Several studies have demonstrated a relationship between the density of available striatal D2/D3 receptors and trait impulsiveness. However, in each case, the availability of dopamine receptors was measured during the resting state. This complicates interpretation of previously observed correlations, which could be influenced by either stable variation in receptor density or context-dependent dopamine release. We present evidence uniquely consistent with the former interpretation, providing clarity to the nature of this brain-behavior relationship.
Keywords: dopamine, positron emission tomography, impulsiveness, attention
Abstract
The density (measured at binding potential) of available striatal D2/D3 receptors has been shown to predict trait impulsiveness. This relationship is highly robust and well replicated. In each case, however, the availability of dopamine receptors was measured at rest. More broadly, the extent to which relationships between dopamine receptor availability and behavioral traits hold when participants perform a cognitive task is unclear. Furthermore, the performance of a cognitive task engages fundamentally different neural networks than are maximally engaged during the resting state. This complicates interpretation of previously observed correlations, which could be influenced by two distinct factors. The first is variation in available receptor density, which reflects a stable trait of the individual. The second is variation in context-specific dopamine release, which differentially displaces some dopamine radiotracers (such as raclopride) across individuals. Using an existing data set, we related trait impulsiveness, as measured using the Barratt Impulsiveness Scale (BIS-11), to the density (binding potential) of available striatal D2/D3 receptors as measured using positron emission tomography (PET) with [11C]raclopride. Importantly, the PET scan was completed while participants performed an attention-demanding visual search task. We replicate robust correlations between this measure of receptor availability and trait impulsiveness previously demonstrated during the resting state, extending this relationship to periods of active task engagement. Our results support the idea that this relationship depends on striatal D2/D3 receptor density and not on context-dependent dopamine release.
NEW & NOTEWORTHY Several studies have demonstrated a relationship between the density of available striatal D2/D3 receptors and trait impulsiveness. However, in each case, the availability of dopamine receptors was measured during the resting state. This complicates interpretation of previously observed correlations, which could be influenced by either stable variation in receptor density or context-dependent dopamine release. We present evidence uniquely consistent with the former interpretation, providing clarity to the nature of this brain-behavior relationship.
understanding the neurobiology underlying variation in behavioral traits is a fundamental issue in the field of human cognitive neuroscience. One important predictor that has arisen from research on this topic is receptor density within neurotransmitter systems as measured using positron emission tomography (PET). The availability of striatal D2/D3 receptors in particular has been linked to a variety of behavioral measures (e.g., Kim et al. 2011; Kohno et al. 2015; Robertson et al. 2015; Volkow et al. 2003). One of the most consistently replicated of these measures is impulsiveness, which is negatively correlated with the density of available D2/D3 receptors within the striatum (e.g., Buckholtz et al. 2010; Lee et al. 2009; see Dalley et al. 2007 for an analogous finding using a rat model of impulsiveness).
In prior PET studies relating D2/D3 receptor availability to impulsiveness (Buckholtz et al. 2010; Dalley et al. 2007; Lee et al. 2009), as is common in the PET literature on individual differences (e.g., Kim et al. 2011; Kohno et al. 2015; Robertson et al. 2015; Volkow et al. 2003), the PET measurements were acquired while participants were in a resting state, without performing a particular cognitively demanding task. This complicates interpretation of the observed correlations, which could be influenced by two distinct factors. The first is variation in receptor density, which reflects a stable trait of the individual. The second is variation in context-specific neurotransmitter release, which differentially displaces the radiotracer across individuals.
This complexity is made especially salient by findings that a distinct neural network is active while participants are in a resting state, what has come to be referred to as the default mode network (DMN; Raichle et al. 2001; Shulman et al. 1997). The DMN can be contrasted with task-positive networks—that is, networks preferentially engaged in the service of performing a cognitive operation or task, such as the dorsal and ventral attention networks (Corbetta and Shulman 2002; Corbetta et al. 2008). Abnormal functioning of the DMN has been linked to impulsiveness and related attention deficit/hyperactivity disorder symptomology (e.g., Broyd et al. 2009; Shannon et al. 2011). During the resting state, therefore, individual differences in the availability of D2/D3 receptors could reflect individual differences in the engagement of the DMN and related neural processing, in addition to the raw density of receptors in a particular brain region. Context-dependent changes in D2/D3 receptor availability have also been linked to behavioral traits (e.g., Anderson et al. 2016).
In light of this complexity, it would be useful to examine whether the relationship between the density of available striatal D2/D3 receptors and impulsiveness extends to situations in which the PET measurements are acquired while participants perform an attention-demanding task. A replication of a predictive relationship under these conditions would suggest that this relationship is robust to task-dependent changes in neural dynamics associated with the engagement of task-positive brain networks, reflecting individual differences in the actual density of available D2/D3 receptors rather than differences in context-dependent levels of endogenous dopamine (DA).
In a prior PET study using [11C]raclopride, we compared the density of available D2/D3 receptors, measured from binding potential (BPND), across two scans in which participants performed a baseline attention task and an otherwise identical attention task that included previously reward-associated distractors (Anderson et al. 2016). Differences in the binding potential of [11C]raclopride between scans for each person were used to assess changes in endogenous DA levels attributable to the presence of the distractors. Importantly, the Barratt Impulsiveness Scale (BIS-11; Patton et al. 1995), scores on which have previously been linked to D2/D3 receptor availability as measured using PET (e.g., Buckholtz et al. 2010; Lee et al. 2009), was administered as a part of a standard assessment battery during screening, and was not analyzed in our prior study. In the present study, we related this measure of impulsiveness to individual differences in the availability of D2/D3 receptors as measured during performance of the baseline attention task, which comprised visual search for a shape-defined target (see Fig. 1). For the sake of comparison, we also related trait impulsiveness to the task-dependent difference in the availability of D2/D3 receptors resulting from the reward manipulation (i.e., reward-related DA release, the main measure of interest in Anderson et al. 2016).
Fig. 1.
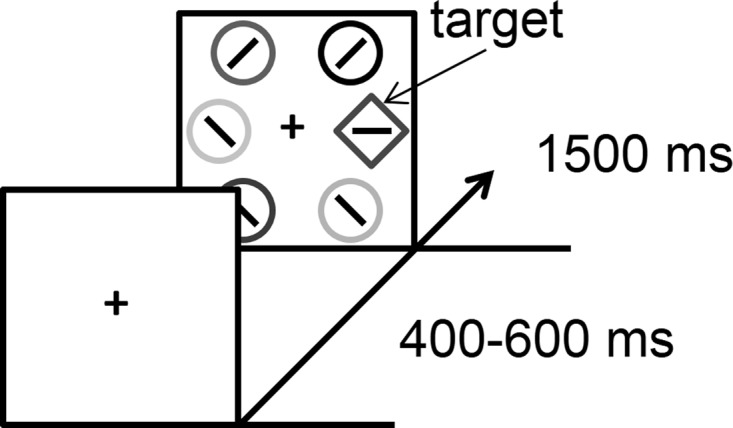
Time course and trial events for the attention task performed during the PET scan. Note that the stimuli are shown in grayscale in the figure, but each stimulus was rendered in a unique color in the actual experiment.
MATERIALS AND METHODS
Participants
Twenty (10 female) healthy adult volunteers (18–31 yr of age, mean = 23.4 yr) who were free of medical or neuropsychiatric disorders participated in the experiment. Screening criteria included a negative drug test and the exclusion of major medical or neuropsychiatric disorders past or present. All participants received a detailed physical exam including vital signs, 12-lead ECG, blood for complete blood count with differential, complete metabolic panel, blood clotting parameters, and creatinine (CPK) for muscle toxicity, urine for urinalysis, and toxicology for drugs of abuse and alcohol breathalyzer before the PET scans. Informed consent was obtained from all participants, and all procedures were approved by the Institutional Review Board of the Johns Hopkins University School of Medicine and conformed to the principles outlined in the Declaration of Helsinki. Data for one participant were not analyzed because of technical problems with presenting the experimental task.
BIS-11
Participants completed the BIS-11 during screening, 6–61 days before the PET scans (mean = 30 days). As in prior studies (Buckholtz et al. 2010; Lee et al. 2009), we extracted the total score as a measure of trait impulsiveness.
Attention Task
Analyses focused on the PET scan during which participants performed the baseline attention task, which comprised visual search for a shape-defined target among neutral nontarget stimuli (see Fig. 1). Participants viewed the stimuli on an LCD monitor by using prism mirrors that allow horizontal viewing in the supine position. The experiment was run on a Dell Latitude E6400 computer running MATLAB software with Psychophysics Toolbox extensions (Brainard 1997), and behavioral responses were made with the use of a modified keyboard with all keys except “z” and “m” removed.
Each of 1,200 trials consisted of a fixation display (400–600 ms), a search array (1,500 ms), and an intertrial interval during which the fixation cross was visible for 400 ms and then removed for 100 ms. Participants were instructed to search for a uniquely shaped target, either a circle among diamonds or a diamond among circles, and report the orientation of a bar within the target as either vertical or horizontal via a button press (“z” and “m,” respectively).
Each shape in the search array was ~3.4° x 3.4° visual angle in size. The middle of the three shapes on each side of the screen was presented 10° center-to-center from fixation, and the two other circles were presented 8° from the vertical meridian, 6° above and below the horizontal meridian. The six stimuli in the search array were all distinct, salient colors. The target was equally often a diamond and a circle, and appeared in each position equally often, with the order of trials randomized. Participants were provided with a brief rest period every 60 trials.
Acquisition of Neuroimaging Data
MRI.
Anatomical MRI scans were obtained for each participant on a day before PET scanning using a 3T Siemens Trio MRI. A T1-weighted SPGR (spoiled grass sequence; TR = 2,110 ms, TE = 2.7 ms, 0.8-mm cubic voxels) covering the whole brain was used to define volumes of interest (VOIs).
PET.
PET was performed on a high-resolution research tomograph (HRRT) in three-dimensional (3-D) mode with a 2.5-mm resolution at the center of the field of view (Sossi et al. 2005). Approximately 20 mCi of [11C]raclopride were administered intravenously as a bolus injection (mean ± SD injected radioactivity: 19.0 ± 1.6 mCi; mean ± SD injected nonradioactive mass of raclopride: 1.2 ± 0.4 μg). The head was stabilized for both PET and MRI by an individualized thermoplastic mask and Velcro straps. A laser light in the PET scanner was used to line up an axial line on the mask, and the scanner bed and participant head tilt were monitored by the PET technologist for the entire scan.
Definition of VOIs
VOIs were defined from the MRI data using the 3-D interactive segmentation mode of a locally developed VOI-defining tool (VOILand), as previously reported (Oswald et al. 2005), and using published segmentation guidelines (Diedrichsen et al. 2009; Oswald et al. 2005; Yushkevich et al. 2006). Striatal VOIs were then subdivided according to the model advanced by Mawlawi et al. (2001) to the ventral striatum and to the anterior/posterior putamen and caudate nucleus (5 subdivisions per side) using a semiautomated method that incorporated anatomical guidance based on postmortem human materials (Baumann et al. 1999; Oswald et al. 2005). VOIs were transferred from MRI to PET space according to MRI-to-PET coregistration parameters obtained with the coregistration module (Ashburner and Friston 2003) in SPM5 (The Statistical Parametric Mapping 5; The Wellcome Trust Centre for Neuroimaging) and applied to PET frames to obtain regional time (radio-)activity curves (TACs).
Reconstruction of PET Data
As in our prior study (Anderson et al. 2016), emission PET scans were reconstructed using the m9 version of the iterative ordered-subset expectation-maximization algorithm correcting for attenuation, scatter, random events, and dead time (Rahmim et al. 2005) and including interframe head motion correction including transmission-emission alignment for the individual frames (Keller et al. 2012). The radioactivity was corrected for physical decay to the injection time. Reconstructions included dynamic PET frames of 256 (left to right) by 256 (nasion to inion) by 207 (neck to cranium) voxels with 1.22-mm isotropic dimensions. The frame schedules were four 15-s, four 30-s, three 1-min, two 2-min, five 4-min, and twelve 5-min frames.
Data Analysis
The “density of available DA receptors” (i.e., the number of receptors not occupied by endogenous DA and assuming the affinity of the receptors is unchanged across subjects and conditions) nondisplaceable binding potential (BPND; Innis et al. 2007) of [11C]raclopride was obtained by the reference tissue graphical analysis (RTGA; Logan et al. 1996) for striatum subdivisions. Within each of the striatal VOIs, we tested for a correlation (Pearson's r) across participants of the magnitude of an individual's measured nondisplaceable binding potential to the total score on the BIS-11, as has been used in prior PET studies of impulsiveness (e.g., Buckholtz et al. 2010; Lee et al. 2009). Correlations obtained using Pearson's r were further scrutinized via a randomization test in which the probability of each correlation was estimated nonparametrically by randomly shuffling the xy pairings (n = 10,000 iterations) using custom code written in MATLAB.
Reward-Related DA Release
For the sake of comparison, we also correlated trait impulsiveness with a measure of task-dependent, reward-related DA release as reported in our prior study (Anderson et al. 2016). Specifically, we computed the difference in BPND between the scan described above and an otherwise equivalent scan in which one of the nontargets was rendered in the color of a previously reward-associated stimulus (as experienced during a learning task conducted the day before scanning) on 50% of trials. Reward-related DA release was calculated as percent increase or decrease from the baseline scan (see Anderson et al. 2016).
RESULTS
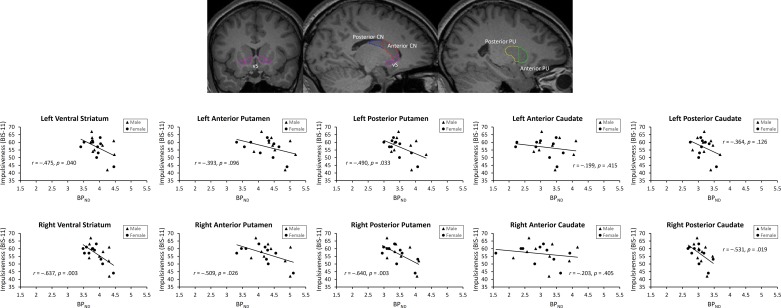
Across all striatal subdivisions, a significant correlation between BPND and trait impulsiveness was observed (r = −0.471, P = 0.042; randomization test: P = 0.020). Within individual subdivisions, significant correlations were observed in bilateral ventral striatum, right anterior putamen, bilateral posterior putamen, and the right posterior caudate (see Fig. 2). In the right ventral striatum and right posterior putamen, these correlations were sufficiently robust to survive Bonferroni correction for multiple comparisons (P values <0.005).
Fig. 2.
Visual depiction of VOIs and observed correlations between trait impulsiveness, as measured using the total score on the BIS-11, and the binding potential of [11C]raclopride (BPND) across VOIs. vS, ventral striatum; CN, caudate nucleus; PU, putamen.
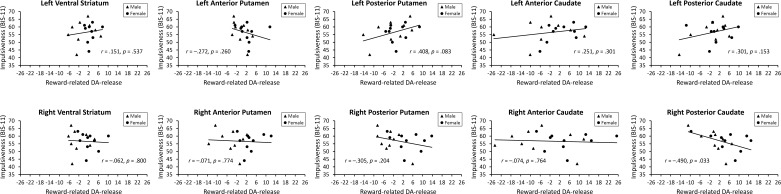
On the other hand, reward-related DA release, a measure of uniquely task-dependent changes in DA receptor availability, was uncorrelated with trait impulsiveness across all striatal subdivisions (r = −0.013, P = 0.958). In no individual subdivision was the correlation significant when corrected for multiple comparisons (P values >0.03, uncorrected; see Fig. 3). Correspondingly, a similar pattern of results was obtained by using BPND from the scan with the reward manipulation rather than the baseline scan (significant correlations in the left and right ventral striatum, left posterior putamen, and left posterior caudate; no significant differences between scans in any VOI, P values >0.12, uncorrected).
Fig. 3.
Observed correlations between trait impulsiveness, as measured using the total score on the BIS-11, and reward-related DA release across VOIs.
DISCUSSION
The results of the present study are straightforward. We replicate a robust relationship between the density of available D2/D3 receptors within the striatum and impulsiveness (Buckholtz et al. 2010; Dalley et al. 2007; Lee et al. 2009), extending this relationship to measurements taken during the performance of an attention-demanding task. Our results demonstrate that this relationship cannot be explained by individual differences in endogenous DA release during the resting state resulting from differential engagement of the default mode network.
Further consistent with this interpretation, between-scan changes in DA release resulting from a reward manipulation were unrelated to trait impulsiveness. This stands in contrast to our prior study using this same data set, in which a behavioral measure of attentional capture was correlated with this exact same measure of task-dependent DA release but not baseline receptor availability (Anderson et al. 2016). This prior result confirms that our experimental approach produces genuine task-related changes in DA release with a range of individual differences, providing a useful basis for comparison to the baseline measure.
It is important to note that impulsiveness was measured in the present study using self-reporting. It is unclear whether the observed relationships would generalize to performance-related measures of impulsiveness such as stop signal reaction time (e.g., Robertson et al. 2015). Our findings provide evidence supporting a stable, task-independent role for the striatal DA system in mediating impulsiveness, likely reflecting individual differences in the measurement of the density of the available DA receptors rather than endogenous DA release, which confirms interpretations of the same relationship that have been observed during the resting state (Buckholtz et al. 2010; Dalley et al. 2007; Lee et al. 2009).
GRANTS
The reported research was supported by National Institutes of Health Grants R01 DA013165 (to S. M. Courtney), S10 RR017219 (to D. F. Wong), and S10 RR023623 (to D. F. Wong). The funding sources played no role in the study beyond financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.A.A. conceived and designed research; B.A.A. performed experiments; B.A.A. and H.K. analyzed data; B.A.A., H.K., D.F.W., and S.M.C. interpreted results of experiments; B.A.A. and H.K. prepared figures; B.A.A. drafted manuscript; B.A.A., H.K., D.F.W., and S.M.C. edited and revised manuscript; B.A.A., H.K., D.F.W., and S.M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We especially thank E. Gean, PhD, J. Roberts, PhD, and Ayon Nandi, MS, of the Wong laboratory, who helped carry out the PET scans, and A. Crabb, MS, and A. Rahmim, PhD, who oversee the HRRT technical scanner reconstructions and physics.
REFERENCES
- Anderson BA, Kuwabara H, Wong DF, Gean EG, Rahmim A, Brašić JR, George N, Frolov B, Courtney SM, Yantis S. The role of dopamine in value-based attentional orienting. Curr Biol 26: 550–555, 2016. doi: 10.1016/j.cub.2015.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid body registration. In: Human Brain Function (2nd ed.), edited by Frackowiak RS, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, and Penny WD. San Diego, CA: Academic, 2003. [Google Scholar]
- Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci 11: 71–78, 1999. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33: 279–296, 2009. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science 329: 532, 2010. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324, 2008. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270, 2007. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46, 2009. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539, 2007. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Keller SH, Sibomana M, Olesen OV, Svarer C, Holm S, Andersen FL, Højgaard L. Methods for motion correction evaluation using 18F-FDG human brain scans on a high-resolution PET scanner. J Nucl Med 53: 495–504, 2012. doi: 10.2967/jnumed.111.095240. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol 87: 164–167, 2011. doi: 10.1016/j.biopsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, Mandelkern MA, London ED. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex 25: 236–245, 2015. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29: 14734–14740, 2009. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16: 834–840, 1996. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057, 2001. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brašić J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 30: 821–832, 2005. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774, 1995. doi:. [DOI] [PubMed] [Google Scholar]
- Rahmim A, Cheng JC, Blinder S, Camborde ML, Sossi V. Statistical dynamic image reconstruction in state-of-the-art high-resolution PET. Phys Med Biol 50: 4887–4912, 2005. doi: 10.1088/0031-9155/50/20/010. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682, 2001. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED. Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci 35: 5990–5997, 2015. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci USA 108: 11241–11245, 2011. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663, 1997. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sossi V, de Jong HW, Barker WC, Bloomfield P, Burbar Z, Camborde M-L, Comtat C, Eriksson LA, Houle S, Keator D, Knob C, Krais R, Lammertsma AA, Rahmim A, Sibomana M, Teras M, Thompson CJ, Trebossen R, Votaw J, Walker M, Wienhard K, Wong DF. The second generation HRRT: a multi-centre scanner performance investigation. 2005 IEEE Nuclear Science Symposium Conference Record, Fajardo, Puerto Rico, October 23–29, 2005, p. 2195–2199. doi: 10.1109/NSSMIC.2005.1596828 [DOI] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, Pappas N. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord 33: 136–142, 2003. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31: 1116–1128, 2006. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]