Summary
Background
Antiretroviral therapy (ART) and harm reduction services have been cited as key contributors to the control of the HIV epidemic, however the specific contribution of the latter has been questioned due to uncertainty in the true efficacy of ART on HIV transmission through needle sharing. Using provincial data on OAT uptake and needle distribution volumes, we aimed to isolate the independent effects of harm reduction services and ART on HIV transmission via needle sharing in British Columbia, from 1996–2013.
Methods
Using comprehensive linked population-level data, we populated a dynamic, compartmental transmission model to simulate the HIV/AIDS epidemic in BC from 1996–2013. HIV incidence, mortality, and quality-adjusted life years (QALYs) were estimated. We estimated scenarios designed to isolate the independent effects of harm reduction services and ART (assuming 50% (10%–90%) efficacy) in reducing HIV incidence through needle sharing. Structural and parameter uncertainty was investigated.
Findings
We estimated that 3204 (2402–4589) incident HIV cases were averted between 1996 and 2013 as a result of the combined effect of the expansion of harm reduction services and ART coverage on HIV transmission via needle sharing. In a hypothetical scenario assuming ART had zero effect on transmission through needle sharing, we estimated harm reduction services alone would have accounted for 77% (62%–95%) of averted HIV incidence. In a separate hypothetical scenario where harm reduction services remained at 1996 levels, we estimated ART alone would have accounted for 44% (10%–67%) of averted HIV incidence. Due to high distribution volumes, needle distribution predominantly accounted for incidence reductions attributable to harm reduction, however OAT provided substantially greater QALY gains.
Interpretation
If the true efficacy of ART in preventing HIV transmission through needle sharing is closer to its efficacy in sexual transmission, ART’s impact on incident cases averted may be greater than that of harm reduction. Nonetheless, harm reduction services played a vital role in reducing HIV incidence in BC, and should be viewed as critical and cost-effective tools in combination implementation strategies to reduce the public health and economic burden of HIV/AIDS.
Keywords: highly active antiretroviral therapy, harm reduction, British Columbia, Canada, HIV transmission, models, theoretical
Introduction
The landmark HPTN-052 study confirmed the ability of combination antiretroviral therapy (ART) to prevent heterosexual transmission of HIV,1 previously described in observational studies.2,3 The PARTNER study subsequently replicated this finding among serodiscordant couples of men who have sex with men (MSM) in a large observational study.4 Most recently, the Strategic Timing of AntiRetroviral Treatment (START) study demonstrated the individual health benefits of immediate ART initiation, regardless of baseline CD4 cell count levels,5 further supporting the case for initiating ART immediately upon HIV diagnosis.6 International efforts are now focused on identifying and implementing evidence-based interventions to maximize population-level diagnosis, ART access and, ultimately, viral suppression to enhance the control of HIV and AIDS in terms of disease progression to AIDS and death, as well as HIV transmission.7
Harm reduction interventions to address substance use disorders (SUD) have direct HIV prevention benefits and are among the most efficient means of optimizing HIV testing and linking people living with HIV (PLHIV) with SUD to ART. Strathdee et al (2010) estimated that HIV incidence could be reduced by 30–60% by addressing the unmet need for opioid agonist treatment (OAT), sterile needle distribution, and ART provision.8 Needle distribution programs (NDP) have proven to be effective and cost-effective components of public health strategies to contain the spread of HIV and viral hepatitis among people who use injection drugs (PWID)9. The site of an explosive outbreak of HIV infection beginning in the mid-1990s, the Downtown Eastside neighbourhood of Vancouver, British Columbia (BC), Canada, now features widespread access to a range of harm reduction services and has, since the mid-1990s, demonstrated significant progress in reducing the incidence of HIV among PWID. Despite increasing HIV testing rates,10 the number of new HIV diagnoses among PWID has fallen drastically in BC, from a high of 352 in 1996 to 25 in 2013.10
Nonetheless, access to harm reduction services is highly constrained in many settings within North America and internationally.11 To some extent this may be attributable to the uncertainty around the specific magnitude of the efficacy of ART in preventing HIV transmission through needle sharing. Establishing this efficacy figure through an experimental trial may be ethically challenging given prior findings for ART’s strong protective effect in preventing sexual transmission.1,4 Further, given the expected duration of such a trial, awaiting its results to set policy directions would come with an enormous opportunity cost.
We took advantage of extensive linked population-level data and a validated dynamic transmission model to simulate the HIV epidemic in British Columbia. Our primary objective was to isolate the independent effects of harm reduction services, and the secondary preventive benefits of ART through needle sharing, on HIV incidence in British Columbia, from 1996–2013. While the broader spectrum of harm reduction services includes services such as psychosocial treatment, housing supports and mental health care, we used available data on uptake of OAT and needle distribution volumes to proxy the combined effect of these services on the reduction of shared injections. We otherwise compared deaths averted, as well as quality adjusted life-years (QALYs) gained as a result of these services to further define the total public health benefits of harm reduction services and ART among PWID and the broader population of PLHIV in British Columbia.
Methods
Model Design
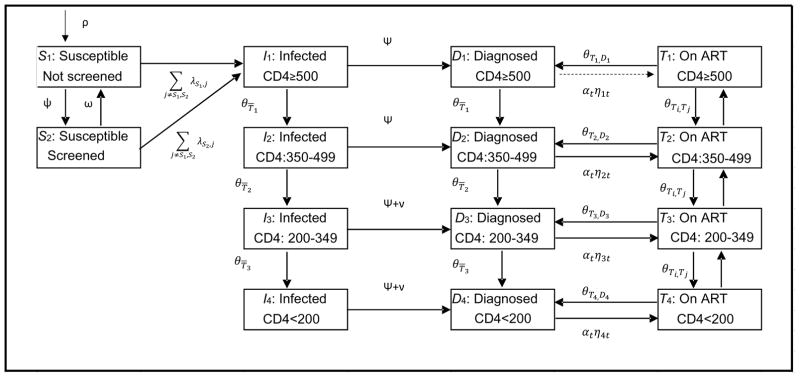
We adapted and extended an existing deterministic transmission model previously used to estimate the health benefits and costs of expanded HIV screening and HAART in the United States and BC.12,13 In brief, we partitioned the adult population of BC aged 15–64 into compartments on the basis of HIV risk behavior (MSM, PWID, MSM-PWID, and heterosexual) screening status (screened in past 12 months or not) and HIV infection status. Among those HIV-infected, individuals were further classified as infected, diagnosed, and on ART, and partitioned according to the CD4 cell count (CD4≥500 cells/μL, 350–499, 200–349, <200; Figure 1). Disease progression was differentiated among those on ART and not on ART, and estimated as a function of CD4 count, stratified into the four categories noted above. Health state transitions occurred at monthly intervals.
Figure 1. Flow chart of the model.
ART=antiretroviral treatment; The model shows movement for individuals in each of the risk behaviour strata (MSM, PWID, MSM/PWID, Heterosexual). Individuals can transition to mortality from any of the model states (transitions not shown). A more comprehensive description of the model, with specific references to parameters used for each transition, is provided in the appendix.
HIV transmission occurred through heterosexual sexual contact, homosexual sexual contact and needle sharing associated with injection drug use. We assume proportional mixing, in which persons with many sexual partners are more likely to select a partner who similarly has many partners. The probability of HIV transmission between two persons depends on the infected person’s HIV risk behavior classification, disease status, and treatment status and the uninfected person’s HIV risk behavior classification. Specifically, the rate of heterosexual and homosexual transmission was a function of the number of sexual partnerships, condom use and transmission probability per partnership. The model captures HIV transmission through needle sharing in a similar manner, as a function of the annual number of injections, average needle-sharing rates and probability of transmission per shared needle. These probabilities were time-dependent, with change in risk behaviours over time, according to proxies of injection and sexual risk behavior,14 as previously implemented. We used the non-HIV sexually transmitted infection rate14 to proxy changes in condom use rates in homosexual and heterosexual HIV transmission.
Annual coverage of needle distribution was defined as the ratio of distributed needles (data available from 2006 to 2013) to the calibrated number of illicit drug injections, and was extrapolated to cover the study timeline using the best fitting regression curve. Annual OAT coverage was defined as the ratio of the total number of person-years on OAT to the population of PWID, with available data (2001–2013) similarly extrapolated to the complete study period. The estimated population-level coverage of these services provided time-dependent adjustments on (fixed, and calibrated figures for) the monthly probability of needle sharing and the monthly number of injections, respectively, using the following formula for the monthly number of shared injections per PWID in year t:
where ds0 denoted monthly number of shared injections per PWID in 1996, CNDPt denoted coverage of number of needles dispensed in year t, COAT,t denoted coverage of OAT in year t and Δd denoted reduced injections due to OAT. These parameters drive the force of HIV infection through injection drug use, which is defined by the following formula:
where i, j, k correspond to compartments of PWID (uninfected, infected and any (either uninfected or infected) compartments, respectively), γijt denoted the needle-sharing sufficient contact rate between uninfected individuals in compartment i and infected individuals in compartment j at time t, Xkt denoted number of PWID in compartment k at time t, and dsktdenoted monthly number of shared injections per PWID in compartment k at time t, taking into account the impact of harm reduction services. The parameter τij represents probability of infection transmission per shared injection between an uninfected individuals in compartment i and an infected individual j. The term in brackets, , corresponds to the probability of selecting a needle-sharing partner in compartment j, based on the proportional mixing assumption.
Given available RCT data, the efficacy of ART in preventing HIV transmission via sexual contact (heterosexual and homosexual) was fixed at 96%, while underlying transmissibility varied by CD4 cell count strata. In the absence of RCT data, previous HIV transmission modeling studies have typically assumed the efficacy of ART in preventing HIV transmission of 50% via needle sharing, with a range of 10% – 90%.13,15 We use the mode (50%), minimum and maximum of this range in all modeling scenarios to define the secondary preventive effect of ART on HIV transmission.
Data Sources
The model was parameterized using comprehensive linked individual health administrative and registry data for the population of diagnosed PLHIV. Details regarding the construction and composition of the HIV-positive cohort and available databases are provided elsewhere.16 To inform changes in the frequency of injection, and the probability of needle sharing, we used publicly-available records of the number of needles dispensed17 (available from 2006–2013) and the number of person-years on OAT18 (available from 2001–2013) (Table A4 and A5 in the appendix, page 10). The study was approved by the University of British Columbia/Providence Health Care’s research ethics board.
Analysis
We estimated six scenarios to fulfill our objectives, with scenarios listed as S1-S6 in the top panel of Table 1. The difference of scenarios S1 and S4 was used to determine the joint effects of harm reduction services and ART on the number of incident cases averted, while the differences in scenarios S3 and S4 and S2 and S4 were used to isolate the independent effects of ART and harm reduction, respectively. Furthermore, the differences in scenarios S5 and S4 and S6 and S4 were used to attempt to further isolate the independent effects of OAT and needle distribution, respectively. The ratio of the respective independent effects and joint effect was then calculated to determine the percentage of incident HIV cases that would have been averted with only ART and harm reduction services.
Table 1.
Results: Six scenarios of harm reduction scale up and ART efficacy in preventing HIV transmission
| Scenario | Incidence, PWID | Incidence, PLHIV | Deaths among PLHIV | Total QALYs | |
|---|---|---|---|---|---|
| Observed scale-up of harm reduction, 50% ART efficacy | S1 | 2,324 | 7,987 | 3,720 | 52,570,329 |
| Observed scale-up of harm reduction, 0% ART efficacy | S2 | 2,861 | 8,718 | 3,856 | 52,569,855 |
| No scale-up in harm reduction, 50% ART efficacy | S3 | 3,811 | 9,782 | 3,980 | 52,561,200 |
| No scale-up in harm reduction, 0% ART efficacy | S4 | 4,927 | 11,191 | 4,181 | 52,560,486 |
| Observed scale-up of OAT, 0% ART efficacy, no NDP | S5 | 4,498 | 10,701 | 4,084 | 52,569,012 |
| Observed scale-up of NDP, 0% ART efficacy, no OAT | S6 | 3,001 | 8,885 | 3,929 | 52,563,104 |
|
| |||||
| Difference in Scenarios | Difference in Scenarios | Difference in Scenarios | Difference in Scenarios | ||
|
| |||||
| Estimated effect of harm reduction and ART, 50% efficacy | S4 - S1 | 2,602 | 3,204 | 461 | 9,843 |
| Estimated effect of harm reduction, with no ART efficacy | S4 - S2 | 2,065 | 2,473 | 325 | 9,369 |
| Estimated effect of ART in the absence of harm reduction, 50% ART efficacy | S4 - S3 | 1,116 | 1,409 | 200 | 714 |
| Estimated effect of OAT, with no NDP and no ART efficacy | S4 - S5 | 429 | 490 | 96 | 8,526 |
| Estimated effect of NDP, with no OAT and no ART efficacy | S4 - S6 | 1,925 | 2,307 | 252 | 2,618 |
Efficacy of ART in the prevention of parenteral HIV transmission only; effects on disease progression and sexual (homo- and heterosexual) transmission are according to published standards; NDP represents needle distribution program.
We adjusted the ART initiation rate to manually calibrate the baseline model to annual ART uptake levels and subsequently ensure each of the 11 validation targets we’ve plotted in Figure A8 in the appendix were matched. For each of the alternate ART efficacy levels for transmission via needle sharing (10%–90%), we calibrated on the number of injections per month to achieve the same degree of fit on each of these indicators, targeting specifically HIV incidence among PWID.
We report the range of effects for ART efficacy=10% to ART efficacy=90%, with ART efficacy=50% as the baseline, both within the total population of PLHIV and among HIV-positive PWID. We re-calibrated the model to re-create the HIV epidemic, as observed (i.e. Scenario 1) for each level of assumed ART efficacy, from 10–90%. These formed a common basis to estimate results, allowing us to provide a range for each scenario. In other words, the model calibrated for ART efficacy=50% was used as the basis of all scenarios presented in Table 1, with ART efficacy subsequently set to zero for scenarios S2, S4, S5 and S6 and access to harm reduction services constrained at 1996 levels in scenarios S3-S6.
We emphasize that only the secondary preventive benefits of ART via needle sharing were constrained in these alternate scenarios; the primary individual-level benefits of delayed disease progression and death, as well as the preventive benefits through sexual transmission are all still included in these hypothetical scenarios. Thus, our analyses are designed to isolate the independent effects of the secondary preventive benefits of ART and harm reduction services in reducing HIV incidence through needle sharing among PWID and at the population-level.
Outcomes
Our primary outcome was the number of incident HIV cases averted as a result of access to harm reduction services and ART, respectively, in isolation of one another. Figures are presented in absolute terms, as well as a percentage of the total number of incident HIV cases averted as a result of the preventive benefits of both ART and harm reduction services. We reiterate that our focus is on HIV incidence attributable to needle sharing only; in all scenarios, we maintained an efficacy of 96% for ART in protecting against sexual transmission of HIV.
Secondarily, we considered the total number of deaths averted, as well total QALYs gained as a result of the preventive benefits of harm reduction and ART. QALYs are a measure of health status that combine patients’ responses on health functioning with societal preferences for different levels of health functioning. We executed a range of sensitivity analyses to determine the robustness of our results to uncertainty in several key aspects of our model. First, given that estimates of HIV prevalence are externally-determined by the Public Health Agency of Canada, we considered the lower- and upper-bounds of these estimates. We also considered alternate prevalence estimates for the population of PWID, different rates of HIV transmissibility per shared injection contact and a narrower timeframe (2006–2013) during which true OAT and needle distribution figures were available.
Role of the funding source
This study was supported by funding from the BC Ministry of Health-funded ‘Seek and treat for optimal prevention of HIV & AIDS’ pilot project, Genome Canada (grant no. 147HIV) as well as the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) under award number R01DA041747. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication.
Results
The model was calibrated to match the number of individuals on ART at the midpoint of each calendar year within 10 individuals (mean 0.1% difference; Figure A5 in the appendix, page 20), and validated against population-level estimates of overall HIV prevalence, the prevalence of diagnosed PLHIV, HIV incidence (overall and by risk group), the number of deaths among PLHIV by calendar year and the size of the HIV-negative population (Figure A8 in the appendix, page 23–28). Estimated prevalence in the model was within 1.6% of the Public Health Agency of Canada’s independently estimated prevalence during the study period. Overall model-based population estimated incidence was within 0.1% of the BC Centre for Disease Control (BC-CDC) independently-estimated incidence, and risk group-specific year-to-year estimates were on average 1.9% lower for PWID, 0.4% higher for MSM, and 1.4% higher for heterosexuals. A detailed account of the model validation is provided in the supplementary appendix (page 4, 23–28).
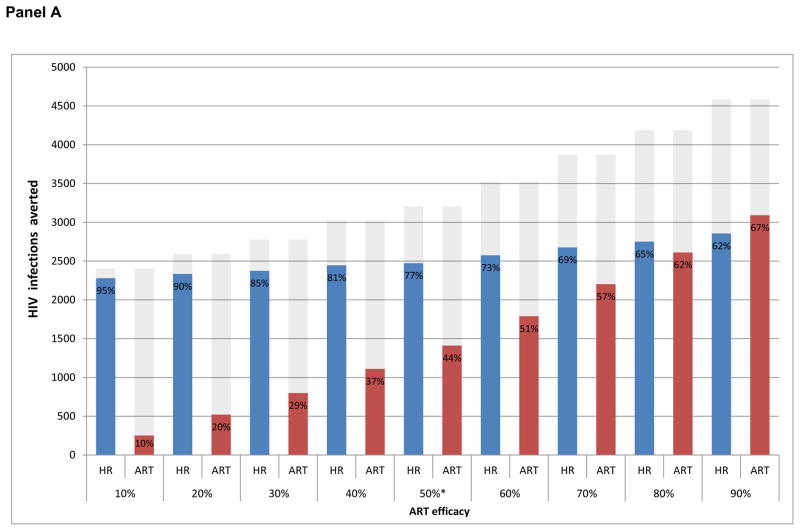
We estimated that 3204 (2402–4589) incident HIV cases were averted in British Columbia between 1996 and 2013 as a result of both harm reduction services and ART (Table 1, scenarios: (S4-S1)). Assuming that ART had no preventive benefits against onward transmission through needle sharing provided a hypothetical counterfactual scenario isolating the effects of harm reduction services on averted incident HIV cases. In this scenario, we estimated harm reduction services averted 2473 (2279 – 2855) incident cases across the province (scenarios (S4-S2)). A contrasting counterfactual scenario holding harm reduction services constant at 1996 levels provided an estimate of the isolated effects of ART in reducing HIV transmission through needle sharing. In this scenario, we estimated that ART averted 1409 (251 – 3090) incident cases across the province (scenarios (S4-S3))
As a result, we estimate harm reduction services alone averted 77% (62% – 95%) of the 3204 (2402–4589) incident cases that would have been observed with efficacious ART and harm reduction, while ART alone would have reduced HIV incidence by 44% (10% – 67%) as a result of prevention of HIV transmission through needle sharing (Figure 2, Panel A). We note that 2,065 of the 2,473 (83.5% (83.3% – 88.8%)) estimated incident cases averted via harm reduction were among PWID, while 1,116 of 1,409 (79.2% (79.2% – 79.4%)) estimated incident cases averted through the effect of ART via needle-sharing were among PWID (Table 1).
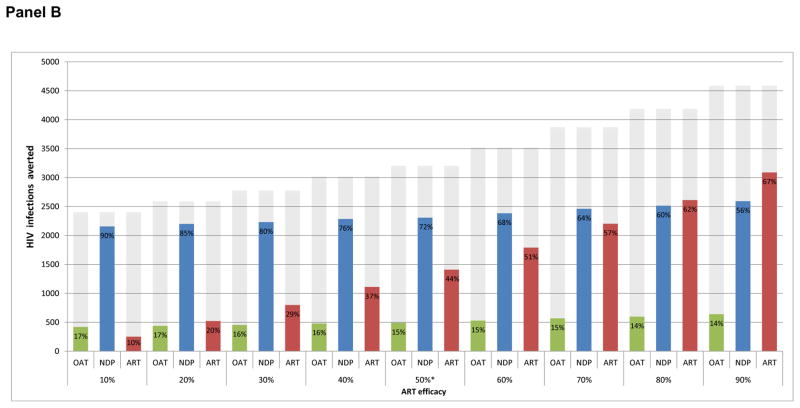
Figure 2. Percentage of incident HIV infections averted attributable to harm reduction services and the preventive benefits of ART via needle sharing.
Panel A: Estimated independent effects of harm reduction services and ART on HIV incidence among PLHIV
Panel B: Estimated independent effects of needle distribution and OAT on HIV incidence among PLHIV
Grey-shaded area represents the combined effect of harm reduction (including NDP and OAT) and ART (Scenario S1) * indicates baseline ART efficacy assumption in reducing HIV transmission via needle sharing.
Otherwise, we estimated harm reduction and ART’s effect on reducing HIV transmission through needle sharing resulted in a total of 461 deaths averted among PLHIV during the study period, and an additional 9,843 QALYs at the population-level (Table 1). Harm reduction services alone accounted for 325 deaths among PLHIV averted, and 9,369 QALYs gained, with ART accounted for 200 deaths among PLHIV averted and 714 QALYs gained (not accounting for QALY gains to those accessing ART).
We further decomposed the potential effects of OAT and needle distribution services, estimating that needle distribution alone accounted for 72% (56% – 90%) of incident cases averted, while OAT accounted for 15% (14% – 17%) amongst all PLHIV (Figure 2, panel B). While needle distribution had a greater impact on HIV incidence and deaths averted, OAT accounted for a greater increase in QALYs as a result of the health benefits attributable to HIV-negative PWID.
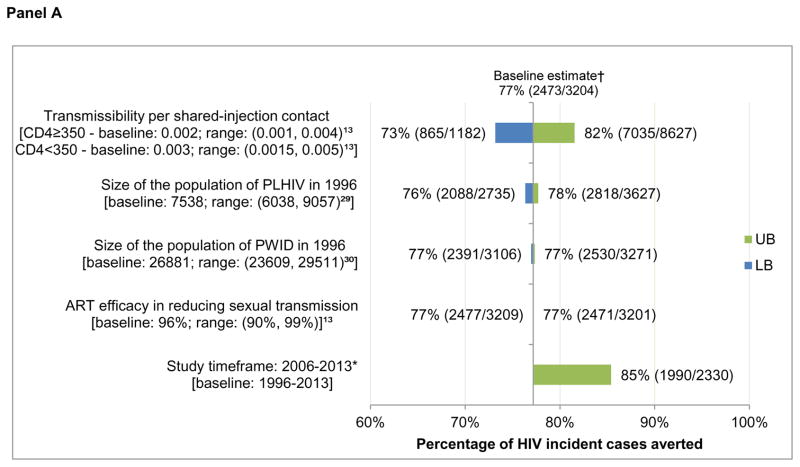
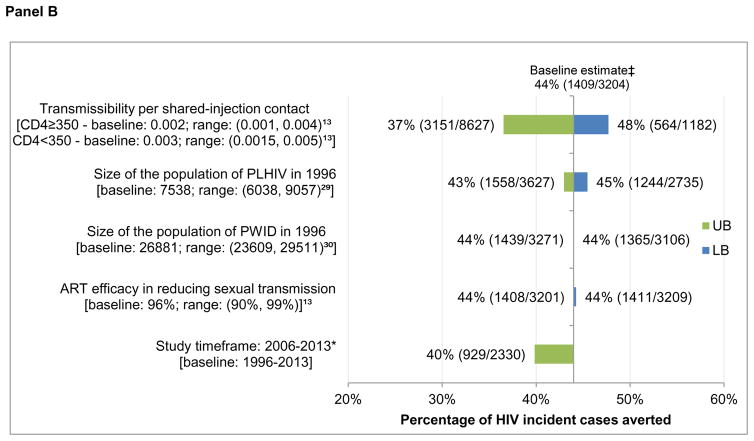
In sensitivity analysis, adjusting HIV transmissibility per shared injection contact according to a previously-defined range had the greatest impact on model estimates (Figure 3), with the proportion of averted incident cases attributable to harm reduction services varying from 73% to 82% among all PLHIV (baseline estimate: 77%) and the ART estimate varying from 37% to 48% (baseline: 44%) among all PLHIV. Further, restricting the timeframe in which both needle distribution and OAT data were available (2006–2013) led to variation in the independent effects of both harm reduction (85% vs. 77%) and ART (40% vs. 44%). Focusing on uncertainty in total provincial HIV prevalence and adjusting the size of the population of PWID had little impact on model results.
Figure 3. Sensitivity analysis on the percentage of incident cases averted among PLHIV and HIV-positive PWID 1996–2013, attributable to ART and harm reduction services at 50% ART efficacy.
Panel A: One-way sensitivity analysis on the effect of harm reduction services among PLHIV
Panel B: One-way sensitivity analysis on the effect of ART among PLHIV
PLHIV: people living with HIV/AIDS; PWID: people who inject drugs. UB: upper bound of the sensitivity scenario range; LB: lower bound of the sensitivity scenario range. * Where observed data was available for both OAT and needle distribution services. †77% = 2473/3204 = (S4-S2)/(S4-S1); ‡44% = 1409/3204 = (S4-S3)/(S4-S1).
Discussion
We found the provision of ART and harm reduction services each had profound independent impacts on the HIV epidemic in BC. Independent of any preventive benefit of ART, OAT and needle distribution would have accounted for 77% (62% – 95%) of the observed decrease in HIV incidence. Conversely, we estimated ART would have accounted for 44% (10% – 67%) of the observed decrease in incidence in absence of any scale-up in OAT or syringe provision programming. These independent effects, generated from separate hypothetical scenarios, add up to more than 100% of the estimated incident HIV cases averted because the synergies between the provisions of these services are no longer captured when their effects are estimated in isolation. Further, we emphasize that our results were based on an assumed, and likely conservative estimate of 50% efficacy of ART in reducing HIV transmission through needle sharing. If the true efficacy of ART in preventing HIV transmission through needle sharing is closer to its efficacy in sexual transmission, ART’s impact on incident cases averted may be greater than that of harm reduction services.
A recently-published article considering a similar question focusing on PWID in Vancouver estimated a stronger comparable independent effect of harm reduction (90%), but an implausibly low estimate of the impact of ART (3%).19 This study compared 2007 HIV incidence to 1996 levels, rather than total cumulative incidence, obscuring comparability to our results, which considered counterfactual cumulative incidence levels with no scale-up in harm reduction services and ART efficacy via needle sharing =0 (i.e. Table 1, scenario S4) throughout the study period as the baseline scenario for our analysis. We believe our consideration of total cumulative incidence, explicit modeling of sexual HIV transmission amongst different HIV risk groups, incorporating data on harm reduction services and accurately modeling ART engagement, dropout and re-engagement20,21 provided a more accurate depiction of the true relative contributions of these vital health services to provincial HIV incidence. Otherwise, our results are concordant with a previously-published individual-level multiple regression analysis of the effect of ‘community viral load’ on HIV incidence within a prospective cohort of Vancouver-area PWID.2 While this earlier study used an aggregate-level measure of exposure to the HIV virus – termed community viral load, and intended to represent the level of exposure to HIV within the local population – the longitudinal analysis controlled for individual-level reports of recent (i.e. occurring in the 180 days prior to each study interview) syringe sharing (likely to be highly correlated with access to needle distribution services and OAT); recent unsafe sex; ethnicity; recent cocaine and heroin use (≥ daily versus < daily); and being homeless or living in marginal housing (yes vs. no). While the study could not provide a point estimate on the efficacy of ART, it established the independent relationship subsequently confirmed in other settings, study designs and patient populations.20,22,23 On a population-level, the association between increased ART coverage and decreased number of new HIV diagnoses found in previous ecological studies were upheld.3
We reiterate that while OAT and needle distribution likely combined to explain much of the impact that harm reduction services have had on the HIV epidemic in BC, our use of OAT and needle distribution figures serve as a proxy for the broader spectrum of harm reduction services, including, psychosocial, housing and mental health programmes available across the province. Currently there are over 50 unique service providers operating in Vancouver’s downtown core alone, offering a spectrum of services including short-term inpatient detoxification, integrated and non-integrated housing supports, peer navigation networks, supervised injection and other targeted services.24 While accounting for the direct or indirect contributions of each of these many services in relation to the current analysis is not feasible, it is important to acknowledge potential impact of these interventions.
Our estimates of the collective impact of harm reduction services on the BC HIV epidemic underline the priority of maintaining and further expanding access to these critical services. BC was the epicenter of the HIV/AIDS epidemic in Canada in 1980s and 1990s, however new cases per 100,000 population has since fallen below most Canadian provinces.25 Harm reduction services played an integral role in this achievement. Several key behavioural and structural factors however distinguish BC from other settings across Canada and internationally. First, universal coverage of antiretroviral medications in BC, over-and-above national universal coverage of primary care, is unique in Canada, borne out of the intense escalation of AIDS-related deaths in the late 1980s to mid-1990s.3 Otherwise, BC, and the greater metropolitan area of Vancouver in particular, have featured extensive, overlapping epidemics of misuse of heroin, crack cocaine, methamphetamine, and most recently synthetic, powder-form fentanyl.26 Death rates from accidental overdose among people in BC with SUD have rivaled that of some of North America’s worst urban drug use epidemics for much of our study period, and were the highest in the world prior to the opening of North America’s first medically supervised injection facility.27 As a result, it is likely that the relative and absolute magnitude of benefits of harm reduction services were greater than would have been observed in settings with less extensive, and possibly more dispersed drug use epidemics. Intuitively, it should be straightforward that the benefits of OAT and needle distribution on HIV incidence will be proportional to the magnitude of the drug use epidemic in a given location.
The current study indicates that the provision of ART and harm reduction services each had substantial independent impacts on the HIV epidemic in BC; these results also may have implications globally, particularly in settings with severe deficits in access to treatment for opioid dependence or sterile syringes. In the US, for example, access to OAT with methadone has been shown to be more restricted than other high-income country settings and significant gaps exist between OAT demand and capacity.28 Syringe provision programs are deemed illegal in approximately half of the states in the US, and there has been a ban on federal funding of these programs for most of the past 30 years. A global review of interventions to prevent HIV infections among PWID estimated that injections covered by needle distribution programs were perhaps 5% and that 8% [range: 6% to 12%] of PWID had access to OAT. ART access was similarly inadequate with coverage estimated to be only 4% [range: 2% to 18%] for PWID.11
This analysis is not without limitations. First, we have not set out to determine ART efficacy through needle sharing in this study. While this is clearly a relevant question, a more appropriate study design would capture individual-level behavior within drug use networks, supported by phylogenetic data on transmitted HIV strains. As a modeling analysis with uncertainty on the size of the population of PWID and mean drug use frequency for this population, our focus was on the relative impacts of harm reduction and ART, allowing for the wide range of inherent uncertainty. It is possible that a multivariate calibration routine may have provided a better fit to the key indicators of the BC HIV epidemic, however we felt the model fit on 11 indicators of the HIV epidemic in BC provided both an accurate representation of the epidemic in BC and results in our analysis. Second, while OAT access was not modeled explicitly, it entered into the needle sharing transmission equation, and adjustments on both the probability of mortality and QALY gains were made for the number of HIV-infected and uninfected PWID accessing OAT in each calendar year. A focal point moving forward will be to fully quantify the impacts of OAT and needle distribution in terms of public health and economic benefits. Third, while the use of STI rates and OAT and needle distribution volumes are indirect proxies of changes in sexual and injection risk behaviors, they are nonetheless population-based measures which allowed us to reproduce HIV incidence with a high degree of accuracy in our model. Further research is required to determine the independent effect of needle distribution on HIV seroconversion in this setting in particular, as our estimates were higher than those found elsewhere9. Otherwise, we’ve previously outlined limitations due to infectivity being modeled indirectly through CD4-based stages of disease progression and no explicit account of higher infectivity in the 6 months following seroconversion.12 Our model was nonetheless able to produce risk-group specific incidence estimates and reproduce key aspects of the HIV epidemic at the population-level in BC with a high degree of precision.
The scale-up of harm reduction services had an impact on HIV incidence comparable to that attributable to ART access. Harm reduction services such as needle distribution and OAT should be viewed as key tools within a combination implementation strategy to reduce the public health and economic burden of HIV/AIDS globally.
Supplementary Material
Research-in-Context.
Previous Evidence
The deterministic transmission model adapted for this research was previously used to estimate the health benefits and costs of expanded HIV screening and ART in the United States and British Columbia. To construct this model, we searched British Columbia provincial data sources and reports to populate and calibrate our model to show the dynamics of the HIV epidemic in British Columbia, Canada, during the study period. This included an extensive search of PubMed for papers published in English between January, 1995, and December, 2014 (keywords “British Columbia”, “Vancouver”, “HIV”, “AIDS”, “antiretroviral therapy”, “highly active antiretroviral therapy”, “injection drug use”, and “men who have sex with men”). Furthermore, we searched the literature for any similar modelling analyses to inform model selection and development (PubMed MESH keywords: “Antiretroviral therapy, highly active [MeSH Term],” and (“Harm Reduction [MeSH Term] OR Needle-Exchange Programs [MeSH Term] OR Opiate Substitution Treatment [MeSH Term]). We included all English language articles published between January1996 and May 2016. Our searches retrieved one comparable study modelling the relative impact of ART and harm reduction services on the HIV epidemic among PWID, however this study was focused strictly on PWID in Vancouver. The study reported similar results for harm reduction services, but lower estimates of the hypothetical effect of ART as a result of the design of their analysis and restricted focus.
Added value of this study
As a result of the unknown efficacy of ART in preventing HIV transmission via needle sharing, the population-level impact of harm reduction services and the secondary preventive benefits of antiretroviral therapy (ART) have yet to be independently estimated. Using a previously-validated dynamic HIV transmission model, we incorporated provincial rates of opioid agonist treatment (OAT) utilization and syringe distribution volumes to isolate the independent effects of ART on transmission via needle-sharing and harm reduction services in reducing HIV incidence in British Columbia, Canada. We compared deaths averted, as well as quality adjusted life-years (QALYs) gained as a result of these services to further define the total public health benefits of harm reduction services and ART.
Implications of all the available evidence
The provision of ART and harm reduction services each had profound independent impacts on the HIV epidemic in British Columbia. Our results also have implications globally, particularly in settings with severe deficits in access to treatment for opioid dependence or sterile syringes. Harm reduction services such as needle distribution and OAT should be viewed as key tools within a combination implementation strategy to reduce the public health and economic burden of HIV/AIDS globally.
Acknowledgments
Funding: BC Ministry of Health; National Institutes of Health (R01DA041747); Genome Canada (142HIV).
Footnotes
Author Contributions: Nosyk designed the study, assisted with analysis and wrote the first draft of the article. Zang, Min and Krebs conducted analyses, and contributed to manuscript development. Lima. Milloy, Shoveller, Barrios, Harrigan, Kerr, Wood and Montaner aided in study design, interpretation of results, and provided critical revisions to the article. Montaner led the procurement of the database. All authors approved the final draft.
Declaration of Interests: Dr. Montaner has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, the MAC AIDS FUND, and ViiV Healthcare. Dr. Milloy has received unstructured funds, paid to his institution, from NG Biomed of Richmond. Dr. Harrigan has received grants from, served as an ad hoc advisor to, or spoke at various events sponsored by: Pfizer, Glaxo-SmithKline, Abbott, Merck, Tobira Therapeutics, Virco and Quest Diagnostics and served as a consultant for ViiV Health Care, Tobira Therapeutics, Selah Genomics Inc, and Quest Diagnostics; He holds stock in Merck and EKF Diagnostics. Dr. Barrios reports personal fees from Gilead Sciences, personal fees from MSD, outside the submitted work. Outside of the work, authors report grants from CIHR, the Michael Smith Foundation, NIDA, NIH and the BC Ministry of Health. All other authors have no conflicts of interest to declare.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood E, Kerr T, Marshall BDL, et al. Longitudinal community plasma HIV-1-RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ. 2009;338:1191–4. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. Lancet. 2010;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodger A, Bruun T, VC, et al. CROI. Boston: 2014. HIV transmission risk through condomless sex if HIV positive partner is on supressive ART: PARTNER study. [Google Scholar]
- 5.Health NIo. [accessed June 11th 2015];Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. 2015 http://www.nih.gov/news/health/may2015/niaid-27.htm.
- 6.Marrazzo JM, Del Rio C, Holtgrave DR, et al. HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society–USA Panel. JAMA. 2014;312(4):390–409. doi: 10.1001/jama.2014.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HIV/AIDS JUNPo, HIV/Aids JUNPo. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 8.Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–84. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;18(11):2144–55. doi: 10.1093/ije/dyt243. [DOI] [PubMed] [Google Scholar]
- 10.BC Centre for Excellence in HIV/AIDS. HIV Monitoring Quarterly Report for British Columbia, Fourth Quarter 2014. Vancouver: BC Centre for Excellence in HIV/AIDS; 2014. [Google Scholar]
- 11.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376(9737):285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 12.Nosyk B, Min JE, Lima VD, Hogg RS, Montaner JS group SHAs. Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study. The Lancet HIV. 2015;2(9):e393–e400. doi: 10.1016/S2352-3018(15)00127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153(12):778–89. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BC Centre for Disease Control. HIV and Sexually Transmitted Infections 2010. Vancouver: BC Centre for Disease Control; 2010. [Google Scholar]
- 15.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath K, Samji H, Nosyk B, et al. Cohort profile: seek and treat for the optimal prevention of HIV/AIDS in British Columbia (STOP HIV/AIDS BC) International journal of epidemiology. 2014;43(4):1073–81. doi: 10.1093/ije/dyu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanner Z, Matsukura M, Ivkov V, Amlani, Buxton J. British Columbia Drug Overdose and Alert Partnership report: British Columbia Centre for Disease Control. 2014. [Google Scholar]
- 18.British Columbia Office of the Provincial Health Officer. BC Methadone Maintenance System: Performance Measures 2011/2012 (Technical Appendix), 2013.
- 19.Fraser H, Mukandavire C, Martin NK, Hickman M, Cohen MS, Miller WC, Vickerman P. HIV treatment as prevention among people who inject drugs–a re-evaluation of the evidence. International Journal of Epidemiology. 2016 Aug 14;:dyw180. doi: 10.1093/ije/dyw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosyk B, Min JE, Lima VD, Yip B, Hogg RS, Montaner JS. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. Journal of acquired immune deficiency syndromes (1999) 2013 Aug 15;63(5):653. doi: 10.1097/QAI.0b013e3182976891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosyk B, Lourenço L, Min JE, Shopin D, Lima VD, Montaner JS STOP HIVAIDS Study Group. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn’. AIDS. 2015 Aug 24;29(13):1681–9. doi: 10.1097/QAD.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk G, Galai N, Astemborski J, et al. Decline in community viral load strongly associated with declining HIV incidence among IDU. 18th conference on retroviruses and opportunistic infections; 2011; 2011. [Google Scholar]
- 23.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vancouver Coastal Health. Downtown Eastside Second Generation Health System Strategy: Design Paper. 2015. [Google Scholar]
- 25.Hogg GS, Nosyk B, Harrigan PR, et al. Rates of new infections in British Columbia continue to decline at a faster rate than in other Canadian regions. HIV Medicine. 2013;14:581–2. doi: 10.1111/hiv.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKee G, Amlani A, Buxton J. Illicit fentanyl: An emerging threat to people who use drugs in BC. BCMJ. 2015;57(6):235. [Google Scholar]
- 27.Martins SS, Sampson L, Cerdá M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. American journal of public health. 2015;105(11):e29–e49. doi: 10.2105/AJPH.2015.302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. American journal of public health. 2015;105(8):e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Personal Communication. BC Centre for Disease Control; Feb 12, 2014. [Google Scholar]
- 30.McInnes CW, Druyts E, Harvard SS, Gilbert M, Tyndall MW, Lima VD, et al. HIV/AIDS in Vancouver, British Columbia: a growing epidemic. Harm Reduct J. 2009;6:5. doi: 10.1186/1477-7517-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.