Abstract
Objective
The objective of this study is to compare maternal and neonatal outcome of opioid-dependent women maintained on buprenorphine or methadone throughout pregnancy in a randomized double-blind double-dummy clinical trial (CT) with a comparison group undergoing a structured standard protocol (SP) at the Medical University of Vienna, Austria.
Methods
One hundred and fourteen subjects were included in the analysis, with 77 in SP (n=51 methadone, n=26 buprenorphine), and 37 in CT (n=19 methadone, n=18 buprenorphine), comparing maternal concomitant consumption during third trimester, demographic birth data, duration of treatment for neonatal abstinence syndrome (NAS), morphine dose for NAS treatment and length of hospital stay (LOS).
Results
Both study groups yielded healthy neonates with no significant demographic differences and equivalently low rates of positive maternal urine toxicologies. However, NAS parameters were significantly better in CT regarding total medication dose administered to neonates (p=0.014) and LOS (p=0.015). Superior results were achieved in buprenorphine compared with methadone-exposed neonates regarding gestational age at birth (p=0.003), birth weight (p=0.011), total morphine dose administered (p=0.008), NAS treatment duration (p=0.008) and LOS (p=0.001).
Conclusions
Comparably favorable outcome for mothers and infants and efficacy and safety of opioid medications were shown in both treatment approaches. Neonatal care could benefit from transferring successful CT procedures into clinical practice. Copyright © 2011 John Wiley & Sons, Ltd.
Keywords: opioid dependence, pregnancy, buprenorphine, methadone, neonatal abstinence syndrome, treatment setting
INTRODUCTION
The use of illicit substances during pregnancy constitutes a major public health challenge due to the large number of affected women and the wide range of clinical and socio-economic problems entailed. Gyarmathy et al. (2009) estimate—on the basis of recent European Monitoring Centre for Drugs and Drug Addiction-compiled data—that 30,000 pregnant women in Europe use opioids each year and the number of pregnant women using other drugs being equally high. The potentially detrimental effects for mother and child are caused by the opioid consumption itself, concomitant use of other harmful substances, physical and psychiatric co-morbidities and the social environment and life conditions the pregnant patients face. A number of experts advocate a comprehensive multi-disciplinary treatment approach targeting the individual problem areas of these women and offering medical as well as psycho-social interventions in order to address the complex character of illicit substance use during pregnancy (Kaltenbach et al., 1998; Fischer, 2000; Rohrmeister et al., 2001; Kraigher et al., 2001; Jones et al., 2008; Winklbaur et al., 2008).
However, there is a severe lack of studies meeting current scientific standards that prospectively examine different treatment settings, therapeutic approaches and pharmacological interventions due to the general reluctance in the scientific community to include pregnant patients in clinical trials (Goldkind et al., 2010). Therefore, only limited data exist that support different treatment strategies, and thus, practices that had turned out to be effective have built the state-of-the-art interventions. For opioid-dependent pregnant women, pharmacological maintenance therapy with methadone, embedded in a multi-disciplinary outpatient treatment setting, has become the state-of-the-art treatment in industrialized countries (Sullivan et al., 1972; Fischer et al., 1998a), whereas maintenance therapy with buprenorphine has also led to promising results regarding maternal and particularly neonatal outcome in recent years (Fischer et al., 1998b; Fischer et al., 2000; Schindler et al., 2003; Unger et al., 2010a). Consequences for the fetus and the newborn have been of particular concern in clinical research, especially the neonatal abstinence syndrome (NAS) that emerges in 55–94% of neonates exposed to opiates in utero (American Academy of Pediatrics Committee on Drugs, 1998), but there are still no internationally approved standardized treatment interventions or guidelines for the treatment of infants suffering from NAS; hence, multiple substances such as paregoric, phenobarbital, morphine sulfate or morphine hydrochloride are in use, whereof morphine solutions have shown to be superior regarding rapid alleviation of neonatal withdrawal (Ebner et al., 2007). Non-pharmacological interventions, such as comfort measurements and rooming-in, which have been shown to account for a considerable reduction in the necessity and duration of NAS treatment and length of stay, are recommended in a complementary manner (Velez and Jansson, 2008; Saiki et al., 2010).
The first trials aiming to investigate methadone versus buprenorphine maintenance throughout pregnancy in randomized controlled studies were conducted by Jones et al. (2005) and Fischer et al. (2006); these small-scale pilot studies, applying a rigorous methodology with daily prenatal visits and double-blind, double-dummy design, showed that buprenorphine was an effective and safe alternative to methadone for mothers and infants, yielding even superior neonatal outcome in the study of Jones et al. (2005) in regard to required morphine dose for NAS treatment and length of stay in hospital. The results of both randomized controlled studies served as the basis for the development of the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study, a double-blind, double-dummy, flexible-dosing and parallel-group clinical trial involving eight sites in North America and Europe, which was designed to prove efficacy and safety of both agents in a larger sample, as well as the standardized application of morphine for neonatal withdrawal (Jones et al., 2010).
The protocols of these pioneering studies on a pregnant high-risk population were implemented successfully at the Addiction Clinic of the Medical University of Vienna where the study procedures were incorporated in the standardized program of the clinic, which has established a comprehensive care model since 1994, specialized in treating opioid-dependent pregnant women and involving a team of multi-disciplinary professionals. This special treatment model involves not only opioid maintenance treatment, medical and psycho-social care for pregnant opioid-dependent women, but neonatal pharmacological treatment and supportive care as well.
This analysis targeted the evaluation of the outcome of our patients undergoing a standard protocol (SP) and to compare their results with those of the Viennese MOTHER study (see Jones et al., 2010) participants (clinical trial (CT)). Its findings should however yield new insight and knowledge about crucial factors leading to the most beneficial results for mothers and children. Moreover, the outcome of the methadone-exposed group should be compared with the buprenorphine-exposed group within and across the two treatment groups.
The uniqueness of this analysis lies in the special selection of patients—the inclusion of a prospectively followed comparison group not eligible for or not willing to participate in the CT, a patient sample representative for the field population of opioid-dependent pregnant women seeking treatment in Austria, and its comparison with the study population.
METHODS
Patient sample
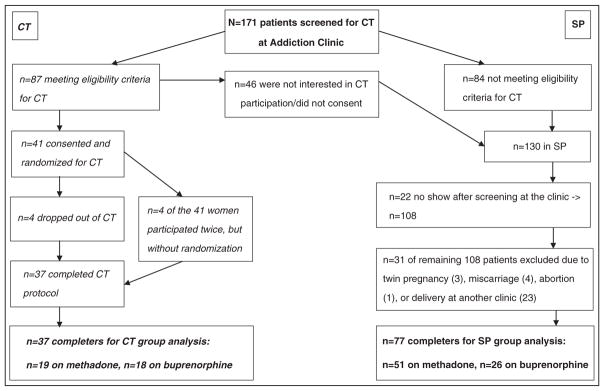
All subjects included in our analysis (N=114) were opioid-dependent (Diagnostic and Statistical Manual of Mental Disorders-IV 304.0) pregnant women (American Psychiatric Association, 1994) undergoing pharmacological maintenance therapy with either methadone or buprenorphine throughout pregnancy and the post-partum period at the Addiction Clinic of the Medical University of Vienna between June 2005, when the first patient was included in the CT, and December 2009, when the last patient was enrolled. All pregnant women attending the clinic in this time frame (N=171) underwent standardized assessment procedures as well as a specific screening by means of a questionnaire developed for the CT to examine their eligibility. Figure 1 displays screening, randomization and treatment completion data for both groups.
Figure 1.
Screening, randomization and treatment completion of both groups.
This figure displays details on the composition of the two treatment groups. The clinical trial group is depicted on the left, the standard protocol group on the right side.
Clinical trial group
The patients eligible for the clinical trial had to be between 18 and 41years of age, and between week 6 and 30 of their single-fetus pregnancy. Pending legal issues, current benzodiazepine and alcohol use disorders as well as serious medical illnesses (e.g., HIV, acute psychosis) and language impairments were exclusion criteria.
The screening results revealed that 15.8% (n=27) of the 171 screened women were benzodiazepine abusers at that time, 12.9% (n=22) stopped attending our clinic after screening and another 12.9% (n=22) did not meet the eligibility criteria due to late pregnancy; other reasons were heterogeneous and individual (Unger et al., 2010b). Eighty-seven women (50.9%) fulfilled the eligibility criteria, whereof 41 (24.0%) were willing to participate in the CT and were blindly randomized to either the methadone or the buprenorphine condition; four patients participated twice—they were not randomly assigned to the opioid medication, and therefore excluded from our analysis. Four buprenorphine-maintained women dropped out of the trial (three of them after transition to the study medication due to medication dissatisfaction and one woman because of no show), and therefore, the first group of the study sample consists of 37 patients (21.6% of screening sample), whereof 19 received methadone and 18 buprenorphine.
Standard protocol—comparison group
From the 130 patients not randomized for the CT (76.02% of screening sample), 108 (63.2%) of the screened women continued attending the Addiction Clinic, where they were offered standardized treatment according to the ethical standards in Austria requiring that all patients who do not meet eligibility criteria or refuse study participation, are offered standard care. Thirty-one of these 108 patients could not be included in our analysis due to abortion, miscarriage, multiple-fetus pregnancy or decision to deliver at another clinic (see Figure 1 for details). Therefore, 77 women constitute the final comparison group (45.0% of screening sample)—they were aged between 18 and 41years, had a single-fetus pregnancy and were no dropout of the CT; 51 of them were maintained on methadone and 26 on buprenorphine.
Procedures
All pregnant women attending the Addiction Clinic are offered comprehensive care by a multi-disciplinary team, including medical doctors, psychologists, nurses and social workers, as well as cooperation with other contributing departments such as the Department of Obstetrics and Gynecology and the Department of Pediatrics. Moreover, the establishment of an extensive network, including external institutions for addiction treatment, youth welfare services, child care institutions and authorities, allows the delivery of individually tailored continuous comprehensive care during and after pregnancy and throughout childhood if needed.
All 171 patients attending the clinic in the designated period of time underwent standardized psychiatric assessment, a laboratory examination (full blood count and infection status, urine toxicology) and completed a comprehensive interview where basic demographic and clinical data regarding their history of addiction and treatment were collected and standardized questionnaires were completed (European Addiction Severity Index (Gsellhofer et al., 1999), German Version of Lancashire Quality of Life Profile (Priebe et al., 1999) and Fagerström Test for Nicotine Dependence (Fagerström and Schneider, 1989). Subsequently, the women were screened and informed about the clinical trial. After inclusion in the SP or CT group, patients underwent the respective standardized procedures (see subsequent sections) according to the specific protocols until post partum, when all mothers completed post-partum questionnaires and were then referred—after an overall mean duration of 21weeks of treatment at the Addiction Clinic—to general practitioners or another treatment institution in Vienna.
For both groups, breastfeeding was generally supported as long as no contraindications, such as concomitant consumption of illegal substances or prescription drugs that are medically contraindicated during lactation and hepatitis (HBV or HCV) with high viral load, were present.
Procedures in clinical trial
After randomization and transfer to study medication, the patients had to attend the clinic daily until 28 days post partum and take their active medication/the placebo under supervision; urine toxicologies for opioids (other than the administered methadone and buprenorphine) and other illicit drugs were conducted three times a week and linked to a behavioral reinforcement strategy (contingency management)—the patients received vouchers, which they could use in local stores in Vienna, for negative urine samples (escalating voucher system), as well as for participation at specific examinations in the study. All study patients gave birth at the Department of Obstetrics and Gynecology, where they had to stay at least 10 days post partum with their neonates (rooming-in with the mother), independent of NAS treatment requirement.
Neonatal abstinence syndrome was assessed by trained nurses and pediatricians (blinded for intra-uterine medication exposure) every 4h using an especially for MOTHER developed modified version of the Finnegan scale (for details see Jones et al., 2010), comprised of 19 opioid withdrawal signs to determine the need for treatment. For comparison, NAS scores and the amount of medication administered over the entire course of NAS treatment were totaled for each day. NAS treatment was initiated when the NAS score was equal to or greater than 9, and conducted with morphine drops (0.05% morphine HCl), dosed according to the total NAS score (Jones et al., 2010).
The women completed their study participation 28 days after delivery and then were transferred to their preferred opioid medication.
Procedures of the standard protocol
In contrast to the CT, the opioid maintenance medication was individually determined and chosen according to medical and personal criteria (medical judgment, previous experiences with medication, personal preference for methadone or buprenorphine), and the frequency of visits was arranged individually according to the patient’s condition and living situation, but ranged on average between two and three visits per week. Patients received take-home doses of their medications for the days they did not attend the clinic. Supervised urine toxicologies were taken regularly, but at least two times a week to test for concomitant consumption of opioids, benzodiazepines and cocaine. Close cooperation with the Department of Obstetrics and Gynecology at the Medical University of Vienna had been established, where patients delivered and received antenatal and postnatal care. After delivery, immediate care of the newborns was assured by experienced and trained staff from the Department of Pediatrics.
Symptoms of NAS in neonates were scored by the same staff as in the CT, every 4h, using a modified version of the Finnegan scale (Finnegan et al., 1975). Neonates with scores exceeding 10 points received NAS treatment with morphine hydrochloride (morphine syrup 0.05%; 1ml=0.5mg; preparation: 1ml Vendal® (Lannacher (GL Pharma), Lannach, Austria) oral solution (5mg/ml) and syrup complex 10% ad 10ml) with a start dose of 0.05mg/kg three times daily, which was subsequently adjusted to the infant’s body weight and NAS intensity. Neonates in the SP were treated in a separate ward and could be transferred to another hospital in Vienna where their treatment was continued and completed; they were discharged after being at least 48 hours medication-free. The mothers were discharged from hospital upon medical indication, but could visit their infants daily.
STATISTICS
Because the investigation of specific neonatal/NAS parameters constituted one of our primary study aims, a completer-analysis was conducted.
Outcome measures of primary interest included maternal concomitant consumption of opioids (other than the administered methadone or buprenorphine), cocaine and benzodiazepines in the third trimester of pregnancy, gestational age at birth (≥37 completed weeks of pregnancy=full-term, <37weeks of pregnancy= pre-term delivery), demographic neonatal data (birth weight, body length, head circumference and Apgar scores at 1min (<8) and 5min (<9) after birth), total morphine dose required to treat NAS, duration of NAS treatment and LOS.
Further outcome measures comprised the number of daily smoked cigarettes (reported by patient in third trimester of pregnancy), mode of delivery (vaginal/Cesarean section), parity, dose of opioid medication at time of delivery, as well as the association of outcome parameters according to opioid medication (buprenorphine/methadone) and daily medication dose at time of delivery.
Statistical analysis was conducted using SPSS 16.0 for Windows. Chi-square tests or Fisher’s exact tests were applied for categorical data, the latter in cases of four-field schemes if at least one cell showed an expected value of five or less. If significant results were detected, standardized residuals were examined. Comparisons of continuous data between the two treatment groups were conducted by the calculation of t-tests for independent samples. In case of combination of maternal and neonatal outcome variables, two-way analysis of variance was applied, with “opioid medication” and “treatment approach” (standard protocol versus clinical trial) as independent variables. p-values are provided for the two main effects as well as for the interaction effect between opioid medication and treatment condition. For analytic purposes, an alpha of p<0.05 was considered significant.
ETHICS
All patients gave informed consent either to have their own and the data of their child—collected within the course of treatment—analyzed and/or to participate in the clinical trial, prior to treatment initiation. Both study protocols, for this analysis and the clinical trial, were approved by the Institutional Review Board (IRB) of the Medical University of Vienna (IRB-no. 514/2008, IRB-no. 451/1998).
RESULTS
Maternal characteristics
No significant differences regarding age, parity, number of daily smoked cigarettes or time of treatment initiation between the two treatment groups (SP/CT) or the two opioid medication groups (methadone/buprenorphine) were found (see Table 1). A comparison of relationship status revealed no difference regarding opioid medication group or treatment approach either (SP single 16% in buprenorphine group, 16% in methadone group, CT single 22% in buprenorphine group, 21% in methadone group, χ2 methadone= 0.245, p methadone=0.725, χ2 buprenorphine=0.268, p buprenorphine=0.701).
Table 1.
Maternal characteristics of the study sample (N=114)
| Standard protocol (n=77)
|
Clinical trial (n=37)
|
Significance*
|
|||||
|---|---|---|---|---|---|---|---|
| Meth (n=51) | Bup (n=26) | Meth (n=19) | Bup (n=18) | pTR | pOM | pIA | |
|
|
|
||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Maternal age | 23.94 (4.51) | 24.81 (5.25) | 26.37 (5.89) | 23.78 (4.48) | 0.488 | 0.392 | 0.088 |
| Parity | 1.55 (0.83) | 1.38 (0.75) | 1.63 (0.76) | 1.61 (1.09) | 0.375 | 0.595 | 0.679 |
| Cigarettes/day | 11.24 (6.67) | 10.81 (6.54) | 12.53 (5.96) | 13.89 (5.81) | 0.098 | 0.724 | 0.493 |
| Treatment initiation | |||||||
| Pregnancy week | 18.56 (7.70) | 20.67 (8.94) | 20.47 (7.16) | 21.12 (5.99) | 0.483 | 0.414 | 0.664 |
Meth, methadone; Bup, buprenorphine; SD, standard deviation.
This table shows maternal age in years, total parity, and number of daily smoked cigarettes reported by the patients in the third trimester of pregnancy, listed according to treatment approach applied and opioid medication administered (methadone/buprenorphine).
Significance of comparisons (p-values) was calculated in three ways: pTR indicates the significance regarding treatment approach (standard protocol versus clinical trial), pOM indicates the significance regarding opioid medication (buprenorphine versus methadone) and pIA indicates the significance taking into account the interaction between treatment approach and opioid medication.
The medication match was equally distributed in the two treatment conditions (SP methadone=66%, SP buprenorphine=34%, CT methadone=51%, CT buprenorphine=49%, χ2=2.536, p=0.111), and the daily opioid medication doses at time of delivery were comparable in both groups (methadone: mean dose SP=74.16mg (standard deviation (SD)=39.26mg, range:8–200mg), mean dose CT=63.68mg (SD= 36.09mg, range: 20–130mg), t=1.011, p=0.315; buprenorphine: mean dose SP=9.85 (SD=6.24, range: 1–24mg), mean dose CT=13.44mg (SD=6.28mg, range: 6–28mg), t=−1.876, p=0.068).
The two groups did not differ regarding concomitant consumption of opioids, cocaine and benzodiazepines during the last trimester of pregnancy (see Table 2).
Table 2.
Percentages of positive urine toxicologies collected during the third trimester of pregnancy
| Urine toxicology positive for | Standard protocol
|
Clinical trial
|
χ2 Meth | p Meth | χ 2 Bup | p Bup | χ2 TR | p TR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meth (n=51) | Bup (n=26) | SP (n=77) | Meth (n=19) | Bup (n=18) | (CT n=37) | |||||||
| Opioids | 36% | 19% | 30% | 37% | 28% | 32% | 0.007 | 0.932 | 0.416 | 0.706 | 0.056 | 0.812 |
| Cocaine | 7% | 9% | 8% | 16% | 6% | 11% | 1.239 | 0.355 | 0.178 | 1.000 | 0.311 | 0.719 |
| Benzodiazepines | 34% | 18% | 29% | 16% | 17% | 16% | 2.178 | 0.140 | 0.016 | 1.000 | 2.039 | 0.153 |
| Any additional substance | 52% | 23% | 42% | 47% | 33% | 41% | 0.132 | 0.717 | 0.559 | 0.498 | 0.026 | 0.871 |
SP, standard protocol; CT, clinical trial; Meth, methadone; Bup, buprenorphine; TR, treatment approach. χ2-test results are separately presented for each medication group (methadone/buprenorphine) and for the treatment approaches (SP/CT).
In the CT group, contingency management had been applied; comparable voucher sums for negative urine samples were paid to the women maintained on buprenorphine ($1490.47) and methadone ($1631.34), respectively (t=0.595, p=0.556).
Delivery parameters and demographic data of neonates
Regarding characteristics of delivery, no statistically significant differences between the two treatment groups were revealed in reference to pre-term (<37weeks of pregnancy) versus full-term deliveries (≥37weeks of pregnancy, see Table 3). However, mothers maintained on methadone delivered 1week earlier compared with those treated with buprenorphine (methadone: mean=38.53weeks (SD=1.98), buprenorphine: mean=39.68weeks (SD=1.94), p= 0.003, see Table 4). Women receiving methadone showed a slightly higher rate of Cesarean sections (58%) in the CT group compared with those in the SP group (33%, p=0.062, see Table 4).
Table 3.
Distribution of sexes in neonates (male/female), date (full-term/pre-term) and mode of delivery (vaginal/Cesarean section) and proportion of infants receiving pharmacological treatment for NAS
| Standard protocol (n=77)
|
Clinical trial (n=37)
|
χ2 Meth | p Meth | χ2 Bup | p Bup | |||
|---|---|---|---|---|---|---|---|---|
| Meth (n=51) | Bup (n=26) | Meth (n=19) | Bup (n=18) | |||||
| Male sex | 25 (49%) | 13 (50%) | 10 (53%) | 7 (39%) | 0.072 | 0.788 | 0.530 | 0.467 |
| Full-term | 45 (88%) | 25 (96%) | 16 (84%) | 17 (94%) | 0.200 | 0.696 | 0.072 | 1.000 |
| Vaginal delivery | 34 (67%) | 17 (65%) | 8 (42%) | 11 (61%) | 3.480 | 0.062 | 0.084 | 0.772 |
| Apgar 1 (<8) | 4 (8.2%) | 0 (0.0%) | 1 (5.3%) | 0 (0%) | 0.169 | 1.000 | ||
| Apgar 5 (<9) | 3 (6.1%) | 0 (0.0%) | 1 (5.3%) | 1 (6%) | 0.018 | 1.000 | 1.422 | 0.419 |
| NAS treatment | 31 (65%) | 15 (58%) | 13 (68%) | 15 (83%) | 0.089 | 0.766 | 3.223 | 0.073 |
Meth, methadone; Bup, buprenorphine; NAS, neonatal abstinence syndrome.
This table shows selected characteristics of the newborns according to treatment approach (standard protocol versus clinical trial) and opioid medication the mothers received throughout pregnancy (buprenorphine versus methadone).
χ2-test results are separately presented for each medication group (methadone/buprenorphine).
Table 4.
Demographic birth data and neonatal abstinence syndrome (NAS) parameters
| Standard protocol (n=77)
|
Clinical trial (n=37)
|
Significance*
|
|||||
|---|---|---|---|---|---|---|---|
| Meth (n=51) | Bup (n=26) | Meth (n=19) | Bup (n=18) | pTR | pOM | pIA | |
|
|
|
||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| GA at delivery (weeks) | 38.59 (1.92) | 39.65 (1.62) | 38.37 (2.17) | 39.72 (2.37) | 0.851 | 0.003 | 0.722 |
| Birth weight (g) | 2729.06 (510.48) | 3151.08 (541.64) | 2861.58 (402.59) | 2966.72 (504.16) | 0.800 | 0.011 | 0.124 |
| Body length (cm) | 48.26 (3.30) | 50.23 (3.28) | 48.66 (2.76) | 49.11 (2.65) | 0.572 | 0.059 | 0.236 |
| Head circumference (cm) | 32.93 (1.72) | 33.56 (1.75) | 33.61 (1.70) | 33.61 (1.97) | 0.319 | 0.383 | 0.392 |
| Total morphine dose (mg) | 21.61 (26.64) | 4.30 (7.30) | 5.18 (6.94) | 2.02 (1.71) | 0.014 | 0.008 | 0.061 |
| NAS treatment (days) | 21.25 (21.22) | 6.62 (8.07) | 9.53 (9.14) | 7.33 (4.46) | 0.079 | 0.008 | 0.048 |
| Length of stay (days) | 29.36 (17.94) | 13.92 (7.33) | 16.74 (6.78) | 13.67 (2.63) | 0.015 | 0.001 | 0.019 |
Meth, methadone; Bup, buprenorphine; SD, standard deviation; GA, gestational age.
This table shows routine birth data as well as NAS characteristics for the whole study sample (N=114), listed according to treatment approach (standard protocol versus clinical trial) and opioid medication the mothers received throughout pregnancy (buprenorphine versus methadone).
Significance of comparisons (p-values) was calculated in 3 ways: pTR indicates the significance regarding treatment approach (standard protocol versus clinical trial), pOM indicates the significance regarding opioid medication (buprenorphine versus methadone) and pIA indicates the significance taking into account the interaction between treatment approach and opioid medication.
No significant differences between the two treatment groups emerged in basic demographic birth parameters. However, neonates exposed to buprenorphine had a higher birth weight (p=0.011) than newborns of methadone-treated mothers (Table 4).
Neonatal abstinence syndrome
The proportion of neonates that needed treatment for NAS did not differ between the two treatment groups (47 infants (62.7%) in SP vs. 28 (75.7%) in CT, χ2=1.895, p=0.169), neither when taking into account the administered opioid medication (see Table 3). However, significant differences between the two treatment groups were detected in crucial NAS parameters. The neonates in the SP group needed higher total morphine treatment doses (p=0.014) and their LOS was significantly longer compared with the CT group (p=0.015). Moreover, considerable differences between the two medication groups were found (Table 4); methadone-exposed newborns received higher doses of morphine drops in total (p=0.008), needed pharmacological treatment for a significantly longer period of time (p=0.008) and had a longer LOS (p=0.001). Significant interaction effects were found between opioid medication and treatment approach for duration of treatment (p=0.048) and LOS (p=0.019).
Additionally, correlations between maternal opioid medication dose at time of delivery and NAS parameters were calculated for both treatment conditions (CT/SP), but neither the correlation between daily opioid dose at delivery and occurrence of NAS, or peak NAS scores, total morphine dose administered to neonates, nor the correlation between daily maternal dose and duration of NAS treatment in days revealed any significant result (Table 5).
Table 5.
Correlations between opioid medication dose at time of delivery and neonatal abstinence syndrome (NAS) parameters
| Correlation of daily dose with | Standard protocol (n=77)
|
Clinical trial (n=37)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean maternal methadone dose (n=51) | Mean maternal buprenorphine dose (n=26) | Mean maternal methadone dose (n=19) | Mean maternal buprenorphine dose (n=18) | |||||
|
|
|
|
|
|||||
| r | p | r | p | r | p | r | p | |
| Occurrence of medicated NAS | −0.03 | 0.851 | −0.21 | 0.298 | 0.23 | 0.338 | 0.35 | 0.154 |
| Peak NAS score | 0.01 | 0.936 | −0.14 | 0.501 | 0.12 | 0.613 | 0.33 | 0.176 |
| Total morphine dose for NAS (in mg) | −0.04 | 0.789 | 0.13 | 0.528 | 0.36 | 0.129 | 0.30 | 0.225 |
| Duration of NAS treatment (in days) | 0.11 | 0.466 | 0.06 | 0.783 | 0.35 | 0.145 | 0.36 | 0.142 |
DISCUSSION
This analysis represents the first comparison of prospectively collected data from two groups of opioid-dependent pregnant women receiving the same opioid maintenance medications within two different treatment settings—a CT group versus a group undergoing a structured SP, comprising the patients who were not eligible or not agreeing to participate in the clinical trial—at the same multi-disciplinary center over the same time frame. Patients in the CT (21.6% of screening sample) were subjected to a strict selection process and highly rigid structures, whereas patients in SP (45.0% of screening sample) were representative for the opioid-dependent pregnant women seeking treatment, reflecting the “real world situation”. Despite this a priori existing difference between the two groups of patients—entailed by the selection process, the women and their neonates showed comparable outcome, except for specific NAS parameters.
Assignment to the opioid medication group represents a central difference between the two groups. Although blinded randomization and standardized dosing were implemented in the CT (Jones et al., 2010), allocation to either buprenorphine or methadone was based on an individual decision by the physician together with the patient in SP. Therefore, the opioid agent and the according dose were corresponding to the patient’s addiction severity in SP, maintaining the more severely affected women on methadone and the more stable patients on buprenorphine. Because benzodiazepine use disorders were exclusion criteria in the CT, the women fulfilling these conditions were treated in SP—a fact that has to be taken into account when interpreting the findings of this analysis; the higher rate of benzodiazepine consumption in SP, particularly in the methadone group seems to be an artifact of the SP treatment approach. Moreover, the SP group contained twice the number of patients treated with methadone than maintained on buprenorphine, reflecting the higher number of more severely addicted women.
A further clear difference between the two treatment settings and thus limitation of results was that the staff assessing and treating the infants in SP was the same as in the CT, but not blinded for the opioid medication the mothers had been administered throughout pregnancy.
The comparison of the SP group with the CT group yielded no significant differences regarding major maternal characteristics such as age, parity, time of treatment initiation or relationship status.
Surprisingly, concomitant consumption of opioids, cocaine and benzodiazepines in the last trimester of pregnancy did not differ significantly between the two treatment groups, despite the lower frequency of visits in SP and the use of contingency management in the CT group. This result is not in line with previous studies reporting successful implementation of contingency management in regard to concomitant consumption of illicit substances or nicotine (e.g., Heil et al., 2008). However, it has to be stressed that the rates of concomitant consumption were generally low to moderate compared with illicit drug use rates up to 80% reported by recent studies investigating methadone-maintained pregnant women in a similar investigation period (e.g., Dryden et al., 2009). This outcome for both patient groups could be associated with the high staff stability in the multi-disciplinary team at the clinic—it has been multiply addressed before that stability in treatment teams improves retention and compliance (Goeb et al., 2000; Schroder et al., 2009).
The comparison of neonatal outcome between the two treatment groups revealed no statistical difference in demographic data, but clearly superior results in the CT group regarding NAS parameters. These findings might be linked to the fact that SP contained the more severely addicted patients that gave birth to children with a more severe course of NAS being reflected in higher morphine doses, longer treatment durations and LOS. However, the results could refer to the different NAS treatment protocols with the CT protocol involving blinded assessment and treatment of the newborns, or the supportive care measures, and the mandatory rooming-in with the mother for at least 10 days after delivery in the CT group. The assessment procedures and the modified Finnegan scoring systems were very similar and only differed marginally. Although the cutoff point for treatment initiation differed by one point, we attribute hardly any differences in our results to the scoring scales; moreover, we did not include any direct NAS score comparisons between SP and CT in this analysis to avoid biased findings in regard to modified Finnegan scores. Velez and Jansson (2008) and Saiki et al. (2010) already stressed that the presence of the mother had an alleviating effect on NAS in neonates. Another possible explanation for the better outcomes in the CT group could be inherent in the treatment condition; the women had daily contacts with the study staff with whom they had already built a relationship with, which may already be considered as a psychological intervention possibly having a positive influence on the well-being of the patient and her unborn child. Morton and Konrad (2009) also stress the importance of building relationships with addicted mothers in health care settings.
When taking into account the opioid medication infants had been exposed to throughout pregnancy, buprenorphine yielded significantly better results in regard to gestational age at delivery and birth weight. Additionally, the neonates needed less morphine for NAS treatment and had a shorter hospital stay—these results remained significant, even when taking into account the interaction effect of the “treatment approach” and “opioid medication” variables. These clearly beneficial results for neonates whose mothers had been maintained on buprenorphine rather than methadone are in line with previous research results favoring the application of buprenorphine throughout pregnancy in opioid-dependent women (Fischer et al., 1998; Jones et al., 2005; Fischer et al., 2006; Ebner et al., 2007; Jones et al., 2010). At this point, it has to be stressed that buprenorphine is not suitable for all opioid-dependent pregnant women; Jones et al. (2010) found a significantly higher dropout rate due to medication dissatisfaction in patients maintained on buprenorphine compared with those receiving methadone. It may have weaker agonistic effects in reducing craving and withdrawal symptoms, as well as causing a higher subjective feeling of “clarity” (Fischer et al., 1999) that seems to be responsible for the reluctance in some patients to take it—thus, for a part of this patient population methadone might be more suitable.
Regarding the relation between pre-partum maternal opioid medication dose and the severity or duration of NAS, research has yielded inconsistent results regarding methadone as well as buprenorphine so far, frequently suggesting a strong interaction with concomitant consumption of other illicit or licit substances, high nicotine consumption and poly-drug use, respectively (Jones et al., 2005; Fischer et al., 2006; Dashe et al., 2002; Berghella et al., 2003; Lim et al., 2009; Kakko et al., 2008; Kahila et al., 2007; Marquet et al., 2002; Simmat-Durand et al., 2009; Cleary et al., 2010). In our analysis, none of the calculated correlations between opioid medication dose at time of delivery and any NAS parameter yielded any significant result, neither in the SP group, nor in the CT group, and therefore our findings do not suggest any opioid medication dose–NAS severity association.
In fact, newborns of both treatment groups remained in hospital for a considerable time (7–8days) beyond the treatment period. This gap might be explained by psycho-social reasons typically affecting this specific patient population—frequently, a decision on child care matters is pending, but it cannot be attributed to the treatment protocols. However, this procedure may be put into perspective for economical reasons.
CONCLUSION
Overall, our findings confirm that patients, who are responding well to buprenorphine, should be maintained on this agent throughout pregnancy because it clearly yields a superior neonatal outcome regarding gestational age at birth, birth weight, total morphine dose needed for NAS treatment, duration of NAS treatment and length of stay in hospital. However, both opioid medications should be available in order to adjust the opioid agent to the patient’s health status. Furthermore, successfully implemented NAS procedures from the clinical trial, including blinded assessment and postnatal supportive care as well as rooming-in, could be considered for integration into standard care in the sense of the argument of Halbreich et al. (2008) that well-delineated procedures inherent in clinical trials could contribute to improved quality of care and treatment outcome. Finally, our findings show that this vulnerable patient population clearly benefits from intense comprehensive care, creating the necessary environment for experiencing a less stressful pregnancy and preparing for motherhood.
Acknowledgments
We thank our nurses, social workers, midwives, psychologists and physicians of the Department of Psychiatry and Psychotherapy, the Department of Obstetrics and Gynecology and the Department of Pediatrics and Adolescent Medicine for the clinical care, and of course the patients for their participation.
Special thanks to the dedicated work of the principal investigators of the MOTHER study sites and our coordinating center: Hendree E. Jones, PhD, Johns Hopkins University; Amelia Arria, PhD, Center for Substance Abuse Research; Sarah H. Heil, PhD, University of Vermont; Karol Kaltenbach, PhD, Jefferson University; Barry M. Lester, PhD, Brown University; Peter M. Martin, MD, Vanderbilt University; Peter Selby, MD, University of Toronto; and Susan Stine, MD, PhD, Wayne University.
This research was supported by the following grants from the National Institute on Drug Abuse R01DA018417 (MOTHER study) and the Austrian National Bank Jubiläums-fonds project no. 13637 (analysis of standard protocol group).
Footnotes
CONFLICT OF INTEREST
Travel support to present data at conferences has been provided by Mundipharma, Reckitt Benckiser, Lannacher (GL Pharma), Roche and Schering Plough.
References
- American Academy of Pediatrics Committee on Drugs. Neonatal withdrawal. Pediatrics. 1998;101:1079–1087. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—DSM-IV. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Berghella V, Lim PJ, Hill MK, Cerpes J, Chennat J, Kaltenbach K. Maternal methadone dose and neonatal withdrawal. Am J Obstet Gynecol. 2003;189:312–317. doi: 10.1067/s0002-9378(03)00520-9. [DOI] [PubMed] [Google Scholar]
- Cleary BJ, Donnelly J, Strawbridge J, Gallagher PJ, Fahey T, Clarke M, Murphy DJ. Methadone dose and neonatal abstinence syndrome—systematic review and meta-analysis. Addiction. 2010;105(12):2071–2084. doi: 10.1111/j.1360-0443.2010.03120.x. [DOI] [PubMed] [Google Scholar]
- Dashe JS, Sheffield JS, Olscher DA, Todd SJ, Jackson GL, Wendel GD. Relationship between maternal methadone dosage and neonatal withdrawal. Obstet Gynecol. 2002;200:1244–1249. doi: 10.1016/s0029-7844(02)02387-6. [DOI] [PubMed] [Google Scholar]
- Dryden C, Young D, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG. 2009;116(5):665–671. doi: 10.1111/j.1471-0528.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- Ebner N, Rohrmeister K, Winklbaur B, Baewert A, Jagsch R, Peternell A, Thau K, Fischer G. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87(2–3):131–138. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. J Behav Med. 1989;12:159–181. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug dependent mother. International J Clin Pharm Biopharmacy. 1975;12:19. [PubMed] [Google Scholar]
- Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95(8):1141–1144. doi: 10.1046/j.1360-0443.2000.95811411.x. [DOI] [PubMed] [Google Scholar]
- Fischer G, Eder H, Jagsch R, Lennkh C, Habeler A, Aschauer HN, Kasper S. Maintenance therapy with synthetic opioids within a multidisciplinary program—a stabilizing necessity for pregnant opioid dependent women. Archives of Women’s Mental Health. 1998a;1:109–116. [Google Scholar]
- Fischer G, Etzersdorfer P, Eder H, Jagsch R, Langer M, Weninger M. Buprenorphine maintenance in pregnant opiate addicts. European Addiction Research. 1998b;4(S 1):32–36. doi: 10.1159/000052040. [DOI] [PubMed] [Google Scholar]
- Fischer G, Gombas W, Eder H, Jagsch R, Peternell A, Stühlinger G, Pezawas L, Aschauer HN, Kasper S. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94(9):1337–1347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, Langer M, Aschauer HN. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95(2):239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, Aschauer H. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- Goeb JL, Coste J, Bigot T, Ferrand I. Prospective study of favorable factors in follow-up of drug addicted patients—apropos of 257 patients of the Cassini Center in Paris. Encephale. 2000;26(6):11–20. [PubMed] [Google Scholar]
- Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research—lessons from the H1N1 influenza pandemic. N Engl J Med. 2010;362(24):2241–2243. doi: 10.1056/NEJMp1003462. [DOI] [PubMed] [Google Scholar]
- Gsellhofer B, Küfner H, Vogt M, Weiler D. European Addiction Severity Index (Europ-ASI)—Manual für Training und Durchführung. Schneider-Verlag; Hohengehren: 1999. [Google Scholar]
- Gyarmathy VA, Giraudon I, Hedrich D, Montanari L, Guarita B, Wiessing L. Drug use and pregnancy—challenges for public health. Euro Surveill. 2009;14(9):33–36. [PubMed] [Google Scholar]
- Halbreich U, Smail N, Tu X, Halbreich J. Participation in clinical trials may improve care of acute schizophrenia inpatients in a general hospital. CNS Spectr. 2008;13(9):757–761. doi: 10.1017/s1092852900013870. [DOI] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, Badger GJ, Lynch ME. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, Crocetti M, Dudas R, Harrow C, Huestis MA, Jansson LM, Lantz M, Lester BM, Milio L. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79(1):1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome following methadone or buprenorphine exposure. NEJM. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, Stine SM, O’Grady KE, Arria AM, Fischer G. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35(3):245–259. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M, Halmesmaki E. A prospective study on buprenorphine use during pregnancy: effects on maternal and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86:185–190. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug and Alcohol Dependence. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25(1):139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Kraigher D, Schindler S, Ortner R, Fischer G. Pregnancy and substance dependency. Gesundheitswesen. 2001;63(Suppl 2):S101–S105. doi: 10.1055/s-2001-16419. [DOI] [PubMed] [Google Scholar]
- Lim S, Prasad M, Samuels P, Gardner D, Cordero L. High-dose methadone in pregnant women and its effect on duration of neonatal abstinence syndrome. American Journal of Obstetrics and Gynecology. 2009;200(1):70e1–70.e5. doi: 10.1016/j.ajog.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Marquet P, Lavignasse P, Gaulier J-M, Lachatre G. Case study of neonates born to mothers undergoing buprenorphine maintenance treatment. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Human Press; Totowa, NJ: 2002. pp. 125–135. [Google Scholar]
- Morton J, Konrad SC. Introducing a caring/relational framework for building relationships with addicted mothers. JOGNN. 2009;38(2):206–213. doi: 10.1111/j.1552-6909.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- Priebe S, Gruyters T, Heinze M, Hoffmann C, Jakel A. Subjektive evaluationskriterien in der psychiatrischen versorgung—erhebungsmethoden für forschung und praxis. [Subjective evaluation criteria in psychiatric care—methods of assessment for research and general practice]. [German] Psychiatrische Praxis (Berlin) 1999;22(4):140–144. [PubMed] [Google Scholar]
- Rohrmeister K, Bernert G, Langer M, Fischer G, Weninger M, Pollak A. Opiate addiction in gravidity—consequences for the newborn. Results of an interdisciplinary treatment concept. Z Geburtshilfe Neonatol. 2001;205(6):224–230. doi: 10.1055/s-2001-19054. [DOI] [PubMed] [Google Scholar]
- Saiki T, Lee S, Hannam S, Greenough A. Neonatal abstinence syndrome—postnatal ward versus neonatal unit management. Eur J Pediatr. 2010;169(1):95–98. doi: 10.1007/s00431-009-0994-0. [DOI] [PubMed] [Google Scholar]
- Schindler SD, Eder H, Ortner R, Rohrmeister K, Langer M, Fischer G. Neonatal outcome following buprenorphine maintenance during conception and throughout pregnancy. Addiction. 2003;98(1):103–110. doi: 10.1046/j.1360-0443.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- Schroder R, Sellman D, Frampton C, Deering D. Youth retention: factors associated with treatment drop-out from youth alcohol and other drug treatment. Drug Alcohol Rev. 2009;28(6):663–668. doi: 10.1111/j.1465-3362.2009.00076.x. [DOI] [PubMed] [Google Scholar]
- Simmat-Durand L, Lejeune C, Gourarier L Groupe d’ Etudes Grossesse et Addictions (GEGA) Pregnancy under high-dose buprenorphine. Eur J Obstet Gynecol Reprod Biol. 2009;142(2):119–123. doi: 10.1016/j.ejogrb.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Sullivan RD, Fischbach AL, Hornick FW. Treatment of a pregnant opiate addict with oral methadone. Ariz Med. 1972;29(2):129–130. [PubMed] [Google Scholar]
- Unger A, Jung E, Winklbaur B, Fischer G. Gender issues in the pharmacotherapy of opioid-addicted women: buprenorphine. J Addict Dis. 2010a;29(2):217–230. doi: 10.1080/10550881003684814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger AS, Martin PR, Kaltenbach K, Stine SM, Heil SH, Jones HE, Arria AM, Coyle MG, Selby P, Fischer G. Clinical characteristics of Central European and North American samples of pregnant women screened for opioid agonist treatment. Eur Addict Res. 2010b;16(2):99–107. doi: 10.1159/000284683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez M, Jansson LM. The opioid dependent mother and newborn dyad: non-pharmacologic care. J Addict Med. 2008;2(3):113–120. doi: 10.1097/ADM.0b013e31817e6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103(9):1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]