Abstract
Low social status is frequently associated with heightened exposure to social stressors and altered glucocorticoid regulation by the hypothalamic-pituitary-adrenal (HPA) axis. Additionally, personality differences can affect how individuals behave in response to social conditions, and thus may aggravate or protect against the effects of low status on HPA function. Disentangling the relative importance of personality from the effects of the social environment on the HPA axis has been challenging, since social status can predict aspects of behavior, and both can remain stable across the lifespan. To do so here, we studied an animal model of social status and social behavior, the rhesus macaque (Macaca mulatta). We performed two sequential experimental manipulations of dominance rank (i.e., social status) in 45 adult females, allowing us to characterize personality and glucocorticoid regulation (based on sensitivity to the exogenous glucocorticoid dexamethasone) in each individual while she occupied two different dominance ranks. We identified two behavioral characteristics, termed ‘social approachability’ and ‘boldness,’ which were highly social status-dependent. Social approachability and a third dimension, anxiousness, were also associated with cortisol dynamics in low status females, suggesting that behavioral tendencies may sensitize individuals to the effects of low status on HPA axis function. Finally, we found that improvements in dominance rank increased dexamethasone-induced acute cortisol suppression and glucocorticoid negative feedback. Our findings indicate that social status causally affects both behavioral tendencies and glucocorticoid regulation, and that some behavioral tendencies also independently affect cortisol levels, beyond the effects of rank. Together, they highlight the importance of considering personality and social status together when investigating their effects on HPA axis function.
Keywords: Social subordination, Dominance rank, Psychosocial stress, Animal personality, Hypothalamic-pituitary-adrenal axis, Nonhuman primates
1. Introduction
In many mammals, including humans, social groups are organized into dominance hierarchies in which an individual’s position in the hierarchy has important consequences for reproductive success, access to resources, and, in some cases, health and mortality risk. These effects are thought to arise in part through unequal distribution of exposure to socio-environmental stressors (Sapolsky, 2005). Stressful experiences in mammals activate the hypothalamic-pituitary-adrenal (HPA) axis, triggering a neuroendocrine cascade that produces glucocorticoids (GC) (e.g. cortisol) and is adaptive in the short-term, but pathological when chronically activated (Cavigelli and Chaudhry, 2012; Miller et al., 2007). Notably, while low status is often associated with chronic stress, some low status individuals do not exhibit elevated cortisol levels (Dowd et al., 2009). This observation has motivated an increased research focus on factors that may interact with social experience to affect physiology (Capitanio, 2011; Hodes et al., 2014), including individual differences in behavior or temperament (e.g. “personality”). However, because many social behaviors are status-dependent, disentangling the effects of personality on GC regulation from those of status presents a major challenge to understanding vulnerability to stress exposure.
Group-living nonhuman primates provide a valuable opportunity to address this gap. Like humans, many nonhuman primate species must navigate complex social relationships, including strictly enforced dominance hierarchies, to survive and reproduce. Within these groups, dominance rank has been associated with altered neuroendocrine function (reviewed in Sapolsky, 2005) and survival (Blomquist et al., 2011), although the magnitude and directionality of rank effects vary depending on the social dynamics of the species, population, and sex (Abbott et al., 2003; Gesquiere et al., 2011; Michopoulos et al., 2012), as well as the statistical power and design of the study (Cavigelli and Caruso, 2015). Further, within species, the effects of occupying a particular rank can vary across individuals. For example, in species in which low rank predicts hypercortisolemia, engagement in affiliative behaviors (e.g. grooming, contact, huddling) has been hypothesized to moderate (“socially buffer”) this effect (reviewed in Hostinar et al., 2014). If rates of affiliative behavior reflect stable behavioral tendencies, they may therefore help explain why some animals are more resilient to social status-induced stress than others.
In support of this idea, factor analytic studies of nonhuman primate behavior suggest that high rates of affiliative behavior can reflect stable behavioral tendencies (e.g., “sociability”) (reviewed in Freeman and Gosling, 2010). However, affiliative behaviors are often collinear with dominance rank (e.g., higher-ranking individuals engage in more affiliation and have stronger bonds than low-ranking individuals: Seyfarth et al., 2013; Snyder-Mackler et al., 2016; but see Archie et al., 2014; Silk et al., 2010). Thus, attributing affiliation rates to an individual’s personality (or other intrinsic factors, such as age and social history), as opposed to dominance rank, remains challenging. Other dimensions of personality encounter the same problem. For instance, although self-grooming rates can be temporally stable (Maestripieri, 2000) and have been argued to capture anxious temperament (Fairbanks and Jorgensen, 2011), they can also be rank-dependent, with low-ranking individuals tending to self-groom more frequently than high-ranking individuals (Pavani et al., 1991). The ability of personality traits to moderate the response to rank-induced social stressors thus depends on the degree to which they themselves are affected by rank (McGuire et al., 1994), as opposed to stable across social situations (Uher et al., 2013).
A number of studies in nonhuman primates have reported associations between personality and cortisol levels, though few have investigated them jointly in the context of dominance rank. Generally, prosocial behaviors that load onto a “sociability” dimension are associated with lower cortisol (Seyfarth et al., 2012), whereas more aggressive and anxious temperaments tend to be associated with higher cortisol (Capitanio et al., 2004; Erickson et al., 2005). These effects could be explained if personality traits exert direct effects on neuroendocrine function (overlapping brain regions govern both emotional behavior and the physiological stress response: Short et al., 2014), or if they indirectly influence cortisol levels by shaping how individuals cope with acute stressors (Taylor et al., 2015), particularly aggression received from more dominant social partners (Capitanio, 2011). However, while a handful of studies have considered both personality and dominance rank effects on cortisol levels in primates (Anestis et al., 2006; Seyfarth et al., 2012), differentiating between the two remains a challenge. For example, Seyfarth et al. found that wild adult female baboons with stronger bonds had lower fecal GC levels, but these females also tended to be higher-ranking than females with weak social bonds (Seyfarth et al., 2012).
In this study, we attempt to overcome this challenge by assessing the relative contributions of dominance rank and personality to GC regulation in group-housed adult female rhesus macaques. We consider three indices of GC regulation by the HPA axis: diurnal cortisol, GC negative feedback, and sensitivity to acute GC challenge. Captive primate models provide a translational opportunity to explore the link between personality, dominance rank, and physiology because social group membership can be systematically rearranged and monitored in ways that are impossible in research with humans or wild nonhuman primates. To take advantage of this possibility, we first constructed 9 new social groups of adult females (5 per group), and then employed a mid-study social group rearrangement in which the same females were reorganized into new social groups in which almost all of them occupied new positions (Snyder-Mackler et al., 2016). This approach enabled us to examine each female in two different social environments across time. We hypothesized that: (1) behavioral tendencies would be causally affected by dominance rank, but exhibit partial stability across social contexts, indicative of “personality” (Funder and Colvin, 1991); (2) the effects of low dominance rank on cortisol levels (Michopoulos et al., 2012) would be moderated by prosocial behavioral tendencies, such that highly affiliative, low ranking females would exhibit fewer signs of GC dysregulation than less affiliative, low ranking females; (3) the effects of high rank would be moderated by anxious tendencies, such that high anxiety, high ranking females would exhibit more signs of GC dysregulation than low anxiety, high ranking females; and (4) improvements in social status would improve HPA sensitivity and responsiveness to GCs, as suggested by prior studies on the plasticity of responses to social conditions (Shively et al., 1997; Snyder-Mackler et al., 2016; Tung et al., 2012).
2. Methods
2.1. Study subjects
Study subjects were 45 adult female rhesus macaques housed in nine, mixed-age social groups of five females each at the Yerkes National Primate Research Center (YNPRC; see Table S1 for detailed demographic information). Group formation initially began in January 2013 using a previously established protocol (described in Snyder-Mackler et al., 2016). Briefly, sexually mature (age range = 3–20 years, median age at the start of the study = 6.8 yr), reproductively intact females at the YNPRC Field Station were serially introduced to indoor run housing (10 m × 10 m) over 2–15 weeks until all groups included five unrelated adult females (Table S1). Females were randomized into groups and order of introduction, with the following exceptions: we avoided co-housing females who had previously had social contact with one another (of 180 total co-housed dyads throughout the study, 97% had no prior social contact), and we avoided co-housing females who were close kin (e.g., full sibling, half sibling, parent-offspring, grandparent-grandoffspring). In this paradigm, females introduced earlier tend to subsequently occupy higher dominance ranks. Animals had unrestricted access to typical low-fat, high-fiber nonhuman primate diet throughout the study, and the Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals.”
The present study was divided into two phases: Phase 1 (February 2013–March 2014) and Phase 2 (April 2014–March 2015). Starting dates for each group and phase were defined by the date of completion of group formation (after addition of the fifth and final female into each group; note that start of group formation was staggered for logistical reasons: see Table S1). Phase 1 groups were formed as described above, whereas Phase 2 groups consisted of the same 45 individuals systematically reorganized into new groups (Table S1). Specifically, groups in Phase 2 were comprised of females who all shared the same or similar dominance ranks in Phase 1 (maximum difference of 1 ordinal rank value), a strategy that altered the ordinal dominance ranks of the majority (36 of the 45) of subjects across the two phases. In both phases, order of introduction strongly predicted Elo rating (Phase 1: r = −0.54, p < 0.001; Phase 2: r = −0.68, p < 0.001), a measure of dominance rank in which higher scores correspond to higher status (Albers and de Vries, 2001; Elo, 1978; Neumann et al., 2011), such that females entering into the group earlier occupied higher ranks by the time of group stabilization. As intended by our study design, an individual’s rank in Phase 1 was uncorrelated with her rank in Phase 2 (r = 0.063, p = 0.68). Female age was correlated with dominance rank in Phase 1 (r = 0.56, p < 0.001), but not in Phase 2 (r = 0.27, p = 0.07); however, we included age as a covariate in all of our analyses for both phases.
2.2. Behavioral characterization
Behavioral data were collected weekly during 30 min focal observations. During these observations, a trained observer recorded the behavioral activities of all five individuals residing in the “focal” group according to an established ethogram (Snyder-Mackler et al., 2016; because of the small group sizes, observers could effectively watch all animals in a group at one time). We collected a total of 398 h of focal observations on the 18 groups (mean per group = 22.1 h, range = 14.5–27.5 h; totals = 223.5 h in Phase 1, and 175 h in Phase 2). To control for differences in hours observed across groups, we calculated all behavioral frequencies and durations per hour observed. Inter-observer reliability among three trained observers exceeded 0.9. In total, we analyzed 10 behavioral measurements. Two captured dominance interactions: the frequency of aggressive behaviors, defined by threats, slaps, grabs, bites, and chases, and the frequency of submissive behaviors, which included grimaces, withdrawals, and screams. Two captured anxiety-like, “displacement” behaviors (Aureli and Whiten, 2009): time spent self-grooming and the frequency of self-scratching bouts. Finally, six captured affiliation-related behaviors: time spent in passive, physical contact with groupmates that did not involve grooming or aggression; time spent grooming one or more groupmates; time being groomed by one or more groupmates; the frequency of approaches in which the focal female initiated a proximity behavior toward another female (defined as sitting less than 1 m away for >3 s); the frequency of approaches received by the focal female from groupmates; and time spent alone, a negative measure of affiliation during which the focal female was more than 1 m away from any groupmate (i.e., not in proximity to others).
Data from Phase 1 and Phase 2 were analyzed separately such that each female had a measure of each behavior during Phase 1 and a separate measure during Phase 2. Only behavioral data collected following the completion of group formation was used (i.e. after the fifth and final female had been introduced into a group). Data were recorded on a notebook computer using a data acquisition program (“WinObs”) that records behavior in an actor-behavior-recipient format (Graves and Wallen, 2006).
2.3. Dominance rank assignment
We assigned dominance ranks using the Elo rating method, in which higher ratings correspond to higher rank/social status (Elo, 1978; Neumann et al., 2011). The Elo method updates an individual’s rating after each dominance interaction based on the pre-interaction probability that she would win or lose the encounter. We opted to use Elo ratings, as opposed to ordinal ranks, because they distinguish adjacently ranked individuals that are matched in relative dominance (e.g. Elo ratings of 1500 versus 1490) from those that are more clearly differentiated (e.g. Elo ratings of 1500 versus 1000). We determined Elo ratings from all dyadic dominance interactions that took place after each group was fully formed. Each individual’s initial Elo rating was set at 1000, and the baseline number of points gained or lost during a dominance interaction (k) was set to 100. This number was then weighted for each interaction by the expected probability of that individual winning or losing, based on a logistic function that was updated following each dominance interaction (Albers and de Vries, 2001). Dominance hierarchies were rapidly established after group formation and highly stable within each study phase. Specifically, Elo ratings at the end of each study phase were significantly correlated with Elo ratings at 10 weeks post-group formation, for both phases (r88 = 0.89, P < 0.001). Final Elo ratings within each study phase were converted to z-scores for statistical analyses, unless otherwise specified, and all Elo computations were performed using the EloRating package (v 0.43) in R (R Development Core Team, 2014).
2.4. Behavioral analysis
To represent correlated behaviors using a minimum number of independent dimensions, we carried out principal components analysis (PCA) using the prcomp function in R. We performed PCA on a 90 × 10 matrix of behavioral data, with a row for each female-study phase combination (45 females × 2 phases) and a column for each of the 10 behaviors we studied. We used a bootstrapping procedure with 10,000 iterations to generate 95% confidence intervals (CI) of the eigenvalues of each principal component (PC). From the resulting PCA, we retained only PCs where the lower bound of the 95% CI was ≥ 1 (Table 1), and applied an orthogonal varimax rotation to generate standardized factor loadings and component scores using the principal function in the R package psych (Revelle, 2015). We used linear mixed effects models (LMM) to examine whether the three resulting standardized component scores for each subject were associated with dominance rank or chronological age (fixed effects), with social group modeled as a random effect. To generate rank, age, and social group-independent measures of the three dominant behavioral tendencies, we extracted residual component scores from each LMM. These values were used to ask whether personality attributes that could not be explained by rank or age (i.e. were orthogonal to rank and age) explain GC regulatory differences (see Section 2.7). Finally, to test for causal effects of changes in dominance rank on behavioral tendencies, we modeled the change between Phase 2 and Phase 1 component scores as a function of change in Elo rating (ΔElo), age, and phase 1 component score. This approach took advantage of the longitudinal nature of our study design, complementing our cross-sectional analyses on females in Phase 1.
Table 1.
Mean ± SEM levels of diurnal serum cortisol (ug/dl) for LHPA axis assessments.
| Time of day | 0800 | 1100 | 1700 |
|---|---|---|---|
| A | |||
| Diurnal Rhythm | |||
| Phase 1 | 14.64 ± 0.48 | 12.05 ± 0.45 | 9.91 ± 0.60 |
| Phase 2 | 14.89 ± 0.99 | 15.67 ± 0.83 | 10.92 ± 0.89 |
| B | |||
| Dexamethasone Suppression Test | |||
| Phase 1 | – | 5.33 ± 0.44 | – |
| Phase 2 | – | 6.21 ± 0.96 | – |
|
| |||
| Time of day | 0800 | 0930 | 1230 |
|
| |||
| Ca | |||
| Dexamethasone Challenge Test | |||
| Phase 1 | 5.71 ± 0.36 | 5.00 ± 0.33 | 2.39 ± 0.20 |
| Phase 2 | 20.94 ± 0.90 | 16.86 ± 0.58 | 6.92 ± 0.30 |
DCT cortisol measurements were systematically lower in Phase 1 than Phase 2. See Section 2.5 for additional details.
2.5. Sampling and assay procedures
All animals were habituated to removal from their groups for conscious venipuncture using established procedures (Michopoulos et al., 2012). The order in which females from a group entered the venipuncture caging was unrelated to rank, and all individuals from the same group were sampled on the same day. Blood samples were obtained using Vacutainer serum separator tubes within 10 min from entering the animal area to minimize arousal, and females were back in their group within 10 min after completing the sampling procedure. Serum separator tubes were immediately placed on ice and centrifuged at 2000 × g for 15 min using an Allegra 6R refrigerated centrifuge (Beckman Coulter, Inc.). Sera were stored at −20 °C until assayed in duplicate. All groups were sampled 9–12 months from the beginning of group formation and cortisol assessments for a given animal were completed within a mean window of 61 days (range: 14–102 d). Blood samples were collected at the end of the typical breeding season in both study phases (January–March).
For cortisol and dexamethasone quantitation, we used LC/MS instead of antibody-based assays, such as ELISA, as the latter are known to cross-react with off-target ligands and metabolites, whereas the former precisely quantifies the ligand of interest and is considered the gold standard for quantification of steroid hormone analysis (Soldin and Soldin, 2009). For cortisol quantification, serum samples (100 μl) were placed into a 96-well block with 10 μl of internal standard (d4-cortisol) and were extracted using an ISOLUTE SLE+ 200 plate (Biotage, Sweden), then reconstituted in 100 μl of LC solvent (0.1% formic acid in H2O:0.1% formic acid in methanol, 65:35, V:V). 10 μl extraction solution was analyzed by LC-ESI-tandem mass spectrometry using a Discovery 5 cm × 2.1 mm C18 column (Supelco, PA) eluted at flow rate of 0.5 ml/min. Cortisol and d4-Cortisol were identified at m/z pairs of 363.1/121.1 and 367.3/125.2 by AB Sciex TripleQuad 6500. For dexamethasone quantification, serum samples (250 μl) were placed into a 96-well block with internal standard (flumethasone) and were extracted using an Oasis HLB 96-well plate (Waters, MA), then reconstituted in 100 μl of LC solvent (2 mM Ammonium Acetate, 0.1% formic acid in H2O:2 mM Ammonium Acetate, 0.1% formic acid in methanol, 55:45, V:V). 20 μl extract was analyzed by LC-ESI-tandem mass spectrometry using a Waters BEH C18, 50 × 2.1 mm. Dexamethasone and flumethasone were identified at m/z pairs of 393.0/354.9 and 411.1/253.0 by AB Sciex TripleQuad 6500. Cortisol and dexamethasone concentrations for each sample were calculated using linear regression analysis of a standard curve. The quantification ranges for the cortisol and dexamethasone assays were 0.1–100 μg/dl and 1.0–100 ng/ml, respectively. For each run, calibration standards were prepared at concentrations of 0, 0.1, 0.5, 1, 5, 10, 20, 50, 75, 100 μg/dl for cortisol, and 0, 0.1, 1.0, 5.0, 10, 20, 50, 75, 100 ng/ml for dexamethasone, and three fortified quality control samples were also analyzed in duplicate in each run. The intra- and inter-assay percentage coefficients of variation (%CV) for cortisol and dexamethasone were 1.21% and 5.78%, and 3.82% and 10.1%, respectively. All assays were performed by the Yerkes NPRC Biomarkers Core Laboratory.
For the diurnal cortisol and dexamethasone suppression tests, cortisol measurements were largely in agreement between the two study phases (Table 1). For the dexamethasone challenge test (DCT), absolute values of cortisol systematically differed between phase 1 and phase 2. The source of this difference is unknown, as the samples from both phases were collected at the same time of year (Jan–March), by the same technicians, and assayed by the same laboratory technician using the same LC/MS procedure, and quality control parameters for the assays did not differ. Importantly, however, all statistical tests were conducted on the change in values from baseline measured in the same phase.
2.6. Diurnal cortisol and responsiveness to dexamethasone
Diurnal cortisol levels were assayed for all females from serum samples collected 1 h after sunrise (0800 h), at 1100 h, and 1 h before sunset (1700 h) on the same day. This assessment was repeated once in each study phase, resulting in six total, unstimulated cortisol measurements per subject. We also evaluated subjects after two independent 0.125 mg/kg intramuscular (IM) doses of dexamethasone (Dex). The first Dex assessment measured GC negative feedback using a Dex suppression test (DST), conducted over a 24 h timescale to measure escape from Dex suppression. Specifically, Dex injection for the DST occurred immediately after the final diurnal cortisol sample was collected at 1700 h. This allowed us to use the 1100 h diurnal cortisol sample from the same day as the baseline measurement for the DST, with another 1100 h sample collected at 1100 h the following day (24 h later) to quantify cortisol levels following injection. The second Dex injection, for a dexamethasone challenge test (DCT), was used to measure short-term sensitivity to suppression of endogenous cortisol (mimicking the response to an acute stressor) and conducted over a shorter timescale. Baseline serum samples collected at 0800 h were immediately followed by Dex injection. Serum was then collected at 1.5 h and 4.5 h post-injection to measure circulating levels of cortisol (see Fig. S1 for a visual schematic of all cortisol assessment procedures; see Table S2 for raw cortisol values).
To control for individual differences in Dex metabolism, which may depend on age or other variables (Pasquali et al., 2002), we measured serum Dex concentration in the same samples (Table S2) and used this value as a covariate in the DST and DCT analyses (Table S3).
2.7. Statistical analysis of cortisol measures
Diurnal cortisol
To analyze the diurnal cortisol data as a function of dominance rank and/or behavioral tendencies (controlled for rank and age), we performed two analyses. First, we modeled raw serum cortisol levels (3 values for each female) using linear mixed models (LMM) in the lme4 package, including time (in h) and age as fixed effects and a random effect of study subject (Bates et al., 2014). Second, we modeled diurnal cortisol slope (1700 h–0800 h cortisol)/9 h, which we summarized as a single value per female per phase, using linear models (LM). To control for possible correlations between slope and intercept, we included 0800 h cortisol levels as a covariate for the slope analysis (Table S3).
Dexamethasone suppression test
For all DST analyses of rank and behavioral tendencies (controlled for rank and age), we used LMs to analyze the difference between pre-Dex and post-Dex serum cortisol levels as the outcome variable, controlling for pre-Dex serum cortisol concentration and age as a covariate (Egbewale et al., 2014) (Table S3).
Dexamethasone challenge test
For the DCT, we used LMs to analyze the effects of rank and behavioral tendencies (controlled for rank and age) on both an “immediate” response to Dex, captured by the difference between pre-Dex and 1.5 h post-Dex serum cortisol levels, and the short-term, but more delayed response, captured by the difference between pre-Dex and 4.5 h post-Dex cortisol levels. As for diurnal cortisol, we controlled for chronological age and slope-intercept correlations (by including pre-Dex cortisol levels as a covariate in each model) (Table S3).
Effects of changes in rank on glucocorticoid regulation
In the three sets of analyses above, we performed a cross-sectional analysis on data from Phase 1 only. However, we also collected parallel data in Phase 2 to test whether improvements (or declines) in rank across phases causally affected glucocorticoid regulation in a longitudinal analysis within individuals. To do so, we implemented linear models of between-phase changes in (a) diurnal cortisol slope; (b) cortisol suppression by Dex (DST); and (c) change in sensitivity to acute Dex at 1.5 h and 4.5 h (DCT), in all cases as a function of change in Elo rating (ΔElo) across phases. We included age, Phase 1 Elo rating (which affects the possible values of ΔElo), and cortisol levels from parallel tests of GC regulation in Phase 1 as model covariates (Table S4). To evaluate the possibility that more complex, nonlinear models better describe the relationships between rank and cortisol, we also implemented generalized additive models (GAMs) using the mgcv package in R (Wood, 2011). GAM results did not qualitatively differ from the linear model results so are shown in Table S4 instead of the main text.
We conducted all statistical analyses using R (v3.1.0). Model residuals were visually inspected for homoscedasticity, and normality was assessed using the Wilks-Shapiro test (all p-values >0.05). Standardized residuals with an absolute value >3 were excluded from the final models. Variance inflation factors (VIF) were <3 for all predictors of interest. Model degrees of freedom (df), t-statistics, and p-values for fixed effects in LMMs were calculated using the lmerTest package (Kuznetsova et al., 2015). Goodness-of-fit chi-squared statistics and P-values were determined using the lrtest function in the lmtest package (Zeileis and Hothorn, 2002) (Table S5).
3. Results
3.1. Behavioral characterization
Principal components analysis (PCA) on the ten behaviors recorded from all females indicated that the first three principal components (PC) cumulatively explained 58% of the total variance in the correlation matrix (N = 90, df = 18, χ2 = 109.2, P < 0.001). All of the behavioral variables loaded onto at least one of the three PCs with an absolute value >0.49 (Table 2). According to the specific behaviors that loaded onto each PC, individuals who scored high on component 1 (PC1) spent less time alone and were more likely to be groomed, approached by, and spend time in physical contact with groupmates. Individuals who scored high on component 2 (PC2) were more aggressive and less submissive, as well as more likely to approach and groom groupmates. Individuals scoring high on component 3 (PC3) had the highest rates of self-scratching and spent more time self-grooming. Based on the component factor loadings and prior studies of rhesus macaque personality (Freeman and Gosling, 2010; Weiss et al., 2011), we conceptualized PC1 as an individual’s social approachability, PC2 as confidence and impulsivity, and PC3 as the expression of anxiety. We used the term “social approachability” for PC1 because it specifically captures approaches and grooming directed to the focal animal, as opposed to the directionally agnostic terms ‘sociability,’ ‘social integration,’ or ‘composite sociality’ used in the broader behavioral literature on nonhuman primates (Capitanio, 1999; Archie et al., 2014; Silk, 2007); note that approaches and grooming initiated by the focal animal are captured more strongly by PC2. For ease of discussion, we refer to PC1 as “social approachability,” PC2 as “boldness,” and PC3 as “anxiousness.”
Table 2.
Standardized, varimax-rotated factor loadings of social behaviors in principal components analysis (PCA).
| Category | Behavior | PC1 | PC2 | PC3 |
|---|---|---|---|---|
|
|
||||
| (Social Approachability) | (Boldness) | (Anxiousness) | ||
| Dominance | Aggression (F) | −0.01 | 0.72 | −0.17 |
| Submissive Gestures (F) | −0.16 | −0.70 | 0.26 | |
| Anxiety | Self-Scratching Bouts (F) | 0.13 | 0.03 | 0.79 |
| Self-Grooming (D) | −0.13 | −0.05 | 0.49 | |
| Affiliation | Alone (D) | −0.81 | −0.47 | 0.05 |
| Approaches Given (F) | 0.40 | 0.51 | 0.21 | |
| Approaches Received (F) | 0.78 | −0.18 | 0.05 | |
| Contact (D) | 0.71 | 0.09 | −0.25 | |
| Grooming Received (D) | 0.58 | 0.40 | 0.14 | |
| Grooming Given (D) | 0.05 | 0.75 | 0.33 | |
| Eigenvalue | 3.19 (2.71, 3.82) | 1.40 (1.34, 1.87) | 1.20 (1.10, 1.48) | |
| Cumulative% Variance | 32 | 46 | 58 | |
Bold typeface indicates the strongest factor loadings (|r| >0.50).
Eigenvalues with confidence intervals are shown, calculated from a bootstrapped distribution (k = 10,000).
D: duration (min/h); F: frequency (events/h).
3.1.1. Predictors of behavioral tendencies
We tested the hypothesis that a female’s age and dominance rank would be associated with her social approachability (PC1), boldness (PC2), and anxiousness (PC3) scores. Across the two study phases, we found that older females were less socially approachable (PC1: βage = −0.078, t87 = −2.89, P = 0.005), less bold (PC2: βage = −0.069, t84 = −3.58, P < 0.001), and less anxious (PC3: βage = −0.079, t85 = −3.02, P = 0.003) than younger females, and that higher-ranking females were more socially approachable (PC1: βElo = 0.36, t87 = 3.15, P = 0.002) and bolder (PC2: βElo = 0.85, t77 = 11.0, P < 0.001), but not less anxious (PC3: βElo = −0.03, t75 = −0.250, P = 0.80), than lower-ranking females. Because rates of aggression differed across social groups, we also tested whether group-level aggression predicted higher rates of anxiety among resident females, but did not find any association (PC3: βgroup-aggression/hr = −0.20, t88 = −1.32, P = 0.19). The results of this analysis suggest that rank and age affect behavioral tendency, and that apart from levels of anxiety, “personality” attributes are primarily dependent on rank.
3.1.2. Causal effects of social context on behavioral tendencies
Based on our analyses of rank and behavioral tendencies in phase 1, we took advantage of the social group rearrangement at the study midpoint to test whether social approachability, boldness, and anxiousness would change in tandem with changes in dominance rank. Controlling for age and PC scores in phase 1, we found that experimentally manipulated changes in a female’s Elo rating between phase 1 and phase 2 (ΔElo) were positively associated with changes in her social approachability (PC1phase2: βΔElo = 0.35, t41 = 2.28, P = 0.028) and boldness (PC2phase2: βΔElo = 0.96, t41 = 8.03, P < 0.001) in phase 2. In other words, improvements in rank increased component scores on PC1 and PC2 (Fig. 1), suggesting that social approachability and boldness were causally affected by an individual’s social status. Improved rank was not associated with changes in anxiousness (PC3phase2: βΔElo = −0.06, t41 = −0.46, P = 0.65), supporting the idea that some, but not all, of an individual’s behavioral tendencies are status-dependent.
Fig. 1.
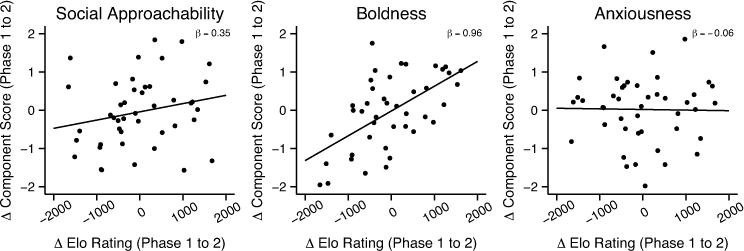
Relationships between changes in Elo rating and changes in behavioral tendency. Changes in Elo rating from phase 1 to phase 2 were positively associated with changes inbehavioral component scores from phase 1 to phase 2 for(A) social approachability(PC1: P = 0.028) and (B) boldness (PC2: P = 6.0 × 10−10), but not (C) anxiousness (PC3: P = 0.65), adjusted for age and phase 1 component score. More positive values along the x-axis reflect larger increases in rank, whereas more negative values reflect larger decreases in rank between phase 1 and phase 2.
3.1.3. Stability of behavioral tendencies across social contexts
No study to our knowledge has tested for cross-situational behavioral consistency in rhesus macaques after moving the same animals to a new social group where they occupied different dominance ranks. We therefore tested whether behavioral tendencies would be stable across the two study phases when controlling for rank and age effects (i.e., by using component scores that were orthogonal to rank and age). We found that boldness in phase 1 predicted phase 2 boldness (βPC2-phase1 = 0.45, t40 = 2.92, P = 0.006), and that anxiousness scores were weakly correlated across phases (βPC3-phase1 = 0.36, t40 = 1.82, P = 0.077). Social approachability was not correlated within-subjects (βPC1-phase1 = 0.08, t40 = 0.52, P = 0.61). Interestingly, after controlling for rank and age, social approachability and boldness were inversely related (r = −0.36, P < 0.001) throughout the study, whereas anxiousness was not associated with social approachability (r = −0.10, P = 0.36) or boldness (r = 0.04, P = 0.67).
3.2. Effects of rank and behavioral tendency on glucocorticoid regulation
3.2.1. Diurnal cortisol
As expected, cortisol significantly decreased from morning to late afternoon (βtime = −0.46, t85 = −8.14, P < 0.001), consistent with the well-established diurnal cortisol rhythm. We did not find a main effect of rank or age on serum cortisol (βtime:Elo = 0.04, t85 = 0.68, P = 0.50; βage = 0.16, t85 = 1.23, P = 0.23); however, higher-ranking females who scored high on anxiousness (PC3) had elevated cortisol levels (i.e., increased output) throughout the day (βElo:PC3 = 1.33, t36 = 2.07, P = 0.046) (Fig. 2A). In addition, females who scored higher on social approachability than expected for their rank and age had a blunted diurnal rhythm, as shown by a smaller dynamic range across the 3 timepoints (βtime:PC1 = 0.16, t85 = 0.68, P = 0.010) (Fig. 2B) and a somewhat shallower slope from 0800 to 1700 h (βPC1 = −0.12, t35 = −1.77, P = 0.085). Boldness was not associated with cortisol output or diurnal cortisol rhythm (Table S3).
Fig. 2.
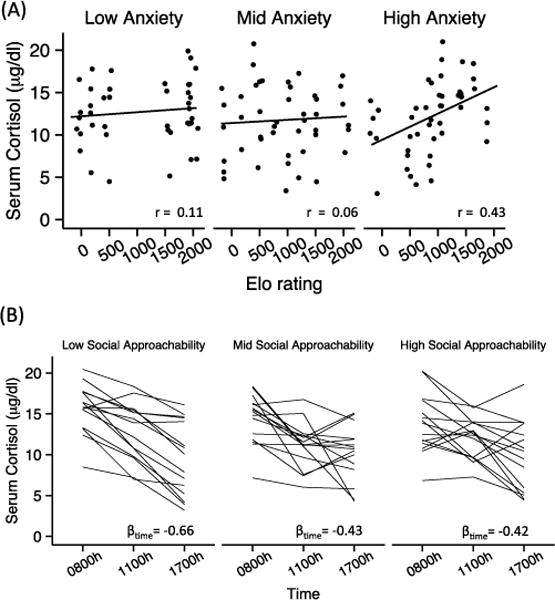
Relationships between Elo rating, behavioral tendency, and diurnal serum cortisol in Phase 1. (A) Among females with higher anxiousness (residual PC3 score; right panel) higher rank predicted increased cortisol output (βElo*PC3 = 0.16, t88 = 2.63, P = 0.010). (B) Females who scored higher in social approachability (residual PC1 score; middle and right panels) had smaller diurnal decreases in cortisol than females with low residual PC1 scores (βPC1*time=0.16, t88 = 2.63, P=0.010; left panel). component scores are split into tertiles for visualization only; statistical models reported in the main text were fit using continuously distributed component scores (Table S3), but Pearson correlation (A) and βtime (B) for each tertile are shown in each panel to provide a summary of the stratified data.
3.2.2. Glucocorticoid negative feedback (DST)
As expected, Dex administration significantly reduced circulating cortisol levels (t80 = −11.5, P < 0.001). Both higher pre-Dex serum cortisol levels and serum Dex levels at the 24 h time point, which reflects differences in Dex metabolism across subjects, predicted larger decreases in cortisol between pre- and post-Dex (24 h) samples (βCortisol-preDex = 0.60, t34 = 3.87, P < 0.001 and βDex = 1.62, t34 = 3.42, P = 0.002). However, after adjusting for serum Dex and pre-Dex cortisol, we found no significant associations between negative feedback and age, dominance rank, or any of our three measures of behavioral tendency (Table S3).
3.2.3. Sensitivity to acute glucocorticoid challenge (DCT)
Serum cortisol levels were lower at 1.5 h (t43 = −3.04, P = 0.004) and 4.5 h after Dex administration (t43 = −8.26, P < 0.001). After controlling for serum Dex concentration and baseline cortisol levels, we found that Elo rating was positively associated with changes in cortisol at 4.5 h post-Dex (βElo = 0.70, t34 = 2.14, P = 0.039), though not at 1.5 h post-Dex (βElo = 0.26, t34 = 1.01, P = 0.32), providing some evidence for heightened Dex sensitivity among higher-ranking females. Age was not associated with changes in cortisol in this test (Table S3). Rank effects on Dex sensitivity at both timepoints differed somewhat depending on a female’s social approachability score (1.5 h: βElo:PC1 = 0.43, t34 = 2.24, P = 0.031; 4.5 h: βElo:PC1 = 0.43, t34 = 2.03, P = 0.051), such that only in more socially approachable females was higher rank associated with increased Dex sensitivity (Fig. 3A). We also found that more anxious females were more sensitive to Dex at 1.5 h (βPC3 = 0.66, t34 = 2.54, P = 0.016) (Fig. 3B), though this association was not significant at 4.5 h (βPC3 = 0.51, t34 = 1.68, P = 0.10). Decreases in cortisol in the DCT were not associated with glucocorticoid negative feedback in the DST (1.5 h: r = 0.01, P = 0.94; 4.5 h: r = −0.10, P = 0.53).
Fig. 3.
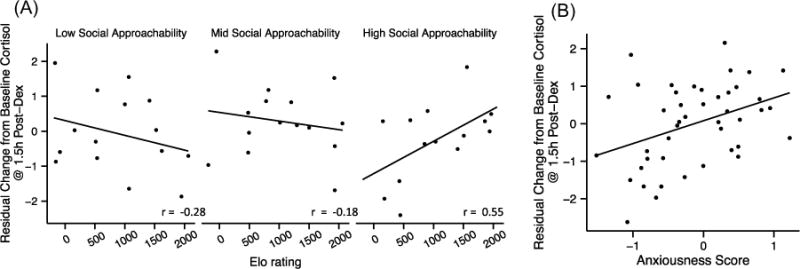
Relationships between Elo rating, behavioral tendency, and the cortisol response to acute dexamethasone challenge (DCT) in Phase 1. Dexamethasone (0.125 mg/kg) was administered at 0800 h immediately following baseline serum collection. Adjusting for other model predictors (Table S3), more positive y-axis values (i.e., larger change from baseline) indicate greater suppression of cortisol by Dex. (A) At 1.5 h post-Dex administration, social approachability (residual PC1 scores) had rank-dependent effects on cortisol suppression (βElo*PC1 = 0.43, t34 = 2.24, P = 0.031): rank predicted sensitivity to Dex only among females who scored high on PC1. (B) At 1.5 h post-Dex, anxiousness (residual PC3) was associated with increased cortisol suppression by Dex (1.5 h: βPC3 = 0.67, t34 = 2.54, P = 0.016). Component scores are split into tertiles for visualization only; statistical models reported inthe maintext were fit using continuously distributed component scores (Table S3), but Pearson correlations (A) for each tertile are shown to provide a summary of the stratified data.
3.3. Causal effects of dominance rank on glucocorticoid regulation
The cross-sectional analyses above vary in their support of rank effects on measures of GC regulation, which is in part expected because different testing protocols capture different phases of HPA axis function (Herman et al., 2016). However, they are also less powerful than longitudinal analyses within females, when occupying different ranks. Thus, we reassessed our cortisol measures in each female at the end of the second phase of the study and modeled the effect of AElo on changes in cortisol across phases. We found that improvements in rank led to increased glucocorticoid negative feedback (DST: βΔElo = 3.11, t40 = 2.22, P = 0.032) and increased sensitivity to acute Dex suppression at both timepoints (DCT: 1.5 h: βΔElo = 1.36, t39 = 2.20, P = 0.034; 4.5 h: βΔElo = 2.56, t39 = 2.87, P = 0.007) (Fig. 4). Diurnal cortisol slope was not affected by rank change (βΔElo = 0.05, t41 = 0.21, P = 0.83), suggesting that improved rank causally improved stress-related regulation of cortisol by the HPA axis, but not basal cortisol output under unstimulated conditions.
Fig. 4.
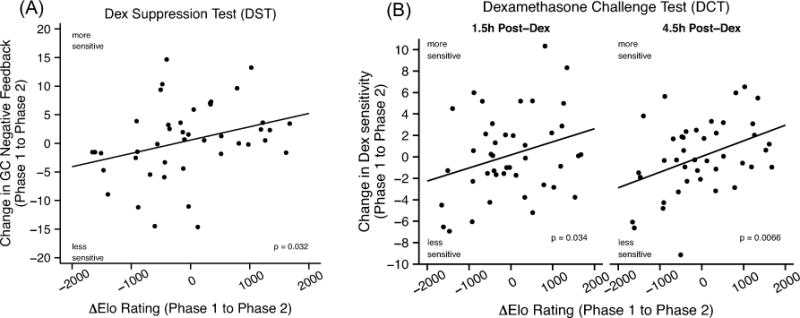
Changes in dominance rank causally affected glucocorticoid regulation. Improved Elo rating (i.e. rank) in phase 2 was associated with increased glucocorticoid negative feedback, as measured by (A) dexamethasone suppression of cortisol, and (B) increased sensitivity to acute dexamethasone challenge at both 1.5 h and 4.5 h post-Dex administration. Change in glucocorticoid negative feedback values from phase 1 to phase 2 (A) and change in sensitivity to acute Dex challenge (B) shown on the y-axis, adjusted for phase 1 values and phase 1 Elo rating. Significance (p-values) tests based LM results shown in Table S4.
4. Discussion
4.1. Behavioral characterization
To our knowledge, this study is the first to characterize behavioral tendencies (‘personality’) in rhesus macaques living in two completely different social environments (i.e., groups with nonoverlapping composition, where study subjects occupied different social ranks in each group). Our results show that dominance rank is a major driver of several dimensions of personality, especially boldness and social approachability, although some intra-individual stability of boldness and anxiousness is detectable across groups and ranks. After controlling for rank, we also found that age predicted behavioral tendency, which may reflect overall age-associated declines in activity level (Moscrip et al., 2000) or more specific behavioral fluctuations that have been reported to occur with age in pigtail macaques (Macaca nemestrina) (Sussman et al., 2014). Together, these findings suggest that some behavioral tendencies in female rhesus macaques are more stable and trait-like (e.g. anxiousness), others are fluid and rank-dependent (e.g. social approachability), and others maybe both trait-like and plastic (e.g. boldness) (Brent et al., 2014).
By definition, personality connotes behavioral stability across time and context. However, few studies of primate behavior have closely considered the interdependence between behavior and social context when attributing “personality” or temperament to an individual (Freeman and Gosling, 2010). Our results therefore pose a unique challenge for defining primate personality, since determinations of personality have tended to rely upon observational data gathered from individuals living in relatively stable social contexts (although see McGuire et al., 1994). Even if standardized testing is used to assess behavioral responses to fixed stimuli (e.g. approach-avoidance, human intruder) and observations are conducted without peers to limit social constraints, an individual’s behavioral repertoire likely remains under the influence of their present social conditions, especially dominance rank. In particular, because rhesus macaques are thought to be highly despotic relative to other macaque species (James Adams et al., 2015), behavioral tendencies in rhesus macaques may be more influenced by dominance rank than in other species. Additionally, behavioral tendencies in wild nonhuman primates may also act as significant determinants of rank change (Konečná et al., 2012). Our study was not designed to address this aspect of interdependency between rank and behavioral tendencies, since it was structured to specifically identify the causal effects of experimentally manipulated rank. However, this question will be important to address in future work, for instance by structuring social group formation based on standardized behavioral testing outcomes, or by exploring behavioral factors that predict deviation from matrilineally inherited dominance rank in wild populations (Lea et al., 2014).
4.2. Effects of rank and behavioral tendencies on glucocorticoid regulation
We performed three assessments of glucocorticoid regulation: one focused on diurnal cortisol levels, one on short-term sensitivity to Dex suppression (DCT), and one on sensitivity to Dex suppression over 24 h (DST). Scores on these three measures were uncorrelated with each other, supporting the notion that regulation of cortisol by the HPA axis involves distinct physiological processes (e.g. reactivity, recovery) that cannot be indexed by a single measurement or test (Herman et al., 2016). However, the results from the DCT and DST tests – the two that simulate the HPA response to cortisol release rather than cortisol output throughout the day – converge to support a contribution of dominance rank on glucocorticoid sensitivity. We observed this pattern in both the effects of rank changes across study phases (DST and DCT, Fig. 4) and in the effects of rank on Dex suppression in Phase 1 data alone (DCT). In agreement with previous studies (Michopoulos et al., 2012; Shively et al., 1997), low ranking, presumably chronically stressed females, were consistently less sensitive to Dex suppression in all three analyses. In contrast, the lack of clear evidence for rank effects on diurnal cortisol dovetails with recent arguments that rank-baseline GC correlations, even when present, require multiple repeated measurements per individual to detect (Cavigelli and Chaudhry, 2012). Furthermore, it is possible that more complex, non-linear relationships between rank and glucocorticoid regulation exist (Cavigelli and Caruso, 2015; Gesquiere et al., 2011), which necessitates further exploration with larger sample sizes.
Behavioral tendencies orthogonal to rank and age additionally contributed to glucocorticoid regulation in our sample. Females who scored higher on our anxiousness dimension (PC3) exhibited some evidence for increased sensitivity to Dex in the DCT (Fig. 3B), while females who scored higher on the social approachability dimension (PC1) showed blunted diurnal cortisol rhythms throughout the day (Fig. 2B). Further, we identified some evidence that behavioral tendencies moderate rank effects on glucocorticoid regulation. Specifically, we found that lower-ranking females who had higher scores on social approachability (PC1) than expected for their rank were less sensitive to acute Dex challenge, and lower-ranking females who had higher scores on anxiousness (PC3) than expected for rank secreted less cortisol throughout the day. These findings were unexpected and contrary to our initial predictions, as they seem to contradict reports that social affiliation is stress buffering (Ditzen and Heinrichs, 2014; Sanchez et al., 2015; Young et al., 2014), and that anxiousness is associated with increased cortisol output (Shackman et al., 2013).
We believe this apparent contradiction may be resolved by returning to our original definition of behavioral tendencies. Specifically, each of our behavioral components was defined by a composite suite of behaviors that may reflect different psychosocial states depending on a female’s social status. PC1 was largely defined by the frequency and duration of “affiliative” interactions (e.g. groom, approach) that were initiated by a female’s groupmates (Table 2), but not by affiliation per se. Indeed, approaches by higher-ranking individuals can be precursors to received threats or aggression as well as affiliative behavior, whereas approaches by lower-ranking groupmates are more likely to be non-threatening (i.e., the type of interaction that strengthens social bonds: Snyder-Mackler et al., 2016). Similarly, we characterized PC3 as “anxiousness” because self-directed behaviors tend to increase during situations of uncertainty, social tension, or danger in nonhuman primates (reviewed in Aureli and Whiten, 2009). However, our observational methods did not permit us to examine whether these behaviors followed received aggression, so it is not clear whether self-scratching and self-grooming truly capture ‘anxiousness’ in this model. Thus, while our decision to attach simple terms to the behavioral PCs we calculated follows the precedent set by the literature (Freeman and Gosling, 2010), our findings suggest a need for caution in studies of animal ‘personality’–especially in using terms like ‘sociability’ with normative or value-laden connotations. Additionally, future studies on animal behavior should avoid assuming a monotonic relationship between personality and physiology without first considering social context, including the contribution of social hierarchies.
4.3. Limitations
The current study has several limitations. First, because we studied only females, we cannot assess whether our findings generalize to male nonhuman primates, in which rank-GC correlations have been extensively described (Abbott et al., 2003; Cavigelli and Caruso, 2015; Gesquiere et al., 2011; Sapolsky, 1989). Second, our study design did not allow us to take into account variation in individual life histories (e.g. maternal experience, parity, birth weight) as a predictor of either behavior or cortisol: because our sample consisted of females across a large range of ages, differences in life history could be large. Similarly, we were not able to assess the effects of other individual characteristics, such as genotype (although our within-subjects analysis provides some measure of control for this source of variance). Third, because females were housed in varying social and demographic conditions prior to entering the study, we were not able to investigate the potential effects of historical dominance rank. This limitation is offset to a degree by evidence that the effects of rank are largely plastic after rank changes (Snyder-Mackler et al., 2016; Tung et al., 2012), at least in this paradigm. Finally, our behavioral observations were conducted solely within social groups of captive rhesus macaques. Future studies would benefit from combining social group observations with standardized behavioral testing paradigms, such as the human intruder test (Kalin and Shelton, 2003), or by testing whether our findings generalize to natural populations.
Supplementary Material
Acknowledgments
The study was not possible without the expert technical assistance of Jennifer Whitley, Angela Tripp, and Jessica Johnson. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016Zi.psyneuen.2016.09.005.
Footnotes
Conflicts of interest
None.
Disclosure
All authors have materially participated in the research and/or article preparation and have approved the final article for submission.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively Ca, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. http://dx.doi.org/10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Albers PCH, de Vries H. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim Behav. 2001;61:489–495. http://dx.doi.org/10.1006/anbe.2000.1571. [Google Scholar]
- Anestis SF, Bribiescas RG, Hasselschwert DL. Age, rank, and personality effects on the cortisol sedation stress response in young chimpanzees. Physiol Behav. 2006;89:287–294. doi: 10.1016/j.physbeh.2006.06.010. http://dx.doi.org/10.1016/j.physbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014: Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc R Soc Lond B Biol Sci. 2014;281:1261. doi: 10.1098/rspb.2014.1261. http://dx.doi.org/10.1098/rspb.2014.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureli F, Whiten A. In: Emotions and Behavioral Flexibility Primate Psychology. Maestripieri D, editor. Harvard University Press; Cambridge, MA: 2009. pp. 289–323. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Lme4: Linear Mixed-effects Models Using Eigen and S4. 2014 http://lme4.r-forge.r-project.org/
- Blomquist GE, Sade DS, Berard JD. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta) Int J Primatol. 2011;32:193–208. http://dx.doi.org/10.1007/s10764-010-9461-z. [Google Scholar]
- Brent LJN, Semple S, MacLarnon A, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML. Personality traits in rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. Int J Primatol. 2014;35:188–209. doi: 10.1007/s10764-013-9724-6. http://dx.doi.org/10.1007/s10764-013-9724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 2004;29:1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. http://dx.doi.org/10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. Am J Primatol. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. http://dx.doi.org/10.1002/(SICI)1098-2345(1999)47:4<299:AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Individual differences in emotionality: social temperament and health. Am J Primatol. 2011;73:507–515. doi: 10.1002/ajp.20870. http://dx.doi.org/10.1002/ajp.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Caruso MJ. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Philos Trans R Soc B. 2015;370 doi: 10.1098/rstb.2014.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Chaudhry HS. Social status, glucocorticoids, immune function, and health: can animal studies help us understand human socioeconomic-status-related health disparities? Horm Behav. 2012;62:295–313. doi: 10.1016/j.yhbeh.2012.07.006. http://dx.doi.org/10.1016/j.yhbeh.2012.07.006. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Development Core Team; 2014. [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32:149–162. doi: 10.3233/RNN-139008. http://dx.doi.org/10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. http://dx.doi.org/10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Med Res Methodol. 2014;14:49. doi: 10.1186/1471-2288-14-49. http://dx.doi.org/10.1186/1471-2288-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo AE. The Rating of Chess Players, Past and Present. New York Arco 1978 [Google Scholar]
- Erickson K, Gabry KE, Schulkin J, Gold P, Lindell S, Higley JD, Champoux M, Suomi SJ. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Dev Psychobiol. 2005;46:331–339. doi: 10.1002/dev.20061. http://dx.doi.org/10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- Fairbanks L, Jorgensen M. Objective behavioral tests of temperament in nonhuman primates. In: Weiss A, King JE, Murray L, editors. Personality and Temperament in Nonhuman Primates. Springer; New York, NY: 2011. pp. 103–127. [Google Scholar]
- Freeman HD, Gosling SD. Personality in nonhuman primates: a review and evaluation of past research. Am J Primatol. 2010;72:653–671. doi: 10.1002/ajp.20833. http://dx.doi.org/10.1002/ajp.20833. [DOI] [PubMed] [Google Scholar]
- Funder DC, Colvin CR. Explorations in behavioral consistency: properties of persons, situations, and behaviors. J Pers Soc Psychol. 1991;60:773–794. doi: 10.1037//0022-3514.60.5.773. http://dx.doi.org/10.1037/0022-3514.60.5.773. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the top: rank and stress in wild male baboons. Science. 2011;333:357–360. doi: 10.1126/science.1207120. http://dx.doi.org/10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Horm Behav. 2006;49:233–236. doi: 10.1016/j.yhbeh.2005.07.005. http://dx.doi.org/10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. http://dx.doi.org/10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Labonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EHF, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. http://dx.doi.org/10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140:256–282. doi: 10.1037/a0032671. http://dx.doi.org/10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Adams M, Majolo B, Ostner J, Schülke O, De Marco A, Thierry B, Engelhardt A, Widdig A, Gerald MS, Weiss A. Personality structure and social style in macaques. J Pers Soc Psychol. 2015;109:338–353. doi: 10.1037/pspp0000041. http://dx.doi.org/10.1037/pspp0000041. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. http://dx.doi.org/10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Konečná M, Weiss A, Lhota S, Wallner B. Personality in Barbary macaques (Macaca sylvanus): Temporal stability and social rank. J Res Pers. 2012;46:581–590. http://dx.doi.org/10.1016/j.jrp.2012.06.004. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Bojesen Christensen RH. LmerTest: Tests in Linear Mixed Effects Models 2015 [Google Scholar]
- Lea AJ, Learn NH, Theus MJ, Altmann J, Alberts SC. Complex sources of variance in female dominance rank in a nepotistic society. Anim Behav. 2014;94:87–99. doi: 10.1016/j.anbehav.2014.05.019. http://dx.doi.org/10.1016/j.anbehav.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Measuring temperament in rhesus macaques: consistency and change in emotionality over time. Behav Processes. 2000;49:167–171. doi: 10.1016/s0376-6357(00)00083-8. http://dx.doi.org/10.1016/S0376-6357(0000083-8) [DOI] [PubMed] [Google Scholar]
- McGuire MT, Raleigh MJ, Pollack DB. Personality features in vervet monkeys: the effects ofsex, age, social status, and group composition. Am J Primatol. 1994;33:1–13. doi: 10.1002/ajp.1350330102. http://dx.doi.org/10.1002/ajp.1350330102. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm Behav. 2012;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. http://dx.doi.org/10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. http://dx.doi.org/10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Ingram DK, Lane MA, Roth GS, Weed JL. Locomotor activity in female rhesus monkeys: assessment of age and calorie restriction effects. J Gerontol A Biol Sci Med Sci. 2000;55:B373–B380. doi: 10.1093/gerona/55.8.b373. [DOI] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. Assessing dominance hierarchies: validation and advantages ofprogressive evaluation with Elo-rating. Anim Behav. 2011;82:911–921. http://dx.doi.org/10.1016/j.anbehav.2011.07.016. [Google Scholar]
- Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, De Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. http://dx.doi.org/10.1210/jc.87.1.166. [DOI] [PubMed] [Google Scholar]
- Pavani S, Maestripieri D, Schino G, Turillazzi PG, Scucchi S. Factors influencing scratching behaviour in long-tailed macaques (Macaca fascicularis) Folia Primatol. 1991;57:34–38. http://dx.doi.org/10.1017/CBO9781107415324.004. [Google Scholar]
- Revelle W. Sych: Procedures for Personality and Psychological Research. 2015 In: p. http://personality-project.org/r/psych-manual.pdf.
- Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 2015;10:512–526. doi: 10.1080/17470919.2015.1087426. http://dx.doi.org/10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Hypercortisolism among socially subordinate wild baboons originates at the CNS level. Arch Gen Psychiatry. 1989;46:1047–1051. doi: 10.1001/archpsyc.1989.01810110089012. http://dx.doi.org/10.1001/archpsyc.1989.01810110089012. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. http://dx.doi.org/10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Silk JB, Cheney DL. Variation in personality and fitness in wild female baboons. Proc Natl Acad Sci. 2012;109:16980–16985. doi: 10.1073/pnas.1210780109. http://dx.doi.org/10.1073/pnas.1210780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Silk JB, Cheney DL. Social bonds in female baboons: the interaction between personality, kinship and rank. Anim Behav. 2013;87:23–29. http://dx.doi.org/10.1016/j.anbehav.2013.10.008. [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci U S A. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. http://dx.doi.org/10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. http://dx.doi.org/10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Shirtcliff EA, Styner MA, Gilmore JH, Coe CL. Population variation in neuroendocrine activity is associated with behavioral inhibition and hemispheric brain structure in young rhesus monkeys. Psychoneuroendocrinology. 2014;47:56–67. doi: 10.1016/j.psyneuen.2014.05.002. http://dx.doi.org/10.1016/j.psyneuen.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. http://dx.doi.org/10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Silk JB. The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. http://dx.doi.org/10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Kohn JN, Barreiro LB, Johnson ZP, Wilson ME, Tung J. Social status drives social relationships in groups of unrelated female rhesus macaques. Anim Behav. 2016;111:307–317. doi: 10.1016/j.anbehav.2015.10.033. http://dx.doi.org/10.1016/j.anbehav.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin SJ, Soldin OP. Steroid hormone analysis by tandem mass spectrometry. Clin Chem. 2009;55:1061–1066. doi: 10.1373/clinchem.2007.100008. http://dx.doi.org/10.1373/clinchem.2007.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman AF, Mates EA, Ha JC, Bentson KL, Crockett CM. Tenure in current captive setting and age predict personality changes in adult pigtailed macaques. Anim Behav. 2014;89:23–30. doi: 10.1016/j.anbehav.2013.12.009. http://dx.doi.org/10.1016/j.anbehav.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Mustoe AC, Hochfelder B, French JA. Reunion behavior after social separation is associated with enhanced HPA recovery in young marmoset monkeys. Psychoneuroendocrinology. 2015;57:93–101. doi: 10.1016/j.psyneuen.2015.03.019. http://dx.doi.org/10.1016/j.psyneuen.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. http://dx.doi.org/10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher J, Addessi E, Visalberghi E. Contextualised behavioural measurements of personality differences obtained in behavioural tests and social observations in adult capuchin monkeys (Cebus apella) J Res Pers. 2013;47:427–444. http://dx.doi.org/10.1016/j.jrp.2013.01.013. [Google Scholar]
- Weiss A, Adams MJ, Widdig A, Gerald MS. Rhesus macaques (Macaca mulatta) as living fossils ofhominoid personality and subjective well-being. J Comp Psychol. 2011;125:72–83. doi: 10.1037/a0021187. http://dx.doi.org/10.1037/a0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B Stat Methodol. 2011;73:3–36. http://dx.doi.org/10.1111/j.1467-9868.2010.00749.x. [Google Scholar]
- Young C, Majolo B, Heistermann M, Schulke O, Ostner J. Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. Proc Natl Acad Sci. 2014;111:18195–18200. doi: 10.1073/pnas.1411450111. http://dx.doi.org/10.1073/pnas.1411450111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2:7–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


