Abstract
Non-tuberculous mycobacteria (NTM) are being recognized increasingly as the causative agents of opportunistic infections in humans. This study investigated the epidemiologic trends of NTM recovery from various clinical specimens in 2 Korean tertiary-care hospitals. We reviewed the laboratory records of patient samples cultured for mycobacteria between 2009 and 2015 at 2 tertiary-care hospitals in Korea. The medical records for patients with positive NTM samples were also reviewed. During the study period, 144,540 specimens were cultured for mycobacteria. The proportion of NTM-positive samples increased from 23.3% in 2009 to 48.2% in 2015. The 2 most frequently isolated NTM were Mycobacterium intracellulare (38.3%) and M. avium (23.1%). The number of clinically significant diseases caused by NTM in inpatients and outpatients increased from 6.8 to 12.9 per 100,000 patients over the same period. The rates of recovery of NTM from clinical specimens and the number of patients with NTM infections increased significantly (P < 0.001, testing for trend) between 2009 and 2015.
Keywords: Mycobacterium tuberculosis, Non-tuberculous Mycobacteria, Mycobacterium intracellulare, Mycobacterium avium
Graphical Abstract
INTRODUCTION
Non-tuberculous mycobacteria (NTM) are a diverse group of species that are widespread in the environment. In addition to the Mycobacterium tuberculosis complex and M. leprae, more than 140 NTM species have been identified (1). Although the majority of NTM species are considered to be only opportunistic pathogens, some can cause pulmonary and extrapulmonary diseases, such as lymphadenitis, skin and soft tissue infections, and disseminated infections, even in immunocompetent hosts (2). Thus, NTM are now recognized as an increasingly important cause of lung disease in various parts of the world (3,4).
Clinical NTM isolates show geographic and environmental variability, making them potential environmental health concerns (5,6). In Korea, the NTM isolation rate has increased steadily (7). In late 2000, liquid culture methods were introduced in Korea to improve the sensitivity of laboratory diagnoses. However, studies of NTM-associated disease are still insufficient, especially with respect to extrapulmonary infections. To better understand the current epidemiologic trend of NTM-associated diseases, we studied the frequency of isolation and the species distribution of NTM recovered from various clinical specimens over a 7-year period at 2 Korean tertiary-care hospitals.
MATERIALS AND METHODS
Study population
We reviewed 7 years of clinical mycobacterial culture records, including the NTM species, identified at 2 hospitals (Pusan National University Hospital [PNUH] and Pusan National University Yangsan Hospital [PNUYH]) between January 2009 and December 2015. For each isolate, we examined the medical records for information regarding the patient's age and sex, specimen collection date and source, and species identified. Chart reviews were performed for patients in whom NTM isolates were detected to evaluate the clinical significance. We defined “clinically significant infections” as those in which medical interventions such as hospitalization or medication were required.
Pulmonary NTM case definition
Non-tubercular lung disease was defined according to the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) 2007 guidelines (1). In this most recent definition, the bacteriological criteria have been simplified from the 1997 ATS criteria, allowing a shorter period for the diagnosis of NTM lung disease and simplified diagnosis (8). The diagnosis of NTM lung disease required the presence of: 1) compatible respiratory symptoms; 2) compatible radiographic findings, nodular or cavity opacities on chest radiography, or multifocal bronchiectasis with or without multiple small nodules on high-resolution computed tomography; and 3) 2 positive sputum cultures or a single positive bronchial washing fluid culture. We reviewed the clinical, radiologic, and microbiological findings of all culture-positive patients for NTM. Patients with NTM pulmonary disease included only those who met all the criteria presented by the ATS/IDSA in 2007.
Extrapulmonary NTM case definition
We categorized cases of extrapulmonary NTM disease as skin and soft tissue infections, disseminated disease, or lymphadenitis. Disseminated disease was diagnosed if the NTM isolate was recovered from the patient's blood or bone marrow and the patient had compatible symptoms. Lymphadenitis was diagnosed if the culture of a biopsy specimen or lymph node discharge yielded an NTM isolate. Skin and soft tissue infections were diagnosed if a wound discharge or skin, subcutaneous tissue, muscle, synovium, or bone biopsy specimen yielded an NTM isolate. An “other” category was used for isolates recovered from other normally sterile sites. M. gordonae isolates were considered to be contaminants and were excluded from the analysis.
Specimen processing
The preparation of clinical specimens for mycobacterial culture followed the recommended guidelines (9). They were cultured on 3% Ogawa medium (Eiken Chemical, Tokyo, Japan) for 8 weeks and inoculated into liquid medium for 6 weeks. For liquid medium culture, Mycobacteria Growth Indicator Tube (MGIT) medium (Becton Dickinson, Franklin Lakes, NJ, USA) was used at PNUYH. At PNUH, the BacT/ALERT 3D system (Organon Teknika, Boxtel, the Netherlands) was used. All cultures that were positive for mycobacteria were routinely subjected to the polymerase chain reaction (PCR) assay (LG Life Science, Daejon, Korea) and MPT 64 test (Standard Diagnostics, Seoul, Korea) for differentiation between M. tuberculosis and NTM. Further species identification was performed at the Korean Institute of Tuberculosis (KIT, Osong, Korea) at the request of the charge physician. A multiplex PCR restriction fragment-length polymorphism assay was performed for the rpoB gene until 2009, after which the test method was changed to a PCR-based reverse line blot hybridization assay for the ITS gene (AdvanSure Mycobacteria GenoBlot Assay; LG Life Sciences, Seoul, Korea) at the KIT.
Data analysis
The proportion of NTM isolates recovered from positive specimens was calculated for each year. The proportion of patients with clinically significant disease caused by NTM was calculated as the annual number of patients with NTM-associated lung disease divided by the total number of patients who visited the 2 participating hospitals over the course of that year, including both inpatients and outpatients. The data were analyzed using SPSS, version 22 (IBM Corp., Chicago, IL, USA). The linear-by-linear association exact test was used to test for significant trends.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of Pusan National University Yangsan Hospital, and informed consent was waived by the committee (05-2016-028).
RESULTS
Annual numbers of patients and specimens
During the study period, 144,540 specimens from 57,311 patients were submitted for mycobacterial culture. The number of samples cultured per year for this purpose increased from 19,497 to 21,044 over the same period. Among the specimens, 35,081 (24.3%) were collected from extrapulmonary sites.
Changes in the proportions of NTM recovered
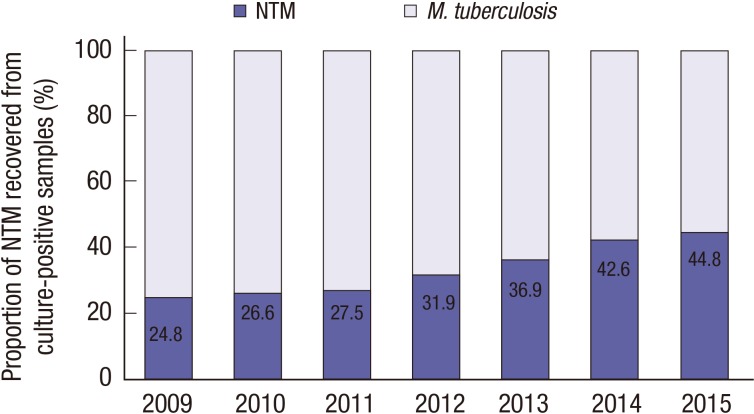
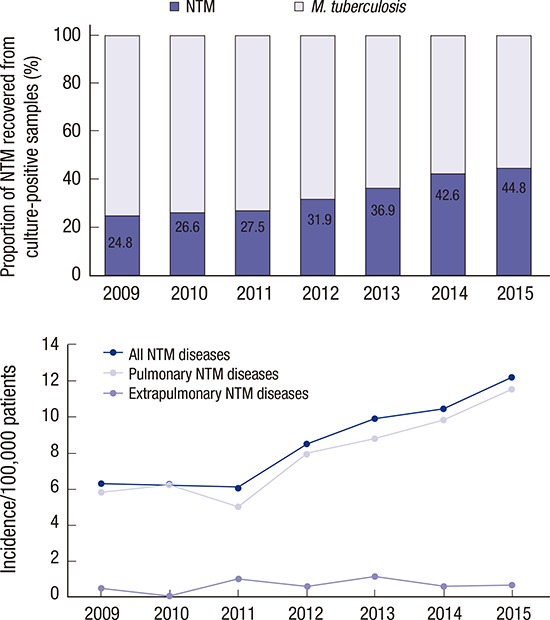
Among the cultured specimens, 16,188 (11.2%) from 7,624 patients were positive for mycobacteria, including M. tuberculosis. Among the culture-positive specimens, 5,558 from 4,379 patients were positive for NTM. The proportion of NTM recovered from those samples increased significantly, from 24.8% in 2009 to 44.8% in 2015 (P < 0.001; test for trend) (Fig. 1).
Fig. 1.
Proportion of M. tuberculosis and NTM in mycobacterial cultures at PNUH and PNUYH from 2009 through 2015.
NTM = non-tuberculous mycobacteria, PNUH = Pusan National University Hospital, PNUYH = Pusan National University Yangsan Hospital.
Prevalence of NTM species
Among the 5,558 NTM cultures, species identification was requested for only 1,069 specimens from 924 patients (19.2%). Except for the case in which the same species was isolated repeatedly from the same patient, the most frequently isolated NTM was M. intracellulare (38.3%), followed by M. avium (23.1%) and M. abscessus (8.4%) (Table 1). A total of 8.1% of the cases in which 2 or more positive bands were detected at the same time after the identification test was changed by the line probe assay method in 2010. The most common mixed-infection species were M. intracellulare and M. avium (2.5%), followed by M. intracellulare and M. abscessus (1.7%) and M. intracellulare and M. massiliense (0.9%). According to the sample collection site, the specimens were divided into 2 groups: pulmonary or extrapulmonary. Among the pulmonary specimens, NTM identification was requested for 1,046 specimens from 900 patients (1,046/5,433; 19.3%). From these samples, the most frequently isolated NTM was M. intracellulare (38.9%), followed by M. avium (23.1%), M. abscessus (8.4%), and M. kansasii (7.7%). Among the extrapulmonary specimens (tissue, body fluid, urine, and cerebrospinal fluid), NTM identification was requested for 24 specimens from 24 patients (24/125; 19.2%), and the most frequent isolates were M. avium (25.0%) followed by M. fortuitum complex (20.9%) and M. intracellulare (16.7%).
Table 1. Distribution of NTM species recovered at PNUH and PNUYH between 2009 and 2015.
| Identified species | % of total isolates |
|---|---|
| M. intracellulare | 38.3 |
| M. avium | 23.1 |
| M. abscessus | 8.4 |
| M. fortuitum complex | 7.8 |
| M. kansasii | 7.8 |
| M. massiliense | 5.2 |
| M. lentiflavum or M. genavense | 3.4 |
| M. chelonae | 1.9 |
| M. terrae complex | 1.4 |
| M. aubagnense | 0.2 |
| M. flavescens | 0.2 |
| M. holsaticum | 0.2 |
| M. marinum | 0.2 |
| M. mucogenicum | 0.2 |
| M. phocaicum | 0.2 |
| M. porcinum | 0.2 |
| M. szulgai | 0.2 |
| M. ulcerans | 0.2 |
| M. celatum | 0.1 |
| M. fuerth | 0.1 |
| M. malmoens | 0.1 |
| M. scrofulceum | 0.1 |
| M. senegalense | 0.1 |
| M. septicum | 0.1 |
| M. shinjnkuens | 0.1 |
| M. xenopi | 0.1 |
NTM = non-tuberculous mycobacteria, PNUH = Pusan National University Hospital, PNUYH = Pusan National University Yangsan Hospital.
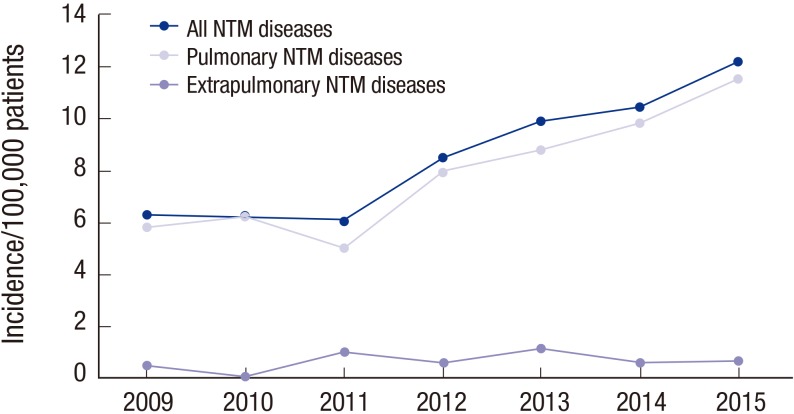
Numbers of patients with clinically significant disease caused by NTM
The total number of inpatients and outpatients who visited the hospitals in one year increased from 1,681,930 to 2,115,986 over the study period. The number of patients with clinically significant disease caused by NTM increased from 6.8 to 12.9 per 100,000 patients. The increase was associated predominantly with pulmonary disease (93.1%; Table 2 and Fig. 2), followed by skin and soft tissue infection (6.6%).
Table 2. Clinically significant disease caused by NTM at PNUH and PNUYH between 2009 and 2015.
| Diseases | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|---|
| Pulmonary disease | 106 | 117 | 109 | 168 | 202 | 219 | 258 | 1,179 (93.1) |
| Skin and soft tissue infection | 8 | 1 | 16 | 11 | 22 | 12 | 13 | 83 (6.6) |
| Disseminated disease | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 (0.2) |
| Lymphadenitis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.1) |
| Other | 0 | 0 | 2* | 0 | 0 | 0 | 0 | 2 (0.2) |
| Total | 114 | 118 | 125 | 179 | 225 | 232 | 272 | 1,267 (100.0) |
Values are presented as number (%).
NTM = non-tuberculous mycobacteria, PNUH = Pusan National University Hospital, PNUYH = Pusan National University Yangsan Hospital.
*Includes one patient with peritonitis and one with meningitis.
Fig. 2.
Number of NTM pulmonary and extrapulmonary diseases per 100,000 patients at PNUH and PNUYH from 2009 through 2015.
NTM = non-tuberculous mycobacteria, PNUH = Pusan National University Hospital, PNUYH = Pusan National University Yangsan Hospital.
DISCUSSION
We found that the annual proportion of NTM recovered from clinical specimens and the prevalence of NTM-associated lung disease increased significantly between 2009 and 2015, similar to previous reports from Korea. Lee et al. (10) reported that the annual percentage of NTM isolations had increased steadily and that the proportion of patients from whom NTM were isolated increased from 21.4% (2006) to 55.0% (2010). Similarly, Koh et al. (11) reported that the proportion of NTM, among all positive mycobacterial respiratory specimen cultures, increased from 43% in 2001 to 70% in 2011. Further, Park et al. (12) reported that the proportion of NTM among positive mycobacterial cultures increased from 22.2% in 2002 to 45.9% in 2008.
Despite the increasing tendency of NTM-associated disease in our study, there was no significant change in the most commonly isolated species. These species were M. avium and M. intracellulare, followed by M. abscessus, similar to other reports from Korea (12). The prevalence of particular mycobacterial species that are responsible for different diseases differs markedly by geographic region. For example, in the United States and Japan, M. avium, M. intracellulare, and M. kansasii are the most common disease-associated species (1,13), whereas in England and Scotland, M. kansasii and M. malmoense, respectively, are the most common (14).
In our study, the most common NTM species isolated from extrapulmonary specimens was M. avium (25.0%). M. intracellulare is the most common species isolated from localized pulmonary infections. M. abscessus was commonly found in skin and soft tissue infections, consistent with findings from Taiwan (15). However, among the extrapulmonary isolates, M. abscessus comprised 8.3% of the total, representing only a 0.9% higher proportion of extrapulmonary isolates than of pulmonary specimens.
Identification of clinical isolates to the species level is crucial because the pathogenic potential (virulence) differs among species. The various species also differ in the clinical spectrum of disease they cause, as well as in their susceptibility to antimicrobial drugs. M. kansasii, M. avium, M. intracellulare, M. abscessus, and M. massiliense show relatively high pathogenic potential, whereas M. fortuitum does not. M. gordonae is one of the least pathogenic NTM, and its isolation from the respiratory tract is typically considered to represent contamination rather than infection (16). This means that when NTM are isolated from clinical specimen, species identification is as important as the fact that an NTM was isolated. However, during our study period, the species was identified in only 19.2% of NTM-positive cultures. Given the current ‘fee-for-service’ rule in our healthcare insurance reimbursement system, the identification of NTM to the species level can be performed only at the request of the clinician with the consent of patient. Although in cases where NTM was designated the cause of clinically significant infections requiring medical intervention, the species were not always identified. This tendency was particularly pronounced in the extrapulmonary specimens, particularly when NTM was detected in skin and soft tissue infection-positive specimens from 24 patients. When NTM is detected in skin and soft tissue infections, many surgeons administer empiric antibiotics when their patients have positive mycobacterial cultures, without a request for identification of isolates or antimicrobial susceptibility tests. However, the need for identification to the species level is increasing to guide precise diagnosis and treatment. Therefore, it is necessary to identify the species in every case when NTM is isolated in the mycobacterial culture. In addition, this low rate of requests for identification of NTM to the species level suggests that clinicians do not consider NTM to be important pathogens. These organisms are widely distributed in the environment, including in soil and water (17). Therefore, the isolation of NTM from respiratory specimens does not imply that the patient has an NTM-associated disease. When NTM are isolated from clinical specimens, the percentage of patients with clinically significant NTM-associated disease seems to differ according to the diagnostic criteria used and the global region. In America and European countries, 40%–50% of culture-positive patients are found to have NTM-associated disease (12,18), whereas only 30%–40% of culture-positive patients in Korea have confirmed NTM-associated disease.
The number of patients with newly diagnosed NTM-associated lung disease also increased steadily during our study period. Several explanations can be offered. The most popular ones are improved laboratory techniques and the increased proportion of the population at risk for such disease (19). The laboratory techniques probably enhance the sensitivity of mycobacterial recovery. For example, the introduction of liquid culture methods enabled us to detect both M. tuberculosis and NTM with greater sensitivity than did the solid-medium method. Although we did not compare the results of solid and liquid culture methods, Lee et al. (10) reported that NTM positivity was significantly higher for liquid culture methods. Globally, the increasing number of individuals with immunodeficiencies, chronic debilitating conditions, and advanced age are contributing to the growing awareness of the pathogenic importance of NTM. Thus, the growing number of patients with NTM infections over the course of our study might be attributable to better diagnosis and to the growing at-risk population.
A variety of clinical information is required to confirm that NTM have caused a significant infection. Since the applicable clinical diagnosis criteria were established in 2007, healthcare utilization and computed tomography imaging of the chest have increased. These increases may also contribute to the more frequent diagnosis of NTM-related lung disease.
The limitations of this study are that only 19.2% of all NTM strains were identified to the species level. The results therefore should be interpreted with caution, as they may not reflect the overall distribution of NTM species accurately. Because this study was retrospective, we could not evaluate all the specimens in which the NTM species were not identified. The reason only a small portion of the isolates were sent for species identification was that all medical activities are fee-for-service, and identification is requested only when patients revisited and agreed to pay for the service. This system resulted in late diagnosis and underestimated the significance of NTM diseases. Kim et al. (20) pointed out these problems and suggested a solution for our health insurance reimbursement system services. But the system has not changed.
In conclusion, we found that the annual percentage of NTM recovered from clinical specimens and the prevalence of patients with NTM infections both increased significantly from 2009 to 2015. The proportion of NTM isolates also has been rising steadily, accounting for 44.8% of all mycobacteria recovered in 2015 at the 2 study hospitals. Further, the number of clinically significant diseases caused by NTM is rising. The most frequently isolated species were M. intracellulare and M. avium.
Footnotes
Funding: This work was supported by the annual clinical research grant from Pusan National University Yangsan Hospital.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Kim N, Yi J, Chang CL. Data curation: Kim N. Formal analysis: Kim N. Investigation: Kim N, Yi J, Chang CL. Writing - original draft: Kim N. Writing - review & editing: Yi J, Chang CL.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Saleeb P, Olivier KN. Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocompromised patients. Curr Infect Dis Rep. 2010;12:198–203. doi: 10.1007/s11908-010-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306–313. doi: 10.1164/rccm.200702-201OC. [DOI] [PubMed] [Google Scholar]
- 4.Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis. 2009;15:1562–1569. doi: 10.3201/eid1510.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson RM. NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–1583. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JL. Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br Med Bull. 2010;96:45–59. doi: 10.1093/bmb/ldq035. [DOI] [PubMed] [Google Scholar]
- 7.Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, Park YK, Kang S. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal. 2008;22:415–420. doi: 10.1002/jcla.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chae DR, Kim YI, Kee SJ, Kim YH, Chi SY, Ban HJ, Kwon YS, Oh IJ, Kim KS, Kim SO, et al. The impact of the 2007 ATS/IDSA diagnostic criteria for nontuberculous mycobacterial disease on the diagnosis of nontuberculous mycobacterial lung disease. Respiration. 2011;82:124–129. doi: 10.1159/000320254. [DOI] [PubMed] [Google Scholar]
- 9.Pfyffer GE. Mycobacterium: general characteristics, laboratory detection, and staining procedures. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington, D.C.: ASM Press; 2015. pp. 536–569. [Google Scholar]
- 10.Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis. 2012;44:733–738. doi: 10.3109/00365548.2012.681695. [DOI] [PubMed] [Google Scholar]
- 11.Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, Ki CS, Kwon OJ. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis (Seoul) 2013;75:199–204. doi: 10.4046/trd.2013.75.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010;14:1069–1071. [PubMed] [Google Scholar]
- 13.Sakatani M. The non-tuberculous mycobacteriosis. Kekkaku. 2005;80:25–30. [PubMed] [Google Scholar]
- 14.Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 2000;55:210–218. doi: 10.1136/thorax.55.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding LW, Lai CC, Lee LN, Hsueh PR. Disease caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 1997–2003. Epidemiol Infect. 2006;134:1060–1067. doi: 10.1017/S0950268805005698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simner PJ, Stenger S, Richter E, Brown-Elliott BA, Wallace RJ, Wengenack NL. Mycobacterium: laboratory characteristics of slowly growing mycobacteria. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington, D.C.: ASM Press; 2015. pp. 570–594. [Google Scholar]
- 17.Falkinham JO., 3rd Ecology of nontuberculous mycobacteria--where do human infections come from? Semin Respir Crit Care Med. 2013;34:95–102. doi: 10.1055/s-0033-1333568. [DOI] [PubMed] [Google Scholar]
- 18.Hong KS, Ahn JH, Choi EY, Jin HJ, Shin KC, Chung JH, Lee KH. Microbiologic distribution and clinical features of nontuberculous mycobacteria in the tertiary hospital in Daegu. Yeungnam Univ J Med. 2015;32:71–79. [Google Scholar]
- 19.Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest. 2008;133:243–251. doi: 10.1378/chest.07-0358. [DOI] [PubMed] [Google Scholar]
- 20.Kim CK, Sung H, Park YJ, Chang CL. A proposal for laboratory workflow changes for efficient tuberculosis control. Ann Clin Microbiol. 2013;16:61–68. [Google Scholar]