 months post-stroke in 86% of 21 patients post-stroke aphasia48. Combined use of lesion and functional imaging data deserves a separate, full review. Other lesion-deficit mapping approaches, such as transcranial magnetic stimulation49 or cortical stimulation7 to evaluate the effect of temporary lesions can also provide important, complementary evidence regarding networks of cortical regions underlying language and cognitive processes.
This review of lesion studies evaluating the role of left pSTG in auditory comprehension illustrates the types of evidence and their limitations that should be considered in mapping brain-behavior relationships from patients with neurological disease. Most importantly, given some unavoidable weaknesses in all such studies (e.g. individual variability in brain-behavior relationships50), converging positive (rather than null) evidence from a variety of sources yields stronger support for lesion-deficit associations than individual experiments.
Acknowledgments
We are grateful to the individuals who participated in this research, as well as the lab members who collected and analyzed data, including Hinnah Shahid and Amy Wright. The research reported in this paper was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through awards R01 DC05375 and P50 DC014664. The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
Author contributions:
AEH conceived the review and wrote the initial draft, CR edited the paper and assisted with image analysis of the new data, JF edited the paper and provided new data from chronic stroke.
Potential Conflicts of Interest: Nothing to report
References
- 1.Rorden C, Karnath H. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):812–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 2.Fellows LK, Heberlein AS, Morales DA, et al. Method matters: An empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17(6):850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- 3.Sarubbo S, Latini F, Sette E, et al. Is the resection of gliomas in wernicke’s area reliable? Acta Neurochir. 2012;154(9):1653–1662. doi: 10.1007/s00701-012-1416-z. [DOI] [PubMed] [Google Scholar]
- 4.Ochfeld E, Newhart M, Molitoris J, et al. Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke. 2010;41(2):325–330. doi: 10.1161/STROKEAHA.109.570374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorden C, Fridriksson J, Karnath H. An evaluation of traditional and novel tools for lesion behavior mapping. Neuroimage. 2009;44(4):1355–1362. doi: 10.1016/j.neuroimage.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernicke K. Text book of cerebral diseases. Berlin, Germany: 1881. [Google Scholar]
- 7.Lesser RP, Luders H, Morris HH, et al. Electrical stimulation of wernicke’s area interferes with comprehension. Neurology. 1986;36(5):658–663. doi: 10.1212/wnl.36.5.658. [DOI] [PubMed] [Google Scholar]
- 8.Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138(Pt 8):2423–2437. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozeat S, Ralph MAL, Patterson K, et al. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 10.Mummery CJ, Patterson K, Price C, et al. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. [PubMed] [Google Scholar]
- 11.Peelen MV, He C, Han Z, et al. Nonvisual and visual object shape representations in occipitotemporal cortex: Evidence from congenitally blind and sighted adults. J Neurosci. 2014;34(1):163–170. doi: 10.1523/JNEUROSCI.1114-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- 13.Selnes OA, Knopman DS, Niccum N, et al. Computed tomographic scan correlates of auditory comprehension deficits in aphasia: A prospective recovery study. Ann Neurol. 1983;13(5):558–566. doi: 10.1002/ana.410130515. [DOI] [PubMed] [Google Scholar]
- 14.Naeser MA, Helm-Estabrooks N, Haas G, et al. Relationship between lesion extent in ‘wernicke’s area’ on computed tomographic scan and predicting recovery of comprehension in wernicke’s aphasia. Arch Neurol. 1987;44(1):73–82. doi: 10.1001/archneur.1987.00520130057018. [DOI] [PubMed] [Google Scholar]
- 15.Hart J, Gordon B. Delineation of single‐word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- 16.Leff AP, Schofield TM, Crinion JT, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: Evidence from 210 patients with stroke. Brain. 2009;132(Pt 12):3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yourganov G, Smith KG, Fridriksson J, Rorden C. Predicting aphasia type from brain damage measured with structural MRI. Cortex. 2015;73:203–215. doi: 10.1016/j.cortex.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kertesz A. The western aphasia battery - revised. New York: Grune & Stratton; 2007. [Google Scholar]
- 19.Yourganov G, Smith KG, Fridriksson J, Rorden C. Predicting aphasia type from brain damage measured with structural MRI. Cortex. 2015;73:203–215. doi: 10.1016/j.cortex.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard D, Patterson KE. The pyramids and palm trees test: A test of semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- 21.Hillis AE, Barker PB, Beauchamp NJ, et al. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55(6):782–788. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- 22.Olsen TS, Bruhn P, Oberg RG. Cortical hypoperfusion as a possible cause of ‘subcortical aphasia’. Brain. 1986;109(Pt 3):393–410. doi: 10.1093/brain/109.3.393. [DOI] [PubMed] [Google Scholar]
- 23.Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain. 2002;125(Pt 5):1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- 24.Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain. 2002;125(Pt 5):1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- 25.Damasio H, Damasio AR. Lesion analysis in neuropsychology. Oxford University Press; USA: 1989. [Google Scholar]
- 26.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41(12):2817–2821. doi: 10.1161/STROKEAHA.110.594432. [DOI] [PubMed] [Google Scholar]
- 27.Hillis AE, Kane A, Tuffiash E, Beauchamp NJ, Barker PB, Jacobs MA, Wityk RJ. Neural substrates of the cognitive processes underlying spelling: Evidence from MR diffusion and perfusion imaging. Aphasiology. 2002;16(4–6):425–38. [Google Scholar]
- 28.Rorden C, Bonilha L, Fridriksson J, et al. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillis AE, Heidler J. Mechanisms of early aphasia recovery. Aphasiology. 2002;16(9):885–895. [Google Scholar]
- 31.Hillis AE, Ulatowski JA, Barker PB, et al. A pilot randomized trial of induced blood pressure elevation: Effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis. 2003;16(3):236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- 32.Hillis AE, Wityk RJ, Tuffiash E, et al. Hypoperfusion of wernicke’s area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50(5):561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- 33.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(Pt 5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 34.Newhart M, Ken L, Kleinman JT, et al. Neural networks essential for naming and word comprehension. Cogn Behav Neurol. 2007;20(1):25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- 35.Wise R, Chollet F, Hadar U, et al. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114(Pt 4):1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- 36.Booth JR, Burman DD, Meyer JR, et al. Modality independence of word comprehension. Hum Brain Mapp. 2002;16(4):251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp DJ, Scott SK, Wise RJ. Retrieving meaning after temporal lobe infarction: The role of the basal language area. Ann Neurol. 2004;56(6):836–846. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- 38.York GK., III Localization of language function in the twentieth century. J Hist Neurosci. 2009;18(3):283–290. doi: 10.1080/09647040802025979. [DOI] [PubMed] [Google Scholar]
- 39.Von Monakow C. Dielokalisation im grosshirn und der de abbau der funktion durch kortikale herde. Wiesbaden: J.F Bergmann; 1914. [Google Scholar]
- 40.Robson H, Keidel JL, Ralph MAL, Sage K. Revealing and quantifying the impaired phonological analysis underpinning impaired comprehension in wernicke’s aphasia. Neuropsychologia. 2012;50(2):276–288. doi: 10.1016/j.neuropsychologia.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(Pt 10):3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proceedings of the National Academy of Sciences. 2012 Feb 21;109(8):E505–14. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faria AV, Sebastian R, Newhart M, et al. Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: Potential clinical significance. Aphasiology. 2014;28(8–9):948–963. doi: 10.1080/02687038.2014.911241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in primary progressive aphasia. J Neurosci. 2012;32(23):7986–7991. doi: 10.1523/JNEUROSCI.6241-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crinion J, Holland AL, Copland DA, et al. Neuroimaging in aphasia treatment research: Quantifying brain lesions after stroke. Neuroimage. 2013;73:208–214. doi: 10.1016/j.neuroimage.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen R, Hillis AE, Pawlak M, Herskovits EH. Voxelwise bayesian lesion-deficit analysis. Neuroimage. 2008;40(4):1633–1642. doi: 10.1016/j.neuroimage.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saur D, Ronneberger O, Kummerer D, et al. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain. 2010;133(Pt 4):1252–1264. doi: 10.1093/brain/awq021. [DOI] [PubMed] [Google Scholar]
- 49.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience–virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 50.Steinmetz H, Seitz RJ. Functional anatomy of language processing: Neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29(12):1149–1161. doi: 10.1016/0028-3932(91)90030-c. [DOI] [PubMed] [Google Scholar]


