Abstract
Purpose
To compare several contemporary urodynamic criteria for diagnosing detrusor underactivity (DU) and estimate how well they coincide with each other.
Materials and Methods
From our prospective urodynamic database we identified nonneurogenic lower urinary tract symptoms (LUTS) patients older than 60 years between 2003 and 2014. Patients were reclassified based on four and three contemporary criteria for DU among men and women. Each criterion was compared with the others using the McNemar test.
Results
Urodynamic data of 4,372 patients (3,357 men and 1,015 women) were analyzed. In men, the prevalence of DU was estimated to be 56%, 17%, 5%, and 10% based on bladder contractility index, Abrams-Griffith number, maximal detrusor pressure at maximal flow rate (PdetQmax) 30, and bladder voiding efficiency (BVE) criteria. In women, 14.9%, 9.6%, and 6.4% of patients were classified as having DU based on maximal flow rate/postvoid residual (Qmax/PVR), PdetQmax 30, and BVE criteria. For individual subjects, all 4 criteria for men were significantly different from each other, while PdetQmax 30 and BVE criteria for women did not differ significantly (p=0.065). Additionally, BVE criterion for men and PdetQmax 30 and BVE criteria for women could distinguish the differences of patient age, free Qmax and free PVR between patient with and without DU.
Conclusions
Each urodynamic criterion for men does not coincide with each other in the diagnosis of DU within individual subjects. On the other hand, PdetQmax 30 criteria and BVE criteria for women could be appropriately applied to clinical practice when diagnosing DU in women with LUTS.
Keywords: Detrusor underactivity, Diagnosis, Urodynamics
INTRODUCTION
The most of clinical conditions causing lower urinary tract symptoms (LUTS) in the elderly are ascribed to an overactive bladder or stress urinary incontinence in women, and benign prostatic hyperplasia in men. On the other hand, another age-related change in the lower urinary tract, detrusor underactivity (DU) has been recognized in 10% to 48% of the community-dwelling elderly [1] and becomes more prevalent with patient age [2].
DU is defined as a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying within a normal time span by the International Continence Society (ICS) in 2003 [3]. However, despite its obvious prevalence, DU has been underresearched on the whole and the diagnosis and clinical implications of DU in the elderly population remain largely unknown since the ICS published its definition more than a decade ago. This is because the standard diagnostic criteria have not been developed and proper management of this condition has not been met in clinical practice.
As for the diagnostic criteria of DU, unlike an overactive bladder, most of published studies used only the urodynamic definition because clinical symptoms and other characteristics are not different between subjects with and without DU [2]. The majority of published criteria concentrate on detrusor strength with the combinations of maximal flow rate (Qmax) and maximal detrusor pressure at Qmax (PdetQmax) [1,2,4,5,6], resulting in incomplete consequence with regard to definitional perspective. Detrusor activity during the voiding phase is affected by contraction speed and duration as well as contraction strength. At present, some other parameters such as postvoid residual (PVR) volume [7] or bladder voiding efficiency (BVE: voided volume/[voided volume+PVR volume]×100%) [8] has been added to the urodynamic measures of DU, but the clinical usefulness of these ancillary parameters in the diagnosis of DU has not been proven.
In the present study, we compared several contemporary urodynamic criteria for diagnosing DU published in the literature using the large-scale urodynamic database registry from SEOUL (SNU-Experts-Of-Urodynamics-Leading) Study group and estimated how well they coincide with each other and their clinical significance. To the best of our knowledge, this study is the first to compare the published criteria of DU with respect to their clinical concordance and implications.
MATERIALS AND METHODS
1. Patients and clinical data
A database of a consecutive series of patients with LUTS aged ≥60 years who received an urodynamic study between 2003 and 2014 was created from the urodynamic database registry of Seoul National University Hospital and Seoul National University Bundang Hospital (the SEOUL Study Group). The Institutional Review Board of the institutions approved the study protocol based on the Declaration of Helsinki and obtaining the informed consent was waived upon consideration of the retrospective analyses of the database (approval number: B-1608/358-104 and J-1701-058-823). All personal identifiers were eliminated from the database and all data were anonymously analyzed.
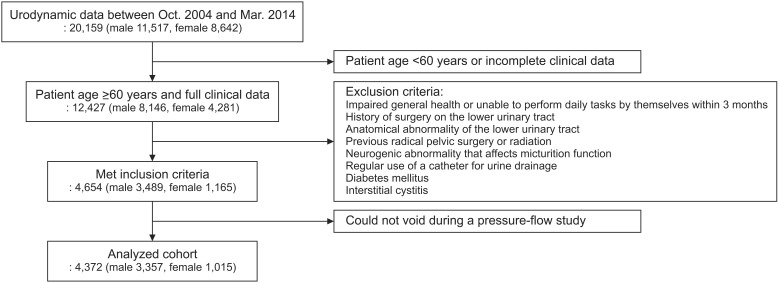
Fig. 1 shows the patient selection process from database registry. After excluding patients with suspected neurological or anatomical conditions, history of surgery or radiation therapy on the lower urinary tract/pelvic cavity, impaired general health or unable to perform daily tasks by themselves within 3 months, regular use of a catheter for urine drainage, diabetes mellitus or interstitial cystitis, 4,654 patients were enrolled in the initial analyses.
Fig. 1. Flow chart for patient selection process from urodynamic database registry.
In practice, patients presenting with LUTS provided a detailed history of LUTS and underwent physical examination, free uroflowmetry and PVR measurement. Also, they documented an International Prostate Symptom Score and a 3-day frequency-volume chart. A free uroflowmetry (DABA, Endo tech, Seongnam, Korea) along with a measurement of PVR volume (BladderScan BVI-3000, Diagnostic Ultrasound, Bothell, WA, USA) were performed prior to urodynamic evaluation. The result with a higher Qmax was chosen from 2 sets of uroflowmetry with a voided volume over 150 mL.
2. Urodynamic evaluation
Urodynamic procedures were in accordance with the guidelines of the ICS [9] and a multichannel urodynamic study (UD-2000, Medical Measures Systems B. V., Enschede, the Netherlands), including a pressure-flow study (PFS), was conducted following the discontinuation of the potential medications that could possibly affect detrusor function for at least 3 days. Pelvic electromyography was performed with surface electrodes attached near the anus at the 3 and 9 o′clock positions. A 6-Fr double-lumen and a 9-Fr balloon catheters were used in all of the urodynamic studies in the measurement of the intravesical and abdominal pressures. Intravesical pressure was determined under conditions of room-temperature saline infusion at 20–50 mL/min depending on the bladder condition. Detrusor compliance was recorded as an actual value (Δinfusion volume/Δdetrusor pressure mL/cmH2O) and was considered impaired when the ΔV/Δpdet was ≤20 mL/cmH2O. Detrusor overactivity was regarded as positive when spontaneous or provoked involuntary detrusor contraction was observed in the filling cystometry regardless of urine leakage [9].
During a PFS, the patient was instructed to void in a standing or sitting position under quiet and relaxed circumstances. If the first voiding trial failed, additory trials were conducted to allow for the probability that the failure was from cortical inhibition. However, if patients were unable to generate measurable urine flow, the events were documented and they were excluded from the analyses in spite of the presence of detrusor contraction (n=282). The degree of bladder outlet obstruction (BOO) during voiding assessed by Abrams-Griffith (AG) number [10] and a formulated bladder contractility index (BCI) [4] were applied to only male patients in the present study.
3. Criteria of DU
Previously proposed urodynamic criteria for diagnosing DU in the literature are summarized in Table 1. For male patients, 4 criteria were applied, including (1) BCI <100 (BCI criteria) [4], (2) AG number<20 and free uroflow Qmax<12 mL/s (AG number criteria) [5], (3) PdetQmax<30 cmH2O and PFS Qmax<10 mL/s (PdetQmax 30 criteria) [6] and (4) BCI<100 and AG number<20 and BVE%<90 (BVE criteria) [8] while female patients were classified based on 3 criteria for DU, including (1) Qmax<12 mL/s with ≥100 mL voided or PVR volume>150 mL on 2 or more free flow readings (Qmax/PVR criteria) [7], (2) PdetQmax<30 cmH2O and PFS Qmax<10 mL/s (PdetQmax 30 criteria) [6] and (3) PdetQmax<20 cmH2O and PFS Qmax<15 mL/s and BVE%<90 and absence of clinical obstruction (BVE criteria) [8]. All patients were reclassified and analyzed based on 4 and 3 contemporary criteria for DU among men and women.
Table 1. Previously proposed urodynamic criteria for diagnosing detrusor underactivity in the literature.
| Study | Target population | Diagnostic criteria | Byname in the present study |
|---|---|---|---|
| Abrams (1999) [4] | Male | BCI <100 | BCI criteria |
| Nitti et al. (2002) [5] | Male | AG number<20 and free uroflow Qmax<12 mL/s | AG number criteria |
| Abarbanel and Marcus (2007) [6] | Male | PdetQmax<30 cmH2O and PFS Qmax<10 mL/s | PdetQmax 30 criteria |
| Gammie et al. (2016) [8] | Male | BCI<100 and AG number<20 and BVE%<90 | BVE criteria |
| Groutz et al. (1999) [7] | Female | Qmax<12 mL/s with ≥100 mL voided or PVR volume>150 mL on 2 or more free flow readings | Qmax/PVR criteria |
| Abarbanel and Marcus (2007) [6] | Female | PdetQmax<30 cmH2O and PFS Qmax<10 mL/s | PdetQmax 30 criteria |
| Gammie et al. (2016) [8] | Female | PdetQmax<20 cmH2O and PFS Qmax<15 mL/s and BVE%<90 and absence of clinical obstruction | BVE criteria |
BCI, bladder contractility index; AG number, Abrams-Griffith number; Qmax, maximum flow rate; PdetQmax, detrusor pressure at maximal flow rate; PFS, pressure-flow study; BVE, bladder voiding efficiency; PVR, postvoid residual.
4. Statistical analysis
The collected data are presented as mean±standard deviation or as a percentage. The linear by linear association analysis for categorical variables and the Student t-test for continuous variables were used to identify the significance between subjects with and without DU. In addition, the McNemar test was used to assess how well each criteria for diagnosing DU correlated with each other. Last, to determine the clinical significance of each criteria, we compared patient age, free Qmax and free PVR volume between patients with and without DU according to each criteria. All statistical analyses were conducted with IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) and MedCalc version 9.6 (MedCalc, Ostend, Belgium) and a 2-tailed p-value <0.05 was determined to indicate statistical significance.
RESULTS
A total of 4,372 patients (men, 3,357; women, 1,015) were included in the final cohort after excluding those who could not void during a PFS. Table 2 depicts patient demographic and urodynamic characteristics. The mean age was 69.3 and 68.1 years and patients ≥70 years accounted for 45% and 38% for men and women, respectively. In a cystometry, 45% and 38% of men and women presented with detrusor overactivity and 7.7% and 6.8% had impaired detrusor compliance.
Table 2. Patient demographic and urodynamic characteristics.
| Characteristic | Men | Women |
|---|---|---|
| No. of patients | 3,357 (76.8) | 1,015 (23.2) |
| Age (y) | ||
| 60–64 | 789 (23.5) | 327 (32.2) |
| 65–69 | 1,055 (31.4) | 301 (29.7) |
| 70–74 | 874 (26.0) | 245 (24.1) |
| 75–79 | 467 (13.9) | 106 (10.4) |
| ≥80 | 172 (5.1) | 36 (3.5) |
| Year of performing an urodynamic test | ||
| 2003–2005 | 337 (10.0) | 191 (18.8) |
| 2006–2008 | 903 (26.9) | 463 (45.6) |
| 2009–2011 | 1,000 (29.8) | 220 (21.7) |
| 2012–2014 | 1,117 (33.3) | 141 (13.9) |
| Body mass index (kg/m2) | 24.0±2.9 | 24.3±3.0 |
| Free uroflowmetry | ||
| Qmax (mL/s) | 9.7±5.8 | 16.0±10.1 |
| Voided volume (mL) | 172.8±119.0 | 191.8±137.6 |
| PVR volume (mL) | 57.2±86.8 | 39.8±72.6 |
| Bladder sensation and capacity during filling CMG | ||
| FDV (mL) | 265.0±105.4 | 254.0±104.9 |
| SDV (mL) | 333.4±112.0 | 313.4±112.7 |
| MCC (mL) | 363.9±121.5 | 367.6±111.9 |
| Involuntary detrusor contraction | 1,510 (45.0) | 385 (38.0) |
| Bladder compliance | ||
| ≤20 mL/cmH2O | 258 (7.7) | 69 (6.8) |
| Pressure-flow study | ||
| Qmax (mL/s) | 9.4±5.4 | 17.9±9.0 |
| Pdet open (cmH2O) | 52.9±26.2 | 20.7±16.9 |
| PdetQmax (cmH2O) | 52.6±23.3 | 24.3±19.6 |
| Pdet clos (cmH2O) | 35.8±17.8 | 18.3±13.6 |
| Bladder contractility index | 99.5±31.4 | - |
| AG number | 33.7±27.9 | - |
Values are presented as number (%) or mean±standard deviation.
Qmax, maximal flow rate; PVR, postvoid residual; CMG, cystometry; FDV, first desire to void; SDV, strong desire to void; MCC, maximum cystometric capacity; Pdet open, opening detrusor pressure; PdetQmax, detrusor pressure at maximal flow rate; Pdet clos, closing detrusor pressure; AG number, Abrams-Griffith number.
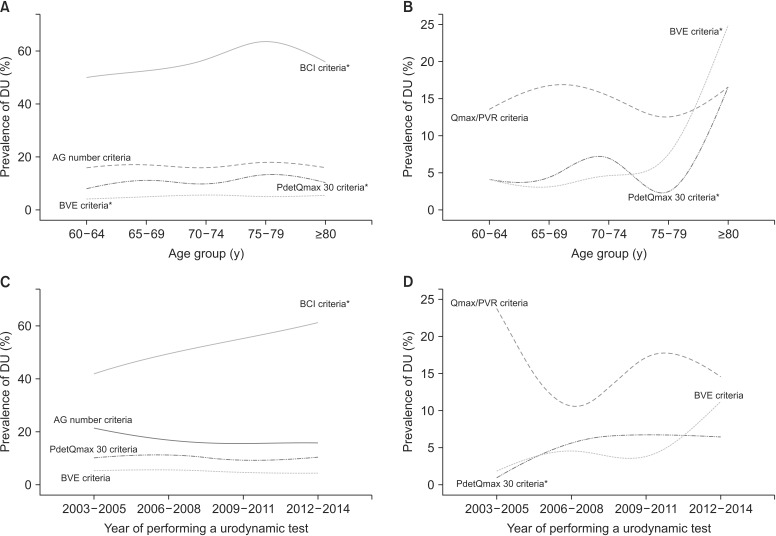
In men, a total of 1,873 patients (55.8%) had DU by BCI criteria, 557 (16.6%) had DU by AG number criteria, 181 (5.4%) had DU by PdetQmax 30 criteria and 345 (10.3%) had DU by BVE criteria (Table 3). For women, 151 patients (14.9%) had DU by Qmax/PVR criteria, 97 (9.6%) had DU by PdetQmax 30 criteria and 65 (6.4%) had DU by BVE criteria. The prevalence of DU significantly increased with patient age in all criteria except AG number criteria for men and Qmax/PVR criteria for women and was consistent across the years in most of the criteria (Fig. 2).
Table 3. Prevalence of detrusor underactivity in the present cohort with nonneurogenic lower urinary tract symptoms based on each urodynamic criteria.
| Urodynamic criteria | No. (%) |
|---|---|
| Men (n=3,357) | |
| BCI criteria | 1,873 (55.8) |
| AG number criteria | 557 (16.6) |
| PdetQmax 30 criteria | 181 (5.4) |
| BVE criteria | 345 (10.3) |
| Women (n=1,015) | |
| Qmax/PVR criteria | 151 (14.9) |
| PdetQmax 30 criteria | 97 (9.6) |
| BVE criteria | 65 (6.4) |
BCI, bladder contractility index; AG number, Abrams-Griffith number; Qmax, maximal flow rate; PdetQmax, detrusor pressure at Qmax; BVE, bladder voiding efficiency; PVR, postvoid residual.
Fig. 2. Prevalence of detrusor underactivity by age group and year of performing an urodynamic study. (A) In men, the prevalence of detrusor underactivity significantly increased with patient age in BCI criteria (p<0.001), PdetQmax 30 criteria (p=0.029), and BVE criteria (p=0.042). (B) In women, the prevalence of detrusor underactivity significantly increased with patient age in PdetQmax 30 criteria (p=0.004) and BVE criteria (p<0.001). (C) In men, the prevalence of detrusor underactivity was consistent across the years in most of the criteria except BCI criteria (p<0.001). (D) In women, the prevalence of detrusor underactivity was consistent across the years except in PdetQmax 30 criteria (p=0.005). *indicates the statistical significance. DU, detrusor underactivity; BCI, bladder contractility index; AG number, Abrams-Griffith number; Qmax, maximal flow rate; PdetQmax, detrusor pressure at Qmax; BVE, bladder voiding efficiency; PVR, postvoid residual.
Individual criteria were compared as the standard with the others (Table 4). For the diagnosis of DU in individual subjects, all 4 criteria for men were significantly different from each other, while PdetQmax 30 criteria and BVE criteria for women did not differ from each other (p=0.065). BCI criteria for men tended to overestimate DU compared with the others.
Table 4. Statistical comparisons of each urodynamic criteria for the diagnosis of detrusor underactivity.
| Criteria | No. of positive | Concordance of each criteria | Positive fraction ratio | p-value | |
|---|---|---|---|---|---|
| No. of positive | No. of negative | ||||
| Men | |||||
| BCI criteria vs. | |||||
| AG number criteria | 557 | 371 | 1,336 | 3.36 | <0.001 |
| PdetQmax 30 criteria | 181 | 181 | 1,482 | 10.35 | <0.001 |
| BVE criteria | 345 | 344 | 1,520 | 5.43 | <0.001 |
| AG number criteria vs. | |||||
| BCI criteria | 1,873 | 371 | 1,336 | 0.3 | <0.001 |
| PdetQmax 30 criteria | 181 | 114 | 2,745 | 3.08 | <0.001 |
| BVE criteria | 345 | 253 | 2,708 | 1.61 | <0.001 |
| PdetQmax 30 criteria vs. | |||||
| BCI criteria | 1,873 | 181 | 1,482 | 0.1 | <0.001 |
| AG number criteria | 557 | 114 | 2,745 | 0.32 | <0.001 |
| BVE criteria | 345 | 98 | 2,946 | 0.52 | <0.001 |
| BVE criteria vs. | |||||
| BCI criteria | 1,873 | 344 | 1,520 | 0.18 | <0.001 |
| AG number criteria | 557 | 253 | 2,708 | 0.62 | <0.001 |
| PdetQmax 30 criteria | 181 | 98 | 2,946 | 1.91 | <0.001 |
| Women | |||||
| Qmax/PVR criteria vs. | |||||
| PdetQmax 30 criteria | 97 | 9 | 822 | 1.56 | <0.001 |
| BVE criteria | 65 | 13 | 829 | 2.32 | <0.001 |
| PdetQmax 30 criteria vs. | |||||
| Qmax/PVR criteria | 151 | 9 | 822 | 0.64 | <0.001 |
| BVE criteria | 65 | 22 | 886 | 1.49 | NS |
| BVE criteria vs. | |||||
| Qmax/PVR criteria | 151 | 13 | 829 | 0.43 | <0.001 |
| PdetQmax 30 criteria | 97 | 22 | 886 | 0.67 | NS |
BCI, bladder contractility index; AG number, Abrams-Griffith number; Qmax, maximal flow rate; PdetQmax, detrusor pressure at Qmax; BVE, bladder voiding efficiency; PVR, postvoid residual; NS, not significant.
In the assessment of the clinical significance of each criteria with discernment ability among patient age, free Qmax and free PVR volume, only BVE criteria for men and both of PdetQmax 30 criteria and BVE criteria for women could distinguish the differences of these clinical parameters between patient with and without DU (Table 5).
Table 5. Comparisons of age and cystometric characteristics between patients with and without detrusor underactivity in each criteria.
| Parameter | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| BCI | AG number | PdetQmax 30 | BVE | Qmax/PVR | PdetQmax 30 | BVE | |
| Age (y) | 69.7 vs. 68.8 | - | 70.3 vs. 69.2 | 69.9 vs. 69.0 | - | 69.6 vs. 67.9 | 70.6 vs. 67.5 |
| p-value | <0.001 | - | 0.011 | 0.015 | - | 0.007 | <0.001 |
| Free Qmax (mL/s) | 8.7 vs. 11.0 | 7.1 vs. 10.2 | - | 8.8 vs. 9.8 | 12.0 vs. 23.0 | 9.9 vs. 16.6 | 10.9 vs. 16.4 |
| p-value | <0.001 | <0.001 | - | <0.001 | <0.001 | <0.001 | <0.001 |
| Free PVR (mL) | - | 56.9 vs. 42.2 | 74.4 vs. 56.2 | 72.3 vs. 53.4 | 107.2 vs. 28.9 | 69.9 vs. 37.2 | 71.5 vs. 33.7 |
| p-value | - | <0.001 | 0.044 | <0.001 | <0.001 | 0.045 | 0.030 |
Values are presented as mean values in patients with detrusor underactivity vs. without detrusor underactivity.
BCI, bladder contractility index; AG number, Abrams-Griffith number; Qmax, maximal flow rate; PdetQmax, detrusor pressure at Qmax; BVE, bladder voiding efficiency; PVR, postvoid residual.
DISCUSSION
Recent studies on DU in the community-dwelling elderly shows that it may be a common geriatric condition [1,2,4,5,6,7,8], although it has received little scientific attention. In addition, based on our findings, the prevalence of DU increases with age, thus making this condition more important as the pathophysiology of LUTS in the elderly. As the normal aging process, the contraction power of bladder may become impaired with age, and this may result in the development of DU in both sexes. Structural changes of the detrusor muscle, a reduced detrusor to collagen ratio, decreased axonal content and changes in muscarinic receptors are usually associated with the development of DU [11].
To date, there is no widely accepted urodynamic criteria of both sexes but several criteria have been proposed in the literature [1,4,5,6,7,8]. Some criteria are based on the combinations of both Qmax and PdetQmax in a PFS, and others are built upon free Qmax/PVR and BVE, as well as Qmax/PdetQmax. In the current study, we identified how well these criteria correlate with each other and whether they show the clinical significance with regard to the discernment ability among patient age, free Qmax and free PVR volume. We think this approach may develop a comprehensive understanding and lay a foundation for further researches on the clinical diagnosis of DU. The prevalence of DU significantly increased with patient age in all criteria except AG number criteria for men and Qmax/PVR criteria for women and was consistent across the years in most of the criteria. These findings are in concordance with the prior research [2].
Overall, there was considerable variation (5.4%–55.8%) in the diagnosis of DU in individual subjects and all 4 criteria were significantly different from each other when applied for male patients. Particularly, BCI criteria tended to overestimate DU compared with other criteria. The reason for this finding might not be easily explained because other urodynamic criteria also involve one of parameters based on the combinations of both Qmax and PdetQmax from a PFS. However, unlike BCI criteria, other 3 criteria are comprised of 1 or 2 additional parameters such as free/PFS Qmax or BVE% other than sole parameters. Especially, BCI has been criticized that it does not consider conceptually the coexistence of DU and BOO [1]. Therefore, a combination of Qmax/PdetQmax from a PFS and other parameters such as free/PFS Qmax or BVE% appears to be the best approach to diagnose DU for men, instead of application of the sole parameters. As for the discernment ability of 3 clinical parameters, only BVE criteria could distinguish the differences of all the parameters between men with and without DU. For now, thus, BVE criteria seems to be most appropriate to utilize in clinical practice, albeit more researches on this field needed.
In women, the prevalence of DU ranged from 6.4% to 14.9% according to each urodynamic criteria and PdetQmax 30 criteria and BVE criteria did not differ from each other, showing the significant concordance between the both. In addition, these 2 criteria could distinguish the differences of all the clinical parameters tested between women with and without DU. Therefore, at present, both criteria could be appropriately applied to clinical practice when diagnosing DU in women with LUTS.
Among the criteria tested in the present study, only Qmax/PVR criterion for women is based on the parameters can be obtained from outpatient practice without performing an urodynamic testing. This criterion was identified to be significantly different from other two criteria based on the urodynamic measures for the diagnosis of DU in individual subjects. Considering this and previous research that reported that clinical symptoms and other characteristics were not different between subjects with and without DU [2], no other tools besides urodynamics, at present, could be used to diagnose the present of DU in real practice.
We chose subjects who were 60 years or more and able to perform daily tasks by themselves. All enrolled patients did not have neurogenic abnormalities or diabetes mellitus and all other conditions that could affect the bladder function were eliminated to create the cohort to the community-dwelling elderly, as opposed to the elderly from chronic care facilities where over the two-thirds of the subjects have been identified to have DU [12]. Thus, around 10% of prevalence of DU in both sexes shown in the present study might reflect the prevalence of the real world. If the known conditions that cause DU such as neurogenic abnormalities or diabetes are included in the target population, the prevalence of DU in our study might increase and we could not get enough of the comparisons between each criteria. In addition, most of diagnostic criteria we compared are based on the population without neurogenic bladder components.
Unlike male patients, female patients with DU were shown to have lower maximum cystometric capacity and higher rate of the impaired detrusor compliance than those without DU in our cohort (data not shown), in agreement with our previous findings [2]. Based on our previous report, it may be inferred that DU is related to reduced bladder compliance, subsequently resulting in a lower maximum cystometric capacity within the female elderly, because more women with DU also had reduced compliance compared with those without DU [2]. However, as multifactorial factors may be involved for to the development of DU in men and women, it is not easy to speculate about the reasons for the differences of pathophysiologic processes of DU between men and women at present.
The current study has some limitations. First, lack of consensus on urodynamic definitions of DU presents a challenge in clinical studies on DU although the only accepted modality for diagnosing DU is an urodynamic study. As previously noted, each criteria are quite variable from study to study [1,4,5,6,7,8] and there are a lot of opinions debating on whether it is best to focus on the strength, speed, or sustainability of detrusor contractility [1]. Second, we did not classify the clinical symptoms regarding LUTS. Although we previously reported that types of LUTS were not significantly different between patients with and without DU in either sex [2], a recent study has shown that there are signs and symptoms that can distinguish men and women patients with DU from patients with either normal urodynamic studies or with BOO. Thus, further studies on this issue may provide useful information about the clinical diagnosis of DU. Furthermore, all patients were referred for detailed assessment of lower urinary tract function as they might not respond to initial treatments, which could resulting in selection bias.
At present, DU remains a poorly established and unclearly understood bladder dysfunction. In addition it is not easy to identify the index patient as multifactorial factors can contribute to the development of DU. We hope our study will help promote more researches on this condition and make further consensus and refinement on the diagnostic criteria of DU.
CONCLUSIONS
DU is an important part of the pathophysiologies of LUTS in the elderly population, with the substantial prevalence. The present study demonstrates that each urodynamic criteria for men shows considerable variation in the diagnosis of DU in individual subjects and especially BCI criteria tend to overestimate DU compared with the others. On the other hand, PdetQmax 30 criteria and BVE criteria for women show the significant concordance and could be appropriately applied to clinical practice when diagnosing DU in women with LUTS. We believe that our study is a first step to improve the comprehensive approach to this poorly understood clinical condition.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389–398. doi: 10.1016/j.eururo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012;53:342–348. doi: 10.4111/kju.2012.53.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 4.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14–15. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 5.Nitti VW, Lefkowitz G, Ficazzola M, Dixon CM. Lower urinary tract symptoms in young men: videourodynamic findings and correlation with noninvasive measures. J Urol. 2002;168:135–138. [PubMed] [Google Scholar]
- 6.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007;69:436–440. doi: 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Groutz A, Gordon D, Lessing JB, Wolman I, Jaffa A, David MP. Prevalence and characteristics of voiding difficulties in women: are subjective symptoms substantiated by objective urodynamic data? Urology. 1999;54:268–272. doi: 10.1016/s0090-4295(99)00097-7. [DOI] [PubMed] [Google Scholar]
- 8.Gammie A, Kaper M, Dorrepaal C, Kos T, Abrams P. Signs and symptoms of detrusor underactivity: an analysis of clinical presentation and urodynamic tests from a large group of patients undergoing pressure flow studies. Eur Urol. 2016;69:361–369. doi: 10.1016/j.eururo.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 10.Tubaro A, La Vecchia C Uroscreening Study Group. The relation of lower urinary tract symptoms with life-style factors and objective measures of benign prostatic enlargement and obstruction: an Italian survey. Eur Urol. 2004;45:767–772. doi: 10.1016/j.eururo.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Hotta H, Morrison JF, Sato A, Uchida S. The effects of aging on the rat bladder and its innervation. Jpn J Physiol. 1995;45:823–836. doi: 10.2170/jjphysiol.45.823. [DOI] [PubMed] [Google Scholar]
- 12.Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. N Engl J Med. 1989;320:1–7. doi: 10.1056/NEJM198901053200101. [DOI] [PubMed] [Google Scholar]