While the orderly representation of major body parts along the precentral gyrus has been known for decades, questions have been raised about the possible existence of additional more detailed aspects of somatotopy. In this study, we have investigated this question with respect to muscles of the arm and show consistent features of within-arm (intra-areal) somatotopic organization. For the first time we also show maps of how inhibitory output from motor cortex is organized.
Keywords: forelimb, ICMS, mapping motor cortex, EMG
Abstract
Stimulus-triggered averaging (StTA) of forelimb muscle electromyographic (EMG) activity was used to investigate individual forelimb muscle representation within the primary motor cortex (M1) of rhesus macaques with the objective of determining the extent of intra-areal somatotopic organization. Two monkeys were trained to perform a reach-to-grasp task requiring multijoint coordination of the forelimb. EMG activity was simultaneously recorded from 24 forelimb muscles including 5 shoulder, 7 elbow, 5 wrist, 5 digit, and 2 intrinsic hand muscles. Microstimulation (15 µA at 15 Hz) was delivered throughout the movement task and individual stimuli were used as triggers for generating StTAs of EMG activity. StTAs were used to map the cortical representations of individual forelimb muscles. As reported previously (Park et al. 2001), cortical maps revealed a central core of distal muscle (wrist, digit, and intrinsic hand) representation surrounded by a horseshoe-shaped proximal (shoulder and elbow) muscle representation. In the present study, we found that shoulder and elbow flexor muscles were predominantly represented in the lateral branch of the horseshoe whereas extensors were predominantly represented in the medial branch. Distal muscles were represented within the core distal forelimb representation and showed extensive overlap. For the first time, we also show maps of inhibitory output from motor cortex, which follow many of the same organizational features as the maps of excitatory output.
NEW & NOTEWORTHY While the orderly representation of major body parts along the precentral gyrus has been known for decades, questions have been raised about the possible existence of additional more detailed aspects of somatotopy. In this study, we have investigated this question with respect to muscles of the arm and show consistent features of within-arm (intra-areal) somatotopic organization. For the first time we also show maps of how inhibitory output from motor cortex is organized.
somatotopic organization, defined as the orderly and systematic representation of body parts across the cortex, has historically been used to describe the organization of primary motor cortex (Phillips and Porter 1977; Porter and Lemon 1993). Understanding this organization has relevance for understanding the contribution of M1 to voluntary movement. The classic electrical stimulation studies of Penfield and Boldrey (1937) in humans and Woolsey (1958) in monkeys demonstrated the basic plan of motor organization across the precentral gyrus with the foot represented most medially, followed by the trunk, then the upper extremity and hand, and finally the face most laterally. To represent this organization, the raw data consisting of cortical sites stimulated and corresponding evoked movements was integrated into composite figurine maps of the motor representation of body parts as a continuous map across the precentral gyrus in which the sizes of body parts were drawn in relation to the amount of cortical tissue devoted to them. This distorted anatomical depiction is termed a homunculus for humans and simunculus for monkeys. With advances in electrophysiological techniques, mapping studies have further refined our understanding of the organization within motor cortex. Studies of movements evoked with high-frequency, short-duration (HFSD) intracortical microstimulation (ICMS; HFSD-ICMS) have suggested that the forelimb representation is organized as concentric rings where the central core of distal forelimb muscle representation is surrounded by a proximal muscle representation (Kwan et al. 1978a, 1978b; Strick and Preston 1978, 1982). More recently, using stimulus-triggered averaging (StTA or single-pulse ICMS) of electromyographic (EMG) activity from 24 simultaneously recorded forelimb muscles, we reported a distinct zone of proximal and distal muscle cofacilitation separating a horseshoe-shaped proximal zone surrounding a core distal forelimb muscle representation (Park et al. 2001). This horseshoe-shaped proximal representation surrounding a core distal representation parallels the maps of proximal and distal forelimb representation reported by He et al. (1993), Fig. 18) based on retrograde tracer labeling of corticospinal neurons. Features of this upper extremity representation within motor cortex have also been confirmed in humans based on functional magnetic resonance imaging (fMRI) work by Graziano and colleagues (Meier et al. 2008). Together these studies support the existence of a somatotopic organization that goes beyond the well-known basic representation of major body parts described above as the simunculus to include consistent features in the representation of muscles within a major body part (the forelimb) referred to as the intra-areal representation (Schieber 2001).
While our previous work (Park et al. 2001) lumped muscles together as either distal or proximal, the goal of this study was to systematically map the representation of numerous individual forelimb muscles within M1 cortex to further delineate the extent of intra-areal somatotopic organization. Another goal of particular interest was the location of individual muscle maps relative to the division of M1 into rostral and caudal zones. Rathelot and Strick (2006, 2009) found that corticomotoneuronal (CM) cells with monosynaptic connections to motoneurons based on the timing of virus labeling, were almost exclusively located in the anterior bank of the precentral gyrus (caudal M1, cM1) whereas rostral M1 (rM1) contained very few (4–10%) CM cells. Yet another goal of the study was to map, for the first time, the inhibitory representation of forelimb muscles in M1 for comparison with excitatory maps obtained in the same animals. We should emphasize that the data set for this study is the same as that in two previous publications (Park et al. 2001, 2004).
METHODS
Behavioral task.
Data were collected from the left M1 of two male rhesus monkeys (Macaca mulatta; ~9 kg, 6 yr old). The monkeys were trained to perform a reach-to-grasp task requiring coactivation of multiple proximal and distal forelimb muscles in natural, functional synergies. Training procedures and the behavioral task have been described in detail previously (Belhaj-Saïf et al. 1998; McKiernan et al. 1998). During each data collection session, the monkey was seated in a custom primate chair and placed inside a sound-attenuating chamber. The left forelimb of the monkey was restrained during task performance, whereas the right forelimb was free to move.
The reach-to-grasp task consisted of four different phases, which were guided in performance by audio and video cues provided by custom computer software. The task began with the monkey’s right hand resting on a home plate device at waist height with its elbow flexed at ~90 degrees. Holding the hand on the plate for a preprogrammed length of time triggered the release of a food reward and a “go” signal. In the second phase of the task, the monkey reached into the target cylinder to grasp the food pellet with its fingers using a precision grip. The target cylinder was located at shoulder level, a little less than one arm’s length away and oriented ~20 degrees from vertical. During this phase, the arm was fully extended. In the third phase, the monkey flexed its elbow and wrist to bring the pellet to its mouth. Finally, in the last phase of the task, the monkey returned its hand to the home plate starting position.
Surgical procedures.
On completion of training, each monkey was implanted with a cortical recording chamber and EMG electrodes. For all implant surgeries, the monkeys were tranquilized initially with ketamine (10 mg/kg), administered atropine, and subsequently anesthetized with isoflurane gas. Both monkeys received prophylactic antibiotic before and after surgery and analgesic medication postoperatively. All surgeries were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care using full sterile procedures. All procedures conformed to the Guide for the Care and Use of Laboratory Animals, published by the United States Department of Health and Human Services and the National Institutes of Health, and were approved by the local Institutional Animal Care and Use Committee.
A magnetic resonance imaging (MRI) compatible plastic chamber allowing exploration of a 30-mm-diameter cortical area was stereotaxically implanted over the forelimb area on the left hemisphere of each monkey using procedures fully described previously (Kasser and Cheney 1985; McKiernan et al. 1998; Mewes and Cheney 1991). The chambers were centered stereotaxically at anterior 21.6 mm, lateral 11.4 mm (monkey M) and at anterior 16.0 mm, lateral 7.4 mm (monkey D), at a 30-degree angle to the midsagittal plane. For MRI compatibility, titanium screws (Bioplate, Los Angeles, CA) and titanium restraining nuts (McMaster-Carr, Chicago, IL) were used. In addition, a titanium screw (Synthes, Monument, CO) in contact with the dura served as a reference ground for electrophysiology.
EMG activity from 24 muscles of the forelimb was recorded using pairs of multistranded stainless steel wires (Cooner Wire, Chatsworth, CA) implanted during a sterile surgical operation. One monkey was implanted using a modular subcutaneous implant technique and the other was implanted using a cranial subcutaneous implant technique. These procedures are described in detail in Park et al. (2000). Briefly, for both techniques, pairs of wires for each muscle were tunneled subcutaneously to their target muscles. The modular subcutaneous implant technique used four connector modules (ITT Canon, New Britain, CT), two placed above and two below the elbow. The cranial subcutaneous implant technique used one circular connector module (Wire Pro, Salem, NJ) placed near the cortical recording chamber. The wire insertion points for specific muscles were identified on the basis of external landmarks and palpation of the muscle bellies. The wires of each pair were bared of insulation for ~2 mm at the tip and inserted into the muscle with a separation of ~5 mm. Proper placement was tested by stimulating through the wires with short trains or single pulses while observing the evoked movements. The wires were removed and reinserted if necessary.
EMGs were recorded from five shoulder muscles, seven elbow muscles, five wrist muscles, five forearm digit muscles, and two intrinsic hand muscles (Table 1). At regular intervals throughout recording, the monkeys were tranquilized with ketamine and the implants were tested with stimulation to confirm electrode location.
Table 1.
Categorization of recorded forelimb muscles
| Joints | Flexors | Extensors | ||
|---|---|---|---|---|
| Shoulder | pectoralis major | PEC | posterior deltoid | PDE |
| anterior deltoid | ADE | teres major | TMAJ | |
| latissimus dorsi | LAT | |||
| Elbow | biceps short head | BIS | triceps lateral head | TLAT |
| biceps long head | BIL | triceps long head | TLON | |
| brachialis | BRA | dorso-epitrochlearis | DE | |
| brachioradialis | BR | |||
| Wrist | flexor carpi radialis | FCR | extensor carpi radialis | ECR |
| flexor carpi ulnaris | FCU | extensor carpi ulnaris | ECU | |
| palmaris longus | PL | |||
| Digit | flexor digitorum superficialis | FDS | extensor digitorum communis | EDC |
| flexor digitorum profundus | FDP | extensor digitorum 2, 3 | ED23 | |
| extensor digitorum 4, 5 | ED45 | |||
| Not Classified | ||||
| Intrinsic hand | abductor pollicis brevis | APB | ||
| first dorsal interosseous | FDI |
Muscle classification based on Howell and Straus (1933).
Cortical recording.
Single cells in primary motor cortex (M1) were recorded using glass- and Mylar-insulated platinum-iridium electrodes with typical impedances between 0.7 and 1.5 MΩ. The recording electrode was positioned within the chamber using a custom x–y coordinate manipulator and was advanced into the cortex with a manual hydraulic microdrive (FHC, Bowdoin, ME). Electrode orientation was at a right angle to the cortical surface.
Data collection.
Microelectrode penetrations were made systematically in precentral cortex in a 1-mm grid interval. In some areas, electrode tracks were placed in the center of the 1-mm square formed by four adjacent tracks to achieve greater spatial resolution. The electrode was advanced with a manual hydraulic microdrive and stimulation was performed at 0.5-mm intervals throughout the depth of the electrode penetration, starting from the first cortical electrical activity encountered. In some tracks, stimulation was performed at 0.25-mm intervals. Cortical electrical activity and EMG activity were simultaneously monitored along with task-related signals.
While the monkey performed the reach-to-grasp task, stimuli (15 µA at 15 Hz) were applied through the electrode and served as triggers for computing StTAs. Individual stimuli were symmetrical biphasic pulses: a 0.2-ms negative pulse followed by a 0.2-ms positive pulse. EMGs were digitized at a rate of 4 kHz and full-wave rectified for StTA procedures. Averages were generally compiled over a 60-ms epoch, including 20 ms before the trigger to 40 ms after the trigger. Stimuli were applied throughout all phases of the reach-to-grasp task and the assessment of effects was based on StTAs of at least 500 trigger events. Segments of EMG activity associated with each stimulus were evaluated and accepted for averaging only when the average of all EMG data points over the entire 60-ms epoch was equal to or greater than 5% of full-scale input. This prevented averaging segments in which EMG activity was minimal or absent (McKiernan et al. 1998).
At some stimulation sites, averages were computed at 30 µA if no poststimulus effects (PStEs) were obtained at 15 µA. When no PStEs were detected with 30 µA, HFSD-ICMS was performed to determine the motor output representation, if any, from that site. HFSD-ICMS consisted of a train of 10 symmetrical biphasic stimulus pulses (negative-positive with total duration of 0.4 ms) at a frequency of 330 Hz (Asanuma and Rosén 1972) and an intensity of 15–30 µA. Evoked movements and muscle contractions detected with palpation were noted.
Cross talk between EMG electrodes was evaluated by constructing EMG-triggered averages. This procedure involved using the motor unit potentials from one muscle’s EMG recording as triggers for compiling averages of rectified EMG activity for all other muscles. Cross talk peaks in nontrigger muscles were expressed as a percent of the peak in the trigger muscle. In addition to common recording of the same motor unit potentials through different sets of electrodes (electrical cross talk), physiological synchrony between motor units can contribute to cross talk peaks. To adjust for this fact, we allowed cross talk up to and including 15% of the trigger muscle peak. Any muscle showing cross talk greater than 15% was eliminated from the database (Fetz and Cheney 1980). In this study, no poststimulus effects had to be eliminated due to cross talk.
Data analysis.
At each stimulation site, averages were obtained from all 24 muscles. Poststimulus facilitation (PStF) and suppression (PStS) effects were computer measured as described in detail by Mewes and Cheney (1991). First, nonstationary, ramping baseline activity (Lemon et al. 1986) was routinely subtracted from the average using custom analysis software (Windows Averager, Larry Shupe, Seattle, WA). Mean baseline activity and standard deviation (SD) were measured across the first 12.5 ms of each average (20 to 7.5 ms pretrigger) after first taking an average across individual trials (EMG segments associated with each stimulus) in the process of generating the StTA. Because the standard deviation of baseline points should decrease as the square root of the number of trigger events, all standard deviations were normalized to 1,300 trigger events (Belhaj-Saïf et al. 1998), with 1,300 being the approximate mode and median of trigger events for all StTAs collected. StTAs were identified as having a poststimulus effect if the envelope of the StTA crossed a level equivalent to ± 2 SD of the mean of the baseline EMG for a period greater than or equal to 0.75 ms (three points). The onset latency of PStF and PStS effects were generally measured as the point where the envelope of the effect intersected the line representing 2 SD away from the baseline. The magnitude of PStF and PStS was expressed as the number of standard deviations above (facilitation) or below (suppression) baseline (Fig. 1). Peak values were measured as the highest point in the peak of facilitation or lowest point in the trough of suppression. Expressions for these methods of quantifying poststimulus effects have been extensively documented previously (Cheney and Fetz 1985; Cheney et al. 1991; Kasser and Cheney 1985).
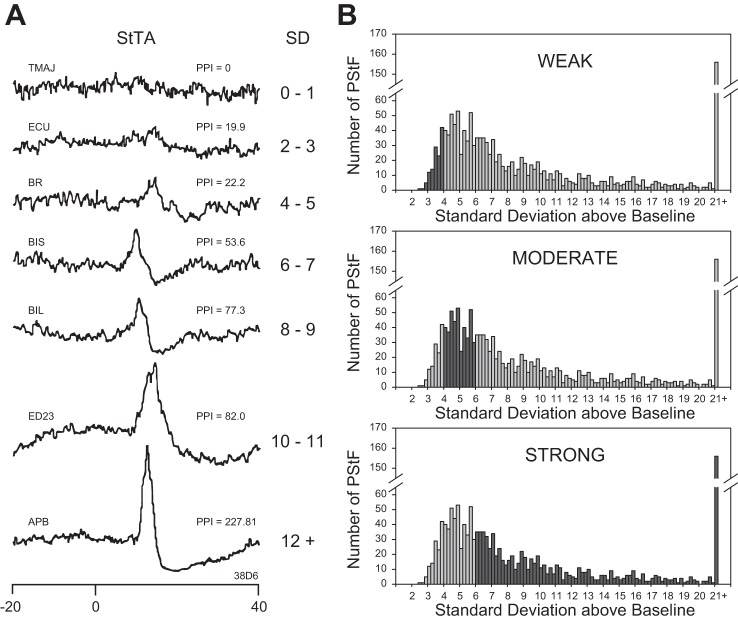
Fig. 1.
A: examples of stimulus-triggered averages (StTA) of rectified EMG activity (15 µA at 15 Hz) illustrating different strength categories of poststimulus facilitation (PStF) effects based on PStF peak magnitude expressed as the number of standard deviations of the peak from the baseline mean (SD or peak-to-noise ratio). Also shown is PStF strength expressed as peak percent increase (PPI) obtained by subtracting the baseline from the peak and dividing by the baseline. Time 0 corresponds to the stimulus event used as a trigger for averaging. A pretrigger period of 12.5 ms (baseline) was used to determine mean baseline activity and SD for each average. Muscle abbreviations are listed in Table 1. B: frequency plots showing the distribution of magnitudes of PStF effects categorized as weak, moderate, and strong (black bars) as a component of the total distribution.
The strength of PStF effects was categorized as follows (Fig. 1). Peaks less than 2 SD of baseline and peaks that remained above 2 SD for less than a 0.75-ms period were considered insignificant and the average was categorized as having no effect. Weak PStF effects had peaks from 2 to 4 SD of mean baseline activity. Moderate and strong peaks had SD ranges of 4–6 and ≥6, respectively (Park et al. 2004). Our maps were based on all effects and their strength measured in SD from baseline after correcting for differences in the number of trigger events. Figure 1 shows frequency distributions for weak, moderate, and strong PStF effects.
MRI analysis and cortical unfolding.
MRI studies were performed ~5 mo after the cortical chamber implant but before the EMG implant. MR imaging protocol is fully described in Park et al. (2001). The monkeys were tranquilized with ketamine and atropine and subsequently anesthetized with isoflurane gas. To give the magnetic resonance (MR) images a reference framework, a custom-designed chamber cap filled with MR opaque marker (liquid vitamin E) was used to identify the x- and y-axes [anterior-posterior (A-P) and medial-lateral (M-L) axes, respectively] of the cortical recording chamber as a cross. Image reconstruction and analysis were performed using Omniview 2D and 3D visualization software (3D Biomedical Imaging, Shawnee Mission, KS), the details of which have been described in detail previously (Park et al. 2001). As a result, a series of oblique parasagittal images of the cortex were obtained with respect to the recording chamber coordinate system. These images were orthogonal to the M-L axis and in register with the chamber coordinate system. Thus the images were parallel to a series of electrode tracks having the same M-L coordinate. For example, an oblique parasagittal image at lateral 4 would represent a slice through the cortex containing all electrode tracks for which the M-L chamber coordinate was lateral 4. These images were then traced to identify gray matter and white matter boundaries in the precentral gyrus. The images and drawings were also used to estimate electrode tracks and stimulation sites.
A two-dimensional rendering of cortical layer V in the anterior bank of the central sulcus required flattening and unfolding its curvature. The process of unfolding has been described in detail by Park et al. (2001). First, all electrode tracks were grouped according to their M-L coordinate. Within each group, the tracks were then ordered according to their A-P coordinate. Briefly, a parasagittal diagram was constructed to represent the cortex that was explored and stimulated, based on the electrophysiological data and observations. This analysis yielded a series of reconstructed parasagittal cortical sections oriented along the A-P axis of the chamber in the plane of the electrode tracks. The serial parasagittal sections were unfolded with respect to the convexity of the precentral gyrus using a reference line similar to the procedure used by Sato and Tanji (1989). The resulting unfolded reference line then provided coordinates for each stimulation site on the two-dimensional map. White matter was identified by a sharp decrease or loss of background unit activity. Sensory cortex was identified by the presence of distinctive spike activity and characteristic receptive fields (Widener and Cheney 1997). For each electrode track, putative layer V sites were identified using a combination of electrode depth, strength of PStF effects, and reconstruction of precentral geometry in relation to MRI sections. While these criteria yielded likely layer V sites, we cannot be certain that all of them were actually layer V. The convexity of the precentral gyrus is drawn in on all maps and corresponds to the boundary between rostral and caudal M1 (Rathelot and Strick 2006, 2009).
Motor output maps.
The strength of PStEs was determined and mapped onto the unfolded two-dimensional map of the cortex as the number of standard deviations above or below baseline. In addition, the center of gravity (COG) (Classen et al. 1998; Karl et al. 2001) of motor output maps of individual muscle representations was calculated using the formula:
where w is the strength of the PStE.
Unfolding the precentral gyrus for mapping yielded a two-dimensional map with a stimulation site density of 1 × 1 mm for sites on the cortical surface and 0.5 × 1.0 mm for sites in the anterior bank of the central sulcus. To adjust for the difference in sampling density, the weight of sites on the cortical surface was doubled.
RESULTS
Data were collected from the left M1 in two rhesus monkeys (Macaca mulatta) with a total of 248 electrode tracks: 115 tracks in monkey D and 133 tracks in monkey M. On the basis of the criteria described earlier, stimulation sites corresponding to cortical layer V were selected and only their PStEs were used for analysis. This yielded 238 sites and 5,712 individual StTA records (140 sites in monkey D and 98 sites in monkey M). The 5,712 individual StTA records yielded 1,398 PStF effects (607 from monkey D; 791 from M) and 679 PStS effects (365 from monkey D; 314 from M). Of the 1,398 PStF effects obtained, 9% were weak, 29% were moderate, and 62% were strong; and of the 679 PStS effects obtained, 18% were weak, 54% were moderate, and 28% were strong according to criteria described in methods.
In addition, HFSD-ICMS was performed at 359 sites, 210 in monkey D and 149 in monkey M. HFSD-ICMS was performed at sites where no PStEs were observed with StTA at intensities up to 30 µA. HFSD-ICMS was used primarily to identify motor output from sites outside the forelimb representation including representations of trunk, hindlimb, and face. Muscles for these representations were not implanted with EMG electrodes and could not be studied with StTA.
Proximal and distal forelimb muscles.
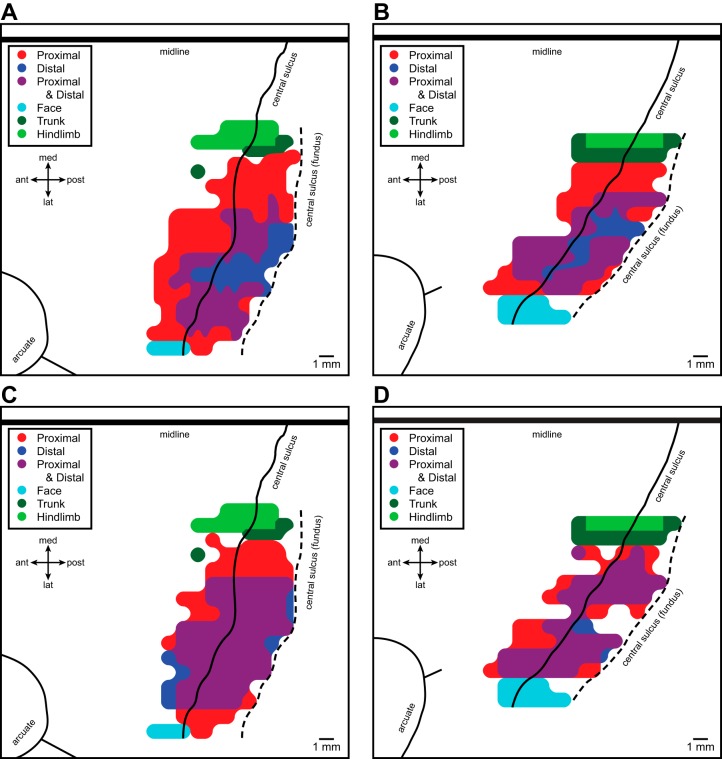
As reported previously (Park et al. 2001), motor output maps (Fig. 2, A and B) constructed based on all PStF effects (strong, moderate, and weak) at layer V sites revealed a central core of distal (wrist, digit, and intrinsic hand) forelimb muscle representation contained largely within the anterior bank of the central sulcus. The core distal representation was surrounded on all sides except at the area 4/3a border by a horseshoe-shaped zone of proximal forelimb muscle representation. The medial and lateral branches of the horseshoe extend posteriorly down the anterior bank of the central sulcus and terminate at the area 4/3a border. Separating the core distal and proximal representations was a proximal-distal cofacilitation zone where StTA produced effects in both proximal and distal forelimb muscles (Park et al. 2001). Motor output maps (Fig. 2, C and D) constructed based on PStS effects revealed a similar pattern of organization but with a larger proximal-distal cofacilitation zone that encroached heavily on the distal only zone and to a lesser extent on the proximal only zone. We will return to PStS maps in a later section.
Fig. 2.
Maps of facilitation (A and B) and suppression (C and D) effects obtained from motor cortex of two monkeys represented in two-dimensional coordinates after unfolding the precentral gyrus. A and B: maps for monkeys D and M, respectively, based on all PStF effects (strong, moderate, and weak) together with high-frequency, short-duration ICMS (HFSD-ICMS)-evoked movements delineating the borders of the forelimb representation with face, trunk, and hindlimb representations (from Park et al. 2001, by permission). C and D: maps for monkeys D and M, respectively, based on all PStS effects, together with HFSD-ICMS-evoked movements delineating the borders of the forelimb representation. Black line is the convexity of the precentral gyrus representing the boundary between rostral and caudal M1 (Rathelot and Strick, 2006, 2009); black dashed line is the fundus of the central sulcus. The midline and arcuate sulcus with spur are also shown.
The boundary of the forelimb representation consisted of no effects anteriorly, sensory cortex or white matter posteriorly (deep into the sulcus), face (lower and upper lip) laterally, and trunk and hindlimb medially. The sites at the anterior boundary yielded no PStE with StTA at 15 and 30 µA, and no evoked movements with HFSD-ICMS at 15 and 30 µA. Stimulation sites extending posteriorly or deep into the sulcus had characteristics of either sensory cortex or white matter (Park et al. 2001). Stimulation sites at the lateral boundary yielded no PStE with StTA at 15 and 30 µA but produced evoked movements of the upper and lower lips with HFSD-ICMS at 15 and 30 µA. Stimulation sites at the medial boundary yielded no PStE with StTA at 15 and 30 µA but produced evoked movements of trunk or hindlimb with HFSD-ICMS at 15 and 30 µA (Fig. 2, A–D).
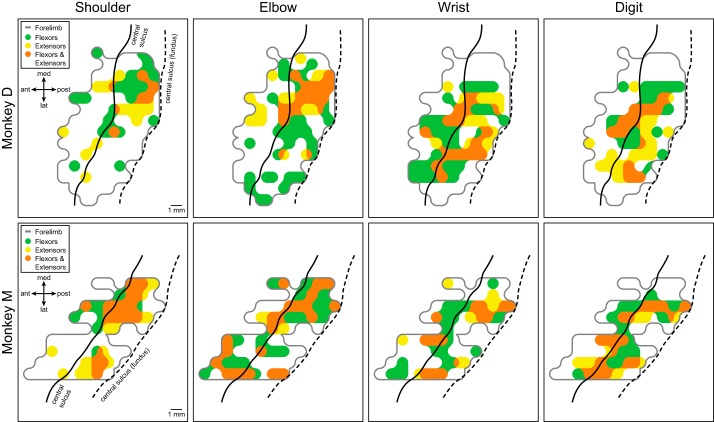
Flexors and extensors.
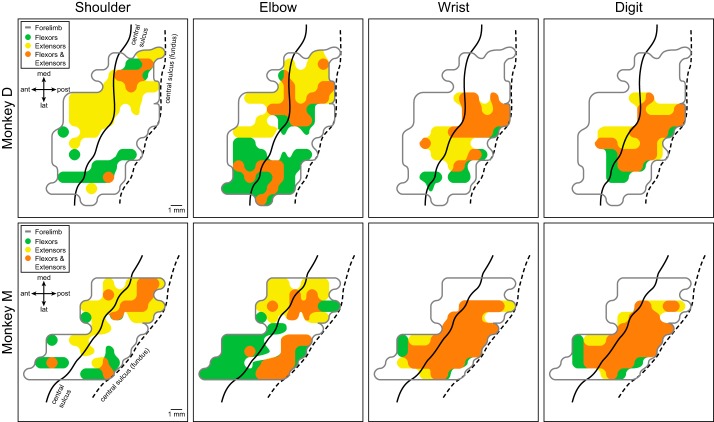
The proximal and distal muscles of the forelimb can be further categorized according to their respective joints and to their function as flexors or extensors (Table 1; see table for definitions of muscle abbreviations). Figure 3 illustrates the cortical motor output maps based on PStF effects in flexor and extensor muscles acting at different joints. Intrinsic hand muscles were excluded from this analysis. Motor output maps of individual muscles grouped according to their flexor or extensor action revealed consistent features of functional organization in both monkeys going beyond our previous findings (Fig. 2, A and B). At proximal joints (shoulder and elbow), the flexor muscles (green, flexor-only effects) were predominantly represented in the lateral branch of the horseshoe-shaped proximal muscle representation, while extensor muscles (yellow, extensor-only effects) were predominantly represented in the medial branch. In addition, within these primary flexor and extensor representations were smaller areas of flexor-extensor cofacilitation (orange). A trend toward this pattern of functional organization is also evident for wrist and digit joints, although it is much less prominent due to large areas of flexor-extensor cofacilitation. In monkey M, for example, the wrist and digit muscle representation is primarily flexor-extensor cofacilitation, whereas the separation of flexor-only and extensor-only representations at the wrist and digits is clearer in monkey D.
Fig. 3.
Primary motor cortex output maps showing the representation of flexor and extensor muscles at different joints in two monkeys based on poststimulus facilitation (PStF). Intrinsic hand muscles were omitted from these maps. Green area corresponds to facilitation of flexors, yellow area corresponds to facilitation of extensors, and orange area corresponds to cofacilitation of both flexors and extensors within the joint. The light gray line encircles the entire forelimb representation as shown in Fig. 2, A and B. The black solid line represents the convexity of the precentral gyrus and the black dashed line represents the fundus of the central sulcus.
Categorizing output effects from cortical sites.
An important aspect of cortical output to muscles concerns the functional pattern of effects on flexor and extensor muscles at different joints (Humphrey and Reed 1983). We categorized the output effects at each joint according to effects on antagonist muscles as no effect, facilitation, or reciprocal suppression (Table 2). In addition, effects consisting of a combination of PStF and PStS in a muscle group (flexors, extensors, or both) were categorized as mixed. Note that this is not a categorization by cortical site; rather it is a categorization by joint so each cortical site may be represented multiple times according to the number of joints with effects. The left columns in Table 2 give the number of effects of each type at each joint summed across both monkeys. The right columns give the corresponding percentages. The most common category (42% overall) was facilitation only. The largest contribution to this category came from sites that facilitated combinations of both flexor and extensor muscles (20.4%). The next most common category (22.5% overall) was mixed where PStF and PStS occurred together in flexor muscles (not the same muscle) or in extensor muscles or in both. This may reflect the complexity of movements supported by cortical output and the need to mix facilitation and suppression in complex combinations that ignore flexor/extensor boundaries. It may also reflect the different ways in which cortical output contributes functionally to a movement (Griffin et al. 2015). The category of suppression only was also relatively common (21.6% overall). Although this suggests that cortical output neurons at some sites may only suppress muscle activity, most of these effects were accompanied by facilitation at another joint. We also assume the presence of undetected facilitation (for instance, in muscles not recorded) in cases where suppression was the only effect observed at all joints. The reciprocal category (13.9% overall) consisted of facilitation of either flexors or extensors and suppression of the antagonist muscles. These effects would be ideally suited to support movements requiring simple activation of either flexors or extensors at a joint aided by suppression of the direct antagonists. In nearly all cases, the overall trends described above were consistent across both monkeys.
Table 2.
Categories of output effects to flexor and extensor muscles and their frequency at individual joints summed across both monkeys D and M
| Number of Cases Observed |
Percent of Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoulder | Elbow | Wrist | Digit | Total | Shoulder | Elbow | Wrist | Digit | Total | |
| PStF only (no PStS) | ||||||||||
| Flexors only | 19 | 33 | 11 | 5 | 68 | 13.2 | 16.9 | 6.9 | 3.4 | 10.5 |
| Extensors only | 37 | 19 | 7 | 9 | 72 | 25.7 | 9.7 | 4.4 | 6 | 11.1 |
| Both flexors and extensors | 11 | 32 | 43 | 46 | 132 | 7.6 | 16.4 | 26.9 | 30.9 | 20.4 |
| PStS only (no PStF) | ||||||||||
| Flexors only | 10 | 19 | 20 | 16 | 65 | 6.9 | 9.7 | 12.5 | 10.7 | 10 |
| Extensors only | 17 | 12 | 10 | 8 | 47 | 11.8 | 6.2 | 6.3 | 5.4 | 7.3 |
| Both flexors and extensors | 6 | 3 | 10 | 9 | 28 | 4.2 | 1.5 | 6.3 | 6 | 4.3 |
| Reciprocal | ||||||||||
| PStF in flexors + PStS in extensors | 7 | 9 | 3 | 10 | 29 | 4.9 | 4.6 | 1.9 | 6.7 | 4.5 |
| PStF in extensors + PStS in flexors | 21 | 13 | 15 | 12 | 61 | 14.6 | 6.7 | 9.4 | 8.1 | 9.4 |
| Mixed PStF + PStS in flexors and/or extensors | 16 | 55 | 41 | 34 | 146 | 11.1 | 28.2 | 25.6 | 22.8 | 22.5 |
| Total | 144 | 195 | 160 | 149 | 648 | 100 | 100 | 100 | 100 | 100 |
Note that this is a tabulation of effects at individual joints. Many individual cortical sites produced effects at multiple joints and the effects at each joint were categorized and entered separately in the appropriate column.
Rather than effects at individual joints, the complete output effects of individual cortical sites can also be categorized along the lines of Table 2. The vast majority of cortical sites produced effects at multiple joints (201/85% vs. 37/15% for single joints). Of 238 cortical sites tested, 46 (19%) produced only PStF; 12 (5%) produced only PStS. Only three sites (1%) produced reciprocal effects at all joints that had effects, but a substantial number of sites produced reciprocal effects in at least one joint (71/30%). The remainder of cortical sites (106, 44.5%), constituting the largest category, produced mixed effects with both PStF and PStS in flexors or extensors or both.
Individual forelimb muscles.
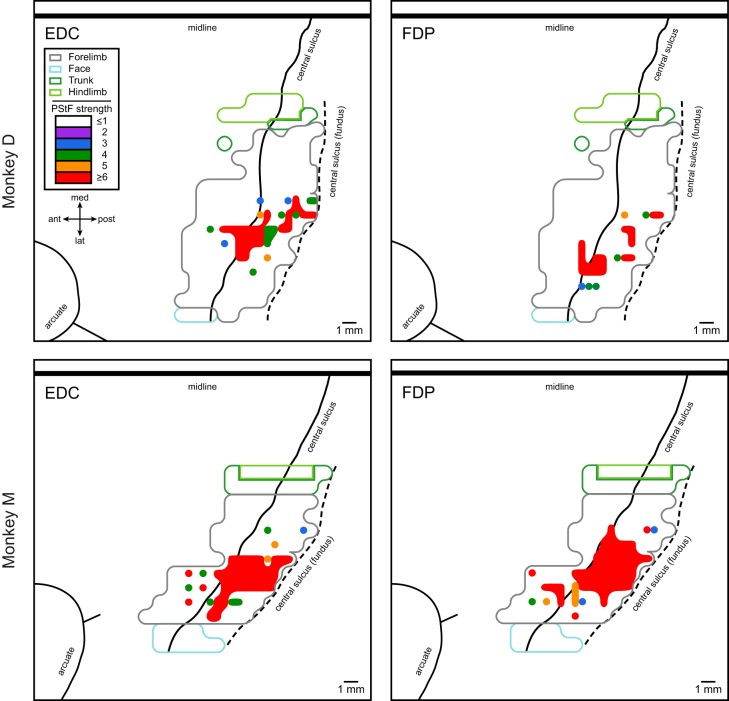
Motor output maps were also constructed for individual muscles in the forelimb. Figure 4 illustrates motor output maps for EDC and FDP from M1 in each monkey based on PStF effects. In this case the maps are color coded according to the strength of effects. Maps of EDC and FDP demonstrate that within a single area of representation there can be multiple foci of strong PStF effects, separated by weaker PStF effects. For example, the EDC map in monkey D and the FDP map in monkey M show two regions of strong PStF effects within one overall area of representation for the muscle. Separated from the principal area of representation, there are also single points with strong effects surrounded by areas with no effects. These results are similar to what others have reported based on a variety of different methods including intracellular recording from motoneurons and movements or EMG responses evoked with repetitive microstimulation in nonhuman primates (Fritz et al. 1985; Gould et al. 1986; Humphrey 1986; Huntley and Jones 1991; Jankowska et al. 1975; Kwan et al. 1978b; Landgren et al. 1962; Schieber 2001; Schieber and Hibbard 1993; Tanji and Wise 1981).
Fig. 4.
Examples of individual muscle representations, EDC and FDP, for two monkeys based on poststimulus facilitation (PStF) effects. The strengths of PStF expressed in standard deviations from baseline are color coded (legend) and mapped onto a background of the summary map outline of all PStF effects (gray line) and HFSD-ICMS-evoked movements identifying the face, trunk and hindlimb representations. Top: EDC and FDP maps in monkey D. Bottom: EDC and FDP maps in monkey M.
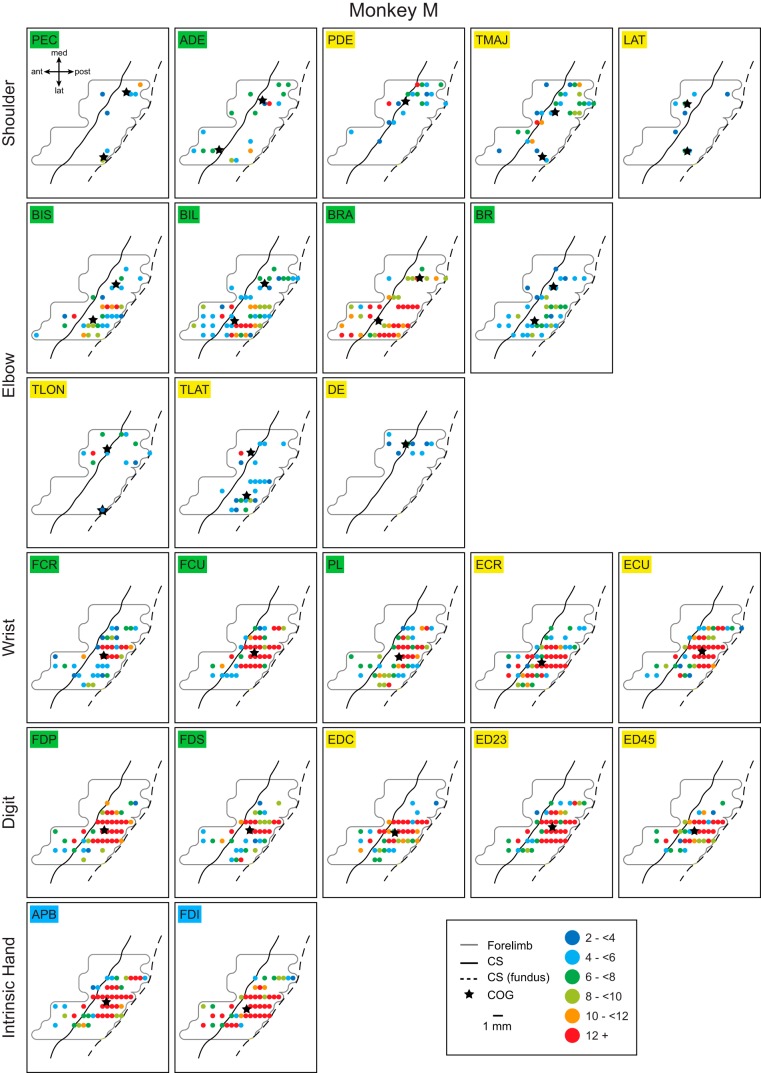
Figures 5 and 6 show the complete set of output maps for all 24 simultaneously recorded muscles for monkeys D and M, respectively. Each point represents one cortical stimulation site color coded for the strength of the effect. The preferential representation of shoulder and elbow extensor muscles medially and flexor muscles laterally is evident in these maps as differences in the number of sites with effects and the strength of effects. An exception would be the shoulder flexor muscles in monkey M. However, when sites that produced effects in both flexor and extensor muscles are distinguished, a clear separation of flexor only and extensor only sites is also evident for the flexor muscles in monkey M (Fig. 3).
Fig. 5.
Individual muscle representations for monkey D based on all PStF effects. The strengths of PStF expressed in standard deviations from baseline are color coded (legend; <2 omitted as insignificant). The full forelimb representation is outlined by the light gray line. The maps are arranged by joint. Black stars within each map represent the centers of gravity (COG) for individual muscle representations. In the case of shoulder and elbow muscles, two COGs are shown: one for the points in the medial branch and one for the lateral branch of the “horseshoe,” as these areas of representation were separated by 3 mm or more. Note that the maps in this figure show the complete representation of each muscle. In addition to PStF in the muscle of interest, PStF generally occurred in other muscles as well including the antagonist muscles. Sites producing cofacilitation of flexors and extensors are shown in maps of Fig. 3. CS, central sulcus.
Fig. 6.
Individual muscle representations for monkey M based on all PStF effects. Same format as Fig. 5.
The greater strength of effects in distal compared with proximal muscles is also evident in Figs. 5 and 6. Nearly all proximal muscles had some representation in both the medial and lateral branches of the horseshoe representation, although as noted above, the organization was preferential for one branch or the other based on whether the muscle was a flexor or extensor. Excitatory output to individual elbow and shoulder muscles encompassed a relatively large fraction of the entire M1 proximal only and proximal plus distal representations. The same was true for individual distal muscle representations. All distal muscles had at least some representation in both the distal only and proximal plus distal zones. Also evident from Figs. 5 and 6 is an overall disparity in the strength of effects in distal muscles of the two monkeys with monkey M having a much larger number of strong effects. However, much of the representation for individual muscles, especially proximal muscles, was relatively weak with generally only one or a few small “hot spots” (red in Figs. 5 and 6). The only exceptions were some of the elbow flexors (BIL, BRA) in monkey M.
Center of gravity analysis.
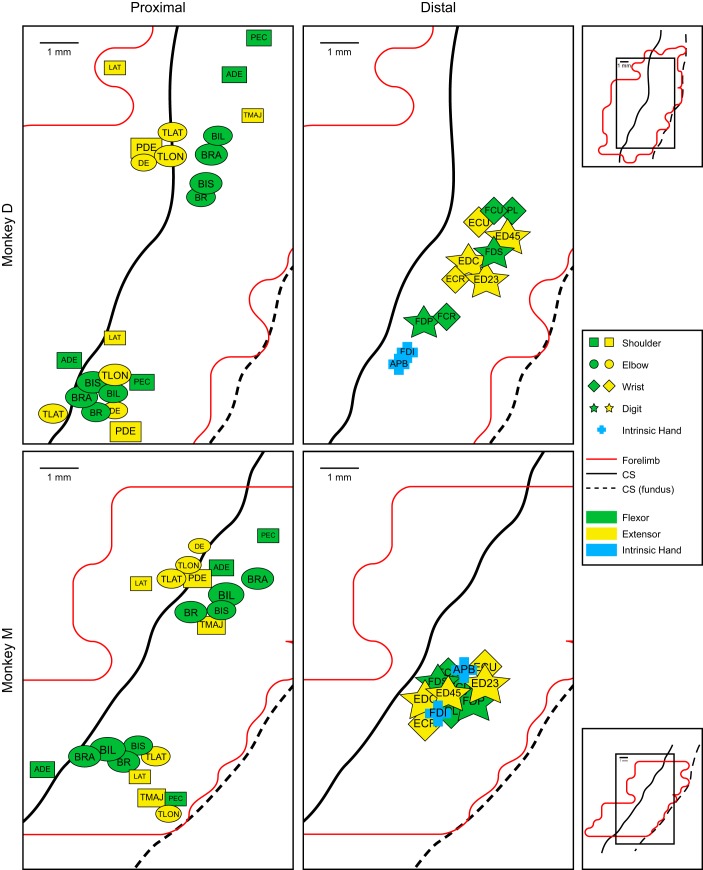
COG can be calculated to determine the geometric center of an area of muscle representation, taking into account the strengths of the PStF at different sites within the representation. COG can be an effective tool for making comparisons, especially in light of the fact that the areas of individual muscle representations are overlapping. By determining a single point capturing the overall area of a specific muscle representation and taking into account the strength of the PStF, we can use the COG as another way to test for possible orderly organization of muscles across the forelimb M1 representation.
Figure 7 plots the COGs for proximal and distal muscles for each monkey. Because the medial and lateral branches of the proximal muscle representations are spatially separated, COGs were calculated and plotted separately for each of these components of the representation. The COG maps reveal some points of consistency in representation across the two monkeys. What is again particularly clear from the COG maps is the absence of any orderly, consistent feature in the spatial distribution of distal muscle COGs. The centers of gravity for distal muscles tend to converge toward a point about midway along the representation in the bank of the precentral gyrus, consistent with the findings of Schieber and Hibbard (1993) based on single unit recording in relation to an individuated finger task. COG convergence for distal muscles is greatest for monkey M and somewhat less for monkey D. Unlike the distal muscles, the proximal muscle COGs do show an interesting additional feature of orderly organization. In both monkeys, the shoulder and elbow extensor muscle COGs in the medial branch of the horseshoe are preferentially represented anteriorly while flexors are represented posteriorly. TMAJ is the only exception to this pattern and in both monkeys its COG is located more posteriorly within the flexor muscle territory. The lateral branch representations did not show any consistent spatial organization of COGs. In monkey M there is a reversal (flexors anterior, extensors posterior) of the pattern observed in the medial branch of the representation; however, this was not as clear in the map for monkey D.
Fig. 7.
Center of gravity (COG) maps for the 24 simultaneously recorded muscles of the forelimb in monkey D (top) and monkey M (bottom). COG was determined for each muscle with symbols representing different joints: shoulder, square; elbow, circle; wrist, diamond; digit, star; and intrinsic hand, cross. Green symbols represent the flexors and yellow symbols represent the extensors. Intrinsic hand muscles are shown in blue. Black solid line is the convexity of the precentral gyrus; black dashed line is the fundus of the central sulcus. Outlines (red) of the full forelimb representation are shown below the COG maps. The area represented by the COG maps relative to the full forelimb representation is given by the small rectangles on the right. The COG calculation was adjusted to take into account the difference in the density of data points between the cortical surface (1 × 1 mm) vs. the anterior bank of the central sulcus (0.5 × 1 mm). Most shoulder and elbow muscles had representations in both the medial and lateral branches of the overall representation. Because these different components were physically separate, we calculated COGs separately for the representations in the medial and lateral branches of the representation.
Although there is clear justification for separating the data for the medial and lateral branches of the proximal muscle representations for COG analysis, an alternative approach would be to base the COG analysis on all the data points for each muscle. When this is done, the results show clear separation between the COGs of shoulder and elbow extensor and flexor muscles with all shoulder extensor muscle COGs located medial to flexor muscle COGs. The same was true of elbow muscles except for PEC, which was located at the same mediolateral position as LAT and TMAJ.
Maps of inhibitory output.
In addition to maps based on PStF effects, PStS was also measured and used to construct maps of inhibitory M1 output to all 24 muscles of the forelimb. PStS effects are believed to be mediated indirectly, most likely through spinal inhibitory interneurons (Cheney and Fetz 1985; Jankowska et al. 1976; Kasser and Cheney 1985; Park et al. 2004). Figure 8 shows the results after lumping together muscles to create shoulder, elbow, wrist, and digit representations with flexors and extensors separated as in Fig. 3. Only pure PStS effects were included. Suppression that was part of a biphasic effect (facilitation followed by suppression) was excluded. Comparing these maps with those in Fig. 3 based on PStF effects, it is clear that the suppression maps are more disorganized than the facilitation maps. As with the maps of PStF, at all joints there are zones where PStS affected only flexors (green), only extensors (yellow), or both flexors and extensors (orange). However, compared with facilitation effects, there is less evidence of a spatial separation in the representation of extensor and flexor muscles. The elbow muscles and wrist muscles in monkey D seem to show an organization similar to the PStF effects with extensors predominantly medial and flexors laterally, but other maps trend the opposite direction (digit muscles in monkey D and shoulder muscles in monkey M). Still other maps fail to show any evidence of spatial segregation of flexor and extensor muscle representation.
Fig. 8.
Maps of inhibitory effects on forelimb flexor (green) and extensor (yellow) muscles from primary motor cortex. Orange area corresponds to cosuppression of both flexors and extensors within the joint indicated. Intrinsic hand muscles were omitted from these maps. The gray line shows the boundary of the full forelimb representation based on PStS (from Fig. 2, C and D). Black solid line is the convexity of the precentral gyrus; black dashed line is the fundus of the central sulcus.
As noted in a previous section, Fig. 2, C and D shows maps of PStS effects lumping together all proximal muscles (red) and all distal muscles (dark blue). Sites yielding PStS in combinations of both proximal and distal muscles are also plotted (purple). The consistent features in the organization of distal (dark blue), proximal (red), and proximal plus distal (purple) muscles evident for maps of excitatory effects (Fig. 2, A and B, from Park et al. 2001) were also present for inhibitory maps but less clear (Fig. 2, C and D). The peripherally located proximal only representation is evident but the distal only representation is almost completely replaced with an extensive proximal-distal representation.
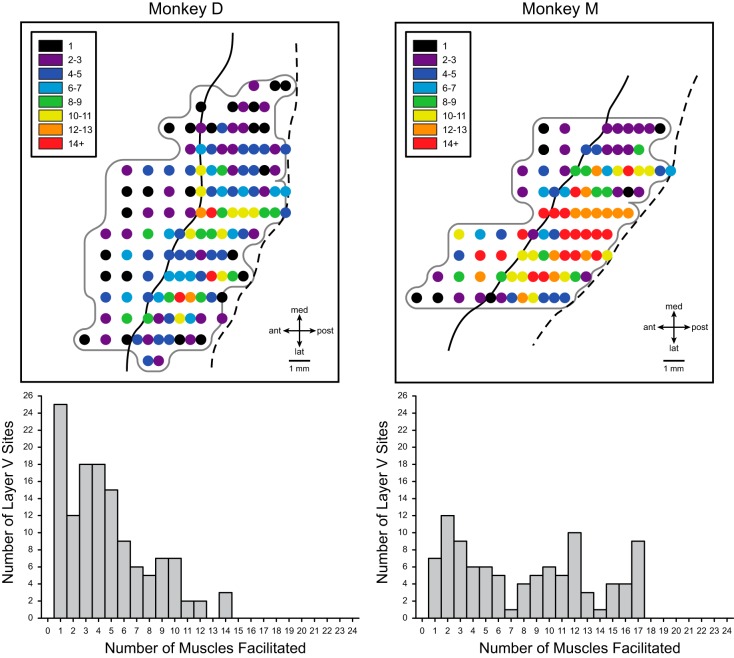
Maps of muscle synergy size.
It is well established that single corticospinal neurons generally contact not one but multiple motoneuron pools that form a functional synergy for executing movement (Fetz and Cheney 1980; Griffin et al. 2015; Lemon et al. 1986; McKiernan et al. 1998; Shinoda et al. 1981). This basic underlying feature of cortical output organization is also reflected in the effects obtained with StTA of EMG activity where facilitation of multiple muscles is the rule rather than the exception (Cheney and Fetz 1985; Lemon et al. 1987; Park et al. 2001, 2004). Given the importance of muscle synergy representation, we were interested in how the size of muscle synergies might be organized across motor cortex. Figure 9 shows maps of muscle synergy size (number of muscles with PStF) for each stimulus site in each of the two monkeys together with corresponding frequency distribution plots. The maps show a centrally located region, mostly in the bank of the precentral gyrus, where the size of muscle synergies was the largest. A small number of additional small “hot spots” are located more peripherally and might be viewed as noncontiguous with the central region. What is especially evident from the maps in Fig. 9 is that the sizes of muscle synergies in monkey D were markedly smaller overall than those in monkey M. This might be a consequence of the fact that the magnitude of PStF in monkey D was also substantially weaker than in monkey M. Consider what happens with increasing stimulus intensity as a way to simulate differences in the strength of PStF effects in the two monkeys. Increasing the intensity of stimulation will yield stronger PStF in the muscles showing effects at weaker intensities, but, in addition, the stronger intensity will generally recruit new muscles (Cheney and Fetz 1985; Widener and Cheney 1997). This is especially true as intensities reach levels well above threshold. These muscles will usually be synergists of existing PStF muscles and will show weaker PStF. Nevertheless, muscle synergy size will increase. This is not to say that synergy size and PStF magnitude are inextricably linked. There were single cortical sites where the synergy size was small but magnitude was high and vice versa. Despite the difference in magnitude of PStF in the two monkeys, both showed a similar pattern of organization of muscle synergy size with a relatively large central region containing the highest muscle counts surrounded peripherally by regions with lower muscle counts. It is also interesting that the frequency distributions of muscle synergy size were not unimodal but showed multiple peaks, especially evident in monkey M, possibly suggesting preferred muscle synergy sizes.
Fig. 9.
Maps of muscle synergy size (total number of muscles facilitated at each cortical site) for monkeys D and M together with frequency distributions of muscle synergy size. Muscle synergy size is color coded as indicated by the inset on each map.
DISCUSSION
Intra-areal somatotopic representation.
In this study, StTA of rectified EMG activity from 24 simultaneously recorded forelimb muscles was used to investigate individual forelimb muscle representation including the extent of intra-areal somatotopic organization within the forelimb area of M1 in rhesus macaques. As reported previously (Park et al. 2001), motor output maps show a central core of distal muscle representation (Kwan et al. 1978b), surrounded by a horseshoe-shaped proximal muscle representation, with a distinct zone of proximal-distal cofacilitation separating the two. Data presented in this paper reveal further consistencies in the M1 intra-areal somatotopic organization of the M1 forelimb representation. Specifically, extensor and flexor muscles of the shoulder and elbow show a separation in the medial-lateral dimension with extensors preferentially represented in the medial branch of the “horseshoe” whereas flexors are preferentially represented in the lateral branch. Furthermore, COG analysis (see results), which specifically take strength of effects into account, confirmed the predominant representation of extensors medially and flexors laterally for the shoulder and elbow muscles.
Distal muscle representations within the core distal forelimb area showed extensive overlap consistent with numerous previous studies in monkeys (Andersen et al. 1975; Armstrong and Drew 1985; Capaday 2004; Capaday et al. 2009, 2013; Fritz et al. 1985; Gould et al. 1986; Humphrey 1986; Huntley and Jones 1991; Jankowska et al. 1975; Kwan et al. 1978b; Landgren et al. 1962; Rathelot and Strick 2006; 2009; Schieber 2001; Schieber and Hibbard 1993; Tanji and Wise 1981). These results are also broadly consistent with a high-resolution fMRI study in humans showing a core distal representation bracketed by proximal musculature of the arm but with a high level of overlap in the representation of movements at different joints (Meier et al. 2008). Extensor and flexor actions were not distinguished in this study.
Although our results suggest that the somatotopy in motor cortex goes beyond the representation of major body parts to include significant intra-areal representations, it does not appear to extend to the digit muscles. In fact, no consistent segregation was observed within the distal muscle (wrist, digit, intrinsic hand) representations, nor was there any consistent separation of flexor and extensor muscles at any of the distal joints. This is in contrast to several studies in human subjects suggesting a nonrandom order to the representation of individual digit movements (e.g., Beisteiner et al. 2001, Dechent and Frahm 2003, Kleinschmidt et al. 1997, Schieber 1999). One conclusion from these studies is that the thumb representation is more lateral than the representation of the little finger, consistent with Penfield’s original summary maps showing the thumb representation more lateral than the representation of the fingers (Beisteiner et al. 2001). Although in monkey D the COG points for the digits (Fig. 7) do seem to follow a progression similar to the imaging studies in humans with thumb muscles lateral to a muscle controlling the little finger (ED4,5), in monkey M this pattern was not upheld and, if anything, was reversed. Similarly, in monkey D the intrinsic hand muscles were located lateral to the wrist and finger muscles, but this was not the case in monkey M. We conclude that, in the rhesus monkey, the data do not support any consistent somatotopy in the representation of individual digits. Instead the representation of digit muscles is highly overlapping with the COGs, in some cases converging on almost a single point, consistent with the findings of Schieber and Hibbard (1993) based on the localization of neurons activated during individuated digit movements and the conclusions of Sanes and Schieber (2001). We should also point out an important difference between our study and the human studies. We have mapped the representation of individual muscles whereas the human studies have mapped the representation of digit movements. It is well known that voluntary movements of individual digits involve the coactivation of several muscles, not just one, and this complicates the comparison of results (Schieber 1995).
Of particular relevance to our findings is the work of Kwan et al. (1978b). They used ICMS trains (15–75 cathodal pulses of 0.2 ms at 300 Hz up to 30-μA current strength) to evoke movements at various joints in the periphery. They described the spatial organization as “nested in such a way that a cortical zone controlling movement of a more distal joint was surrounded by a cortical zone controlling a more proximal joint. Thus, for the entire forelimb, the encirclement would be in the order of shoulder, elbow, wrist and fingers with the shoulder zone at the perimeter and finger zone in the center.” Based on this description, the results are often described as consisting of a concentric circle organization although Kwan et al. did not actually use these terms. In fact, the data presented in their paper does not represent concentric circles but rather the shoulder and elbow representations have a horseshoe-shaped geometry open at the caudal end along the area 4-3a border, mirroring the maps in the present study and anatomical studies of labeled corticospinal neurons (He et al. 1993). These representations surround a core area consisting of wrist and digit muscle representations.
The data from Kwan et al. (1978b) summarizing the organization of evoked movements across the cortex (their Fig. 2) does not show a clear separation of extensor and flexor movements apparent in our results (Fig. 3). However, this is not surprising given the difference in methods and the fact that even in our data the separation of extensor and flexor representations is not complete but preferential.
Comparison with muscle maps derived from virus labeling.
Viral labeling of CM cells making monosynaptic connections with motoneurons of different muscles (Rathelot and Strick 2006) provides an important point of comparison with our data based on StTA of EMG activity. Rathelot and Strick found that CM cells with monosynaptic connections to motoneurons innervating digit muscles (abductor pollicis longus, adductor pollicis, and extensor digitorum communis) were largely buried in the anterior bank of the precentral gyrus (cM1) with only 1–3% located within the rostral part (surface) of the precentral gyrus (rM1). One of these muscles (EDC) was also part of our mapping study (Fig. 4) and provides a meaningful basis for comparison with the viral labeling results. First, our EDC maps show an overall high level of agreement with those of Rathelot and Strick (their Fig. 3). The vast majority of the EDC representation in the maps from both monkeys in our study was in the anterior bank of the central sulcus (cM1) with very little extending rostrally beyond the convexity of the gyrus. Much of this representation is coded in red (Fig. 4) indicating strong effects. In this respect, our results, like those of Rathelot and Strick (2006, 2009), differ significantly from the maps reported by Kwan et al. (1978b) based on high-frequency ICMS trains to evoke movements. Their maps show the distal and proximal muscle representations encompassing not only the anterior bank of the central sulcus (cM1) but also the crest (surface) of the precentral gyrus extending almost 4 mm anteriorly to the border of area 6 (rM1). Our results are also consistent with a recent study showing that excitatory postsynaptic potentials with a definitive monosynaptic latency were only obtained from focal stimulation (ICMS) of cM1 (Witham et al. 2016).
The total size of the EDC representation reported by Rathelot and Strick (2006) based on labeled CM cells was 47.6 mm2 (monkey JA3 in Fig. 3). The total area of the EDC representation in monkey M of our study was 46.1 mm2. However, the representation is somewhat smaller in monkey D (32.9 mm2). It is also possible to compare the size of the EDC representation where CM cells were most dense with the area in our maps where PStF was the strongest (red in Fig. 4). The two-dimensional area of densest cell representation for EDC from Fig. 3 of Rathelot and Strick is ~12.7 mm2, compared with ~13.2 mm2 for monkey M in our study and ~6.9 mm2 for monkey D. The size of these areas obviously depends on the criteria applied for “densest” and “strongest.” Nevertheless, the degree of agreement in results seems noteworthy.
In a later study, Rathelot and Strick (2009) used the viral labeling method to map the distribution of CM cells with monosynaptic connections to motoneurons of two proximal muscles, one of which (lateral head of triceps, TLAT) was also a muscle we mapped with StTA. Most of the viral labeled CM cells for TLAT were located on the medial side of the core distal representation consistent with the general pattern of representation we have reported in which elbow and shoulder extensors are preferentially represented medially to the core distal muscles. In our study, the representation of TLAT in monkey D was also medial to the core distal representation. Two additional elbow extensor muscles (TLON and DE) also show a preferential representation on the medial side of the distal muscle representation in monkey D. In monkey M the picture was not quite as consistent. Two elbow extensor muscles (TLON and DE) showed a preferential representation on the medial side of the distal muscle core, but TLAT actually showed a preferential representation lateral to the distal muscle core. Nevertheless, the viral labeling results for TLAT, an elbow extensor, are consistent with the overall organization we have reported in the current paper in which there exists a preferential representation of elbow and shoulder extensor muscles medial to the core distal representation.
Variability across subjects.
Monkey M’s maps show a larger area of strong representation in virtually all distal muscles compared with monkey D. We have noted large differences in the strength of PStF in previous studies on hindlimb muscles (Hudson et al. 2015; Messamore et al. 2016). The reason for such sizeable differences is unclear, but it does not seem to be related to properties of the EMG recording. For example, the average magnitude of the EMG signal in monkey D was not different than that in monkey M. Because there is no reason to assume any difference in the density of corticospinal neurons in the two monkeys, the difference in magnitude of PStF might suggest a less prominent monosynaptic linkage in monkey D compared with monkey M.
Implications for interpretation of PStF effects.
It is generally assumed that monosynaptic connections with motoneurons should yield the strongest effects with spike and StTA (Fetz and Cheney 1980; Lemon et al. 1986). The fact that the strength of our PStF effects correlates closely with the division of M1 into monosynaptic to motoneurons (cM1) and nonmonosynaptic (rM1) supports this assumption. Nearly all the strong PStF effects we observed [signal-to-noise (S/N) ratio of 12+ at 1,300 trigger events, red points in Figs. 5 and 6] were from sites in cM1 correlating closely with the area of representation containing CM cells with monosynaptic connections to motoneurons reported by Rathelot and Strick (2006, 2009). Of the small number of sites yielding strong PStF (S/N 12+) that were in rM1 (only 24 of 342 total sites with strong PStF across both monkeys), 71% were within 1 mm of the precentral convexity and none were more than 2 mm from the convexity. Based on this, it might be tempting to deduce that PStF effects obtained at 15 μA having a magnitude of ≥12 measured as S/N ratio are likely due to underlying monosynaptic connections whereas PStF with magnitudes <12 would likely be due to nonmonosynaptic connections. This is supported by the fact that only 5/681 (<1%) PStS effects, which are clearly nonmonosynaptic, had a S/N magnitude of 12 or greater. Of course, there were many PStF effects in cM1 that were less than 12 S/N ratio. Some of these might be mediated by nonmonosynaptic connections but it is also possible that the cells producing the effects were on the fringe of the excitatory field of the stimulus and therefore the effect was weak. Accordingly, PStFs weaker than 12 could still be due to underlying monosynaptic connections to motoneurons. The only conclusion that might be drawn from this is that PStEs with a S/N ratio of 12 or greater are likely due to monosynaptic connections. As noted in methods, because S/N ratio increases as the square root of the number of trigger events, we normalized all our magnitudes to 1,300 trigger events so this criterion would apply to stimulus-triggered averages at or normalized to 1,300 trigger events.
Inhibitory motor output map of M1.
One advantage of StTA of EMG activity over ICMS train-evoked movements or EMG responses in mapping motor output is its ability to reveal the existence of inhibitory output to motoneurons. For the first time, we were able to construct complete inhibitory motor output maps for muscles within the forelimb representation of M1. The maps showed some characteristics similar to those constructed from PStF data. The concentric organization of proximal and distal muscles was present, although the proximal-distal muscle representation was much larger as a fraction of the total representation and largely replaced the distal only representation. Inhibitory output maps also demonstrated some evidence of flexor/extensor segregation consistent with maps of facilitation effects.
Overall summary and conclusions.
Motor output maps of M1 based on individual muscle representations revealed a new consistent feature of intra-areal forelimb representation building on previous evidence of a horseshoe-shaped proximal muscle representation surrounding a central core of distal (wrist, digit, and intrinsic hand) representation reported previously (Park et al. 2001). Maps of individual forelimb muscles revealed a preferential separation of shoulder and elbow flexor and extensor muscles in which the flexors are largely represented in the lateral branch of the horseshoe whereas the extensors are largely represented in the medial branch. Center of gravity calculations support this organization. For the first time, maps of inhibitory effects from M1 cortex were also obtained and show some of the same features as the maps of excitatory output. Muscle synergy size was mapped across M1 and was found to vary by location with the largest synergies located in the bank of the precentral gyrus. The pattern of effects was analyzed by joint. The most common pattern was facilitation only followed next by mixed facilitation and suppression, then suppression only, and reciprocal as the least common category.
GRANTS
This work was supported by NIH Grants NS051825 and NS39023, the Kathleen M. Osborn Endowment, and NIH Center Grant HD02528.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.M.H., M.C.P., and P.D.C. analyzed data; H.M.H., M.C.P., A.B.-S., and P.D.C. interpreted results of experiments; H.M.H. and M.C.P. prepared figures; H.M.H., M.C.P., and P.D.C. drafted manuscript; H.M.H., M.C.P., and P.D.C. edited and revised manuscript; H.M.H., M.C.P., A.B.-S., and P.D.C. approved final version of manuscript; M.C.P. and A.B.-S. performed experiments.
ACKNOWLEDGMENTS
The authors thank Ian Edwards for technical assistance.
Present address for M. C. Park: Department of Neurosurgery, University of Minnesota School of Medicine, 420 Delaware St. SE, MMC 96, Minneapolis, MN 55455.
REFERENCES
- Andersen P, Hagan PJ, Phillips CG, Powell TP. Mapping by microstimulation of overlapping projections from area 4 to motor units of the baboon’s hand. Proc R Soc Lond B Biol Sci 188: 31–36, 1975. doi: 10.1098/rspb.1975.0002. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Electromyographic responses evoked in muscles of the forelimb by intracortical stimulation in the cat. J Physiol 367: 309–326, 1985. doi: 10.1113/jphysiol.1985.sp015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res 14: 243–256, 1972. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B, Moser E, Deecke L. Finger somatotopy in human motor cortex. Neuroimage 13: 1016–1026, 2001. doi: 10.1006/nimg.2000.0737. [DOI] [PubMed] [Google Scholar]
- Belhaj-Saïf A, Karrer JH, Cheney PD. Distribution and characteristics of poststimulus effects in proximal and distal forelimb muscles from red nucleus in the monkey. J Neurophysiol 79: 1777–1789, 1998. [DOI] [PubMed] [Google Scholar]
- Capaday C. The integrated nature of motor cortical function. Neuroscientist 10: 207–220, 2004. doi: 10.1177/107385403262109. [DOI] [PubMed] [Google Scholar]
- Capaday C, Ethier C, Brizzi L, Sik A, van Vreeswijk C, Gingras D. On the nature of the intrinsic connectivity of the cat motor cortex: evidence for a recurrent neural network topology. J Neurophysiol 102: 2131–2141, 2009. doi: 10.1152/jn.91319.2008. [DOI] [PubMed] [Google Scholar]
- Capaday C, Ethier C, Van Vreeswijk C, Darling WG. On the functional organization and operational principles of the motor cortex. Front Neural Circuits 7: 66, 2013. doi: 10.3389/fncir.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res 87: 213–252, 1991. doi: 10.1016/S0079-6123(08)63054-X. [DOI] [PubMed] [Google Scholar]
- Classen J, Knorr U, Werhahn KJ, Schlaug G, Kunesch E, Cohen LG, Seitz RJ, Benecke R. Multimodal output mapping of human central motor representation on different spatial scales. J Physiol 512: 163–179, 1998. doi: 10.1111/j.1469-7793.1998.163bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechent P, Frahm J. Functional somatotopy of finger representations in human primary motor cortex. Hum Brain Mapp 18: 272–283, 2003. doi: 10.1002/hbm.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol 44: 751–772, 1980. [DOI] [PubMed] [Google Scholar]
- Fritz N, Illert M, Kolb FP, Lemon RN, Muir RB, van der Burg J, Wiedemann E, Yamaguchi T. The cortico-motoneuronal input to hand and forearm motoneurons in anesthetized monkey. J Physiol 366: 20P, 1985. [Google Scholar]
- Gould HJ III, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247: 297–325, 1986. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hoffman DS, Strick PL. Corticomotoneuronal cells are “functionally tuned”. Science 350: 667–670, 2015. doi: 10.1126/science.aaa8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952–980, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AB, Straus WL Jr. The muscular system. In: The Anatomy of the Rhesus Monkey (Macaca mulatta), edited by Hartman CG, Straus WL Jr. New York: Hafner, 1933, p. 89–175. [Google Scholar]
- Hudson HM, Griffin DM, Belhaj-Saïf A, Cheney PD. Properties of primary motor cortex output to hindlimb muscles in the macaque monkey. J Neurophysiol 113: 937–949, 2015. doi: 10.1152/jn.00099.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DR. Representation of movements and muscles within the primate precentral motor cortex: historical and current perspectives. Fed Proc 45: 2687–2699, 1986. [PubMed] [Google Scholar]
- Humphrey DR, Reed DJ. Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. Adv Neurol 39: 347–372, 1983. [PubMed] [Google Scholar]
- Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey Motor cortex: a correlative anatomic and physiological study. J Neurophysiol 66: 390–413, 1991. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Projections of pyramidal tract cells to α-motoneurones innervating hind-limb muscles in the monkey. J Physiol 249: 637–667, 1975. doi: 10.1113/jphysiol.1975.sp011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol 258: 467–487, 1976. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 21: 3609–3618, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser RJ, Cheney PD. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol 53: 959–978, 1985. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Nitschke MF, Frahm J. Somatotopy in the human motor cortex hand area. A high-resolution functional MRI study. Eur J Neurosci 9: 2178–2186, 1997. doi: 10.1111/j.1460-9568.1997.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Kwan HC, Mackay WA, Murphy JT, Wong YC. An intracortical microstimulation study of output organization in precentral cortex of awake primates. J Physiol (Paris) 74: 231–233, 1978a. [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41: 1120–1131, 1978b. [DOI] [PubMed] [Google Scholar]
- Landgren S, Phillips CG, Porter R. Cortical fields of origin of the monosynaptic pyramidal pathways to some alpha motoneurones of the baboon’s hand and forearm. J Physiol 161: 112–125, 1962. doi: 10.1113/jphysiol.1962.sp006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Mantel GWH, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol 381: 497–527, 1986. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Muir RB, Mantel GW. The effects upon the activity of hand and forearm muscles of intracortical stimulation in the vicinity of corticomotor neurones in the conscious monkey. Exp Brain Res 66: 621–637, 1987. doi: 10.1007/BF00270695. [DOI] [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998. [DOI] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol 100: 1800–1812, 2008. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messamore WG, Van Acker GM III, Hudson HM, Zhang HY, Kovac A, Nazzaro J, Cheney PD. Cortical effects on ipsilateral hindlimb muscles revealed with stimulus-triggered averaging of EMG activity. Cereb Cortex 26: 3036–3051, 2016. doi: 10.1093/cercor/bhv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes K, Cheney PD. Facilitation and suppression of wrist and digit muscles from single rubromotoneuronal cells in the awake monkey. J Neurophysiol 66: 1965–1977, 1991. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Cheney PD. Chronic recording of EMG activity from large numbers of forelimb muscles in awake macaque monkeys. J Neurosci Methods 96: 153–160, 2000. doi: 10.1016/S0165-0270(00)00155-2. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol 92: 2968–2984, 2004. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci 21: 2784–2792, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443, 1937. doi: 10.1093/brain/60.4.389. [DOI] [Google Scholar]
- Phillips CG, Porter R. Corticospinal Neurones: Their Role in Movement. New York: Academic, 1977. [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford: Clarendon, 1993. [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Schieber MH. Orderly somatotopy in primary motor cortex: does it exist? Neuroimage 13: 968–974, 2001. doi: 10.1006/nimg.2000.0733. [DOI] [PubMed] [Google Scholar]
- Sato KC, Tanji J. Digit-muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol 62: 959–970, 1989. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: the roles of extrinsic finger muscles. J Neurosci 15: 284–297, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. Somatotopic gradients in the distributed organization of the human primary motor cortex hand area: evidence from small infarcts. Exp Brain Res 128: 139–148, 1999. doi: 10.1007/s002210050829. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol 86: 2125–2143, 2001. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science 261: 489–492, 1993. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yokota JI, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett 23: 7–12, 1981. [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Multiple representation in the primate motor cortex. Brain Res 154: 366–370, 1978. doi: 10.1016/0006-8993(78)90707-2. [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. I. Motor output organization. J Neurophysiol 48: 139–149, 1982. [DOI] [PubMed] [Google Scholar]
- Tanji J, Wise SP. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 45: 467–481, 1981. [DOI] [PubMed] [Google Scholar]
- Widener GL, Cheney PD. Effects on muscle activity from microstimuli applied to somatosensory and motor cortex during voluntary movement in the monkey. J Neurophysiol 77: 2446–2465, 1997. [DOI] [PubMed] [Google Scholar]
- Witham CL, Fisher KM, Edgley SA, Baker SN. Corticospinal inputs to primate motoneurons innervating the forelimb from two divisions of primary motor cortex and area 3a. J Neurosci 36: 2605–2616, 2016. doi: 10.1523/JNEUROSCI.4055-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN. Organization of somatic sensory and motor areas of the cerebral cortex. In: Biological and Biochemical Basis of Behavior, edited by Harlow HF, Woolsey CN. Madison, WI: University of Wisconsin Press, 1958, p. 63–81. [Google Scholar]