Abstract
Central nervous system diseases are major health issues and are often associated with disability or death. Most central nervous system disorders are characterized by high levels of oxidative stress. Nuclear factor erythroid 2 related factor (Nrf2) is known for its ability to regulate the expression of a series of enzymes with antioxidative, prosurvival, and detoxification effects. Under basal conditions, Nrf2 forms a complex with Kelch-like ECH associated protein 1, leading to Nrf2 inactivation via ubiquitination and degradation. However, following exposure of Keap1 to oxidative stress, Nrf2 is released from Keap1, activated, and translocated into the nucleus. Upon nuclear entry, Nrf2 binds to antioxidant response elements (ARE), thereby inducing the expression of genes such as glutathione s-transferase, heme oxygenase 1, and NADPH quinine oxidoreductase 1. Many dietary phytochemicals have been reported to activate the protective Nrf2/ARE pathway. Here, we review the preventive and protective effects of dietary Nrf2 activators against CNS diseases, including stroke, traumatic brain injury, Alzheimer’s disease, and Parkinson’s disease.
Keywords: Nrf2, dietary, acute brain injury, neurodegenerative disease
1. Introduction to the Nrf2 pathway
Nuclear factor erythroid 2 related factor (Nrf2) is a basic leucine zipper (bZIP) transcription factor with a characteristic Cap ‘n’ collar structure (1). It is one of the key regulator of phase II drug metabolizing enzymes, including glutathione S-transferase (GST), heme oxygenase 1 (HO-1) and NADPH quinine oxidoreductase 1 (NQO-1), all of which play important roles in antioxidant and pro-survival effects and the detoxification of xenobiotics (2–4). As with other nuclear transcription factors, Nrf2 translocates into the nuclear compartment upon activation and binds antioxidant response elements (ARE) that initiate expression of downstream genes. It is generally accepted that Kelch-like ECH associated protein 1 (Keap1) plays an essential role in the inactivation of Nrf2 (5), but there are also additional proteins that regulate the important Nrf2/ARE pathway (6).
1.1 Keap1-dependent pathway
1.1.1 Nrf2 under basal conditions
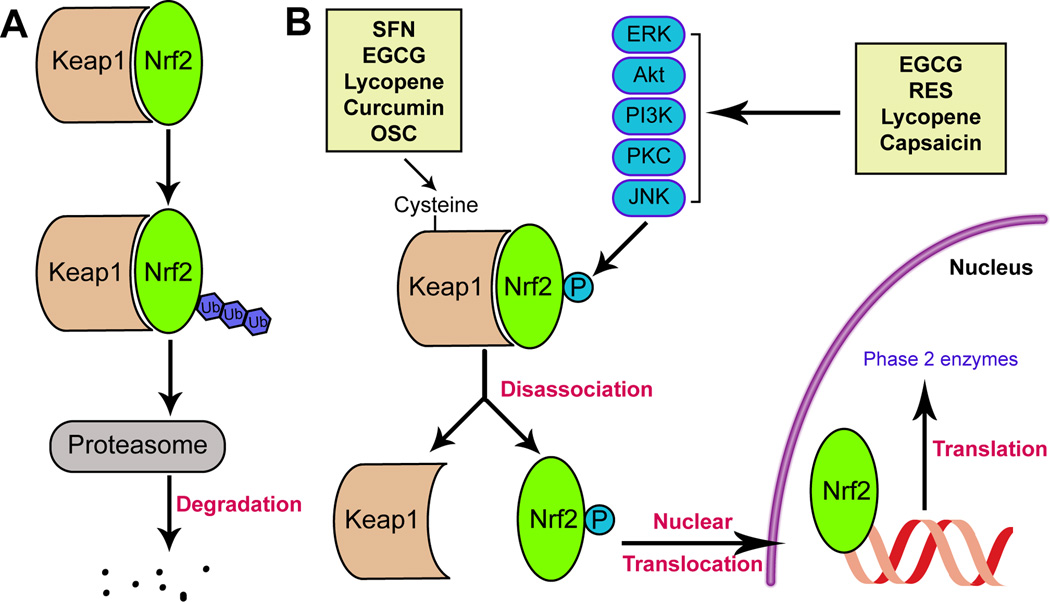
Nrf2 is composed of six functional domains known as Nrf2-ECH homologies (Neh) and designated as Neh1-6. Each Neh domain serves a unique function (7). The Neh1 domain is the CNC-bZIP domain, enabling Nrf2 to form a heterodimer with the ZIP domain of small musculoaponeurotic fibrosarcoma (Maf) proteins (8). The Neh2 domain mediates the binding of Nrf2 to Keap1, which acts as a cytosolic repressor of Nrf2 activation. Keap1 is composed of three functional domains: a bric-a-brac (BTB) domain, an intervening region (IVR), and a Kelch domain (also named DGR domain). Two Kelch domains can bind with high affinity to two motifs located in the Neh2 domain of Nrf2 (9). Under basal conditions, Nrf2 forms a complex with Keap1, which is in turn linked to a functional E3 ubiquitin ligase complex named Rbx1 via an adaptor protein, Cullin3 (10). The formation of this complex facilitates the ubiquitination and degradation of Nrf2 by the ubiquitin-proteasome system (Fig. 1).
Fig 1.
Mechanisms of select dietary Nrf2 activators. (A) Under physiological or baseline conditions, Nrf2 is sequestered by Keap1, leading to its ubiquitination and degradation by the proteasome. (B) Two mechanisms are involved in Nrf2 activation. The first is by modification of the cysteines in Keap1, which leads to conformational changes in this protein and the subsequent release of Nrf2. The second mechanism is by activation of kinases that phosphorylate Nrf2 and thereby free it from Keap1-mediated sequestration. After nuclear translocation, Nrf2 binds to the antioxidant response element (ARE) in the promoter regions of phase 2 enzyme genes. SFN, curcumin, and OSC all activate Nrf2 by the first mechanism, whereas RES and capsaicin function through the second mechanism and EGC and lycopene work through both mechanisms. Keap1: Kelch like ECH associated protein 1; SFN: sulforaphane; EGCG: epigallocatechin gallate; OSC: organosulfur compound; RES: resveratrol.
1.1.2 Dissociation of the Nrf2-Keap1 complex
The dissociation of the Nrf2-Keap1 complex is an essential prerequisite for Nrf2/ARE activation. Specifically, two cysteine residues (Cys273, Cys288) in the IVR domain and one cysteine residue (Cys151) in the BTB domain are essential for Keap1-mediated repression of Nrf2 activity under homeostatic, unstressed conditions (4). The oxidation of these cysteine residues affects the conformation of Keap1 and leads to dissociation of the Nrf2-Keap1 complex, thereby stabilizing and activating Nrf2, and upregulating the expression of phase II genes (10).
1.1.3 Nuclear translocation of Nrf2
All transcription factors must enter the nucleus to be active and induce gene expression. In the case of Nrf2, this process can be completed within 15 min after tert-Butylhydroquinone (t-BHQ) treatment, the prototypical Nrf2 activator (11). The key mediators that regulate nuclear import and export of transcription factors are nuclear localization signals (NLS) and nuclear export sequences (NES). A number of such nuclear shuttling signals have been identified on Nrf2, including three NLS motifs (NLS1, NLS2 and NLS3) and two NES motifs (NES1 and NES2).
The direction of Nrf2 movement is determined by a homeostatic balance between import and export driving forces. Under basal conditions, NES1 is functional, and the import force is overshadowed by the export force, retaining Nrf2 in the cytosol and eliciting its degradation. Following exposure to oxidative or electrophilic stressors, Cys183 of NES1 becomes adducted, reducing its function. The import forces then overwhelm the export forces, culminating in Nrf2 nuclear translocation (12). In addition, Nrf2 nuclear translocation can be enhanced via phosphorylation by specific kinases. One such example is protein kinase C (PKC), which can phosphorylate Ser40 in the Neh2 domain (13), although its role in Nrf2 nuclear shuttling remains controversial. Another candidate kinase is Fyn, which phosphorylates Tyr568 in the Neh3 domain, thereby controlling nuclear export of Nrf2 (11, 14).
After translocation into the nucleus, Nrf2 forms a heterodimer with a group of nuclear bZIP proteins called small Maf proteins (5). This heterodimerization enhances the binding specificity of Nrf2 to the cis-acting enhancer ARE/EpRE (15) located in the promoter region of phase II genes (16, 17). As a consequence of this DNA binding event, Nrf2 is able to initiate the transcription of phase II genes (18).
1.2 Keap1-independent pathway
Recent studies have proposed a Keap1-independent ubiquitination model of Nrf2 degradation (Fig. 1) (19). In this model, glycogen synthase kinase-3β (GSK3β) phosphorylates Ser342 and Ser347 at Neh6 of Nrf2. Phosphorylated Neh6 can bind with the Skp, Cullin, F-box (SCF)-containing ubiquitin ligase adaptor β-TrCP. β-TrCP is a scaffolding protein that directly links Nrf2 to the Cullin1/Rbx1 ubiquitination complex, which is subsequently degraded. This GSK-3β and β-TrCP-dependent Nrf2 degradation model is supported by the finding that GSK-3β inhibitors stabilize Nrf2 in Keap1−/− mouse embryo fibroblasts (MEFs) (14). Additionally, the cancer-chemopreventive agent nordihydroguaiaretic acid can activate Nrf2 and increase HO-1 protein levels by inhibiting GSK-3β phosphorylation in Keap1−/− MEFs (20).
Several other kinases also regulate the Nrf2/ARE pathway. For example, PKC can phosphorylate Nrf2 at Ser40, which located in the Neh2 domain and is critical for its interaction with Keap1. This phosphorylation event results in the disassociation of the Keap1/Nrf2 complex (21). Phosphatidylinositol 3-kinase (PI3K) also positively regulates the transcriptional activity of Nrf2 (22). Finally, the mitogen-activated protein kinase (MAPK) family includes three members—extracellular signal-regulated protein kinase (ERK), c-jun N-terminal kinase (JNK), and p38, all of which may modulate Nrf2 activation (1). However, such effects seem to depend on the cell type. For example, p38 is a negative regulator of Nrf2 in human hepatoma HepG2 and murine hepatoma Hepa1c1c7 cells (23), but it has the opposite role in MCF-7 mammary epithelial cells (24).
Some proteins can directly regulate the Nrf2/ARE pathway in the cytoplasm or in the nucleus. For example, p21 is a cytoplasmic mediator that binds the DLG motif of Nrf2 (the same site that binds to Keap1) through its C-terminal KRR motif. The competition between p21 and Keap1 for Nrf2 binding compromises Keap1-mediated ubiquitination of Nrf2 (25). Another protein, sequestosome-1 (also known as p62), binds and inactivates Keap1 and thus augments the expression of genes regulated by Nrf2 (6). In the nucleus, the BTB domain and CNC homolog 1 (BACH1) heterodimerizes with Maf and occupies ARE sequences to inhibit the expression of phase II genes. When challenged by oxidative insults, BACH1 is phosphorylated and exported to the cytoplasm. Maf proteins are thereby liberated and can form a heterodimer with Nrf2 to enhance the expression of phase II genes (18).
1.3 Self-limitation of the Nrf2 pathway
After exposure to oxidative stress, the activated Nrf2/ARE pathway boosts the expression of phase II genes by Keap1-dependent and independent means. However, there are endogenous regulatory mechanisms to prevent excessive activation of this pathway. First, AREs are located in the promoter region of Cul3, Rbx1, and Keap1 genes. Thus, when the Nrf2/ARE pathway is activated, the transcription of these inhibitory proteins is simultaneously promoted. This negative feedback loop is defined as an autoregulatory arm of the Nrf2/ARE pathway (22). Second, the Keap1-Cul3-Rbx1 complex can translocate into the nucleus, an event that is mediated by prothymosina (ProTα), a Keap1-binding protein with a nuclear localization signal. As a result, 10–15% of the Keap1-Cul3-Rbx1 complex is located in the nucleus. Upon nuclear entry, the Keap1-Cul3-Rbx1 complex releases ProTα and binds Nrf2, leading to the ubiquitination and degradation of nuclear Nrf2 (26). Third, oxidative stressors such as hydrogen peroxide promote the activation of GSK-3β by phosphorylating its tyrosine 216 residue. Activated GSK-3β subsequently phosphorylates Fyn (p-Fyn, a member of Src family) at a threonine residue(s), leading to the accumulation of p-Fyn in the nucleus and subsequent phosphorylation of Nrf2 at tyrosine 568. Subsequently, phosphorylated Nrf2 interacts with Crm1 or exportin 1 and is exported out of nucleus (27), thereby leading to its inactivation and loss of phase II enzyme expression. These mechanisms would all serve to prevent neoplastic transformation of tissue from excessive activation of the prosurvival Nrf2/ARE pathway.
2. The neuroprotective effects of the Nrf2/ARE pathway against CNS diseases
There are more than 200 detoxification and antioxidant genes driven by the Nrf2/ARE pathway. Thus, Nrf2 can elicit neuroprotection against CNS disorders in manifold ways. In this review, we will focus on the protective roles of Nrf2 against acute neurological diseases.
2.1 Acute neurological diseases
2.1.1 Traumatic brain injury (TBI)
TBI is a major cause of death and disability worldwide, especially in children and young adults. Oxidative stress is a major component of the pathogenesis of TBI. Many studies have demonstrated that Nrf2 is capable of reducing the brain damage caused by TBI (28).
Following TBI, the brain exhibits a significant increase in Nrf2 expression and phase II enzymes, such as HO-1 and NQO1 (29). HO-1 catalyzes the first and rate-limiting step of heme catabolism (30). The neuroprotective effects of HO1 can be attributed to two mechanisms: the breakdown of heme and the generation of antioxidants. Hemoglobin, oxidase, and peroxidase are the three main classes of proteins containing heme (31, 32). Breakdown of these proteins causes a net reduction in superoxide and other reactive oxygen species (ROS). The breakdown products of heme also exhibit neuroprotective effects. For example, biliverdin and bilirubin are both potent antioxidants (32, 33). NQO1 itself also has anti-oxidative properties, indicating an important role in neuroprotection (34). The increased expression of these enzymes after traumatic insults suggests that the Nrf2/ARE pathway is an endogenous compensatory adaptation against the damage elicited by TBI.
(−)-Epicatechin, a type of natural flavonol, has also been demonstrated to exert neuroprotective effects via activation of Nrf2/ARE. EC has been shown to decrease TBI lesion volume and neuronal degeneration in mice, and to increase the expression of Nrf2 and HO-1 following TBI. However, the neuroprotective effects of Epicatechin are abolished in Nrf2−/− mice (35). Similar results have also been reported by other groups (36). Taken together, these findings demonstrate that endogenous activation of Nrf2 protects the brain from TBI.
Nrf2 activation can ameliorate blood-brain barrier (BBB) dysfunction. The BBB barrier loses integrity following direct exposure to mechanical or shearing forces and secondary oxidative and inflammatory damage after TBI. Secondary damage causes a loss of endothelial cells and tight junction proteins, further exacerbating BBB dysfunction (37). While direct lesions after trauma cannot be reversed, the secondary damage can perhaps be ameliorated. One approach to ameliorating the secondary oxidative damage is Nrf2 activation. For example, Zhao and colleagues reported that the BBB retains its integrity in wildtype mice treated with an Nrf2 activator, in contrast to Nrf2 knockout mice (37). These findings demonstrate that Nrf2 activation exerts robust BBB protective effects.
2.1.2 Ischemic stroke
Stroke is the leading cause of disability and the third-leading cause of mortality across the world. Ischemic stroke is the most common cause of stroke. The pathological processes of stroke are complex, ranging from excitotoxicity, oxidative stress, and inflammation to mitochondrial dysfunction (38, 39). Nrf2 serves as a natural brake on ischemic injury. Following middle cerebral artery occlusion (MCAO) in rodents, Keap1 levels are naturally decreased, and this loss is paralleled by an increase in Nrf2 and its downstream proteins, such as thioredoxins, glutathione (GSH) synthases, and HO-1 (40). Thioredoxins are 12-kDa enzymes containing a conserved Cys-X-X-Cys motif in their active center. This motif plays an important role in eliminating oxidized proteins via the exchange between cysteine thiols and protein disulfides (41). GSH is the most abundant antioxidant peptide in the CNS and scavenges various reactive oxygen species, including superoxide, nitric oxide, the highly reactive hydroxyl radical, and peroxynitrite (42). GSH can also mitigate toxicity secondary to high cysteine concentrations (43).
Activation of the Nrf2 pathway is critical for scavenging ROS, and this scavenging function contributes to robust neuroprotection against ischemic brain injury. Wu et al. reported that the Nrf2/ARE pathway activator myricetin lessened the production of ROS, decreased infarct volume, and reduced neuronal loss after ischemia (44, 45). This conclusion is supported by another study employing a second Nrf2/ARE pathway activator, Panax notoginseng saponins (46). Finally, Nrf2−/− mice subjected to stroke exhibit higher levels of ROS than wildtype mice (47), supporting the aforementioned notion that Nrf2 is a natural compensatory mechanism.
2.1.3 Hemorrhagic strokes
Hemorrhagic strokes include intracerebral hemorrhages (ICH) and subarachnoid hemorrhages (SAH), which are classified based on the location of bleeding and blood accumulation. That is, ICH involves bleeding into brain parenchyma whereas SAH involves bleeding into the subarachnoid space.
In addition to the intracranial hypertension and brain herniation caused by hematoma and edema, ischemia and oxidative stress also contribute to brain injury after ICH (48). HO-1 is upregulated within 24 hr after ICH, peaking at day 5, and subsiding on day 8, indicating a temporal window of activation of the Nrf2/ARE pathway (49). However, HO-1 elicits detrimental effects in ICH, as shown by smaller injuries in HO-1 knockout mice (50). Post-injury administration of the selective Nrf2 activator tBHQ has been shown to attenuate neurodegeneration and improve neurological outcomes after ICH (51, 52). Consistent with these latter observations, Nrf2−/− mice showed more severe neurological deficits and cellular damage after ICH (53). Although HO-1 induction by tBHQW would be expected to elicit detrimental effects after ICH (see above), the parallel activation of many other antioxidant response genes by Nrf2 may ameliorate the negative consequences of greater heme breakdown by HO-1.
Although SAH per se does not directly lead to parenchymal cerebral damage, it is accompanied by vasospasms that indirectly cause ischemia and acute brain injury (54). In rodent SAH models, there is activation of Nrf2 in cerebral vessels, as shown by increased Nrf2 nuclear translocation and DNA binding in both endothelial and smooth muscle cells of the basilar artery (55). tBHQ treatment also markedly upregulates the expression of Keap1, Nrf2, HO-1, NQO1, and GSTα1 after SAH. Furthermore, activation of the Keap1/Nrf2/ARE pathway is also associated with attenuation of cognitive dysfunction after SAH (56).
2.2 Neurodegenerative Diseases
2.2.1 Alzheimer’s disease (AD)
AD is a progressive neurodegenerative disorder characterized by memory loss and cognitive dysfunction. Pathological hallmarks of AD include the cerebral deposition of amyloid-beta (Aβ) peptides in senile plaques and neurofibrillary tangles of hyper-phosphorylated tau aggregates. Aβ, generated from the cleavage of amyloid precursor protein, is thought to be critical for neuronal cell death in AD (57). Aβ itself has been shown to induce the production of H2O2 in cultured cells (58), revealing that oxidative damage may play a role in AD progression. In turn, mitochondrial ROS production increases Aβ production in a self-amplifying cascade and leads to neurodegeneration in vivo (59). As will be argued below, natural Nrf2/ARE activation may break the progression of AD by reducing oxidative stress-mediated cell death.
Postmortem tissue from AD victims exhibits increased levels of proteins that are downstream of Nrf2. For example, NQO1 is increased in AD brains (60). Similar patterns have been reported for HO-1, p62, and glutathione redox system genes in the hippocampus and cerebellum in AD (61). However, some studies have found a decrease of Nrf2 in AD brains (62). One explanation for these discrepancies may be the stage of disease at the time of tissue collection. There may be upregulation of endogenous defense systems at early stages of the disease but loss of natural protective mechanisms at late stages.
Many studies have demonstrated positive effects of Nrf2 activation on AD phenotypes. An activator of the Nrf2/ARE pathway, 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic acid-Methyl Amide (CDDO-MA) has been shown to improve memory and decrease plaque formation, Aβ, and markers of oxidative stress in Tg19959 transgenic AD mice (63). Similar effects have been reported with other Nrf2 activators, such as tBHQ and curcumin. Administration of Nrf2 activators ameliorates long-term memory loss in addition to the accumulation of ROS or Aβ peptides (62, 64). In hypobaric hypoxia-induced dementia, tropomyosin receptor kinase A expression and ERK phosphorylation were increased by acetyl-L-carnitine, resulting in Nrf2 nuclear translocation. The activated Nrf2/ARE pathway in turn ameliorated memory impairments in this model (65).
2.2.2 Parkinson’s disease (PD)
Mitochondrial dysfunction is believed to be critical in the pathogenesis of PD (66, 67). Parkin and PINK1 genes play important roles in mitochondrial quality control and are mutated in some familial PD cases (68). The mitochondrial inhibitors 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or its metabolite 1-methyl-4-phenylpyridine, 6-hydroxydopamine, and rotenone are commonly used to induce a PD-like phenotype in animals (58). Mitochondrial function can also be inhibited by dopamine (DA) quinone, a by-product of DA oxidation (69). DA quinone itself can induce oxidative stress due to its ability to bind protein cysteine residues and GSH, leading to GSH depletion and changes in cellular function. Furthermore, GSH depletion results in mitochondrial complex I inhibition, which further exacerbates mitochondrial dysfunction (58). Taken together, these findings suggest that oxidative stress is an important factor in the pathogenesis of PD. In addition, there is evidence of oxidative damage to proteins, lipids, and DNA in postmortem tissue from PD victims (70).
Several studies suggest that Nrf2 plays a naturally protective role in PD (71) . The substantia nigra of PD patients exhibits higher levels of Nrf2 downstream genes, such as NQO1 and HO-1 (72). Furthermore, Nrf2−/− mice exhibit increased sensitivity to the dopaminergic toxins MPTP and 6-OHDA (73). Administration of the Nrf2 activators tBHQ and 1,2-dithiole-3-thione ameliorated the PD phenotype in experimental mouse models (25). Chen and colleagues have demonstrated that astrocytes with Nrf2 activation, but not neurons, can reduce the neurotoxicity of MPTP (25), indicating that it is astrocytic Nrf2 that is critical for neuroprotection against PD models.
3. Phytochemicals with Nrf2-ARE activating ability
As discussed above, Nrf2 plays a protective role against acute and chronic brain disorders, making it a promising target for clinical intervention (74). There are several categories of Nrf2 activators. Below we will focus on dietary Nrf2 activators, as they are widely available and safe.
3.1 Sulforaphane
Sulforaphane (1-isothiocyanate-4-methylsulfinyl butane, SFN)—a naturally occurring isothiocyanate compound—is present in cruciferous vegetables such as broccoli, brussel sprouts, cabbage, and cauliflower. SFN has indirect antioxidant properties by virtue of Nrf2 pathway activation and upregulation of genes downstream of ARE (75, 76).
Oral bioavailability is one of key limiting factors for phytochemicals. SFN passively diffuses into cells because of its lipophilicity and small molecular size (77, 78). Within the cell, SFN is conjugate to glutathione (SFN-GSH) in the presence of glutathione-S-transferase (GST), which maintains a concentration gradient across the cell membrane and ensures continued passive diffusion of SFN into the cell (79). SFN is an electrophile that can react with protein thiols to form thionoacyl adducts. Hong and colleagues reported several sensor cysteines in human Keap1 that can be modified by SFN, including Cys-77, −226, −249, −257, −489, −513, −518, and −583 (80). Another sensor cysteine for SFN is Cys151 in mouse Keap1, which is essential for the association of Cul3 ubiquitin ligase (81). By modifying these cysteines, SFN can prevent ubiquitination of Nrf2, thereby leading to Nrf2 stabilization. Stabilized Nrf2 is then free to translocate into the nucleus and initiate the Nrf2/ARE pathway. In addition to targeting Keap1, SFN can also enhance Nrf2 mRNA and protein expression by reducing methylation of the first 15 CpGs of Nrf2 promoters (82). Other targets of SFN, such as DNA methytransferases and histone deacetylase (83) are out of the scope of this review.
Many studies have demonstrated protective effects of SFN against CNS diseases via activation of the Nrf2/ARE pathway. For example, Ping et al. reported that administration of SFN could significantly reduce infarct volume by increasing Nrf2 and HO-1 expression in experimental ischemia models (84). In addition, SFN reduced the numbers of apoptotic neurons, activated microglia, and oxidative parameters after ischemia. In TBI models, SFN is able to preserve BBB function by reducing loss of endothelial cell markers and tight junction proteins. Nrf2 is a key mediator in this process, as SFN administration increases the expression of Nrf2-driven genes, including GSTα3, GPX, and HO-1 in the parietal cortex and brain microvessels (47). SFN also has protective effects against chronic neurodegeneration, such as experimental AD and PD. The accumulation of Aβ peptides in AD is accelerated by oxidative stress, impaired protein-folding in the endoplasmic reticulum, and deficient proteasome- and autophagic-mediated clearance of damaged proteins (85). Kwak et al demonstrated that SFN can increase proteasome function in vivo, which offers a viable strategy to protect neuronal cells from oxidative damage in AD (86). Finally, SFN can inhibit 6-OHDA-induced DA neuron loss by increasing Nrf2 nuclear translocation and HO-1 expression in experimental PD (87, 88). These latter findings have also been confirmed in rat organotypic nigro-striatal co-cultures (89).
3.2 Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a diferuloylmethane derived from the rhizomes of turmeric. Curcumin shows a wide range of beneficial properties, including antioxidant and anti-inflammatory effects. Unlike SFN, the bioavailability of curcumin is poor. Many studies have found that limited tissue distribution, rapid metabolism, and short half-life severely curtail its bioavailability (90–93). Because of its poor bioavailability, the effect of curcumin on Nrf2/ARE pathway is highly dose-dependent. Curcumin treatment at a concentration of 5 µM does not have a significant effect on Nrf2 activation, whereas 15 and 30 µM curcumin does activate Nrf2 (94). Curcumin exerts its protective effects by both Nrf2-dependent and independent means. Curcumin has two electrophilic α, β-unsaturated carbonyl groups, which can modify the thiol groups of Keap1 and release Nrf2, thereby activating the Nrf2/ARE pathway and inducing the expression of phase II enzymes (95, 96). Furthermore, the ability of curcumin to increase the expression of HO-1 has been demonstrated in vascular endothelial cells, astrocytes, and cultured hippocampal neurons (97–99). Finally, curcumin can upregulate NQO1 expression (100).
Although curcumin has poor bioavailability, it is highly lipophilic and may cross the BBB, accumulating in the brain at a sufficient concentration to activate the Nrf2 pathway. Indeed, several studies have shown neuroprotective effects of curcumin. Yang et al. reported that curcumin could protect neurons from brain focal ischemia in vivo through the induction of Nrf2/HO-1 (101). This finding is in agreement with studies on ethanol-induced brain damage (102). Similarly, Li et al. also reported that curcumin can protect the brain against ischemia/reperfusion injury by activating the Nrf2/ARE pathway (103). Furthermore, pre-incubation of cultured neurons with low concentrations of curcumin for 12 hours improves cellular resistance to glucose oxidase-mediated oxidative damage (104). The protective effects of curcumin have also been reported with post-treatment after the onset of the injury (105). Thus, curcumin has both neuroprotective and neuro-preventive properties.
Epidemiological studies suggest that oral curcumin exposure through the curry-rich Indian diet is associated with a 4.4 reduction in the incidence of AD in India compared to the USA (106). However, one limitation of these findings is that they are correlative and that a causal link between curcumin and lower AD risk has not yet been established. It is also unclear if elderly patients with diseases such as AD are diagnosed as readily in underdeveloped countries as they are in developed nations. Nevertheless, Scapagnini et al. suggested that curcumin can protect cortical neurons against apoptotic cell death induced by β-amyloid peptide (99). Similarly, Cole and colleagues reported that 6 months of dietary supplementation of transgenic AD mice (Tg2576) with curcumin reduces inflammation, oxidative cerebral damage, and Aβ-induced cognitive deficits (107, 108). These authors also discovered that curcumin could easily enter the brain and inhibit the formation and toxicity of Aβ oligomers (109). These studies support the view that curcumin exposure via ingestion of the turmeric spice exerts similar beneficial effects.
3.3 Epigallocatechin gallate
Epigallocatechin gallate (EGCG) is the most abundant catechin compound in green tea. It is well established that EGCG is a potent antioxidant and anti-inflammatory agent (110). Epidemiological studies show that consumption 200–300 mg of EGCG per day is beneficial, as it is the most potent Nrf2 activator among all green tea catechins (111).
EGCG has a low absorption rate (probably <5%) and an average Tmax of 2 hours after oral administration (112, 113), due to its instability in the air and inactivation in the gastrointestinal tract and liver (114). Thus, EGCG also has poor bioavailability, similar to curcumin. Despite these pharmacokinetic properties, EGCG exhibits robust diffusion through bodily tissues, including the endothelium of the BBB (115).
EGCG has the capacity to activate Nrf2/ARE and induce HO-1 expression (116). Several studies have shown that EGCG can also interact with more upstream kinases, such as ERK, PI3K, PKC and JNK, causing the disassociation of Nrf2/Keap1 complex. In endothelial cells, EGCG induced HO-1 expression by activating Akt and ERK1/2, whereas in B lymphoblasts, EGCG induced Nrf2 translocation and HO-1 expression by activation of p38 MAPK and Akt (117). As one might expect, the effect of EGCG on MAPKs is dose and time-dependent. Low concentrations of EGCG result in the activation of ERK and induction of ARE-mediated gene expression, whereas higher concentrations cause activation of MAPKs such as JNK, thereby leading to apoptosis (111, 118).
Han et al. have reported protective effects of EGCG against ischemia/reperfusion injury in a rat model of MCAO (119). Administration of EGCG improved neurologic scores, reduced infarct volume, and ameliorated neuronal apoptosis due to increased GSH biosynthesis (via Nrf2 activation) and decreased ROS content. EGCG also can induce the expression of HO-1 in cultured neurons through the Nrf2/ARE pathway, protecting neurons against oxidative stress-induced cell death (120). Furthermore, EGCG is a potent neuroprotective agent in AD models. By inducing the expression of Nrf2 and HO-1, EGCG increases important endogenous antioxidants in microglial cells. EGCG suppresses Aβ-induced cytotoxicity by reducing the activation of the MAPK signal transduction cascade (121).
3.4 Allyl sulfides
Allyl sulfides are organosulfur compounds (OSCs) found abundantly in garlic, and include diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS). OCSs exert health benefits, and have been used as a food and medicinal herb for thousands of years.
OSCs have neuroprotective effects by induction of the expression of phase II enzymes. Due to their lipid solubility, they are easily absorbed, but are nevertheless barely detectable in the blood or urine, even after consumption of large amounts of garlic (122). This disappearance is probably caused by rapid hepatic metabolism as they pass through the liver (123). Within the liver, allicin from garlic shows a remarkable first-pass effect and is very efficiently metabolized into DADS and allyl mercaptan (124). This may prevent OSCs from reaching high concentrations in extrahepatic tissues after dietary delivery. Further studies are needed to determine the bioavailability and pharmacokinetics of OSCs in humans.
DATS is thought to react with Keap1 at Cys288, which would cause the release of Nrf2, and activation of genes downstream of ARE (125). Allyl sulfides also activate Nrf2/ARE in a similar manner as EGCG. Allyl sulfides interact with upstream kinases and lead to the dissociation of the Nrf2-Keap1 complex. Xu et al. reported that DATS protected B35 neural cells against oxygen glucose deprivation-induced cell damage via upregulation of the PI3K/Akt-mediated Nrf2 pathway and the expression HO-1 (126). DAS has also been shown to upregulate antioxidant genes and enzymes by activating Nrf2 through ERK/p38 pathways in lung MRC-5 cells (127) and in renal cells (128).
3.5 Resveratrol
The polyphenol Resveratrol (trans-3,5,4’-trihydroxystilbene, RES) is abundant in grapes and red wine. It is thought to be beneficial against cancer, diabetes, neurodegenerative, and cardiovascular diseases (129–131). As with allyl sulfides, the bioavailability of RES is very poor, approximating <1% when delivered orally (130). Such limited bioavailability can be explained by its low water solubility, limited stability, and rapid metabolism (131).
RES activates the Nrf2/ARE pathway activation by interacting with upstream kinases. Cheng et al. reported that RES activated the Nrf2 pathway and induced HO-1 and glyoxalase via ERK phosphorylation, resulting in suppression of methylglyoxal-induced insulin resistance in HepG2 cells (132). RES pretreatment also has neuroprotective effects against cerebral ischemia/reperfusion injury by activation of Nrf2 and upregulation of HO-1 in vivo (133, 134). In addition, Gaballah and colleagues recently observed that RES increased Nrf2 DNA binding activity in the rotenone model of neuronal oxidative stress in experimental PD (135).
3.6 Lycopene
Lycopene is a polyunsaturated hydrocarbon phytochemical present in tomatoes and carrots. It belongs to the tetraterpene carotenoid family (136) and exhibits health-benefits through its antioxidant activity. There are few studies about the bioavailability of lycopene, though the structural localization of lycopene in chloroplasts of fruit and vegetables is an important limitation in terms of bioavailability (137). The distribution of lycopene among tissues is tissue-specific, with high concentrations in testes, adrenals, liver, and prostate, but low concentrations in others tissues (138).
Several studies have demonstrated that lycopene can stimulate the expression of phase II enzymes by activation of the Nrf2/ARE pathway (139). Lycopene has been shown to elicit Nrf2 translocation and upregulate the ARE system in HepG2 and MCF-7 cells (140). Lycopene pretreatment increases the expression of Nrf2/HO-1 and reduces ischemic injury following global cerebral ischemia, indicating an important role of the Nrf2/ARE pathway in lycopene-medicated neuroprotection. Finally, lycopene also exerts protective effects against Aβ-induced mitochondrial oxidative stress and dysfunction in cultured rat cortical neurons, mitigating neuronal apoptosis and revealing a potential therapeutic approach against Aβ-induced neurotoxicity (141).
3.7 Capsaicin
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), the major active pungent molecule in hot chili pepper, has been shown to have immunomodulatory functions and cytotoxicity towards cancer cells (142). Capsaicin is easily absorbed following percutaneous, oral, gastrointestinal and systemic routes of administration. Due to its low half-life in plasma and rapid metabolism in the liver, capsaicin also exhibits poor bioavailability. The mechanism of Nrf2 regulation by capsaicin has been described by Joung and colleagues (143), who showed that capsaicin induced ROS production by inhibiting NQO1, which in turn caused oxidative stress, activated the PI3K/Akt pathway, and induced dissociation of the Nrf2-Keap1 complex in HepG2 cells. Notably, a capsaicin-rich diet exhibits favorable effects on Aβ levels and cognitive function (144). However, the role of Nrf2 in this process remains unclear.
The activation of the Nrf2/ARE pathway by so many dietary compounds is not coincidental. Plants often produce substances that elicit a robust stress response, as they need to defend themselves from attack by microorganisms, insects, reptiles, birds, and mammals. Many phytochemicals are therefore toxic in high doses. This feature partly explains why cells respond to many phytochemicals with robust activation of the Nrf2/ARE pathway, as this pathway is so intimately involved in detoxification and redox balance. In effect, many phytochemicals may actually be eliciting via Nrf2 a primordial form of preconditioning, which is defined as an adaptive cellular response to mild stress that prepares cells to survive subsequent challenges.
There are numerous other dietary phytochemicals not discussed above that can regulate the Nrf2/ARE pathway and exert neuroprotective effects. A description of every single ARE activator is beyond the scope of this review. However, Table 1 lists additional phytochemicals that exhibit neuroprotective properties against CNS diseases.
Table 1.
Other dietary Nrf2 activators
| Compound | Origin | Molecular Mechanism | Ref |
|---|---|---|---|
| 3H-1,2-dithiole-3- thione (d3t) |
Brussels sprouts, cabbage |
↑ Phosphorylation of Akt | (144) |
| ↑ Nrf2 nuclear translocation | |||
| ↑ HO-1 | |||
| 3-O-caffeoyl-1- methylquinic acid |
Phyllostachys edulis | ↑ Phosphorylation of EKR and JNK | (145) |
| ↑ Nrf2 nuclear translocation | |||
| ↑ HO-1 | |||
| Brazilin | Caesalpinia echinata | ↑ Phosphorylation of ERKs and PI3K/Akt | (146) |
| ↑ HO-1 | |||
| Cafestol | Coffee | ↑ Binding of Nrf2 to ARE | (147) |
| ↑ Expression of NQO1 | |||
| Carnosol | Herb rosemary | ↑ ERK, Akt, p38, MAPK | (148) |
| ↑ HO-1 | |||
| Chaocone | Cardamom | ↑ Expression of HO-1, GPX2 and TrxR1 | (149) |
| ↑ Nrf2 nuclear translocation | |||
| Chlorophyllin | Green vegetables | ↑ Phosphorylation of PI3K/Akt | (150) |
| ↑ HO-1, NQO1 | |||
| Cinnamaldehyde | Cortex Cinnamomi | ↑ Expression of Nrf2 | (151) |
| ↑ Nrf2 nuclear translocation | |||
| ↑ HO-1 NQO1 | |||
| Eupatilin | Artemisia asiaitica | ↑ Phosphorylation of ERKs and PI3K/Akt | (152) |
| ↑ Nrf2 nuclear translocation | |||
| ↑ HO-1 | |||
| Fisetin | Strawberries, apples, Japanese wax tree |
↑ Phosphorylation of PI3K/Akt, PKC and p38 | (153) |
| ↑ HO-1 | |||
| ↓ ROS | |||
| Isoorientin | Gentiana olivieri | ↑ Phosphorylation of PI3K/Akt | (154) |
| ↑ HO-1, NQO1 | |||
| Kahweol | Coffee | ↑ Binding of Nrf2 to ARE | (155) |
| ↑ Activation of pI3K and p38 | |||
| ↑ HO-1 | |||
| Omega-3 fatty acids | Cold water fish, fish oil, flaxseed |
↑ Nrf2 nuclear translocation | (156) |
| ↑ HO-1 | |||
| Piperine | Black pepper | ↑ Phosphorylation of MAPK, | (157) |
| ↑ HO-1, | |||
| ↑ Nrf2 nuclear translocation | |||
| Plumbagin | Plumbago | ↑ HO-1, Phosphorylation of Akt and ERK |
(158) |
| Quercetin | Red kidney bean, caper, radish, onion |
↑ Phosphorylation of p38 | (159) |
| ↑ Nrf2 nuclear translocation | |||
| ↓ Nrf2 ubiquitination, | |||
| ↑ HO-1, NQO1 | |||
| Xanthohumol | Hops | ↑ Expression of phase II enzymes | (152) |
| ↑ activation of Nrf2 via covalent | |||
| modification of Keap-1 at cysteine 27 residue | |||
| Zerumbone | Tropical ginger | ↑ HO-1, | (160) |
| ↑ Nrf2 nuclear translocation |
4. Conclusion
In summary, Nrf2 is sequestered under normal homeostatic conditions when redox balance is properly maintained by other cellular components. However, upon loss of redox equilibrium and other types of injury, the Nrf2 pathway is naturally activated as an endogenous compensatory mechanism. As with a preconditioning stimulus, various dietary phytochemicals can activate the Nrf2/ARE pathway and induce expression of many pro-survival genes, thereby exerting powerful neuroprotective effects against CNS diseases. Therefore, targeting the Nrf2 pathway with rational drug design is a promising therapeutic approach. However, further studies are warranted to identify the mechanism underlying Nrf2 activation by dietary compounds and to establish their protective effects in models of CNS disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS092810 to F.Z.; NS093539 to R.K.L; and NS089534, NS095671 to J.C.), and the Natural Science Foundation of China (81271276 to F.Z.). We thank Patricia Strickler for secretarial support.
Abbreviations
- AD
Alzheimer’s disease
- ARE
antioxidant response elements
- BBB
blood-brain barrier
- BTB
bric-a-brac domain
- bZIP
basic leucine zipper
- CDDO
2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic acid
- CNS
central nervous system
- EGCG
epigallocatechin gallate
- ERK
extracellular signal-regulated protein kinase
- GSH
glutathione
- GPX
GSH peroxidase
- GSK3β
glycogen synthase kinase-3β
- GST
glutathione s-transferase
- HO-1
heme oxygenase 1
- ICH
intracerebral hemorrhages
- IVR
intervening region
- JNK
c-jun N-terminal kinase
- Keap1
Kelch-like ECH associated protein 1
- Maf
musculoaponeurotic fibrosarcoma
- MAPK
mitogen-activated protein kinase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Neh
Nrf2-ECH homologies
- NES
nuclear export sequences
- NLS
nuclear localization signals
- NQO-1
NADPH quinine oxidoreductase 1
- Nrf2
nuclear factor erythroid 2 related factor
- PD
Parkinson’s disease
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3-kinase
- ROS
reactive oxygen species
- SAH
subarachnoid hemorrhages
- SFN
sulforaphane
- TBI
traumatic brain injury
- tBHQ
tert-butylhydroquinone
References
- 1.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008;52:S84–S94. doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 3.Stefanson AL, Bakovic M. Dietary Regulation of Keap1/Nrf2/ARE Pathway: Focus on Plant-Derived Compounds and Trace Minerals. Nutrients. 2014;6(9):3777–3801. doi: 10.3390/nu6093777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang MJ, An CR, Gao YQ, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 7.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of Toxicology. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 8.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends in Molecular Medicine. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, et al. Different Electrostatic Potentials Define ETGE and DLG Motifs as Hinge and Latch in Oxidative Stress Response. Molecular and Cellular Biology. 2007;27(21):7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of Substrate Adaptors Can Facilitate Cullin-mediated Ubiquitylation of Proteins by a “Tethering” Mechanism: A TWO-SITE INTERACTION MODEL FOR THE Nrf2-Keap1 COMPLEX. Journal of Biological Chemistry. 2006;281(34):24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, Bloom DA, Jaiswal AK. Nuclear Import and Export Signals in Control of Nrf2. Journal of Biological Chemistry. 2005;280(32):29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Kong A-N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinogen. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H-C, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-mediated Transcription. Journal of Biological Chemistry. 2002;277(45):42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 14.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/β-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Molecular and Cellular Biology. 2011;31(6):1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. Journal of Biological Chemistry. 1991;266(18):11632–11639. [PubMed] [Google Scholar]
- 16.Nguyen T, Sherratt PJ, Huang H-C, Yang CS, Pickett CB. Increased Protein Stability as a Mechanism That Enhances Nrf2-mediated Transcriptional Activation of the Antioxidant Response Element: DEGRADATION OF Nrf2 BY THE 26 S PROTEASOME. Journal of Biological Chemistry. 2003;278(7):4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 17.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294(1–2):1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 18.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 Competes with Nrf2 Leading to Negative Regulation of the Antioxidant Response Element (ARE)-mediated NAD(P)H:Quinone Oxidoreductase 1 Gene Expression and Induction in Response to Antioxidants. Journal of Biological Chemistry. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 19.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, et al. Structural and Functional Characterization of Nrf2 Degradation by the Glycogen Synthase Kinase 3/β-TrCP Axis. Molecular and Cellular Biology. 2012;32(17):3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojo AI, Medina-Campos ON, Rada P, Zúñiga-Toalá A, López-Gazcón A, Espada S, et al. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free Radical Biology and Medicine. 2012;52(2):473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97(23):12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee OH, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282(50):36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- 23.Correa F, Ljunggren E, Mallard C, Nilsson M, Weber SG, Sandberg M. The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia:Involvement of p38 MAPK. Glia. 2011;59(5):785–799. doi: 10.1002/glia.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275(36):27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 25.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106(8):2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niture SK, Jaiswal AK. Prothymosin-α Mediates Nuclear Import of the INrf2/Cul3·Rbx1 Complex to Degrade Nuclear Nrf2. Journal of Biological Chemistry. 2009;284(20):13856–13868. doi: 10.1074/jbc.M808084200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. Journal of cell science. 2009;122(Pt 24):4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Wu G, Liu Z. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Mediates Neuroprotection in Traumatic Brain Injury at Least in Part by Inactivating Microglia. Med Sci Monit. 2016;22:2161–2166. doi: 10.12659/MSM.896568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan W, Wang HD, Hu ZG, Wang QF, Yin HX. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci Lett. 2008;431(2):150–154. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 30.Ferrandiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14(5):473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- 31.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14(11):495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everse J, Coates PW. Neurodegeneration and peroxidases. Neurobiol Aging. 2009;30(7):1011–1025. doi: 10.1016/j.neurobiolaging.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 34.Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res. 2008;57(5):325–331. doi: 10.1016/j.phrs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Cheng T, Wang W, Li Q, Han X, Xing J, Qi C, et al. Cerebroprotection of flavanol (−)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic Biol Med. 2016;92:15–28. doi: 10.1016/j.freeradbiomed.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong Y, Yan W, Chen S, Sun CR, Zhang JM. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol Sin. 2010;31(11):1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27(38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigo R, Fernandez-Gajardo R, Gutierrez R, Matamala JM, Carrasco R, Miranda-Merchak A, et al. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah A, Liu H, et al. Inflammation in Ischemic Stroke: Mechanisms, Consequences and Possible Drug Targets. CNS Neurol Disord Drug Targets. 2014;13(8):1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian F, Shang J, et al. Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 2011;1370:246–253. doi: 10.1016/j.brainres.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 42.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108(3):227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 43.Janaky R, Varga V, Hermann A, Saransaari P, Oja SS. Mechanisms of L-cysteine neurotoxicity. Neurochem Res. 2000;25(9–10):1397–1405. doi: 10.1023/a:1007616817499. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Yue Y, Peng A, Zhang L, Xiang J, Cao X, et al. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016;7(6):2624–2634. doi: 10.1039/c6fo00419a. [DOI] [PubMed] [Google Scholar]
- 45.Yao Y, Miao W, Liu Z, Han W, Shi K, Shen Y, et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl Stroke Res. 2016;7(6):535–547. doi: 10.1007/s12975-016-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou N, Tang Y, Keep RF, Ma X, Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21(10):1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38(12):3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 48.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Regan RF. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: correlation with oxidative injury. J Neurochem. 2007;103(5):2015–2021. doi: 10.1111/j.1471-4159.2007.04885.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130(Pt 6):1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukumari-Ramesh S, Alleyne CH., Jr Post-Injury Administration of Tert-butylhydroquinone Attenuates Acute Neurological Injury After Intracerebral Hemorrhage in Mice. J Mol Neurosci. 2016;58(4):525–531. doi: 10.1007/s12031-016-0722-y. [DOI] [PubMed] [Google Scholar]
- 52.Zhao XR, Aronowski J. Nrf2 to Pre-condition the Brain Against Injury Caused by Products of Hemolysis After ICH. Transl Stroke Res. 2013;4(1):71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, et al. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med. 2007;43(3):408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Martin RD, Zhang JH. Advances in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110(Pt 1):15–21. doi: 10.1007/978-3-7091-0353-1_3. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Chen G, Zhu WW, Zhou D. Activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the basilar artery after subarachnoid hemorrhage in rats. Ann Clin Lab Sci. 2010;40(3):233–239. [PubMed] [Google Scholar]
- 56.Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z, et al. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One. 2014;9(5):e97685. doi: 10.1371/journal.pone.0097685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24(4):219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 58.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 59.Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012;16(12):1421–1433. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raina AK, Templeton DJ, Deak JC, Perry G, Smith MA. Quinone reductase (NQO1), a sensitive redox indicator, is increased in Alzheimer’s disease. Redox Rep. 1999;4(1–2):23–27. doi: 10.1179/135100099101534701. [DOI] [PubMed] [Google Scholar]
- 61.Tanji K, Maruyama A, Odagiri S, Mori F, Itoh K, Kakita A, et al. Keap1 is localized in neuronal and glial cytoplasmic inclusions in various neurodegenerative diseases. J Neuropathol Exp Neurol. 2013;72(1):18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- 62.Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, et al. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39(3):302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, et al. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J Neurochem. 2009;109(2):502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eftekharzadeh B, Maghsoudi N, Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie. 2010;92(3):245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Barhwal K, Hota SK, Jain V, Prasad D, Singh SB, Ilavazhagan G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009;161(2):501–514. doi: 10.1016/j.neuroscience.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 66.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4(3):267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 67.Sochocka M, Koutsouraki ES, Gasiorowski K, Leszek J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy. CNS Neurol Disord Drug Targets. 2013;12(6):870–881. doi: 10.2174/18715273113129990072. [DOI] [PubMed] [Google Scholar]
- 68.Hattori N, Saiki S, Imai Y. Regulation by mitophagy. Int J Biochem Cell Biol. 2014;53:147–150. doi: 10.1016/j.biocel.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Hwang JH, Ahn JS, Kim SD, Lim JG, Kim YG, Kim KH, et al. The changes of serum soluble intercellular adhesion molecule-1 after systemic steroid treatment in vitiligo. J Dermatol Sci. 1999;22(1):11–16. doi: 10.1016/s0923-1811(99)00035-3. [DOI] [PubMed] [Google Scholar]
- 70.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. discussion S-8. [DOI] [PubMed] [Google Scholar]
- 71.Kumar H, Koppula S, Kim IS, More SV, Kim BW, Choi DK. Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: a promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol Disord Drug Targets. 2012;11(8):1015–1029. doi: 10.2174/1871527311211080012. [DOI] [PubMed] [Google Scholar]
- 72.Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rabano A, Kirik D, et al. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson’s disease. Hum Mol Genet. 2012;21(14):3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- 73.Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27(6):1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 74.Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Negrette-Guzman M, Huerta-Yepez S, Tapia E, Pedraza-Chaverri J. Modulation of mitochondrial functions by the indirect antioxidant sulforaphane: a seemingly contradictory dual role and an integrative hypothesis. Free Radic Biol Med. 2013;65:1078–1089. doi: 10.1016/j.freeradbiomed.2013.08.182. [DOI] [PubMed] [Google Scholar]
- 76.Evans PC. The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. EPMA J. 2011;2(1):9–14. doi: 10.1007/s13167-011-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winiwarter S, Bonham NM, Ax F, Hallberg A, Lennernas H, Karlen A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally, theoretically derived parameters A multivariate data analysis approach. J Med Chem. 1998;41(25):4939–4949. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- 78.Cooper DA, Webb DR, Peters JC. Evaluation of the potential for olestra to affect the availability of dietary phytochemicals. J Nutr. 1997;127(8 Suppl):1699S–1709S. doi: 10.1093/jn/127.8.1699S. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364(Pt 1):301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18(12):1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 81.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243(2):170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 82.Su ZY, Zhang C, Lee JH, Shu L, Wu TY, Khor TO, et al. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev Res (Phila) 2014;7(3):319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- 83.Licznerska B, Szaefer H, Matuszak I, Murias M, Baer-Dubowska W. Modulating potential of L-sulforaphane in the expression of cytochrome p450 to identify potential targets for breast cancer chemoprevention and therapy using breast cell lines. Phytother Res. 2015;29(1):93–99. doi: 10.1002/ptr.5232. [DOI] [PubMed] [Google Scholar]
- 84.Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, et al. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010;1343:178–185. doi: 10.1016/j.brainres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 85.Lopez Salon M, Morelli L, Castano EM, Soto EF, Pasquini JM. Defective ubiquitination of cerebral proteins in Alzheimer’s disease. J Neurosci Res. 2000;62(2):302–310. doi: 10.1002/1097-4547(20001015)62:2<302::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 86.Kwak MK, Cho JM, Huang B, Shin S, Kensler TW. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic Biol Med. 2007;43(5):809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 87.Deng C, Tao R, Yu SZ, Jin H. Sulforaphane protects against 6-hydroxydopamine-induced cytotoxicity by increasing expression of heme oxygenase-1 in a PI3K/Akt-dependent manner. Mol Med Rep. 2012;5(3):847–851. doi: 10.3892/mmr.2011.731. [DOI] [PubMed] [Google Scholar]
- 88.Yoon NS, Cho Y, Lee SY, Choi HJ, Hwang O. Inactivation of Aconitase by Tetrahydrobiopterin in DArgic Cells: Relevance to PD. Exp Neurobiol. 2010;19(1):23–29. doi: 10.5607/en.2010.19.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87(7):1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 90.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 91.Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology. 1981;22(4):337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 92.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8(12):761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 93.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 94.Panchal HD, Vranizan K, Lee CY, Ho J, Ngai J, Timiras PS. Early anti-oxidative and anti-proliferative curcumin effects on neuroglioma cells suggest therapeutic targets. Neurochem Res. 2008;33(9):1701–1710. doi: 10.1007/s11064-008-9608-x. [DOI] [PubMed] [Google Scholar]
- 95.Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20(5):911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 96.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98(6):3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28(8):1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 98.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61(3):554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 99.Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8(3–4):395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 100.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8(3):e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Rajakrishnan V, Viswanathan P, Rajasekharan KN, Menon VP. Neuroprotective role of curcumin from curcuma longa on ethanol-induced brain damage. Phytother Res. 1999;13(7):571–574. doi: 10.1002/(sici)1099-1573(199911)13:7<571::aid-ptr494>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44(2):192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu JX, Zhang LY, Chen YL, Yu SS, Zhao Y, Zhao J. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regen Res. 2015;10(3):481–489. doi: 10.4103/1673-5374.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 107.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22(6):993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 109.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 110.Thangapandiyan S, Miltonprabu S. Epigallocatechin gallate effectively ameliorates fluoride-induced oxidative stress and DNA damage in the liver of rats. Can J Physiol Pharmacol. 2013;91(7):528–537. doi: 10.1139/cjpp-2012-0347. [DOI] [PubMed] [Google Scholar]
- 111.Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23(6):605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 112.Williamson G, Dionisi F, Renouf M. Flavanols from green tea and phenolic acids from coffee: critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol Nutr Food Res. 2011;55(6):864–873. doi: 10.1002/mnfr.201000631. [DOI] [PubMed] [Google Scholar]
- 113.Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Lovegrove JA, et al. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol Nutr Food Res. 2012;56(6):966–975. doi: 10.1002/mnfr.201100726. [DOI] [PubMed] [Google Scholar]
- 114.Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int J Mol Sci. 2011;12(9):5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin LC, Wang MN, Tseng TY, Sung JS, Tsai TH. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J Agric Food Chem. 2007;55(4):1517–1524. doi: 10.1021/jf062816a. [DOI] [PubMed] [Google Scholar]
- 116.Kweon M-H, Adhami VM, Lee J-S, Mukhtar H. Constitutive Overexpression of Nrf2-dependent Heme Oxygenase-1 in A549 Cells Contributes to Resistance to Apoptosis Induced by Epigallocatechin 3-Gallate. Journal of Biological Chemistry. 2006;281(44):33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- 117.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69(3):1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 118.Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78(25):2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 119.Han J, Wang M, Jing X, Shi H, Ren M, Lou H. (−)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res. 2014;39(7):1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 120.Romeo L, Intrieri M, D’Agata V, Mangano NG, Oriani G, Ontario ML, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28(Suppl):492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 121.Cheng-Chung Wei J, Huang HC, Chen WJ, Huang CN, Peng CH, Lin CL. Epigallocatechin gallate attenuates amyloid beta-induced inflammation and neurotoxicity in EOC 13.31 microglia. Eur J Pharmacol. 2016;770:16–24. doi: 10.1016/j.ejphar.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 122.Lawson LD, Ransom DK, Hughes BG. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res. 1992;65(2):141–156. doi: 10.1016/0049-3848(92)90234-2. [DOI] [PubMed] [Google Scholar]
- 123.Sun MM, Bu H, Li B, Yu JX, Guo YS, Li CY. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol Res. 2009;31(1):23–27. doi: 10.1179/174313208X332959. [DOI] [PubMed] [Google Scholar]
- 124.Egen-Schwind C, Eckard R, Kemper FH. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Med. 1992;58(4):301–305. doi: 10.1055/s-2006-961471. [DOI] [PubMed] [Google Scholar]
- 125.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, et al. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One. 2014;9(1):e85984. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu XH, Li GL, Wang BA, Qin Y, Bai SR, Rong J, et al. Diallyl trisufide protects against oxygen glucose deprivation -induced apoptosis by scavenging free radicals via the PI3K/Akt -mediated Nrf2/HO-1 signaling pathway in B35 neural cells. Brain Res. 2015;1614:38–50. doi: 10.1016/j.brainres.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 127.Ho CY, Cheng YT, Chau CF, Yen GC. Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. J Agric Food Chem. 2012;60(1):100–107. doi: 10.1021/jf203800d. [DOI] [PubMed] [Google Scholar]
- 128.Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol. 2009;606(1–3):162–1671. doi: 10.1016/j.ejphar.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 129.Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–1014. doi: 10.1016/j.phrs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54(1):7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 131.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 132.Cheng AS, Cheng YH, Chiou CH, Chang TL. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem. 2012;60(36):9180–9187. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- 133.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem Res. 2011;36(12):2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 134.Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke. 2015;46(6):1626–1632. doi: 10.1161/STROKEAHA.115.008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaballah HH, Zakaria SS, Elbatsh MM, Tahoon NM. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem Biol Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 136.Cruz Bojorquez RM, Gonzalez Gallego J, Sanchez Collado P. [Functional properties and health benefits of lycopene] Nutr Hosp. 2013;28(1):6–15. doi: 10.3305/nh.2013.28.1.6302. [DOI] [PubMed] [Google Scholar]
- 137.Schweiggert RM, Kopec RE, Villalobos-Gutierrez MG, Hogel J, Quesada S, Esquivel P, et al. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study. Br J Nutr. 2014;111(3):490–498. doi: 10.1017/S0007114513002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moran NE, Erdman JW, Jr, Clinton SK. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys. 2013;539(2):171–180. doi: 10.1016/j.abb.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. International journal of cancer. 2008;123(6):1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, et al. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4(1):177–186. [PubMed] [Google Scholar]
- 141.Qu M, Jiang Z, Liao Y, Song Z, Nan X. Lycopene Prevents Amyloid [Beta]-Induced Mitochondrial Oxidative Stress and Dysfunctions in Cultured Rat Cortical Neurons. Neurochemical Research. 2016;41(6):1354–1364. doi: 10.1007/s11064-016-1837-9. [DOI] [PubMed] [Google Scholar]
- 142.Beltran J, Ghosh AK, Basu S. Immunotherapy of tumors with neuroimmune ligand capsaicin. J Immunol. 2007;178(5):3260–3264. doi: 10.4049/jimmunol.178.5.3260. [DOI] [PubMed] [Google Scholar]
- 143.Joung EJ, Li MH, Lee HG, Somparn N, Jung YS, Na HK, et al. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K–Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal. 2007;9(12):2087–2098. doi: 10.1089/ars.2007.1827. [DOI] [PubMed] [Google Scholar]
- 144.Li KR, Yang SQ, Gong YQ, Yang H, Li XM, Zhao YX, et al. 3H-1,2-dithiole-3-thione protects retinal pigment epithelium cells against Ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Sci Rep. 2016;6:25525. doi: 10.1038/srep25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kweon MH, In Park Y, Sung HC, Mukhtar H. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2-dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic Biol Med. 2006;40(8):1349–1361. doi: 10.1016/j.freeradbiomed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 146.Choi BM, Kim BR. Upregulation of heme oxygenase-1 by brazilin via the phosphatidylinositol 3-kinase/Akt and ERK pathways and its protective effect against oxidative injury. Eur J Pharmacol. 2008;580(1–2):12–18. doi: 10.1016/j.ejphar.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 147.Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology. 2010;139(5):1699–1710. 710 e1–710 e2. doi: 10.1053/j.gastro.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 148.Shibata S, Ishitobi H, Miyaki S, Kawaoka T, Kayashima T, Matsubara K. Carnosic acid protects starvation-induced SH-SY5Y cell death through Erk1/2 and Akt pathways, autophagy, and FoxO3a. Int J Food Sci Nutr. 2016:1–6. doi: 10.1080/09637486.2016.1208734. [DOI] [PubMed] [Google Scholar]
- 149.De Spirt S, Eckers A, Wehrend C, Micoogullari M, Sies H, Stahl W, et al. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic Biol Med. 2016;91:164–171. doi: 10.1016/j.freeradbiomed.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 150.Kavitha K, Thiyagarajan P, Rathna Nandhini J, Mishra R, Nagini S. Chemopreventive effects of diverse dietary phytochemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes. Biochimie. 2013;95(8):1629–1639. doi: 10.1016/j.biochi.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Wang F, Pu C, Zhou P, Wang P, Liang D, Wang Q, et al. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell Physiol Biochem. 2015;36(1):315–324. doi: 10.1159/000374074. [DOI] [PubMed] [Google Scholar]
- 152.Yao J, Zhang B, Ge C, Peng S, Fang J. Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J Agric Food Chem. 2015;63(5):1521–1531. doi: 10.1021/jf505075n. [DOI] [PubMed] [Google Scholar]
- 153.Seo SH, Jeong GS. Fisetin inhibits TNF-alpha-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int Immunopharmacol. 2015;29(2):246–253. doi: 10.1016/j.intimp.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 154.Lim JH, Park HS, Choi JK, Lee IS, Choi HJ. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Arch Pharm Res. 2007;30(12):1590–1598. doi: 10.1007/BF02977329. [DOI] [PubMed] [Google Scholar]
- 155.Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008;582(17):2655–2662. doi: 10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 156.Zhang MJ, Wang SP, Mao LL, Leak RK, Shi YJ, Zhang WT, et al. Omega-3 Fatty Acids Protect the Brain against Ischemic Injury by Activating Nrf2 and Upregulating Heme Oxygenase 1. J Neurosci. 2014;34(5):1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi BM, Kim SM, Park TK, Li G, Hong SJ, Park R, et al. Piperine protects cisplatin-induced apoptosis via heme oxygenase-1 induction in auditory cells. J Nutr Biochem. 2007;18(9):615–622. doi: 10.1016/j.jnutbio.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 158.Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112(5):1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li C, Zhang WJ, Frei B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016;9:104–113. doi: 10.1016/j.redox.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shin JW, Ohnishi K, Murakami A, Lee JS, Kundu JK, Na HK, et al. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev Res (Phila) 2011;4(6):860–870. doi: 10.1158/1940-6207.CAPR-10-0354. [DOI] [PubMed] [Google Scholar]