Abstract
Few studies of atopic dermatitis (AD) in adult patients have evaluated differences in clinical features of AD according to onset age. We aimed to characterize the clinical features of AD in adult patients according to age of onset. Subjects with AD outpatient visiting the Department of Dermatology at Kangnam Sacred Heart Hospital were recruited for this study. A dermatologist conducted clinical evaluation, a survey of demographics, and onset of AD-associated signs and symptoms for each participant. Total immunoglobulin E (IgE) was also tested. A total of 280 adult AD patients were enrolled, among which 232 patients (82.86%) showed pre-adult-onset (age < 18 years) and 48 patients (17.14%) had adult-onset (age ≥ 18 years) of AD. There were significant differences between the 2 groups in the area of initial involvement (P = 0.017) and in treatment history (P = 0.010). Interestingly, patients with body mass index (BMI) ≥ 25 showed significantly higher Eczema Area and Severity Index (EASI) scores than did patients with BMI < 25 in the pre-adult-onset adult AD group (P = 0.048). On the other hand, there were no significant differences in sex, family history, BMI, EASI, and total IgE between patients with pre-adult-onset AD and patients with adult-onset AD. Our findings suggest that, even though many common features exist, there are significant differences between the clinical characteristics of pre-adult-onset and adult-onset AD subgroups, in adult patients with AD.
Keywords: Atopic Dermatitis, Age of Onset, Clinical Feature
Graphical Abstract
INTRODUCTION
Atopic dermatitis (AD) is a common pruritic, chronic, relapsing, inflammatory skin disease occurring primarily in childhood (1,2,3,4). The prevalence of AD in childhood increased from 2% to 5% before 1960; rose to 9%–12% after 1970; and is currently as high as 20% (5). AD affects 2%–7% of adults worldwide (2,6). AD clears up during infancy or childhood in the majority of these patients (7,8). Many studies indicate that AD has a clearance rate of 50%–70% after 10 years (9,10). Recently, a higher persistence rate of AD after puberty has been reported than in previous studies (at least 40%–60%) (11,12).
AD typically constitutes 3 phases: the infantile phase from 0 to 2 years of age, the childhood phase between 2 and 12 years of age, and the adolescent or adult phase (13). There are few studies on the investigation of the natural course of AD according to the age of onset (7). Garmhausen et al. (7) found that signs of atopy and concomitant atopic disease were significantly more present in patients with early-onset and a chronically persistent course, than in patients with onset of AD after the age of 20 years. Adult-onset AD, for which the symptoms appeared first in adult life, has recently been recognized as a subgroup of AD, first introduced by Bannister and Freeman (14).
So far, there are few published studies about the different clinical characteristics in relation to age of onset. There are also only a few studies focused on adult-onset AD (11,15,16). Therefore, we determined the epidemiology of adult-onset AD, and the differences in clinical characteristics of adult patients with AD, according to age of onset.
MATERIALS AND METHODS
Patient collection
For this study, 280 adult participants with AD (151 male and 129 female; 18–56 years old) were recruited in the Department of Dermatology at Kangnam Sacred Heart Hospital from October 2011 to August 2015. All the following criteria were considered for inclusion: 1) age > 18 years, and 2) with active AD diagnosed by a dermatologist according to the criteria of Hanifin et al. (17).
Clinical features and severity of AD
Patients with AD were divided into the 2 groups depending on the age of onset. The pre-adult-onset AD subgroup had onset of AD between birth and 18th year of life, and the adult-onset AD subgroup, including the 18th year of life and after (3,18,19). Pre-adult-onset AD includes patients with onset age during infantile, childhood, and juvenile phases of life. An experienced dermatologist conducted the questionnaires and physical examinations. The severity of AD was assessed using the Eczema Area and Severity Index (EASI) score (17). Body mass index (BMI) was calculated using weight (kg) and upright height (cm). Using understandable questions in the questionnaire, the dermatologist freely interviewed patients. The questionnaire contained questions about the onset age, occupation, living environment, personal and family history of atopic diseases, previous treatment history, initial involvement area, associated findings (e.g., xeroderma, ichthyosis), Visual Analogue Scale (VAS) score for pruritus, seasonal variation, and aggravating and relieving factors.
Total immunoglobulin E (IgE)
Blood samples were taken to measure the serum-total IgE levels using the paper radioimmunosorbent test (PRIST) kit (Behring, Marbug, Germany).
Statistical analysis
The χ2 test for nominal variables and the Student's t-test for continuous variables were used to determine the significance of differences. For associations of body-site distribution of initial involvement area with disease onset, data were analyzed using the χ2 test and the Fisher exact test, when cells with expected frequencies of less than 5 were more than 20% of the total. Also, because this study is a kind of exploratory study, we did use the χ2 test and the Fisher exact test to confirm the associations, without adjustment for multiple testing. Pearson's correlation analysis and simple linear regression analysis were used. Significance levels for all analyses were set at P < 0.05. All statistical analyses were conducted using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Ethics statement
Informed consent was obtained from each participant. The study protocol was approved by the Institutional Review Board of Kangnam Sacred Heart Hospital (IRB No. 2012-08-77). Data were collected with respect to the confidentiality of patient records.
RESULTS
Patient demographics
Table 1 showed demographic data of the patients. Of the 280 adult patients with AD, 48 (17.14%) had adult-onset (≥ 18 years of age) history of AD and 232 (82.86%) had pre-adult-onset (< 18 years of age).
Table 1. Patient demographics.
| Parameters | Adult AD | P value | |
|---|---|---|---|
| Pre-adult-onset (< 18 yr; n = 232) | Adult-onset (≥ 18 yr; n = 48) | ||
| Sex (M/F) | 122 (52.6)/110 (47.4) | 29 (60.4)/19 (39.6) | 0.322 |
| Age, yr | 25.987 ± 7.250 (range, 18–56) | 32.708 ± 8.570 (range, 18–54) | < 0.001* |
| Age of onset, yr | 5.766 ± 5.289 | 27.375 ± 7.946 | < 0.001* |
| Duration, yr | 20.42 ± 8.85 | 5.54 ± 5.86 | < 0.001* |
| EASI score | 11.098 ± 8.986 | 10.015 ± 10.630 | 0.495 |
| Serum total IgE, kU/L | 803.300 ± 1,116.852 | 1,172.900 ± 1,114.932 | 0.114 |
| Serum total IgE (≥ 200), kU/L | 113/136 (83.09) | 24/29 (82.76) | 1.000 |
| BMI, kg/m2 | 22.274 ± 3.609 | 23.012 ± 3.658 | 0.206 |
| Obesity (BMI ≥ 25) | 42 (18.1) | 13 (27.1) | 0.209 |
| Habitation | 0.636 | ||
| City | 191 (82.3) | 42 (87.5) | |
| Countryside | 3 (1.3) | 0 (0) | |
| Past medical history | |||
| Allergic rhinitis | 109 (46.9) | 22 (45.8) | 0.884 |
| Asthma | 23 (9.9) | 4 (8.3) | 0.799 |
| History of family atopic disease | 116 (50.0) | 20 (41.7) | 0.293 |
| Allergic rhinitis | - | ||
| Parents | 32 (13.8) | 3 (6.3) | |
| Siblings | 21 (9.1) | 6 (12.6) | |
| Asthma | - | ||
| Parents | 1 (0.4) | 4 (8.3) | |
| Siblings | 1 (0.4) | 2 (4.2) | |
| AD | - | ||
| Parents | 8 (3.4) | 4 (8.3) | |
| Siblings | 32 (13.8) | 6 (12.6) | |
Values are presented as number (%) or mean ± standard deviation.
AD = atopic dermatitis, EASI = Eczema Area and Severity Index, IgE = immunoglobulin E, BMI = body mass index.
*P < 0.001.
Details of each patient's habitation (city or countryside) and past medical history of atopy (allergic rhinitis and asthma) are shown in Table 1. Also, shown there is the family history of atopy (allergic rhinitis, asthma, and AD) of each patient. There were no statistically significant differences between the 2 groups in all these categories.
Elevated total IgE level (≥ 200 kU/L) was found in 83.09% (113/139) of pre-adult-onset participants and in 82.76% (24/ 29) of adult-onset participants, with no significant difference between the 2 groups (P = 0.476). At the time of study enrollment, there was no statistically significant difference in the EASI score between the 2 groups (P = 0.495). There were also no significant differences in sex, BMI, and obesity (BMI ≥ 25) between the 2 groups.
The body-site distribution of initial involvement area was different according to onset age in adult patients with AD
The body-site distribution of the areas of initial involvement is shown in Table 2. Regarding the site of onset, a typical flexural (flexor surface of extremities) onset of AD was seen in the majority of cases in pre-adult-onset adult AD (n = 119; 51.3%). On the other hand, the trunk was seen as the most common area involved in adult-onset adult AD (n = 14; 29.2%). This difference in the body-site distribution of the area of initial involvement was statistically significant (P = 0.017). In pre-adult-onset adult AD, the flexor surface of arms and legs was significantly more highly involved than in adult-onset adult AD (P = 0.005), whereas the trunk was significantly more highly involved in adult-onset adult AD than in pre-adult-onset adult AD (P = 0.031). Adult AD patients with adult-onset tended to have head and neck areas initially involved, compared with pre-adult-onset adult AD, but this was not statistically significant (P = 0.278). Specifically, there were no significant differences between the localized parts (at all severities) as determined using the localized EASI score (head and neck, P = 0.852; upper extremities, P = 0.547; trunk, P = 0.138; lower extremities, P = 0.168, Table 3).
Table 2. Body-site distribution of initial involvement area.
| Initial involved area | No. (%) of adult AD patients | P value | |
|---|---|---|---|
| Pre-adult-onset (< 18 yr; n = 232) | Adult-onset (≥ 18 yr; n = 48) | ||
| Total | 0.017* | ||
| Head and neck | 38 (16.4) | 11 (22.9) | 0.278 |
| Flexor surface of arms and legs | 119 (51.3) | 14 (29.2) | 0.005† |
| Trunk | 65 (28.0) | 21 (43.8) | 0.031* |
| Extensors (elbows/knees/other aspects of limbs) | 2 (0.9) | 1 (2.1) | 0.432‡ |
| Hands and feet | 6 (2.6) | 1 (2.1) | 1.000‡ |
| Genital area | 2 (0.9) | 0 (0.0) | 1.000‡ |
AD = atopic dermatitis.
*P < 0.050; †P < 0.010; ‡Data were analyzed using the Fisher exact test. The others were analyzed using the χ2 test.
Table 3. Total and localized EASI score.
| Sites of AD | EASI scores in adult AD | P value | |
|---|---|---|---|
| Pre-adult-onset (< 18 yr; n = 232) | Adult-onset (≥ 18 yr; n = 48) | ||
| Localized | |||
| Head and neck | 1.127 ± 1.198 | 1.165 ± 1.172 | 0.852 |
| Upper extremities | 2.502 ± 1.952 | 2.720 ± 2.679 | 0.547 |
| Trunk | 2.602 ± 3.376 | 3.555 ± 5.036 | 0.138 |
| Lower extremities | 4.594 ± 4.883 | 3.460 ± 3.846 | 0.168 |
| Total | 11.098 ± 8.986 | 10.015 ± 10.630 | 0.495 |
Values are presented as mean ± standard deviation. The data were analyzed using the χ2 test.
EASI = Eczema Area and Severity Index, AD = atopic dermatitis.
Those with pre-adult-onset adult AD tend to get treatment more frequently, compared with those having adult-onset adult AD
The previous treatment history of participants is shown in Table 4. In the questionnaire and interview, previous treatment history was organized into 3 categories: ‘no treatment’ included those who had never been treated before. Those who got treatment only during flare-ups or exacerbation of AD were included in the ‘occasionally’ group, and those who got treatment steadily were included in the ‘continuously’ group. The majority of participants in both groups were in the ‘occasionally’ treated group: adult-onset adult AD, n = 21 (43.8%) vs. pre-adult-onset adult AD, n = 128 (55.2%). Patients with pre-adult-onset adult AD had more frequent treatment than did those with adult-onset adult AD (P = 0.010; Table 2).
Table 4. Previous treatment history.
| Frequency of history | Adult AD | P value | |
|---|---|---|---|
| Pre-adult-onset (< 18 yr; n = 232) | Adult-onset (≥ 18 yr; n = 48) | ||
| Treatment history | 0.010* | ||
| No treatment | 19 (8.2) | 13 (27.1) | < 0.001† |
| Occasionally | 128 (55.2) | 21 (43.8) | 0.149 |
| Continuously | 85 (36.6) | 14 (29.2) | 0.324 |
Values are presented as number (%). ‘No treatment’ included the patients who had never treated before. The patients who got treatment only during the flares or exacerbationof AD were included ‘occasionally’ group and the patients who got treatment steadily and followed up to clinic were included ‘continuously’ group.
AD = atopic dermatitis.
*P < 0.050; †P < 0.001.
For early-onset adult AD, patients who were obese (BMI ≥ 25) showed significantly higher EASI scores than did those who were not obese (BMI < 25)
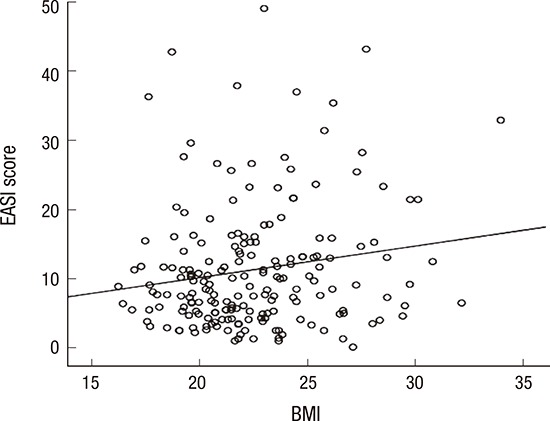
For all the adult AD patients, there was no significant difference (P = 0.784) in the EASI scores of those who were (BMI ≥ 25) and were not (BMI < 25) obese. This was also similar for those in the adult-onset adult AD group (P = 0.784). However, in the pre-adult-onset adult AD group, there was a significant difference between the EASI scores of those who were obese and those who were not obese (P = 0.048). In the Pearson's correlation analysis, there was a significant association between BMI and EASI scores in pre-adult-onset adult AD (r = 0.167; P = 0.021). In the simple linear regression analysis, with the presumption of BMI being an independent variable, a reliable prediction of the dependent variable (EASI score) could be made. A significant proportion of the variance (R2 = 0.028; 2.8%) in the EASI score is thought to be associated with the BMI (Fig. 1; β = 0.167; P = 0.021), although the correlation was quite weak. When the test was repeated with participants separated by sex, women (R2 = 0.048; 4.8%; β = 0.220; P = 0.037), but not men (R2 = 0.010; 1.0%; β = 0.102; P = 0.307); showed statistically significant association between BMI and EASI score in the pre-adult-onset adult AD group.
Fig. 1.
Association between BMI and EASI score in pre-adult-onset adult AD. In pre-adult-onset adult AD, there was a significant association between obesity (BMI ≥ 25) and EASI score by simple linear regression analysis (R2 = 0.028; 2.8%; β = 0.167; P = 0.021).
BMI = body mass index, EASI = Eczema Area and Severity Index, AD = atopic dermatitis.
There were no significant differences in signs of atopy and concomitant symptoms between pre-adult- and adult-onset adult AD groups
Xeroderma was seen in the majority of cases in both groups: pre-adult-onset adult AD (n = 93; 40.4%) vs. adult-onset adult AD (n = 16; 33.3%). Other symptoms noted included pruritus after sweating, orbital darkening, and intolerance to wool, among others. Details of the AD-associated signs and symptoms for each group are described in Table 5. None of these showed significant difference between the 2 groups.
Table 5. AD-associated signs and symptoms.
| Sings or symptoms | Adult AD | P value | |
|---|---|---|---|
| Pre-adult-onset (< 18 yr; n = 232) | Adult-onset (≥ 18 yr; n = 48) | ||
| Xeroderma | 148 (87.6) | 27 (90.0) | 0.707 |
| Pruritus after sweating | 119 (70.4) | 18 (60.0) | 0.256 |
| Nummular eczema | 93 (40.4) | 16 (33.3) | 0.359 |
| Orbital darkening | 86 (50.9) | 15 (50.0) | 0.929 |
| Allergic shiner | 80 (47.3) | 11 (36.7) | 0.280 |
| Intolerance to wool | 80 (47.3) | 14 (46.7) | 0.946 |
| Cheilitis | 65 (38.5) | 9 (30.0) | 0.377 |
| Handfoot dermatitis | 63 (37.3) | 6 (20.0) | 0.067 |
| Palmar hyperlinearity | 38 (22.5) | 3 (10.0) | 0.119 |
| Nipple eczema | 37 (21.9) | 7 (23.3) | 0.861 |
| Nickel allergy | 33 (19.5) | 6 (20.0) | 0.908 |
| Perifollicular accentuation | 32 (18.9) | 4 (13.3) | 0.463 |
| Food intolerance | 32 (18.9) | 7 (23.3) | 0.576 |
| Intolerance to metal | 29 (17.2) | 8 (26.7) | 0.217 |
| Keratosis pilaris | 26 (15.6) | 2 (6.7) | 0.263 |
| Pityriasis alba | 25 (14.8) | 5 (16.7) | 0.784 |
| Photosensitivity | 19 (11.2) | 4 (13.3) | 0.757 |
| Bacterial skin infection | 16 (9.5) | 1 (3.3) | 0.478 |
| Dennie-Morgan line | 12 (7.1) | 2 (6.7) | 0.932 |
| Hertoghe sign | 9 (5.4) | 4 (13.3) | 0.114 |
| Fungal skin infection | 8 (4.8) | 1 (3.3) | 0.729 |
| Ichthyosis | 7 (4.2) | 1 (3.3) | 0.826 |
| White dermographism | 6 (3.6) | 2 (6.7) | 0.353 |
| Molluscum contagiosum | 2 (1.2) | 0 (0.0) | 0.548 |
Values are presented as number (%).
AD = atopic dermatitis.
DISCUSSION
In this study, we evaluated the epidemiology and adult AD-associated features according to onset age. It is meaningful that we especially focused on and discussed the clinical features of adult-onset adult AD (20,21,22,23,24,25). We found that significant differences exist between the pre-adult- and adult-onset groups regarding adult AD-associated features. Between the 2 groups, there were significant differences in the areas of initial involvement and in treatment history. Patients that were obese showed more severe AD than did those that were not obese, but only in pre-adult-onset adult AD.
So far, due to either lack of awareness or non-acceptance of the concept of adult-onset AD, the cut-off age criteria and clinical features of adult-onset disease are still being debated and currently differ, based on several reports (18). The prevalence of adult-onset AD also differs by study (9.0%–24.5%) (3,7,26). Bannister and Freeman (14) found that among adult patients with adult-onset AD, 65% were females and that the age distribution ranged from 20s to 80s, with peak ages in the 50s. The prevalence of adult-onset AD also differs in previous literature in other countries: Singapore (13.6%), Nigeria (24.5%), and so on (3,11,12). It is also true that the cut-off ages differed by study, e.g., over 20 or 21 (3,11,12).
In our research, 17.14% of adult AD patients had a history of late-onset. In several large-scale clinical trials of patients with AD, age eligibility for study participation was set to 18 years or older (27,28,29). For this reason, we decided to define adult-onset as the onset of AD including the 18th year of life and after. The distribution of AD varies according to the patient's age. In the adolescent and adult phases, dermatitis of the face, trunk, and hand is often the primary manifestation (14,18,19). According to previous studies, the generalized involvement occurred in 34%, and the most commonly involved body area was hands (28%), followed by face (26%) in adult-onset AD (14). Kanwar and Narang (18) summarized the phenotype as localized variants of adult-onset AD (n = 46); hand eczema (n = 18; 52.77%), eyelid dermatitis (n = 9; 25%), nipple dermatitis (n = 8; 22.2%), and periorificial dermatitis (n = 5; 13.8%). Kanwar and Narang (18) also reported that adult-onset AD shares many manifestations with air-borne contact dermatitis and parthenium dermatitis, so it is hard to classify symptoms accurately.
In our study, in pre-adult-onset adult AD, the flexor surfaces of arms and legs were significantly more highly involved initially, than in adult-onset adult AD (P = 0.005), whereas the trunk was significantly more highly involved initially in adult-onset adult AD, than in pre-adult-onset adult AD (P = 0.031). Our findings were consistent with those of other studies. On the other hands, there was no significant difference in the initial involvement of hands and feet between adult-onset and pre-adult-onset adult AD group (P = 1.000). Only 6 patients (2.6%) in pre-adult-onset adult AD patients and only 1 patient (2.1%) in adult-onset adult AD patients reported that the lesions were firstly started on the hands and feet. Because this study relies on the memory of the patients, the prevalence of hand or foot eczema can be undervalued. In aspects of the prevalence and the severity of hand dermatitis at the time of study enrollment, we assessed them as AD-associated signs and symptoms. 63 patients (37.3%) in pre-adult-onset adult AD patients and 6 patients (20.0%) in adult-onset adult AD patients had handfoot dermatitis at the time of study enrollment. These findings were similar to those of other studies (14,18), even though our study did not investigate the patients with hand dermatitis separately. However, there was no significant difference in handfoot dermatitis between adult-onset and pre-adult-onset adult AD group (P = 0.067). Additionally, we only evaluated EASI scores and localized EASI scores which consist of ‘head and neck,’ ‘upper extremities,’ ‘trunk,’ and ‘lower extremities’ so the severity of handfood dermatitis was not assessed separately. Those in the adult phase with AD, typically have facial involvement, such as persistent facial erythema (21). Malasezzia (fungal) infection and irritant contact dermatitis of the head and neck could induce the head and neck lesions observed in adult AD (13,14,18). However, our study did not show statistically significant difference of the areas of initial involvement in the head and neck areas, between the pre-adult and adult-onset groups. EASI scores, particularly in the part ‘head and neck’ among the localized EASI scores, at the time of study enrollment might affect the results, but the initial EASI scores at the time of onset are unknown.
Patients with pre-adult-onset adult AD had more frequent treatment than did patients with adult-onset adult AD. Parents of children with AD have a tendency to bring their children to the clinic more often than adult patients visit clinics. In addition, the pre-adult-onset group had longer periods with the disease; so they might have had more chances to go to clinics or get treatment. Disease insight might also be different between the 2 groups due to the duration of the disease.
There are a few studies about the relationship between AD and obesity (20,24). However, there is no study focused on the onset age of AD regarding differences in this association between pre-adult-onset AD and adult-onset AD. Our study showed that obese patients showed significantly higher EASI scores than did patients that were not obese, but only in pre-adult-onset adult AD. Moreover, our study found that there was a significant association between BMI and EASI score only among women in the pre-adult-onset adult AD group.
There have been reports of the positive association of obesity with AD in children and adults (20,30,31). Similar to our results, in a nation-wide study of 5,202 Korean adults, obesity was positively related to the presence of AD in women (20). A possible relationship between obesity and AD can be explained in several ways. There seem to be links between genetic markers of asthma, atopy, and obesity, such as ADRB2, NR3C1, and polymorphisms of the fractalkine receptor gene CX3CR1 (32). The genetic influences could explain a positive relationship between atopy and obesity in some subgroups, for examples, those with polymorphisms of the fractalkine receptor gene CX3CR1, the b2-adrenergic (ADR B2), and the glucocorticoid (NR3C1) receptors genes (32). Obesity has been reported to increase systemic inflammation and its effect on the immune system (32). Adipokines, which are secreted by white adipose tissue, are associated with inflammatory effect, including cytokines, circulating immune cells, and T-cell polarization (32,33,34). Reduced adiponectin caused respiratory inflammation with allergic march (33). Nagel et al. (35) also found associations between high leptin levels and asthma, and between low adiponectin levels and AD. Obesity can influence sex hormones (32), playing a role on the development of atopic disorders (36). Estrogen stimulated interleukin (IL)-3 and IL-4 in monocytes and promoted a Th2 phenotype immune response (32).
We thought about the reason why there was correlation between obesity and AD only in the pre-adult-onset group, but not in the adult-onset group, in several ways. First, there might be genetic differences between those in the early-onset and late-onset adult AD groups (32). Also, due to the limitations of the cross-sectional study, we could not find the onset point of obesity and the weight change in each patient. Silverberg et al. (5) suggested that prolonged obesity beginning early in life is associated with increased risk for, and severity of, AD. The longer duration of obesity among the pre-adult-onset group might cause these results. In addition, in cases of pre-adult-onset adult AD, the higher BMI the patients have, the harder to control the patient circumstances, such as severe secretion of sweat, self-management with topical agents, and the surrounding environment, which could influence the severity of AD, especially when young. More specifically, in the case of adult-onset adult AD, patients can control their conditions themselves because they have become used to managing their disease since the disease started. Actually, previous and recent studies to assess the associations of obesity with AD are mostly case-control studies or epidemiological investigation, so it is hard to indentify causal relationships or causes, apart from relevance (5,20,37). To confirm the association between obesity and AD, further studies are warranted to determine if weight loss may prevent or mitigate AD.
One limitation in our study is the inability to assess the temporal relationship between the exposure and outcome. There are potential reporting biases and recall bias of a cross-sectional study using the questionnaire-based method. Secondly, this study is a kind of preliminary study with small sample size. With small sample size and with participants at a single institution, the tertiary care setting might be a major limitation. Last, because this study was based on patient recall, we could not specifically divide the pre-adult-onset group into real early-onset (before 2 years old), early-childhood onset (before 6 years old), and late childhood onset. Late child-onset or adolescent-onset AD subjects could have very similar features with adult-onset AD subjects. Patients often refer to the onset of disease by randomly distinguishing between the occurrence of the disease in childhood and the occurrence of an adult, so the onset criterion was divided into early onset and late onset. Eventually, pre-adult-onset adult AD patients include both infantile-onset and juvenile-onset AD in our study. Because the major areas of initial involvement differ in those 2 groups, additional research is needed to distinguish these 2 subgroups to capture more objective data.
In summary, we herein describe the characteristics of clinical features of AD according to onset age. Even though there are many common features, there are some significant differences between pre-adult-onset and adult-onset AD subgroups in adult patients with AD. Between the 2 groups, there were significantly differences in the areas of initial involvement and the relation to obesity depending on severity of AD. Thus, our results will contribute to understanding the clinical characteristics of AD and to predicting the course of these diseases.
Footnotes
Funding: This research was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3A04049491, NRF-2015M2A2A6A04044376, and NRF-2017R1A2B4006252). This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI17C0597).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Park CW. Data curation: Son JH. Investigation: Son JH. Validation: Park CW, Kim HO, Chung BY. Writing - original draft: Son JH, Chung BY.
References
- 1.Williams HC. Epidemiology of atopic dermatitis. Clin Exp Dermatol. 2000;25:522–529. doi: 10.1046/j.1365-2230.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Hunter JA, Herd RM. Recent advances in atopic dermatitis. Q J Med. 1994;87:323–327. [PubMed] [Google Scholar]
- 3.Tay YK, Khoo BP, Goh CL. The profile of atopic dermatitis in a tertiary dermatology outpatient clinic in Singapore. Int J Dermatol. 1999;38:689–692. doi: 10.1046/j.1365-4362.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim HO, Cho SI, Kim JH, Chung BY, Cho HJ, Park CW, Lee CH. Food hypersensitivity in patients with childhood atopic dermatitis in Korea. Ann Dermatol. 2013;25:196–202. doi: 10.5021/ad.2013.25.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Kleiman E, Lev-Tov H, Silverberg NB, Durkin HG, Joks R, Smith-Norowitz TA. Association between obesity and atopic dermatitis in childhood: a case-control study. J Allergy Clin Immunol. 2011;127:1180–1186.e1. doi: 10.1016/j.jaci.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Kim H, Park CW, Lee CH. Quality of life in adults with atopic dermatitis. Korean J Dermatol. 2011;49:983–992. [Google Scholar]
- 7.Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, Novak N. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajka G. Essential Aspects of Atopic Dermatitis. [place unknown]: Springer; 2012. [Google Scholar]
- 9.Sandström Falk MH, Faergemann J. Atopic dermatitis in adults: does it disappear with age? Acta Derm Venereol. 2006;86:135–139. doi: 10.2340/00015555-0040. [DOI] [PubMed] [Google Scholar]
- 10.Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015;7:101–105. doi: 10.4168/aair.2015.7.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandström MH, Faergemann J. Prognosis and prognostic factors in adult patients with atopic dermatitis: a long-term follow-up questionnaire study. Br J Dermatol. 2004;150:103–110. doi: 10.1111/j.1365-2133.2004.05711.x. [DOI] [PubMed] [Google Scholar]
- 12.Wüthrich B. Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol. 1999;83:464–470. doi: 10.1016/S1081-1206(10)62852-9. [DOI] [PubMed] [Google Scholar]
- 13.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836–845. doi: 10.1111/all.12619. [DOI] [PubMed] [Google Scholar]
- 14.Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225–228. doi: 10.1046/j.1440-0960.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 15.Roth HL, Kierland RR. The natural history of atopic dermatitis. A 20-year follow-up study. Arch Dermatol. 1964;89:209–214. doi: 10.1001/archderm.1964.01590260047008. [DOI] [PubMed] [Google Scholar]
- 16.Lammintausta K, Kalimo K, Raitala R, Forsten Y. Prognosis of atopic dermatitis. A prospective study in early adulthood. Int J Dermatol. 1991;30:563–568. [PubMed] [Google Scholar]
- 17.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar AJ, Narang T. Adult onset atopic dermatitis: under-recognized or under-reported? Indian Dermatol Online J. 2013;4:167–171. doi: 10.4103/2229-5178.115508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52:579–582. doi: 10.1016/j.jaad.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Han KD, Jung HM, Youn YH, Lee JY, Park YG, Lee SH, Park YM. Association between obesity, abdominal obesity, and adiposity and the prevalence of atopic dermatitis in young Korean adults: the Korea National Health and Nutrition Examination Survey 2008–2010. Allergy Asthma Immunol Res. 2016;8:107–114. doi: 10.4168/aair.2016.8.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon JA, Roh KY, Koh BK, Kim JW. Clinical characteristics of adolescence and adult atopic dermatitis in Korea. Korean J Dermatol. 2004;42:949–954. [Google Scholar]
- 22.Kim KH, Park KC. Clinical characteristics of adult atopic dermatitis. Ann Dermatol. 1998;10:229–232. [Google Scholar]
- 23.Chu H, Shin JU, Park CO, Lee H, Lee J, Lee KH. Clinical diversity of atopic dermatitis: a review of 5,000 patients at a single institute. Allergy Asthma Immunol Res. 2017;9:158–168. doi: 10.4168/aair.2017.9.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KH, Park AY, Kim JS. Factors associated with atopic dermatitis in Korean adults: the Korean National Health and Nutrition Survey 2008. Korean J Rehabil Nurs. 2012;15:83–90. [Google Scholar]
- 25.Chung Y, Kwon JH, Kim J, Han Y, Lee SI, Ahn K. Retrospective analysis of the natural history of atopic dermatitis occurring in the first year of life in Korean children. J Korean Med Sci. 2012;27:723–728. doi: 10.3346/jkms.2012.27.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739–744. doi: 10.1111/j.1365-4632.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 27.Haeck IM, Knol MJ, Ten Berge O, van Velsen SG, de Bruin-Weller MS, Bruijnzeel-Koomen CA. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:1074–1084. doi: 10.1016/j.jaad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 29.Gutgesell C, Heise S, Seubert S, Seubert A, Domhof S, Brunner E, Neumann C. Double-blind placebo-controlled house dust mite control measures in adult patients with atopic dermatitis. Br J Dermatol. 2001;145:70–74. doi: 10.1046/j.1365-2133.2001.04283.x. [DOI] [PubMed] [Google Scholar]
- 30.Koutroulis I, Magnelli L, Gaughan J, Weiner E, Kratimenos P. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713–717. doi: 10.1111/apa.12970. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg JI, Silverberg NB, Lee-Wong M. Association between atopic dermatitis and obesity in adulthood. Br J Dermatol. 2012;166:498–504. doi: 10.1111/j.1365-2133.2011.10694.x. [DOI] [PubMed] [Google Scholar]
- 32.Boulet LP. Obesity and atopy. Clin Exp Allergy. 2015;45:75–86. doi: 10.1111/cea.12435. [DOI] [PubMed] [Google Scholar]
- 33.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–1213. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 34.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 35.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 36.Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006;117:1001–1007. doi: 10.1016/j.jaci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Luo X, Xiang J, Dong X, Cai F, Suo J, Wang Z, Liu M. Association between obesity and atopic disorders in Chinese adults: an individually matched case-control study. BMC Public Health. 2013;13:12. doi: 10.1186/1471-2458-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]



