Abstract
The symbiosis responsible for nitrogen fixation in legume root nodules is initiated by rhizobial signaling molecules [Nod factors (NF)]. Using transgenically tagged microtubules and actin, we dynamically profiled the spatiotemporal changes in the cytoskeleton of living Lotus japonicus root hairs, which precede root-hair deformation and reflect one of the earliest host responses to NF. Remarkably, plant-parasitic root-knot nematodes (RKN) invoke a cytoskeletal response identical to that seen in response to NF and induce root-hair waviness and branching in legume root hairs via a signal able to function at a distance. Azide-killed nematodes do not produce this signal. A similar response to RKN was seen in tomato. Aspects of the host responses to RKN were altered or abolished by mutations in the NF receptor genes nfr1, nfr5, and symRK, suggesting that RKN produce a molecule with functional equivalence to NF, which we name NemF. Because the ability of RKN to establish feeding sites and reproduce was markedly reduced in the mutant lines, we propose that RKN have adapted at least part of the symbiont-response pathway to enhance their parasitic ability.
Keywords: cytoskeleton, NemF, rhizobia, actin, microtubule
Plants engage other organisms in diverse symbioses ranging from mutualistic associations with rhizobia and mycorrhizae (supplying the plant with fixed nitrogen and phosphorous, respectively) to hosting harmful parasites such as root-knot nematodes (RKN, Meloidogyne spp.). Unlike rhizobia, which interact with strictly defined legume species, the RKN host range encompasses essentially all vascular plants, leading to extensive crop loss worldwide (1). The specificity of particular rhizobial–legume associations is largely mediated at the root surface via specific lipochitooligosaccharide signaling molecules (2) synthesized by the bacterium and collectively termed Nod factors (NF). Invasion of the host by rhizobia is a highly orchestrated process, beginning with ion movements (3–5) and changes in cytoskeletal organization (6–8). The resultant signals lead to the first morphologically visible step, which is the disruption of the normal tip-growing pattern and curling of epidermal root hair cells 2–4 h after initial exposure and before bacterial entry (9). NF alone is sufficient to elicit cytoskeletal and ion responses, gene expression changes (10, 11), root-hair deformation, and the formation of pseudonodules, but not infection threads. NF production and host sensitivity both are essential for nodulation (12, 13).
In contrast, RKN apparently enter the host simply by mechanical penetration. Migration into the vascular cylinder is intercellular and nondestructive and, once in the stele, RKN induce characteristic giant cells (GC), from which the developing larvae feed. GC formation invokes host pathways in common with those necessary for nitrogen-nodule formation, including induction of specific transcription regulators (14), early nodulation genes (15, 16), and cytokinin-response pathways (17), suggesting that at the cellular level, RKN and rhizobia exploit a common strategy. Consistent with this, a beet cyst nematode-inducible resistance gene is induced during rhizobial invasion (11). EST sequencing revealed RKN homologues of rhizobial sequences, including genes required for NF synthesis (18, 19). This result led us to investigate whether RKN might produce functionally equivalent signaling molecules capable of inducing rapid cytoskeletal changes similar to those observed after application of NF. We found that RKN invoke an identical subcellular reorganization and root-hair deformation response via a signal able to function at a distance. Evidence that a plant-parasitic nematode signal can induce a plant response is previously undescribed.
Recent studies of Lotus japonicus plants defective in nodulation have identified receptors with roles in NF perception (20–22). The symbiosis receptor-like kinase (SYMRK, defined by symRK SL1951-5), also shown to be involved in mycorrhizal signal perception, is required for root-hair curling in response to rhizobia but not for swelling and branching (20). Two transmembrane receptor kinases (NFR1 and NFR5, defined by the nfr1-2 and nfr5-1 alleles, respectively) are required for the earliest cellular and physiological changes, including membrane depolarization and pH changes, and are strong candidates for defining the primary NF receptors (21); SYMRK is a possible secondary NF receptor (20). A complementary genetic analysis in Medicago truncatula has revealed the equivalent NF-response pathway (23), including the apparent NF receptor (24). We compared root-hair growth in response to nematodes in nfr1, nfr5, and symRK mutants and wild-type plants and show that L. japonicus SYMRK, NFR1, and NRF5 receptors are putatively involved in the perception of nematode-derived signals. We further suggest that equivalent proteins are exploited by RKN in non-legume hosts.
Materials and Methods
Plant Culture and Nematode Inoculation. L. japonicus (Gifu) and Meloidogyne incognita were maintained as described (17). Second-stage RKN larvae (L2) were isolated and hatched by standard means (25), collected after downward migration through filter paper, surface sterilized with 1% NaOCl, and suspended in sterile deionized water. Each root was perfused with ≈10 L2 in suspension. Deionized water was used as a control. Nematodes were killed by incubation in 0.05% sodium azide for 30 min, followed by extensive washing in sterile water.
GFP::MAP4 and GFP::Talin constructs driven by the cauliflower mosaic virus 35S promoter were inserted into binary vectors pBin plus and pWPF 127, respectively, and electroporated into Agrobacterium rhizogenes strain AR10. Hairy roots were induced from 4- to 5-day-old L. japonicus seedlings (26) by using a 2-day-old A. rhizogenes culture grown on solid yeast extract broth (YEB) and suspended in sterile water as inoculum. After inoculation, plants were incubated overnight in darkness and then placed in a 16/8-h light–dark cycle at 22°C. Hairy roots grew from the wound site in 2 weeks. Plants with newly grown hairy roots were excised and placed on 1.2% agar containing 0.5× Gamborg's B5 medium with 300 mg/ml Cefotaxime.
NF Additions. Excised living roots were examined for transgenic protein expression levels, mounted on microscope slides with 0.5× Gamborg's B5 medium, and incubated at room temperature for 30 min. Samples were imaged for 5 min before and 60 min after treatment. Roots were perfused with 10-7 M Mesorhizobium loti R7A NF. Sterile medium was used as a control.
Microscopy. Samples were imaged by using a confocal microscope (Leica, Deerfield, IL) with a ×40 1.2-numerical aperture Zeiss water immersion objective in fluorescent and transmitted light modes. Time-lapsed optical stacks were recorded with GFP excited at 488 nm and emission collected from 500 to 550 nm. Differential interference contrast (DIC) images were recorded concurrently. Images were processed by using Leica confocal software and photoshop 7.0 (Adobe Systems, San Jose, CA). Volume rendering of confocal stacks was performed by using a maximum projection algorithm. Living roots were mounted on microscope slides, covered with no. 1.5 cover glasses, and observed with ×20 and ×40 Neofluar objectives on a Zeiss Axiovert 100 TV inverted microscope by using DIC optics. Images were acquired with an ORCA-ER camera (Hamamatsu, Hamamatsu City, Japan) by using metamorph (Universal Imaging, Downington, PA) and photoshop for image processing.
Results
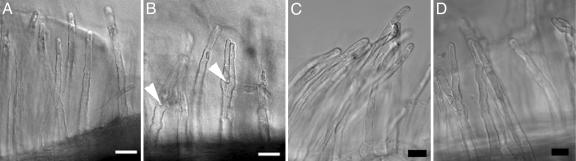
RKN and NF Elicit an Equivalent Root-Hair Effect. In water or culture medium, L. japonicus root hairs are straight and smooth (Fig. 1A). Addition of purified NF elicits the classic wavy and branched appearance within 4 h (not shown). Strikingly, the addition of RKN also caused identical root-hair deformations in developing root hairs, which are apparent 1 h after addition of L2 and are fully manifested as wavy branching root hairs by 4 h (Fig. 1B). RKN larvae do not synthesize structural chitin (27). However, because nematode egg shells contain chitin (the backbone constituent of NF), we used a protocol that required freshly hatched L2 to actively migrate downward through filter paper (leaving the shells behind) before extensive washing to remove any soluble chitin. L2 killed by exposure to sodium azide and washed extensively entirely fail to elicit wavy or branched root hairs (Fig. 1C). At no time throughout the observation period were the nematodes in physical contact with the root; our use of a perfusion chamber permitted released material to rapidly reach the root area. To confirm the implication that live RKN generate a diffusible morphogenic signal in L. japonicus root hairs, we treated roots with an L2-free supernatant harvested from the nematode suspension, producing identically wavy root hairs (Fig. 1D).
Fig. 1.
NF and RKN induce similar morphological effects on L. japonicus root hairs. Roots were exposed to sterile water (A), RKN larvae (B), azide-killed nematodes (C), or nematode supernatant (D). Arrowheads, branching root hairs. (Bar, 50 μm.)
Real-Time Monitoring of the Root-Hair Cytoskeleton. The cytoskeleton plays a central role in signal transduction and regulation of the growth machinery of root hairs and strongly influences their growth pattern (28). To assay cytoskeletal dynamics of microtubule (MT) and actin filaments associated with the observed morphological changes, we constructed L. japonicus hairy roots transgenic for GFP fusions of the MT associated protein, MAP4, and the actin-binding protein, Talin. Individual roots were selected for low-level constitutive expression of the chimeric proteins. Confocal laser-scanning microscopy confirmed that developing transgenic root-hair cells exhibited normal cytoplasmic streaming and healthy cytoarchitecture. Cortical MT of the transgenic roots expressing GFP::Map4 were arranged in a net axial manner along the root hair, and endoplasmic MT extended from the root-hair apex to the nucleus. Root-hair MT displayed a very dynamic retrograde flow, recapitulating what is seen in root hairs of Arabidopsis thaliana and M. truncatula (29). The base of root hairs expressing GFP::Talin contained bundles of actin filaments parallel to the longitudinal axis of the root hair. In the subapical region, these thick bundles were finer, more dispersed, and not parallel. The intense fluorescence seen in the tip area indicated a large amount of diffuse actin. Like the MT, the actins were highly dynamic and exhibited retrograde flow.
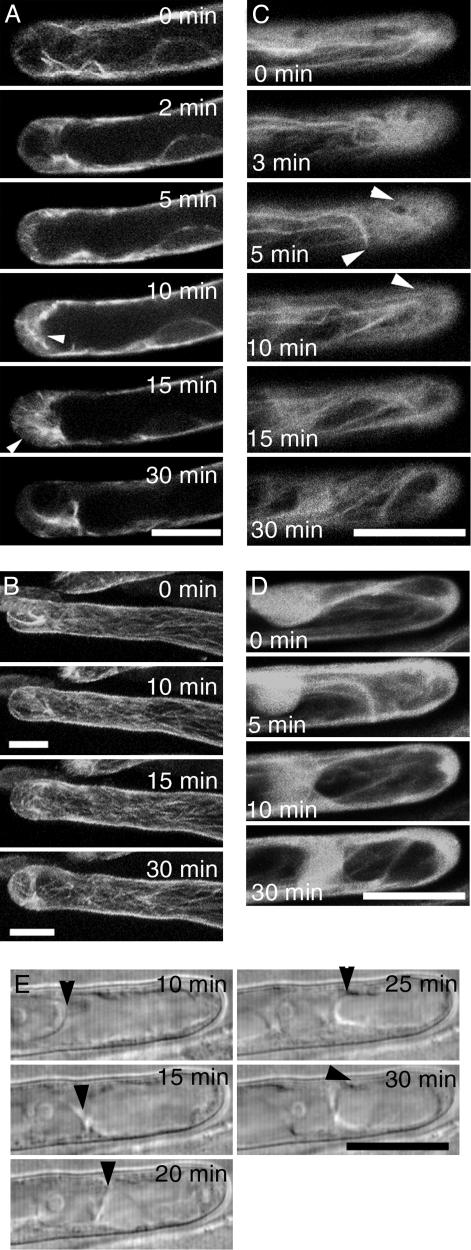
Living roots were imaged continuously before and after perfusion with living RKN (Fig. 2) or 100 nM M. loti NF (Figs. 6 and 7, which are published as supporting information on the PNAS web site). Within 2 min of applying either 5–10 sterile freshly hatched RKN L2 or NF 100–300 μm distal to the transgenic root tip, the finer endoplasmic MT (Movie 1, which is published as supporting information on the PNAS web site) disintegrated, and those that remained became thicker (Fig. 2A). The number of endoplasmic MT increased at 5–10 min. Repolymerization of endoplasmic MT initiated from the very tip of the root hair. In many cases, a ring-like structure, previously observed by immunolocalization in NF-treated root hairs (8), was observed 3–5 μm below the tip (data not shown). Cortical MT showed a gradual increase in intensity and became more fragmented (Fig. 2B and Movie 2, which is published as supporting information on the PNAS web site). A slight swelling in the root-hair tip area became apparent at 30 min after treatments.
Fig. 2.
Confocal and differential interference contrast time-lapse analysis of L. japonicus root-hair cells after exposure to RKN at t = 0 min. (A) Reorganization of endoplasmic MT. MT thickness and amount peaks at 10–15 min after nematode exposure (arrowheads) and declines to preexposure levels by 30 min. (B) Full stack projection shows that the cortical MT increase slightly after exposure to RKN. (C) Cortical fine bundles of actin initially decrease but reappear at 5–10 min (arrowheads). (D) Total projection of confocal stacks from C. (E) Migration of the nucleus (arrowhead) toward the apex of the root hair after exposure to RKN. (Bars, 20 μm.)
Distributional changes of MT and actin filaments appeared to be coordinated. By 3 min after exposure to RKN or NF, the cortical fine bundles of actin decreased, and the diffuse fluorescence increased (Fig. 2C). The fine actin bundles regenerated 5 min after treatment and were especially striking by 10 min (Fig. 2C and Movie 3, which is published as supporting information on the PNAS web site). Diffuse fluorescence around the root-hair apex increased at 5 min, and this persisted for 30 min (Fig. 2D and Movie 4, which is published as supporting information on the PNAS web site). The structure of actin filaments around the nucleus altered just before the initiation of nuclear movement, and the altered pattern persisted throughout the duration of the nuclear movement. The nuclear behavior of nematode-challenged root hairs was identical to that seen in NF-treated root hairs (Fig. 2E and Movie 5, which is published as supporting information on the PNAS web site). The rate of nuclear movement was 6 μm/min (growth rate of root hair, 1.02 μm/min) and 5.16 μm/min (growth rate of root hairs, 1.26 μm/min) for RKN-treated root hairs and NF-treated root hairs, respectively. In some cases, the nuclei displayed initial basipetal movement.
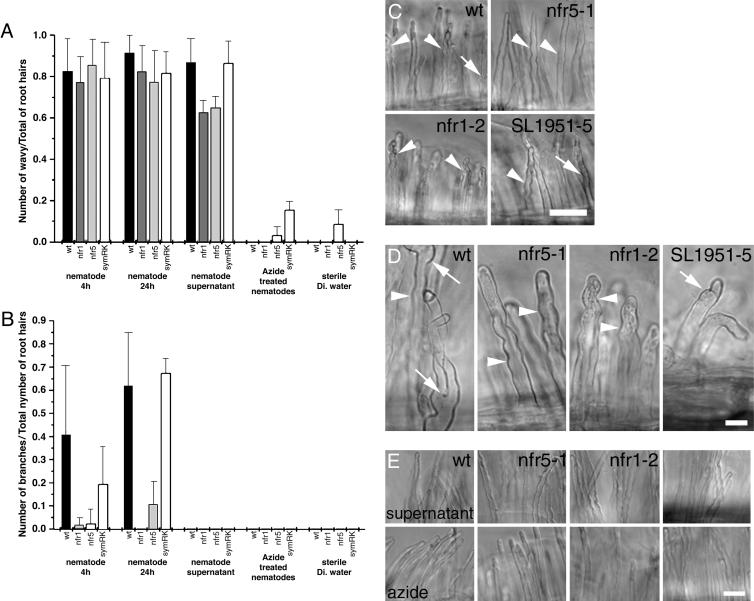
L. japonicus Mutants Altered in NF Perception also Are Altered in RKN Perception. The remarkable parallel between the cytological responses of root-hair cells to NF and RKN led us to investigate whether perception of the signals from these disparate organisms might be mediated by a common or overlapping host signal transduction pathway. Genetic analyses of the rhizobial and mycorrhizal symbioses revealed a suite of candidate NF receptors linked to downstream pathways (23, 30). We compared the response to RKN in root hairs of wild-type plants with nfr1, nfr5, and symRK mutants in at least five independent experiments each. We scored the classical root-hair deformation responses of wavy growth, branching, and bulging as separate features. Although chitooligosaccharides alone can induce rapid proton efflux from tomato cells (31), induction of ENOD40 in soybean (32), and calcium spiking in pea (33), only bona fide NF is known to elicit all three root-hair deformation effects (34).
Wild-type root hairs exposed to RKN for 4 h (Fig. 3 A and B) exhibited a wavy growth pattern with bulging tips (73/90 root hairs examined). On average, 40% of the root hairs per plant also displayed branching (Fig. 3 C and D). Root hairs of all three mutants also displayed a wavy growth pattern after 4-h exposure to RKN L2. However, root-hair branching was essentially absent from nfr5-1 (1/85) and nfr1-2 (7/118) plants and reduced to half that of wild type in the symRK line. Student's t test confirmed the significance of the differences between wild-type and nfr5-1 (P = 0.004) and nfr1-2 (P = 0.005) lines, respectively. Neither wild-type nor mutant root hairs treated with sterile deionized water or azide-killed nematodes exhibited an altered growth pattern.
Fig. 3.
Mutants altered in NF perception also are altered in RKN perception. (A) Proportion of wavy root hairs formed in response to nematode signaling. (B) Root-hair phenotype 4 h after exposure to RKN showing wavy growth (arrowheads). (C) Proportion of branched root hairs formed in response to RKN. (D) Root-hair phenotype 24 h after exposure to RKN showing branching (arrow); nfr mutants display only a wavy growth pattern (arrowhead). (E) Root hairs treated with RKN suspension supernatant display a wavy growth pattern; dead nematodes do not alter polar root-hair growth. The number of wavy root hairs 4 h after exposure to RKN was significantly different (P = 2.68 × 10-7, independent Student's t test) than after exposure to deionized water. (Bars, 50 μm.)
Exposure of wild-type and mutant root hairs to RKN for 24 h resulted in a pattern of waviness recapitulating that seen with a 4-h exposure (Fig. 3). The branching phenotype was slightly different. Approximately 60% of wild-type and symRK root hairs show branching in addition to the wavy growth pattern. In contrast, only 10% of nfr5-1 and 0% of nfr1-2 hairs exhibited branching. Collectively, these results point to a nematode signal independent of the primary NF perception pathway to induce a wavy growth pattern in L. japonicus root hairs, but which requires the NFR1 and NFR5 receptors to induce root-hair branching. SYMRK function is dispensable for root-hair branching induced by long exposure (24 h) to RKN larvae but appears to play a role in mediating the branching process after short exposure to nematodes.
To further investigate the nature of the nematode signal, we exposed wild-type and mutant roots to an RKN-free aliquot of the water in which the L2 had been suspended. On wild-type and mutant plants, this supernatant elicited wavy root hairs equivalent to those induced by L2 (Fig. 3E) but, interestingly, the number was slightly but significantly reduced on nfr5-1 (P = 0.004) and nfr1-2 (P = 0.002) plants compared with these lines exposed to live L2. None of the root hairs exposed only to the supernatant displayed branching.
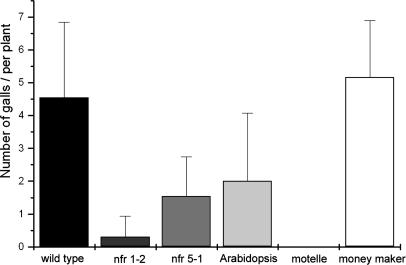
NF-Perception Mutants Alter RKN Feeding Site Formation. For rhizobial and mycorrhizal symbioses, the primary interaction with the host occurs on the root surface. However, the much larger M. incognita L2 (≈400 μm in length × ≈16 μm in diameter) apparently enter the root solely by mechanical penetration, with the “specific” interaction beginning in the vascular cylinder, with the induction of GC and the subsequent root gall (35, 36). We measured the ability of RKN to induce galls on nfr5-1, nfr1-2, and symRK seedlings grown in agar. To benchmark this assay, we included the near-isogenic tomato cultivars, Lycopersicon esculentum cv. Motelle and cv. Moneymaker. Moneymaker is a reliable host for RKN, and Motelle exhibits a high degree of resistance. Wild-type L. japonicus and Moneymaker tomato were found to support robust galling with galls absent on the Motelle plants (Fig. 4). By contrast, gall formation was substantially reduced, although not abolished, in the nfr5-1 and nfr1-2 backgrounds and reduced by about half in symRK plants. Because RKN infections are more efficient in soil, we assessed gall induction on pot-grown nfr5-1, nfr1-2, and symRK plants. As an indirect but effective measure of GC function, we counted the number of sexually mature egg-laying females. Under these conditions, symRK plants were found to support essentially wild-type levels of galling (>50 galls), each with an egg-laying female. In contrast, nfr1-2 mutants exhibited an average of only 7.0 small galls, supporting an average of only 2.6 egg-laying females per plant. Nfr5-1 mutants exhibited an intermediate but variable phenotype, with some plants supporting >20 galls. Subjectively, these galls appeared larger than those seen on nfr1-2 plants, and approximately half of these galls supported mature females. The NFR1 and NFR5 receptors can modulate the ability of RKN to establish feeding sites, although the role for SYMRK is less clear.
Fig. 4.
The number of RKN feeding sites, measured as the number of galls formed per plant, is reduced in plants mutant in NF perception.
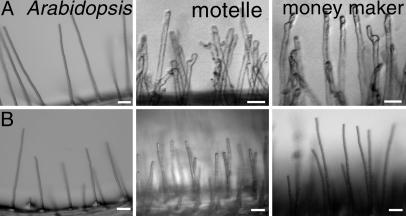
RKN Responses in Non-Legumes. To observe whether similar morphological effects are elicited in root hairs of non-legumes, we exposed wild-type A. thaliana (Columbia) plants and Motelle and Moneymaker tomato cultivars to RKN. A. thaliana is not responsive to any known NF and fails to engage in mycorrhizal interactions. Although A. thaliana can be coaxed to support RKN reproduction, infection rates are very low, and it is not believed to be a natural host in the wild (37). Similarly, L. esculentum is not typically considered to respond in a specific manner to NF, although tomato cells do exhibit rapid alkalinization after exposure to chitooligosaccharides (31). However, like most land plants, tomato actively engages in mycorrhizal symbiosis (30) and is a robust host for RKN (35, 36). Not surprisingly, we found that A. thaliana root hairs treated with nematodes or sterile deionized water did not exhibit altered root-hair morphology (Fig. 5). However, both RKN-sensitive and resistant L. esculentum cultivars exhibited unequivocal root-hair waviness and branching 24 h after exposure to RKN (Fig. 5), demonstrating that the response to RKN is not a feature unique to legumes and possibly implicating a role for components of the NF-response pathway as being more broadly exploited by RKN.
Fig. 5.
Root-hair branching response to RKN is not unique to legumes. (A) Twenty-four hours after exposure to RKN, Arabidopsis root hairs remain straight, whereas RKN-resistant and susceptible cultivars of L. esculentum (Motelle and Moneymaker, respectively) respond by forming branched and wavy root hairs. (B) No root-hair phenotype was observed after exposure to deionized water. (Bars, 50 μm.)
Discussion
Central to successful mutualistic symbiosis by rhizobia and mycorrhizae is the ability of the microbe to communicate with the plant via diffusible signals (2, 12, 38, 39). Based on our cellular and genetic analyses, the same appears to be true for the parasitic symbiosis between RKN and their plant host. Not only do NF and RKN invoke similar cytoskeletal, nuclear, and morphological effects in L. japonicus root hairs, but also specific components of the host perception machinery are involved in both interactions, implying a degree of common function between these prokaryotic and eukaryotic symbionts. The root-hair cells respond in a defined manner both to L2 and nematode perfusates, indicating that the RKN-derived signal functions at a distance. Collectively, these results point to the existence of an RKN-derived signaling molecule(s), which, by analogy with bacterial NF, we call NemF.
First, like NF (2), NemF appears to function at low concentration. Our root-hair assays typically used 10 L2, but an effect is observable with a single individual. Second, the observation that the wavy root-hair phenotype is separable from the branching phenotype in different genetic backgrounds points either to NemF being composed of two or more functional signals or, alternatively, the plant perceives the signal through at least two independent routes, one of which involves the NFR1 and NFR5 receptor kinases. In this regard, NemF also parallels rhizobial NF, which functions through these primary receptors and through SYMRK as a secondary receptor. Third, although NemF clearly is a diffusible water-soluble signal, the supernatant from an RKN suspension is unable to elicit the branched root-hair response. This might suggest that NemF is short-lived, with the lack of branching simply reflecting a concentration effect; live nematodes presumably continue to produce a signal, whereas the levels of signal may drop in the supernatant. Another alternative is that the signal is produced by RKN only after exposure to a plant. It is clear for rhizobia (2, 12), and evidence is mounting for mycorrhizae (38, 39), that the microbes produce NF or Myc Factor, respectively, only once they have perceived a plant signal; it is an intriguing possibility that this is the same for RKN. Our confocal observations of root-hair cytoskeletal rearrangements showed that, although the changes elicited by rhizobia and RKN are spatially identical, the temporal patterns are different, with the response to RKN being retarded. This is consistent with a need for the nematode to respond to the plant before synthesizing and/or releasing NemF.
The cellular responses we observed are rapid and precede actual root contact by the nematode. This result was unexpected, because it previously was believed that the initial interaction with the host was actual penetration of the root by the L2. Further, commitment of the L2 to the parasitic interaction is typically thought of as beginning at the time of GC induction in the vascular cylinder >24 h after root penetration. Our findings do not directly address the primary mechanism of GC induction but clearly indicate a role for the NF/NemF response pathway. Unlike their effect on rhizobial nodulation, mutations in either nfr1 and nfr5 do not abolish RKN feeding site formation, but rather they significantly reduce the number of galls formed. Further, these mutations diminish the fecundity of the resultant adult female nematodes, indicating that those GC that do form are unable to fully sustain the nutritional requirements of the developing nematodes. This strongly argues that the effect of these genes is exerted directly in the GC and not at some other stage of the RKN life cycle. The actual GC inductive process is accompanied by transcriptional events common to the early events of nodule formation by rhizobia (15, 16), suggesting that the sequence of events initiated by NF might be similar to those events that lead to GC formation. Although the chemical nature of NemF remains unknown, in our model, the bacterial, fungal, and nematode signal molecules are sufficiently similar to be recognized by the same receptor complexes (20, 21) and thus initiate similar downstream effects. Further, although mutations in nfr1 exerted a greater influence upon GC formation and root-hair branching in response to NemF than did mutations in the other genes examined, other as-yet-unidentified receptors may play a more central role in NemF perception.
Arabidopsis root hairs do not respond to NemF and qualitatively, RKN infection of the L. japonicus nfr1 mutants strongly resembles RKN infection of wild-type A. thaliana. Perhaps causally related, A. thaliana lacks both SYMRK and, more importantly, the DMI3 calcium- and calmodulin-dependent protein kinase (40) necessary for transduction of NF and mycorrhizal signals (23). Sequencing implies that other non-legumes appear to have an intact NF-response pathway, and consistent with this is our observation that RKN induce root-hair waviness and branching in tomato. Several authors (23, 30) have proposed that the evolutionarily more recent rhizobial symbiosis arose by recruitment of plant functions that coevolved during the ancient development of the plant–mycorrhizal association. We concur with this notion and propose that the evolution of parasitism in RKN was accompanied by conscription of the older symbiotic pathways for RKN pathogenesis. The previous findings that RKN appear to have acquired rhizobial genes via horizontal gene transfer (18, 19), including those associated with NF biosynthesis, suggest a mechanism for such evolutionary adaptation. We have shown that legume root-hair cells respond in a defined, rapid, and stereotypical manner to RKN perfusates before direct contact is made between nematode and plant; models of GC induction should allow for a similar possibility.
Supplementary Material
Acknowledgments
The symRK line was the kind gift of Martin Parniske and Jill Perry (Sainsbury Laboratory, Norwich, U.K.), and nrf1 and nrf5 mutants were generously supplied by Jens Stougaard and Simona Radutoiu (University of Århus, Århus, Denmark). GFP::MAP4 and GFP::Talin constructs were generously supplied by Elison Blancaflor (Samuel Roberts Noble Foundation, Ardmore, OK) and Z. B. Yang (University of California, Riverside, CA), respectively. M. loti R7A NF was the kind gift of A. Serna-Sanz (Sainsbury Laboratory). We thank Jennifer Schaff for maintenance of RKN stocks and for performing mutant infection studies. This work was supported by National Science Foundation Plant Genome Award DBI0077503 (to D.M.B.) and by the North Carolina Research Station [NC AgRe Stat 407050 (to N.S.A.)].
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GC, giant cell(s); L2, second-stage nematode larva(e); MT, microtubule(s); NF, Nod factors; RKN, root-knot nematode; NemF, RKN factor; SYMRK, symbiosis receptor-like kinase.
References
- 1.Koenning, S. R., Overstreet, C., Noling, J. W., Donald, P. A., Becker, J. O. & Fortnum, B. A. (1999) J. Nematol. 31, 587-618. [PMC free article] [PubMed] [Google Scholar]
- 2.Ardourel, M., Demont, N., Debellé, F. D., Maillet, F., de Billy, F., Promé, J. C., Dénarié, J. & Truchet, G. (1994) Plant Cell 6, 1357-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrhardt, D. W., Wais, R. & Long, S. R. (1996) Cell 85, 673-681. [DOI] [PubMed] [Google Scholar]
- 4.Felle, H. H., Kondorosi, E., Kondorosi, A. & Schultze, M. (1996) Plant J. 10, 295-301. [Google Scholar]
- 5.Felle, H. H., Kondorosi, E., Kondorosi, A. & Schultze, M. (1998) Plant J. 13, 455-463. [Google Scholar]
- 6.Allen, N. S., Bennett, M. N., Cox, D. N., Shipley, A., Ehrhardt, D. W. & Long, S. R. (1994) Adv. Mol. Gen. Plant–Microbe Interact. 3, 107-114. [Google Scholar]
- 7.Cárdenas, L., Vidali, L., Domínguez, J., Pérez, H., Sánchez, F., Hepler, P. K. & Quinto, C. (1998) Plant Physiol. 116, 871-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weerasinghe, R. R., Collings, D. A., Johannes, E. & Allen, N. S. (2003) Planta 218, 276-287. [DOI] [PubMed] [Google Scholar]
- 9.Truchet, G., Roche, P., Lerouge, P., Vasse, J., Camut, S., de Billy, F., Promé, J. C. & Dénarié, J. (1991) Nature 351, 670-673. [Google Scholar]
- 10.Horvath, B., Heidstra, R., Lados, M., Moerman, M., Spaink, H. P., Promé, J. C., Van Kammen, A. & Bisseling, T. (1993) Plant J. 4, 727-733. [DOI] [PubMed] [Google Scholar]
- 11.Mitra, R. M., Shaw, S. L. & Long, S. R. (2004) Proc. Natl. Acad. Sci. USA 101, 10217-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long, S. R. (1996) Plant Cell 8, 1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch, A. M., Lum, M. R. & Downie, J. A. (2001) Plant Phyisol. 127, 1484-1492. [PMC free article] [PubMed] [Google Scholar]
- 14.Koltai, H. & Bird, D. McK. (2000) Plant J. 22, 455-459. [DOI] [PubMed] [Google Scholar]
- 15.Koltai, H., Dhandaydham, M., Opperman, C. H., Thomas, J. & Bird, D. McK. (2001) Mol. Plant–Microbe Interact. 14, 1168-1177. [DOI] [PubMed] [Google Scholar]
- 16.Favery, B., Complainville, A., Vinardell, J. M., Lecomte, P., Vaubert, D., Mergaert, P., Kondorosi, A., Kondorosi, E., Crespi, M. & Abad, P. (2002) Mol. Plant–Microbe Interact. 15, 1008-1013. [DOI] [PubMed] [Google Scholar]
- 17.Lohar, D. P., Schaff, J. E., Laskey, J. G., Kieber, J. J., Bilyeu, K. D. & Bird, D. McK. (2004) Plant J. 38, 203-214. [DOI] [PubMed] [Google Scholar]
- 18.McCarter, J. P., Mitreva, M. D., Martin, J., Dante, M., Wylie, T., Rao, U., Pape, D., Bowers, Y., Theising, B., Murphy, C., et al. (2003) Genome Biol. 4, research0026.1-0026.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholl, E. H., Thorne, J. L., McCarter, J. P. & Bird, D. McK. (2003) Genome Biol. 4, research0039.1-0039.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stracke, S., Kistner, C., Yoshida, S., Mulder, L., Sato, S., Kaneko, T., Tabata, S., Sandal, N., Stougaard, J., Szczyglowsi, K., et al. (2002) Nature 417, 959-962. [DOI] [PubMed] [Google Scholar]
- 21.Radutoiu, S., Madsen, L. H., Madsen, E. B., Felle, H. H., Umehara, Y., Grønlund, M., Sato, S., Nakamura, Y., Tabata, S., Sandal, N., et al. (2003) Nature 425, 585-592. [DOI] [PubMed] [Google Scholar]
- 22.Madsen, E. B., Madsen, L. H., Radutoiu, S., Olbryt, M., Rakwalska, M., Szczyglowski, K., Sato, S., Kaneko, T., Tabata, S., Sandal, N., et al. (2003) Nature 425, 637-640. [DOI] [PubMed] [Google Scholar]
- 23.Riely, B. K., Ané, J.-M., Penmesta, R. V. & Cook, D. R. (2004) Curr. Opin. Plant Biol. 7, 408-413. [DOI] [PubMed] [Google Scholar]
- 24.Limpens, E., Franken, C., Smit, P., Willemse, J., Bisseling, T. & Geurts, R. (2003) Science 302, 630-634. [DOI] [PubMed] [Google Scholar]
- 25.Hussey, R. S. & Barker, K. R. (1973) Plant Dis. Rep. 57, 1025-1028. [Google Scholar]
- 26.Stiller, J., Martirani, L., Tuppale, S., Chian, R.-J., Chiurazzi, M. & Gresshoff, P. M. (1997) J. Exp. Bot. 48, 1357-1365. [Google Scholar]
- 27.Veronico, P., Gray, L. J., Jones, J. T., Bazzicalupo, P., Arbucci, S., Cortese, M. R., Di Vito, M. & De Giorgi, C. (2001) Mol. Genet. Genomics. 266, 28-34. [DOI] [PubMed] [Google Scholar]
- 28.Collings, D. A. & Allen, N. S. (2000) in Actin: A Dynamic Framework for Multiple Plant Cell Functions (Kluwer, Dordrect, The Netherlands), pp. 145-164.
- 29.Sieberer, B. J., Timmers, A. C. J., Lhuissier, F. G. P. & Emons, A. M. C. (2002) Plant Physiol. 130, 977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parniske, M. (2004) Curr. Opin. Plant Biol. 7, 414-421. [DOI] [PubMed] [Google Scholar]
- 31.Felix, G., Regenass, M. & Boller, T. (1993) Plant J. 4, 307-316. [Google Scholar]
- 32.Minami, E., Kouchi, H. Carlson, R. W., Cohn, J. R., Kolli, V. K., Day, R. B., Ogawa, T. & Stacey, G. (1996) Mol. Plant–Microbe Interact. 9, 574-583. [DOI] [PubMed] [Google Scholar]
- 33.Walker, S. A., Viprey, V. & Downie, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 13413-13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dénarié, J., Debelle, F. & Promé, J. C. (1996) Annu. Rev. Biochem. 65, 503-535. [DOI] [PubMed] [Google Scholar]
- 35.Bird, D. McK. (1996) J. Parasitol. 82, 881-888. [PubMed] [Google Scholar]
- 36.Hussey, R. S. (1989) Annu. Rev. Phytopathol. 27, 123-141. [Google Scholar]
- 37.Niebel, A., Barthels, N., de Almeida-Engler, J., Karimi, M., Vercauteren, I., van Montague, M. & Gheysen, G. (1994) in Advances in Molecular Plant Nematology, eds. Lamberti, F., De Giorgi, C. & Bird, D. McK. (Plenum, New York), pp. 161-170.
- 38.Kosuta, S., Chabaud, M., Lougnon, G., Gough, C., Dénarié, J., Barker, D. G. & Becard, G. (2003) Plant Physiol. 131, 952-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buee, M., Rossignol, M., Jauneau, A., Ranjeva, R. & Becard, G. (2000) Mol. Plant–Microbe Interact. 13, 693-698. [DOI] [PubMed] [Google Scholar]
- 40.Lévy, J., Bres, C., Geurts, R., Chalhoub, B., Kulikova, O., Duc, G., Journet, E., Ané, J. M., Lauber, E., Bisseling, T., et al. (2004) Science 303, 1361-1364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.