Abstract
Objective
To examine the relationships between growth (birth to age 2 years) and developmental outcomes in children born with very low birthweight (VLBW).
Design
Motor and mental development in children born with VLBW were regressed on anthropometric measurements at birth, 9 months and 2 years using multivariable regression.
Setting
The Early Childhood Longitudinal Study—Birth Cohort, a longitudinal cohort, community sample, designed to be representative of children born across the USA.
Patients
950 children born with VLBW (<1500 g).
Main Outcome Measures
Motor and cognitive scores on the Bayley Scales at 9 months and 24 months chronological age.
Results
A high proportion of children exhibited poor growth, with length-for-age z-scores <−2 (ie, stunting) in 21.3% of children at 9 months (adjusted for prematurity) and 34.2% of children at 2 years. Compared with children having z-scores >−2, children with growth shortfalls in head circumference, length and weight had a higher adjusted OR (aOR) of low Bayley motor scores at 9 months and 2 years (aOR ranging from 1.8 to 3.3, all p<0.05), while low Bayley cognitive scores were predicted by 9-month deficits in length and weight (aOR 2.0 and 2.4, respectively, both p<0.01) and 2-year deficits in length and head circumference (aOR 2.9 and 2.8, both p<0.05).
Conclusion
Anthropometric measures of growth were linked to current and future neurodevelopmental outcomes in children born with VLBW. While careful length measures may be a particularly useful marker, deficits in all anthropometric measures were risk factors for developmental delays.
INTRODUCTION
Cognitive and motor development in early life are tied to later success in school and the workplace.1–3 Contributors to cognitive and motor development are complex and include influences that occur during early development, both before and after birth.4–6 Growth from the prenatal period through the first 3 years, in particular, has been linked to cognitive and motor development both as a surrogate for overall health and development and as the markers of increasing brain size and neurological maturation.7–9 Anthropomorphic measurements taken at birth and in early childhood have been used in studies of child development over the past half century to document this association.10,11 Readily available as part of general paediatric care, weight, head circumference (HC) and length, are assessed frequently during the first 3 years of life.12
Children born with low birthweight (LBW, <2500 g) comprise about 8% of births annually in the USA13 and about 16% of births worldwide.14 In the USA, about 1.5% of children are born with very LBW (VLBW, <1500 g)13 and 11.4% of births were preterm (before 37 weeks gestation) in 2013.13 International estimates of VLBW births vary significantly due to differences in local practice patterns surrounding neonatal resuscitation and infant care in prematurity.14 Children born with VLBWare known to be at particularly high risk of developmental difficulties and poor cognitive and motor outcome.11,15,16
Nutritional support for infants born with VLBW has improved significantly since the introduction of intravenous (IV) nutrition for preterm infants in the 1970’s17 and specialised preterm infant formulas in the 1980s.18 It is assumed that improvements in early growth among infants born with VLBW seen with modern neonatal intensive care translates into improved cognitive and motor performance in childhood and beyond. However no nationally representative, longitudinal study, evaluating the relationship between growth parameters and neurocognitive outcomes of VLBW infants has been completed since improvements in VLBW infant nutrition have become standard of care. Understanding the relationship between easily obtained measures of growth and neurocognitive outcomes could be clinically useful for early identification of children at risk of lower neurological developmental status.
The goal of this study was to evaluate the relationship between longitudinally measured growth parameters (weight, length, HC) and performance on the Bayley Scales of infant and toddler development during the first 2 years in children born with LBW. We analysed data from the Early Childhood and Longitudinal Study—Birth Cohort (ECLS-B), a nationally representative comprehensive cohort study that oversampled children born with VLBW. We tested a hypothesis that early anthropometry and growth would correlate with Bayley scores at both 9 months and 2 years.
METHODS
The ECLS-B is a large multi-source, multi-method study sponsored by the National Center for Education Statistics (NCES), US Department of Education.19,20 It includes a nationally representative sample of children born in 2001 who were selected based on a random sampling of birth certificates and examined longitudinally at birth, at 9 months and at 2 years. The study sampled births within primary sampling units from the National Center for Health Statistics vital statistics programme, stratified by geographical region, median household income, proportion minority population and metro versus non-metro area. The NCES ethics review board approved the study and parents gave informed consent. The researchers obtained a final sample of approximately 10 700 completed interviews (rounded to the nearest 50 in compliance with NCES rules). Of these, approximately 950 children were born with VLBW by birth certificate records. Parents reported how many days their child stayed in the hospital after birth for medical problems and how many days the child was on a ventilator.
Anthropometry
Birthweight data were gathered directly from birth certificates. Direct measurements of anthropometry were obtained by trained researchers using standardised protocols and equipment including a digital scale, stadiometer, and looped tape measure. Children were dressed in light clothing without shoes. Measurements were taken twice, if these were within 5% of each other, their average was used, otherwise a third measurement was taken and the three measurements averaged. At 9 months recumbent length was measured and at 2 years standing height was measured. All anthropometric measurements were converted to age-specific and gender-specific percentiles and z-scores using the 2006 WHO growth charts.21 In addition to weight and height, we used body mass index (BMI) to take into account changes in body mass over time with age.22 For children born <37 weeks, we created a corrected age at the 9-month visit by subtracting the number of weeks the child was born prematurely from the chronological age; we then used this corrected age to create anthropometry z-scores at 9 months. We classified children as being small or large for z-scores <−2 or >2, respectively. To adjust birth weights for gestational age and gender, we used the Fenton z-score calculator.23
Developmental outcomes
Trained researchers administered the Bayley Short Form— Research edition to children at both the 9-month and 2-year waves. Mental scores include problem-solving and language tasks; motor scores include fine and gross motor skills. Using Item Response Theory modelling, the ECLS-B researchers estimated Bayley Scales of infant development scores.19 Standardised t-scores were created for the ECLS-B data to enable individual comparisons with the total sample of 10 700 children studied in the ECLS-B. T-scores were based on age, adjusting for prematurity. The mental and motor t-scores have mean of 50 and SD of 10.
Covariates
Parents identified their child’s gender. NCES calculated socioeconomic status (SES) based on family income, maternal education, maternal occupation, paternal education and paternal occupation.19 Participants were categorised into SES quintiles (lowest=1; highest=5).
Statistical analysis
All analyses were run using SAS (V.9.4; SAS Institute, Research Triangle, North Carolina, USA). We used linear regression analyses to predict Bayley t-scores from anthropometry z-scores. As other studies have found differences between genders,24 we assessed for interaction terms. Next, we completed the analyses in a categorical fashion, looking at those who fall two SDs below the mean for anthropometry assessments, predicting odds of Bayley Scores more than 2 SD below the mean. All regression models were run without adjustment and with adjustment for potential confounders (birthweight, race/ethnicity, sex, SES). We assessed relationships between 2-year Bayley outcomes based on anthropometric measures at 9 months and based on the change in z-scores between 9 months and 2 years.
RESULTS
Anthropometric measurements and Bayley Scores were available for approximately 950 VLBW children at age 9 months and 650 children at 2 years. Of these, 34% were born extremely premature (22 to <28 weeks), 46% were born very premature (28 to <32 weeks), 18% were born moderate-to-late preterm (32 to <37) and 3% were born full term (≥37) (see online supplementary figure S1). Further demographic information is displayed in table 1.
Table 1.
Participant characteristics by birthweight category (N=950*)
| Birthweight category (data provided as percentage by category)
|
||||
|---|---|---|---|---|
| <750 g | 750–<1000 g | 1000–<1250 g | 1250–<1500 g | |
| Percentage by birthweight category | 14 | 24 | 29 | 32 |
| Variable (% by category, columns add to 100%) | ||||
| Gender | ||||
| Male (50%) | 49 | 56 | 43 | 52 |
| Female (50%) | 51 | 44 | 57 | 48 |
| p Value (χ2) | 0.02 | |||
| Gestation | ||||
| Extremely preterm (22 to <28 weeks) (33%) | 82 | 58 | 21 | 7 |
| Very preterm (28 to <32 weeks) (50%) | 13 | 36 | 58 | 56 |
| Moderate-to-late preterm (32–<37 weeks) (17%) | 5 | 5 | 20 | 31 |
| Term | 0 | 1 | 1 | 6 |
| p Value (χ2) | <0.0001 | |||
| Socio-economic status | ||||
| 1. High (22%) | 24 | 17 | 23 | 24 |
| 2. Medium high (22%) | 23 | 22 | 22 | 23 |
| 3. Medium (22%) | 21 | 28 | 19 | 20 |
| 4. Medium low (17%) | 15 | 17 | 19 | 16 |
| 5. Low (17%) | 17 | 16 | 17 | 18 |
| p Value (χ2) | 0.6 | |||
| Parental education | ||||
| 1. Less than high school (17%) | 15 | 15 | 15 | 17 |
| 2. High school (28%) | 29 | 21 | 27 | 31 |
| 3. Some college/vocational (28%) | 31 | 36 | 30 | 22 |
| 4. College degree (17%) | 17 | 18 | 15 | 17 |
| 5. Graduate or doctorate (11%) | 8 | 10 | 13 | 12 |
| p Value (χ2) | 0.1 | |||
| Hospital stay | ||||
| 1–15 days | 1 | 2 | 4 | 6 |
| 15–30 days | 0 | 1 | 14 | 40 |
| 30–60 days | 7 | 32 | 49 | 42 |
| >60 days | 92 | 65 | 33 | 12 |
| <0.0001 | ||||
| Days on ventilator | ||||
| 0 days | 4 | 10 | 29 | 43 |
| 1–15 days | 16 | 34 | 40 | 39 |
| 15–30 days | 13 | 23 | 16 | 7 |
| 30–60 days | 33 | 22 | 11 | 7 |
| >60 days | 34 | 12 | 4 | 4 |
| <0.0001 | ||||
N rounded to the nearest 50 in compliance with National Center for Education Statistics (NCES) guidelines.
NS, not significant (p>0.05).
For each of the anthropometric measures (HC, height, weight and BMI), there was a high proportion of children with z-scores below −2 SD at both 9 months and 2 years (see online supplementary table S1). Greater than 5% of children had z-scores >2 for HC and BMI at 9 months and 2 years.
Motor and mental development as reported by mean Bayley t-scores is shown for each birthweight category in figure 1. These t-scores are standardised for age corrected for gestation. Mean values shown are further adjusted for sex, race/ethnicity and SES. All groups of children born with VLBW had t-score means below the overall mean created to be 50 for the general population. Compared with children in lower birthweight categories, children in higher birthweight categories had higher Bayley t-scores for motor skills at both time points and for cognitive skills at 9 months, even taking into account degree of prematurity and socio-economic factors. Using linear regression assessing association between birthweight (in grams) and Bayley t-scores, each of these categories of development (motor and mental at 9 months and 2 years) was significantly associated (all p<0.05, data not shown). This was also true for relationships between birthweight z-score for gestational age as determined by the Fenton measurements and motor and cognitive scores at 9 months and 2 years (p<0.05).23 In evaluating for odds of low Bayley scores in children born small for their gestational age (SGA) (birthweight for gestation <10th percentile), children born SGA had higher odds of having low scores at 9 months but not 2 years (see online supplementary table S2).
Figure 1.
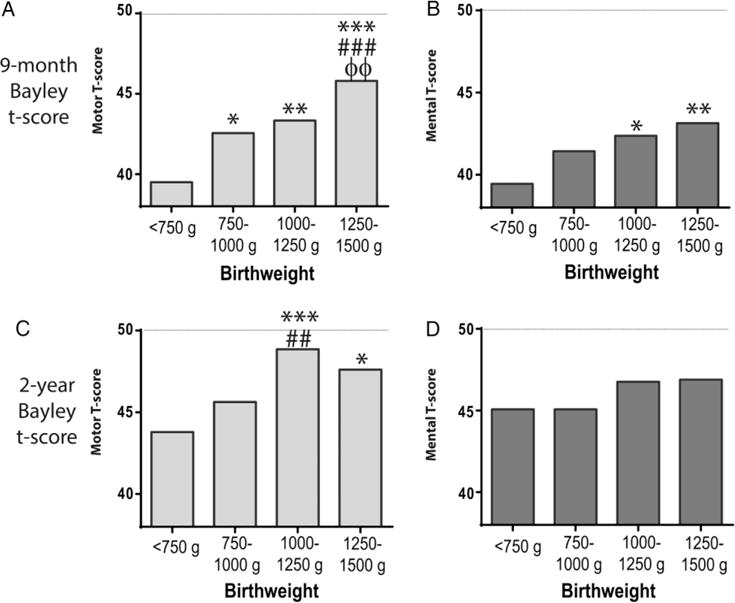
Mean Bayley development scores by birthweight categories. T-scores for motor (A and C) and mental (B and D) development at 9 months and 2 years are adjusted for sex, race/ethnicity, socio-economic status and gestational age. The population mean for these scores is set to 50 with a SD of 10. Comparison to group weighing <750 g: *p<0.05; **p<0.01; ***p<0.001. Comparison to group weighing 750–1000 g: ##p<0.01; ###p<0.001. Comparison to group weighing 100–1250 g: ΦΦ p<0.01.
Table 2 provides results of linear regression analyses examining the relationships between HC, length, weight and BMI on Bayley t-scores. In cross-sectional regression of Bayley t-scores at both time points, when adjusting for birthweight and other covariates, length and weight were strong predictors; HC was a predictor in all models with the exception of cognition at 9 months. BMI was a predictor in adjusted models of motor outcomes at 2 years. In assessing which factors were related to change in Bayley score over time, both length and weight were significantly associated with change in Bayley motor and mental scores, even after adjusting for baseline Bayley score at 9 month.
Table 2.
Linear regression of Bayley motor and cognitive scores by WHO growth parameters
| HC z-score | Length z-score | Weight z-score | Overall | BMI z-score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p Value | Estimate | SE | p Value | Estimate | SE | p Value | p Value | Estimate | SE | p Value | |
| 9-month Bayley score by 9-month anthropometry | |||||||||||||
| Motor | 0.60 | 0.19 | 0.002 | 0.97 | 0.25 | 0.0001 | 0.75 | 0.25 | 0.003 | 0.005 | 0.18 | 0.22 | 0.4 |
| Cognitive | 0.39 | 0.23 | 0.09 | 1.24 | 0.30 | <0.0001 | 1.23 | 0.29 | <0.0001 | 0.004 | 0.48 | 0.25 | 0.06 |
| 2 years Bayley score by 9-months anthropometry | |||||||||||||
| Motor | 0.23 | 0.22 | 0.3 | 0.83 | 0.29 | 0.004 | 1.0 | 0.28 | 0.0003 | 0.02 | 0.45 | 0.24 | 0.06 |
| Cognitive | 0.41 | 0.18 | 0.02 | 0.64 | 0.23 | 0.005 | 0.57 | 0.22 | 0.01 | 0.01 | 0.13 | 0.19 | 0.5 |
| 2-year Bayley score by 2-year anthropometry | |||||||||||||
| Motor | 1.17 | 0.33 | 0.0004 | 1.51 | 0.35 | <0.0001 | 1.57 | 0.32 | <0.0001 | <0.0001 | 0.95 | 0.32 | 0.004 |
| Cognitive | 0.57 | 0.27 | 0.03 | 1.09 | 0.28 | 0.0001 | 0.64 | 0.26 | 0.01 | 0.0006 | 0.36 | 0.27 | 0.2 |
| Change in Bayley by 9-month anthropometry* | |||||||||||||
| Motor | 0.0051 | 0.21 | 1.0 | 0.57 | 0.28 | 0.04 | 0.64 | 0.27 | 0.02 | 0.18 | 0.31 | 0.28 | 0.3 |
| Cognitive | 0.23 | 0.17 | 0.2 | 0.56 | 0.23 | 0.02 | 0.24 | 0.22 | 0.03 | 0.19 | −0.45 | 0.28 | 0.1 |
Relationships shown are for z-scores of each of the anthropometric measures, corrected for gestational age at 9 months but not 2 years. All models adjusted for sex, race/ethnicity, SES and birthweight. Bold face indicates p<0.05.
Analysis regarding change in Bayley further adjusted for 9-month Bayley scores.
Overall p value represents the significance of the combined effects of HAZ, WAZ and HCZ on the Bayley score, adjusting for sex, race/ethnicity, SES and birthweight, and in the final two models, 9 month Bayley score.
BMI, body mass index; HC, head circumference; SES, socio-economic status.
When height for age Z-score (HAZ), weight for age Z-score (WAZ) and head circumference for age Z-score (HCZ) were put into models together, the overall p values were significant for all models except when the outcome was change in Bayley score.
Results for logistic regression analyses are found in table 3. Children who were more than 2 SDs below the mean in anthropometry consistently had increased odds of having developmental delays characterised by Bayley t-scores more than 2 SDs below the mean. For low motor scores at both time points, these odds were statistically significant as related to low z-scores for HC, length and weight. For low cognitive scores, children with shorter length and lower weight at 9 months and those with shorter length and smaller HC at 2 years had higher odds of delay. When HAZ, WAZ and HCZ were put into models together, overall p values were significant for all except those predicting cognitive Bayley scores at 2 years.
Table 3.
Odds of Bayley motor and cognitive scores more than 2 SD below the mean by WHO growth parameters 2 SD below mean
| Head circumference | Length | Weight | Overall | BMI | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (CI) | p Value | OR (CI) | p Value | OR (CI) | p Value | p Value* | OR (CI) | p Value | |
| 9-month Bayley score by 9-month anthropometry | |||||||||
| Motor | 2.71 (1.62 to 4.56) | 0.0002 | 3.02 (1.91 to 4.76) | <0.0001 | 3.03 (1.84 to 4.99) | <0.0001 | 0.002 | 2.18 (1.13 to 4.20) | 0.02 |
| Cognitive | 1.25 (0.71 to 2.19) | 0.4 | 2.01 (1.23 to 3.11) | 0.002 | 2.41 (1.50 to 3.89) | 0.0003 | 0.01 | 2.08 (1.12 to 3.87) | 0.02 |
| 2-year Bayley score by 9-month anthropometry | |||||||||
| Motor | 1.66 (0.77 to 3.55) | 0.2 | 1.36 (0.70 to 2.67) | 0.4 | 2.64 (1.34 to 5.22) | 0.005 | 0.04 | 1.91 (0.77 to 4.76) | 0.2 |
| Cognitive | 1.50 (0.64 to 3.53) | 0.4 | 1.42 (0.70 to 2.87) | 0.3 | 1.71 (0.79 to 3.72) | 0.2 | 0.2 | 1.68 (0.63 to 4.54) | 0.3 |
| 2-year Bayley score by 2-year anthropometry | |||||||||
| Motor | 3.28 (1.61 to 6.67) | 0.0010 | 1.81 (1.01 to 3.24) | 0.05 | 2.59 (1.16 to 5.77) | 0.02 | 0.02 | 3.10 (1.09 to 8.81) | 0.03 |
| Cognitive | 2.75 (1.21 to 6.24) | 0.02 | 2.86 (1.37 to 5.99) | 0.005 | 2.36 (0.93 to 6.00) | 0.07 | 0.008 | 1.23 (0.27 to 5.50) | 0.8 |
Model adjusted for sex, race/ethnicity, SES, birthweight. Bold face indicates p<0.05.
BMI, body mass index; SES, socio-economic status.
p Value represents the overall significance of HAZ, WAZ and HCZ together, adjusting for sex, race/ethnicity, SES and birthweight.
DISCUSSION
Unlike previous cohorts evaluating the relationship between anthropometry and outcome, children in our cohort were born in a time when high calorie IV and enteral nutrition were available from birth. We found in this cohort of children that multiple measures of growth prior to age 2 were linked to early developmental abilities: (1) in cross-sectional analyses, z-scores for height, weight and HC were correlated with Bayley scores of motor and cognitive development at 9 months and 2 years; (2) length and weight measures at 9 months were correlated with that child’s Bayley scores at 2 years and (3) the 9-month length and weight z-scores correlated with the change in Bayley scores from 9 to 24 months. Despite access to high calorie enteral feeding, this cohort remained at high risk for suboptimal postnatal growth. In our sample, 34% of children born with VLBW had height z-scores <−2 at age 2 years. Those children who were smaller had higher risk of developmental delays. Our findings remained true in models adjusted for SES, birthweight and gestational age, arguing for the importance of early growth in development of early motor and language skills—key precursors to later academic success. These data have implications for following children born with VLBW to identify those at risk of developmental deficits.
These findings are relevant to a large and increasing population in the USA and worldwide. Each year approximately 12% of infants in the USA—almost 500 000—are born prior to the 37th post-menstrual week.25 Long-term survival of infants born preterm has risen dramatically. Preterm birth is now a leading cause of neurodevelopmental disabilities in children,26 and the degree of neurodevelopmental disability is inversely correlated with gestational age at birth. Growth during the developmental window spanning the late prenatal and early postnatal period is clearly very important to development.27 Our data support that early measures of growth can be helpful in identifying children at risk of poor neurodevelopmental outcome for whom early intervention could be particularly effective. Linear regression models revealed relationships between 2-year Bayley scores and 9-month measures of HC, length and weight. Logistic regression revealed that children with weight and weight-for-height measures ≥2 SDs below the mean at 9 months were more likely to have low Bayley scores at 2 years.
Weight gain is the most commonly used marker of improved nutrition. Prior studies on this topic have largely focused on birthweight as related to these outcomes.24,27 By 2 years of age, links between current weight and cognitive Bayley scores were less consistent than seen for HC and length at that time point in our study. Throughout, measures of length were consistently linked to Bayley outcomes, suggesting linear growth as a good marker of health and nutrition,28 more so than measurement of weight alone. This may have implications for a lack of further improvements in cognitive scores in the presence of excess weight gain. Further study is needed, but these data raise the potential that at older ages, height and HC may be better indicators of healthy growth and better predictors of cognitive development than weight alone. This is similar to patterns seen around the world, where height at the second birthday is associated with reduced neurodevelopmental capacity.29,30
Prior to the widespread availability of high-calorie infant nutrition, several studies found a relationship between VLBW and developmental outcomes. In the 1970s, Ross et al31 evaluated 86 preterm infants born with LBW for relationships between growth and 12-month Bayley scores, finding that children with normal growth in the first year of life had normal neurobehavioural outcomes (defined as mean Bayley scores >85th), while those with sub-normal growth had delayed neurobehavioural outcomes. Horwood et al32 found cognitive, educational and behavioural deficits in almost 300 children born with VLBW in New Zealand prior to 1990. In-hospital growth velocity has also been shown to correlate with neurodevelopmental outcomes.33
The majority of brain growth happens in the first 1000 days of life. Brain size increases four times during the preschool years, and reaches approximately 90% of adult size by age 6.34 The relationships between nutrition and neural proliferation, growth and pruning are not well understood.35 Undernutrition, particularly during gestation or in the neonatal intensive care unit (NICU) period, can affect the developing brain and cognitive abilities.35 Changes in memory have been found with poor nutrition in mice.36 Studies examining brain structure and function are seeking to examine pathways of cognitive delay such as defects in myelination, disturbances to synaptic proliferation and pruning and neuro-inflammation.29 Brain size correlates with HC and decreased growth velocity of the head may indicate that there is a disruption in neurodevelopment.37 Catch-up weight gain in babies born SGA has been correlated with regional brain volume catch-up as well.38 Another study examined myelination, measured by transcephalic impedance, and found relationships with developmental scores.39 In smaller samples, HC in children born with VLBW was found to correlate with later cognitive abilities.40 More recently, Cheong et al evaluated 202 preterm infants using HC, MRI and Bayley scores at age 2 years. They noted that the percentage of children with microcephaly increased from term to 2 years of age, indicating postnatal brain growth failure. In this study, HC was a marker of overall brain volume, but did not correlate with grey matter abnormalities.37
Another recent study found that children with consistently small heads over the first 2 years of life were seven times more likely to have a neurocognitive disorder.41 Those authors also noted a study in the UK that found that in children whose HC crossed below the second percentile, 40% had neurodevelopmental pathology.42
While this study employed a recruitment approach based on birth certificates to get a sample representative of all children born across the USA, the study was limited by a lack of data regarding details of the NICU stay and severity of medical diagnoses of these predominantly preterm infants. Additionally, the technique of repeating a third measure of anthropometry only if the first two measures were >5% different potentially allowed for a margin of error in the values used. Further research is needed to examine the way medical complexity influences growth.
Our findings are consistent with prior studies linking brain growth to early child development and may have implications underscoring the importance of brain growth for a child’s motor and problem-solving skills. Modern nutritional practices have not modified this association. Children identified with slower growth may be identified by their paediatrician for further evaluation. In the present era of improved nutrition for VLBW infants, there remains a link between childhood growth and neurodevelopment.
References
- 1.Knudsen EI, Heckman JJ, Cameron JL, et al. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc Natl Acad Sci USA. 2006;103:10155–62. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle O, Harmon CP, Heckman JJ, et al. Investing in early human development: timing and economic efficiency. Econ Hum Biol. 2009;7:1–6. doi: 10.1016/j.ehb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danks M, Maideen MF, Burns YR, et al. The long-term predictive validity of early motor development in “apparently normal” ELBW survivors. Early Hum Dev. 2012;88:637–41. doi: 10.1016/j.earlhumdev.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:614S–20S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 5.Grantham-McGregor S, Cheung YB, Cueto S, et al. Child development in developing countries 1—Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fund UNCs, editor. UNICEF. Improving child nutrition: the achievable imperative for global progress. New York, NY: 2013. http://www.unicef.org/gambia/Improving_Child_Nutrition_-_the_achievable_imperative_for_global_progress.pdf. [Google Scholar]
- 7.Gandhi M, Ashorn P, Maleta K, et al. Height gain during early childhood is an important predictor of schooling and mathematics ability outcomes. Acta Paediatrica. 2011;100:1113–18. doi: 10.1111/j.1651-2227.2011.02254.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RA, Nelson CA. Developmental science and the media—Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Walker SP, Wachs TD, Grantham-McGregor S, et al. Child Development 1 Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378:1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 10.Batty GD, Shipley MJ, Gunnell D, et al. Height, wealth, and health: an overview with new data from three longitudinal studies. Econ Hum Biol. 2009;7:137–52. doi: 10.1016/j.ehb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Datar A, Jacknowitz A. Birth weight effects on children’s mental, motor, and physical development: evidence from twins data. Matern Child Health J. 2009;13:780–94. doi: 10.1007/s10995-009-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AAP. Bright futures: prevention and health promotion for infants, children, adolescents, and their families. Elk Grove, IL: American Academy of Pediatrics; 2015. [Google Scholar]
- 13.Martin J, Hamilton B, MJK O, et al. In: Births: final data for 2013. Statistics NCfH, editor. Hyattsville, MD: 2015. (National vital statistics reports). http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf. [PubMed] [Google Scholar]
- 14.UNICEF. Undernourishment in the womb can lead to diminished potential and predispose infants to early death. New York, NY: 2015. http://data.unicef.org/nutrition/low-birthweight.html. [Google Scholar]
- 15.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Stoltzfus RJ, Rasmussen KM. The dangers of being born too small or too soon. Lancet. 2013;382:380–1. doi: 10.1016/S0140-6736(13)61567-6. [DOI] [PubMed] [Google Scholar]
- 17.Meng HC, Stahlman MT, Otten A, et al. Use of a crystalline amino-acid mixture for parenteral nutrition in low-birth-weight infants. Pediatrics. 1977;59:699–709. [PubMed] [Google Scholar]
- 18.Greer FR, Steichen JJ, Tsang RC. Effects of increased calcium, phosphorus, and vitamin-D intake on bone mineralization in very low-birth-weight infants fed formulas with polycose and medium-chain triglycerides. J Pediatr. 1982;100:951–5. [PubMed] [Google Scholar]
- 19.Nord C, Edwards B, Andreassen C, et al. Early Childhood Longitudinal Study, Birth Cohort (ECLS-B), user’s manual for the ECLS-B longitudinal 9-month-2-year data file and electronic codebook. Washington, DC: National Center for Education Statistics; 2006. [Google Scholar]
- 20.DeBoer MD, Agard HE, Scharf RJ. Milk intake, height and body mass index in preschool children. Arch Dis Child. 2015;100:460–5. doi: 10.1136/archdischild-2014-306958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Onis M, Martorell R, Garza C, et al. WHO child growth standards based on length/height, weight and age. Acta Paediatrica. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ. A critique of the NCHS weight for height standard. Hum Biol. 1985;57:183–96. [PubMed] [Google Scholar]
- 23.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streimish IG, Ehrenkranz RA, Allred EN, et al. Birth weight- and fetal weight-growth restriction: Impact on neurodevelopment. Early Hum Dev. 2012;88:765–71. doi: 10.1016/j.earlhumdev.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 26.Behrman R, Butler A, editors. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 27.Ross GS, Krauss AN, Perlman JM. Physical growth and cognitive abilities in concordant versus discordant birth weight twins at three years old. Early Hum Dev. 2012;88:753–6. doi: 10.1016/j.earlhumdev.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–65. doi: 10.1179/2046905514Y.0000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SM, Walker SP, Grantham-McGregor S, et al. Early childhood stunting and later behaviour and school achievement. J Child Psychol Psychiatry. 2002;43:775–83. doi: 10.1111/1469-7610.00088. [DOI] [PubMed] [Google Scholar]
- 31.Ross G, Krauss AN, Auld PAM. Growth achievement in low-birth-weight premature-infants—relationship to neuro-behavioral outcome at one year. J Pediatr. 1983;103:105–8. doi: 10.1016/s0022-3476(83)80791-4. [DOI] [PubMed] [Google Scholar]
- 32.Horwood LJ, Mogridge N, Darlow BA. Cognitive, educational, and behavioural outcomes at 7 to 8 years in a national very low birthweight cohort. Arch Dis Child. 1998;79:F12–20. doi: 10.1136/fn.79.1.f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 34.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitsky DA, Strupp BJ. Malnutrition and the brain—changing concepts, changing concerns. J Nutr. 1995;125:S2212–20. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- 36.Ranade SC, Rose A, Rao M, et al. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience. 2008;152:859–66. doi: 10.1016/j.neuroscience.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Cheong JLY, Hunt RW, Anderson PJ, et al. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121:E1534–40. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- 38.Tzarouchi LC, Drougia A, Zikou A, et al. Body growth and brain development in premature babies: an MRI study. Pediatr Radiol. 2014;44:297–304. doi: 10.1007/s00247-013-2822-y. [DOI] [PubMed] [Google Scholar]
- 39.Ellison P, Heimler R, Franklin S. The relationships of transcephalic impedance, head growth and caloric-intake to outcome in preterm infants. Acta Paediatrica Scandinavica. 1984;73:820–7. doi: 10.1111/j.1651-2227.1984.tb17782.x. [DOI] [PubMed] [Google Scholar]
- 40.Leppanen M, Lapinleimu H, Lind A, et al. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics. 2014;133:63–70. doi: 10.1542/peds.2013-1187. [DOI] [PubMed] [Google Scholar]
- 41.Wright CM, Emond A. Head growth and neurocognitive outcomes. Pediatrics. 2015;135:E1393–8. doi: 10.1542/peds.2014-3172. [DOI] [PubMed] [Google Scholar]
- 42.Baxter PS, Rigby AS, Rotsaet MHEPD, et al. Acquired microcephaly: causes, patterns, motor and IQ effects, and associated growth changes. Pediatrics. 2009;124:590–5. doi: 10.1542/peds.2008-2784. [DOI] [PubMed] [Google Scholar]


