Abstract
We recently identified a novel hypothalamic neuropeptide inhibiting gonadotropin release in quail and termed it gonadotropin-inhibitory hormone (GnIH). Cell bodies and terminals containing the dodecapeptide GnIH are localized in the paraventricular nucleus (PVN) and median eminence, respectively. To understand the physiological role of GnIH, we investigated the mechanisms that regulate GnIH expression. In this study, we show that melatonin originating from the pineal gland and eyes induces GnIH expression in the quail brain. Pinealectomy (Px) combined with orbital enucleation (Ex) (Px plus Ex) decreased the expression of GnIH precursor mRNA and content of mature GnIH peptide in the diencephalon, which includes the PVN and median eminence. Melatonin administration to Px plus Ex birds caused a dosedependent increase in expression of GnIH precursor mRNA and production of mature peptide. The expression of GnIH was photoperiodically controlled and increased under short-day photoperiods, when the duration of melatonin secretion increases. To identify the mode of melatonin action on GnIH induction, we investigated the expression of Mel1c, a melatonin receptor subtype, in GnIH neurons. In situ hybridization of Mel1c mRNA combined with immunocytochemistry for GnIH revealed that Mel1c mRNA was expressed in GnIH-immunoreactive neurons in the PVN. Melatonin receptor autoradiography further revealed specific binding of melatonin in the PVN. These results indicate that melatonin is a key factor for GnIH induction. Melatonin appears to act directly on GnIH neurons through its receptor to induce GnIH expression. This is the first demonstration, to our knowledge, of a direct action of melatonin on neuropeptide induction in any vertebrate class.
Keywords: melatonin receptor, photoperiod, reproduction
In vertebrates, the neuropeptide control of gonadotropin secretion is primarily through the stimulatory action of the hypothalamic decapeptide, gonadotropin-releasing hormone (GnRH). Until recently, an inhibitory neuropeptide of gonadotropin secretion had not been identified in vertebrates, although gonadal sex steroids and inhibin can modulate gonadotropin secretion. We recently identified a novel hypothalamic dodecapeptide (SIKPSAYLPLRF-NH2) that directly inhibits gonadotropin release in the Japanese quail and termed it gonadotropin-inhibitory hormone (GnIH; ref. 1). This was the first demonstration of a hypothalamic neuropeptide inhibiting gonadotropin release in any vertebrate. The novel neuropeptide, GnIH, is located in neurons of the paraventricular nucleus (PVN) and their terminals in the median eminence (ME) (1–3). We further cloned a cDNA encoding the GnIH precursor polypeptide in the quail brain (4). The GnIH precursor encoded one GnIH and two GnIH-related peptides (GnIH-RP-1 and -RP-2), and the GnIH precursor mRNA was expressed only in the PVN (2–4). Thus, the PVN is the sole source of GnIH synthesis. Subsequently, we identified a cDNA encoding GnIH in the brain of the white-crowned sparrow (5). The sparrow GnIH precursor and mature GnIH peptide were also expressed in the PVN. Both quail and sparrow GnIH rapidly inhibited gonadotropin release in vivo (5). Based on these studies (1–5), GnIH is likely an important neuropeptide for the regulation of avian reproduction.
To understand the physiological role of GnIH in avian reproduction, changes in GnIH expression during development and under different physiological conditions require investigation. We recently characterized developmental changes in GnIH expression in the quail hypothalamo-hypophysial system and demonstrated that GnIH begins its function around hatch and acts as a hypothalamic factor to regulate gonadotropin release in quail (3). Until now, a regulatory mechanism(s) governing GnIH expression has remained unclear. Many bird species are photoperiodic, as are many mammals. Photoperiodic mammals rely on the annual cycle of changes in nocturnal secretion of melatonin to drive their reproductive responses (6). In contrast, a dogma has existed that birds do not use seasonal changes in melatonin secretion to time their reproductive effort, and a role for melatonin in birds has remained enigmatic (7, 8). Despite the accepted dogma, there is strong evidence that melatonin is involved in regulation of several seasonal processes, including gonadal activity and gonadotropin secretion (9–15). In light of these reports and considering GnIH's inhibitory effects on gonadotropin secretion (1, 5), we hypothesized that melatonin may be involved in the induction of GnIH expression, thus influencing gonadal activity. Therefore, we investigated the action of melatonin on GnIH expression by using quail, a highly photoperiodic bird species. Because the pineal gland and eyes are the major sources of melatonin in the quail (16), we first analyzed the effects of pinealectomy (Px) combined with orbital enucleation (Ex) (Px plus Ex) on the expression of GnIH precursor mRNA and content of the mature GnIH peptide. Subsequently, melatonin was administered to Px plus Ex birds. We further analyzed the action of endogenous melatonin on GnIH expression by using quail exposed to short-day (SD) and long-day (LD) photoperiods, thus varying the length of endogenous melatonin secretion by varying the length of the night (melatonin is secreted at night) (17, 18). To identify the mode of melatonin action on GnIH expression, we investigated the expression of Mel1c, a melatonin receptor subtype, in GnIH neurons in the PVN. We chose Mel1c because it is expressed in the PVN of the chicken hypothalamus (19); i.e., the corresponding region in which GnIH neurons are located in quail. We cloned a partial cDNA encoding Mel1c in the quail diencephalon and conducted in situ hybridization of Mel1c mRNA combined with immunocytochemistry for GnIH. Finally, melatonin binding in the PVN was investigated by melatonin receptor autoradiography. Here, we show that melatonin is a potent inducer of GnIH expression. Our results provide further evidence that melatonin acts directly on GnIH neurons through Mel1c to regulate GnIH expression.
Materials and Methods
Subjects. Adult male Japanese quail Coturnix japonica at 3 months of age were housed in a temperature-controlled room (25 ± 2°C) under daily photoperiods of 16-h light and 8-h dark (LD; lights on at 0700 hours). All birds were isolated in individual cages, and the experimental protocol was approved in accordance with guidelines prepared by Hiroshima University (Higashi-Hiroshima, Japan).
Experimental Schedules. The pineal gland and eyes are the major sources of melatonin in the quail (16). Thus, in the first experiment, Px and Ex were conducted to determine possible action of melatonin on GnIH expression. As described for Px (20), the skin on the top of the head was cut to expose the skull. An incision (0.25 cm2) was made in the skull above the pineal gland with a dental drill. The pineal gland was removed with fine forceps through an opening made in the meninges. In Sham-operated birds (SH), the pineal gland was exposed but not removed. Px birds were divided into two groups, and one group underwent bilateral Ex (Px plus Ex) as described (16). All surgery was performed under Nembutal anesthesia (40 mg/kg). One week after surgery, SH, Px, and Px plus Ex birds (n = 5 in each group) were terminated by decapitation between 1400–1600 hours for the collection of diencephalic samples. The GnIH precursor mRNA was quantified by means of competitive PCR. GnIH concentration in the diencephalon, which includes GnIH neurons in the PVN and their terminals in the ME, was quantified by means of ELISA. The completeness of Px and Ex was verified at autopsy and confirmed by measuring melatonin in the diencephalon and plasma by means of an RIA. For the measurement of melatonin, separate groups of identically treated birds of SH, Px, and Px plus Ex groups (n = 5 in each group) were also terminated during the dark phase (between 0200 and 0400 hours), when endogenous plasma melatonin is high (16). Thus, we verified that our surgical procedures were effective.
In the second experiment, melatonin was administered to Px plus Ex birds to investigate the action of melatonin on GnIH expression. Two days after surgery, Px plus Ex birds were divided into four groups (n = 5 in each group) and s.c. implanted with a Silastic (silicone type, Dow-Corning, Midland, MI) plate containing melatonin (Sigma) at three different doses (low dose, 2.5 mg per plate; medium dose, 10 mg per plate; and high dose, 40 mg per plate) or vehicle as described (21, 22). One week after the beginning of treatment, all birds (n = 5 in each group) were terminated between 1400 and 1600 hours and diencephalic samples were collected for quantification of GnIH precursor mRNA and mature GnIH peptide.
In the third experiment, we conducted quantitative analyses for the expression of GnIH precursor mRNA and mature GnIH content in PVN neurons in SH, Px plus Ex, and Px plus Ex plus melatonin (10 mg per plate) birds. One week after the beginning of treatment, all birds (n = 5 in each group) were terminated between 1400 and 1600 hours, and the brains were collected. The expression of GnIH precursor mRNA and GnIH content in PVN neurons were analyzed by in situ hybridization and immunocytochemistry, respectively.
In the fourth experiment, we manipulated endogenous melatonin to determine its action on GnIH expression. Birds were exposed to SD (8-h light and 16-h dark; lights on at 0900 hours) or LD (16-h light and 8-h dark; lights on at 0700 hours) photoperiods. Melatonin secretion occurs during the dark phase; thus, SD treatment increases the duration of endogenous melatonin secretion (17, 18). After three weeks of exposure to SD or LD, birds (n = 8 in each group) were terminated between 1400 and 1600 hours, and diencephalic samples were analyzed for GnIH precursor mRNA and GnIH content.
In the fifth experiment, the expression of Mel1c, a melatonin receptor subtype, in GnIH neurons in the PVN was analyzed to investigate the mode of melatonin action on GnIH expression. Expression of Mel1c occurs in the chicken hypothalamus (19), therefore we measured Mel1c expression in the quail diencephalon by cloning a corresponding partial cDNA. Subsequently, we determined the coexpression of Mel1c and GnIH by Mel1c in situ hybridization, combined with GnIH immunocytochemistry.
Finally, melatonin binding in the PVN of the brain (n = 4) was analyzed by melatonin receptor autoradiography.
Competitive PCR Analysis of GnIH Precursor mRNA. To quantify mRNA encoding the GnIH precursor polypeptide in the diencephalon, competitive PCR analysis was performed as described (ref. 3; also see Supporting Methods, which is published as supporting information on the PNAS web site). GnIH precursor mRNA level was normalized with the expression of the housekeeping gene β-actin and expressed as a ratio of GnIH precursor mRNA concentration to β-actin mRNA concentration in the corresponding total RNA derived from each diencephalic sample.
ELISA of GnIH. Peptides were extracted from the diencephalon of each bird, as described (1, 3, 22–24). The extract was subjected to competitive ELISA by using rabbit anti-GnIH serum (1, 3), which is described in Supporting Methods. We previously confirmed that the antiserum cross-reacts with GnIH on the basis of competitive ELISA (1).
RIA of Melatonin. Melatonin was extracted from each diencephalic sample by using chloroform, as described (16). Plasma melatonin was measured directly without extraction. The RIA was performed as described (25), by using rabbit anti-melatonin serum supplied by the Institute for Molecular and Cellular Regulation (Gunma University, Maebashi, Japan).
In Situ Hybridization of GnIH mRNA and Immunocytochemical Analysis of GnIH. In situ hybridization of GnIH mRNA was conducted by using a digoxigenin-labeled antisense RNA probe as described (5, 26–28). After hybridization, the sections were incubated with alkaline phosphatase-labeled sheep anti-digoxigenin antibody (Anti-digoxigenin-AP, Fab fragments; Roche Diagnostics), and immunoreactive product was detected by immersing the sections in a substrate solution (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate stock solution; Roche Diagnostics). Control for specificity of in situ hybridization of GnIH mRNA was performed by using a digoxigenin-labeled sense RNA probe complementary to the antisense probe sequence. Immunocytochemical analysis of GnIH was conducted as described (1–3). The specificity of the GnIH antiserum was assessed by preabsorbing the antiserum with the antigen (10 μg of GnIH per ml).
DNA Sequencing of the Partial Quail Mel1c cDNA. The partial quail Mel1c cDNA was cloned as described in Supporting Methods and used for in situ hybridization of Mel1c mRNA. The cloned partial quail Mel1c cDNA sequence is shown in Fig. 7, which is published as supporting information on the PNAS web site.
In Situ Hybridization of Mel1c mRNA Combined with Immunocytochemical Analysis of GnIH. To examine the expression of Mel1c mRNA in GnIH neurons in the PVN, in situ hybridization of Mel1c mRNA and immunocytochemistry for GnIH (1–3) were conducted. In situ hybridization of Mel1c mRNA was conducted by using a digoxigenin-labeled antisense RNA probe as described (5, 26–28). To detect colocalization of Mel1c mRNA and GnIH in PVN neurons, immunocytochemical analysis of GnIH was further conducted as described (1–3) on the same sections previously labeled by means of in situ hybridization of Mel1c mRNA. After immersing the sections in antiserum directed against GnIH, they were incubated with rhodamine-labeled goat anti-rabbit IgG.
Melatonin Receptor Autoradiography. [125I]iodo-melatonin (IMEL) receptor autoradiography was performed as described (ref. 12; also see Supporting Methods). To assess the relative amount of binding in the PVN, total binding in the PVN was compared with binding in adjacent PVN sections incubated in 20 pM IMEL plus 1 μM melatonin.
Results
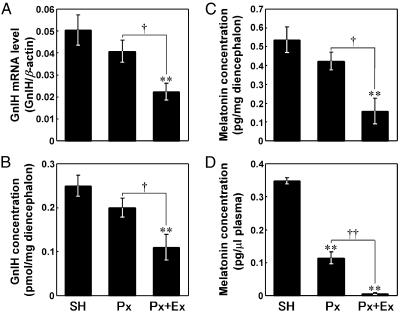
Effects of Px Combined with Ex on the Expression of GnIH Precursor mRNA and Content of GnIH Peptide. Changes in GnIH precursor mRNA levels in the diencephalon after Px plus Ex were measured by means of competitive PCR. The GnIH mRNA level was expressed as a ratio of GnIH mRNA concentration to β-actin mRNA concentration. As shown in Fig. 1 A, the GnIH mRNA level decreased significantly in Px plus Ex birds compared with SH and Px birds (P < 0.01, Px plus Ex vs. SH; P < 0.05, Px plus Ex vs. Px). On its own, Px tended to decrease the GnIH mRNA level, but the effect was not statistically significant (Fig. 1 A). Changes in GnIH concentration in the diencephalon were measured by competitive ELISA, using the antiserum against GnIH. As shown in Fig. 1B, Px plus Ex also induced a significant decrease in the GnIH concentration on a unit weight basis (mg) of diencephalon (P < 0.01, Px plus Ex vs. SH; P < 0.05, Px plus Ex vs. Px). As for the GnIH mRNA, Px alone also failed to induce a significant decrease in the GnIH concentration (Fig. 1B). These changes in GnIH mRNA and GnIH after Px plus Ex were closely related to the change in melatonin in the diencephalon and plasma (Fig. 1 C and D). Px plus Ex was followed by significant decreases in melatonin concentrations in both the diencephalon and plasma (P < 0.01, Px plus Ex vs. SH; P < 0.01 or P < 0.05, Px plus Ex vs. Px; Fig. 1 C and D). The significant decreases in GnIH mRNA (P < 0.01) and GnIH (P < 0.05) expressions in PVN neurons after Px plus Ex were confirmed histologically by in situ hybridization and immunocytochemistry, respectively (Figs. 2 A, B, D, and E and 3).
Fig. 1.
Effects of Px and Px combined with Ex (Px+Ex) on the expressions of GnIH precursor mRNA level (A) and GnIH (B) in the diencephalon and melatonin concentrations in the diencephalon (C) and plasma (D). Each column and the vertical line represent the mean ± SEM (n = 5 samples; one sample from one bird). **, P < 0.01 versus SH; ††, P < 0.01, †, P < 0.05 Px versus Px plus Ex by one-way ANOVA, followed by Duncan's multiple range test.
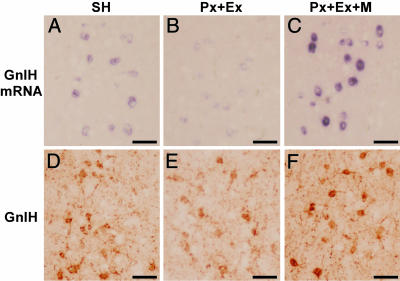
Fig. 2.
Effects of Px combined with Ex and melatonin (10 mg per plate) administration to Px plus Ex (Px + Ex) birds (Px + Ex + M) on the expressions of GnIH precursor mRNA (A–C) and GnIH (D–F) in PVN neurons. (Bars, 50 μm.)
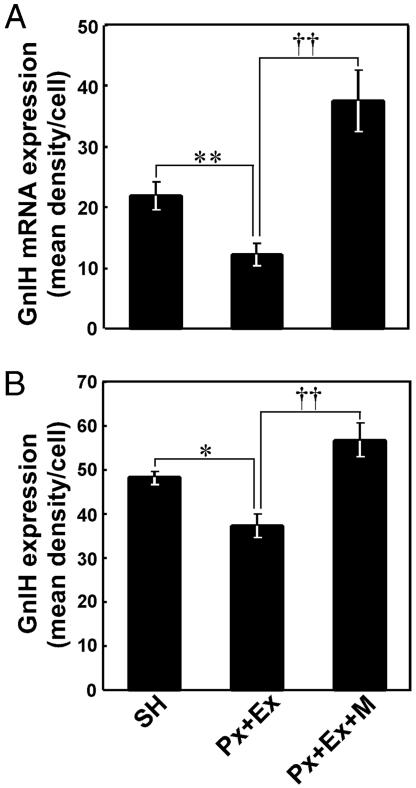
Fig. 3.
Quantitative analyses of the expression of GnIH precursor mRNA (A) and GnIH content (B) in PVN neurons in the SH, Px plus Ex, and Px plus Ex plus melatonin birds. The immunoreactivity of individual neurons was measured as a gray scale value from 0 (white) to 256 (black) and expressed as the mean density per cell, which was obtained by subtracting background gray values. Each column and the vertical line represent the mean ± SEM (n = 5 samples; one sample from one bird). **, P < 0.01; *, P < 0.05, SH versus Px plus Ex; ††, P < 0.01, Px plus Ex versus Px plus Ex plus melatonin by one-way ANOVA, followed by Duncan's multiple range test.
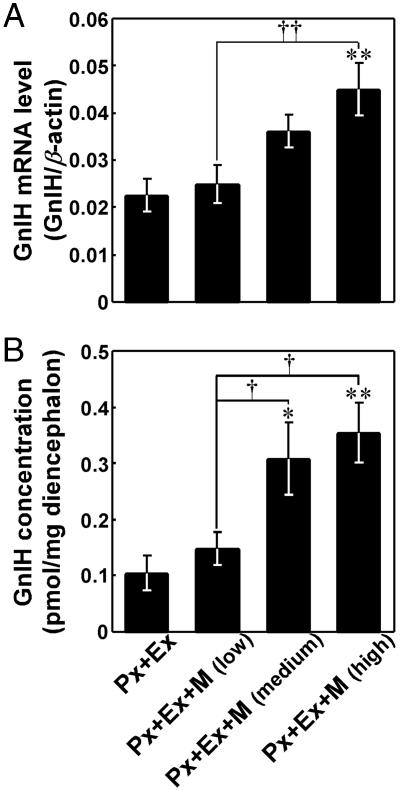
Effects of Melatonin Administration on the Expressions of GnIH Precursor mRNA and GnIH Peptide. To investigate whether melatonin is involved in GnIH induction, the expression of GnIH precursor mRNA and GnIH peptide concentration in the diencephalon were measured after melatonin administration to Px plus Ex birds. As shown in Fig. 4A, melatonin administration for one week to Px plus Ex birds was followed by a significant increase in the GnIH mRNA level in a dose-dependent manner [P < 0.01, Px plus Ex plus melatonin (high dose) vs. Px plus Ex or Px plus Ex plus melatonin (low dose)]. Melatonin administration to Px plus Ex birds also increased GnIH peptide concentration in a dose-dependent manner [P < 0.01, Px plus Ex plus melatonin (high dose) vs. Px plus Ex; P < 0.05, Px plus Ex plus melatonin (medium dose) vs. Px plus Ex; Px plus Ex plus melatonin (high dose) or Px plus Ex plus melatonin (medium dose) vs. Px plus Ex plus melatonin (low dose); Fig. 4B]. The significant increases in GnIH mRNA (P < 0.01) and GnIH (P < 0.01) expressions in PVN neurons by melatonin administration to Px plus Ex birds were confirmed histologically by in situ hybridization and immunocytochemistry, respectively (Figs. 2 B, C, E, and F and 3).
Fig. 4.
Effects of melatonin administration on the expression of GnIH precursor mRNA (A) and GnIH content (B) in the diencephalon. Various doses of melatonin (low dose: 2.5 mg per plate, medium dose: 10 mg per plate, and high dose: 40 mg per plate) were administered to Px plus Ex quail by means of a Silastic plate for 1 week (Px plus Ex plus melatonin; Px+Ex+M). Px plus Ex quail (controls) were implanted with only Silastic adhesive. Each column and the vertical line represent the mean ± SEM (n = 5 samples; one sample from one bird). **, P < 0.01; *, P < 0.05 versus Px plus Ex (control); ††, P < 0.01; †, P < 0.05 versus Px plus Ex plus melatonin (low dose) by one-way ANOVA, followed by Duncan's multiple range test.
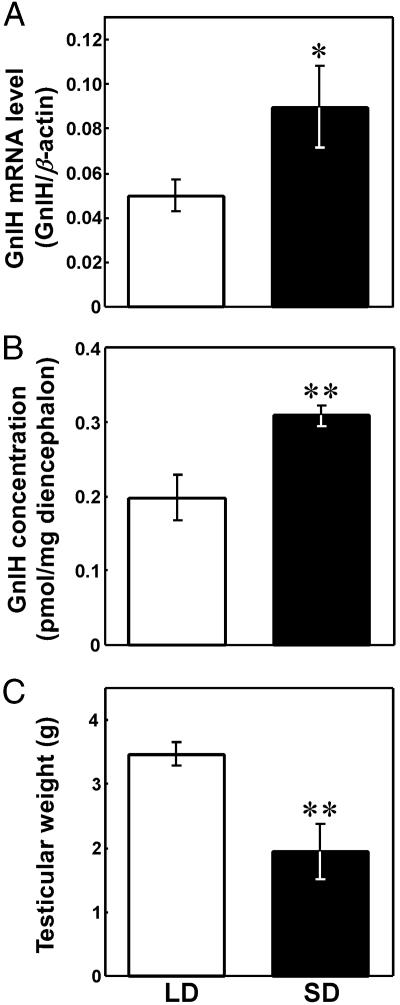
Effect of Photoperiodic Manipulation on the GnIH Expression. The nocturnal secretion of melatonin depends on the length of the dark phase of the light:dark cycle. Thus, birds were exposed to SD and LD photoperiods to manipulate endogenous melatonin secretion. In this way, we were able to analyze the effect of changes in endogenous melatonin on GnIH induction. The expressions of GnIH mRNA and GnIH in the diencephalon increased significantly in birds exposed for 3 weeks to SD compared with that in LD birds (P < 0.05 or P < 0.01, SD vs. LD; Fig. 5 A and B). Importantly, testicular weight also decreased significantly in birds exposed to SD (P < 0.01, SD vs. LD; Fig. 5C).
Fig. 5.
Effect of photoperiodic manipulation on the expression of GnIH precursor mRNA (A) and GnIH content (B) in the diencephalon and combined testicular weight (C). Quail were exposed to either LD or SD photoperiods for 3 weeks. Each column and the vertical line represent the mean ± SEM (n = 8 samples; one sample from one bird). **, P < 0.01; *, P < 0.05 versus LD by Student's t test.
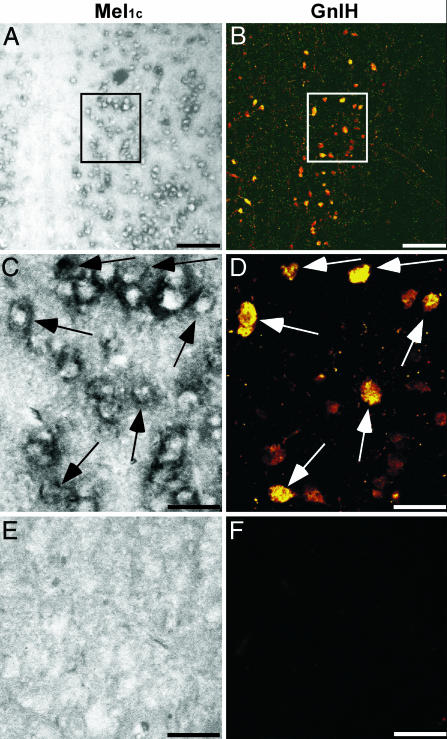
Expression of Mel1c mRNA in the GnIH Neuron. To identify the mode of melatonin action on GnIH induction, we investigated the expression of Mel1c mRNA in GnIH neurons in the PVN. Partial quail Mel1c cDNA was cloned (Fig. 7) and used for in situ hybridization of Mel1c mRNA. An intense expression of Mel1c mRNA was distributed in the PVN of the diencephalon (Fig. 6 A and C). Controls in which the sense RNA probe was substituted for the antisense RNA probe showed no positive signal in the PVN (Fig. 6E). To investigate whether Mel1c mRNA is expressed in the GnIH neuron in the PVN, immunocytochemistry for GnIH was further conducted on the same sections used for in situ hybridization of Mel1c mRNA. As shown in Fig. 6B, an intense immunoreaction with GnIH was also found in the PVN. There was clear cellular colocalization of Mel1c mRNA and GnIH (Fig. 6 C and D). A complete absence of immunoreaction with GnIH in the PVN was observed by preincubation of the antiserum with synthetic GnIH (Fig. 6F).
Fig. 6.
Expression of Mel1c mRNA in the GnIH neuron. In situ hybridization using antisense RNA probe for Mel1c mRNA in the quail PVN (A and C) was followed by immunocytochemistry for GnIH (B and D) on the same section. In situ hybridization using sense RNA probe (E) and immunocytochemistry with the GnIH antiserum preincubated with a saturating concentration of synthetic GnIH (F) served as controls. A and B are at the same low magnification. (Bars, 100 μm.) C–F are at the same high magnification. (Bars, 30 μm.) C and D are at the same high magnification of blocked areas in A and B, respectively. Arrows in C and D indicate identical cells. Similar results were obtained in repeated experiments by using six different birds.
Melatonin Binding in the PVN. There was significant IMEL binding in the PVN as compared with nonspecific binding in the same area on adjacent sections (Fig. 8, which is published as supporting information on the PNAS web site). Binding in the PVN was high compared with its immediate surrounding areas (375.4 ± 47.1% above background), but it was low, relative to other areas of the brain that routinely exhibit extremely high IMEL binding, such as the optic tectum (1,020 ± 121%).
Discussion
GnIH, a dodecapeptide identified in the quail, is the first identified hypothalamic neuropeptide directly inhibiting gonadotropin release in a vertebrate (1). GnIH is expressed in PVN neurons in the diencephalon (1–3). Until now, no mechanism regulating GnIH expression had been determined. We hypothesized that melatonin may be involved in the induction of GnIH expression, thus influencing gonadal activity. We based our hypothesis on evidence that melatonin is involved in regulation of several seasonal processes in birds, including gonadal activity and gonadotropin secretion (9–11, 13–15), and considering GnIH's inhibitory effects on gonadotropin secretion (1, 5). Our hypothesis was confirmed by a combination of experiments involving melatonin manipulation in quail. In the present study, Px combined with Ex and melatonin replacement were performed. A combination of Px plus Ex decreased the expression of GnIH precursor mRNA and GnIH peptide in the diencephalon including the PVN, concomitant with a decrease in endogenous melatonin in the diencephalon and plasma. In a further experiment, melatonin administration to Px plus Ex birds increased, in a dose-dependent fashion, GnIH mRNA expression and GnIH concentration in the diencephalon. Effects of melatonin on the expressions of GnIH mRNA and GnIH in PVN neurons were further confirmed histologically by in situ hybridization and immunocytochemistry, respectively. Furthermore, GnIH mRNA and GnIH peptide in the diencephalon increased under SD photoperiods. In quail, as in all vertebrates, the nocturnal secretion of melatonin is night-length-dependent (17), and the onset of melatonin secretion occurs soon after lights were turned off (18). Thus, the increase in GnIH expression under SD is likely to be due to the increase in the duration of endogenous melatonin secretion. Melatonin administration by means of implants is pharmacological, because they produce elevated levels of melatonin throughout the 24-h period of the light:dark cycle. Endogenous melatonin is only elevated during the dark phase of the light:dark cycle. However, the observed effects of melatonin implants on GnIH mRNA and GnIH peptide were in the same direction as those resulting from changes in endogenous melatonin. Taken together, we conclude that melatonin derived from the pineal gland and eyes acts as a potent GnIH-inducing factor in birds. To the best of our knowledge, this is the first report showing the action of melatonin on neuropeptide induction in the vertebrate brain.
To begin to understand the mode of melatonin action on GnIH induction, it is imperative to gather data on the distribution of melatonin receptor relative to GnIH neurons. Recent molecular studies (19, 29) have led to the identification of three avian melatonin receptor subtypes, Mel1a, Mel1b, and Mel1c, which comprise a distinct subfamily within the superfamily of G-protein-coupled receptors. Because Mel1c mRNA is differentially expressed in the chicken hypothalamus (19), quail Mel1c cDNA was cloned and used for in situ hybridization of Mel1c mRNA, which was combined with GnIH immunocytochemistry. The GnIH antiserum recognized PVN neurons that were positively stained by in situ hybridization of Mel1c mRNA, providing strong evidence that GnIH neurons in the PVN express Mel1c in the quail. We also examined the expressions of Mel1a and Mel1b, other melatonin receptor subtypes, in GnIH neurons by means of in situ hybridization. Although no good evidence for colocalization of Mel1a and Mel1b with GnIH neurons was found, our data (not shown) do not definitively preclude that possibility. Specific binding of melatonin in the PVN was further shown by using melatonin receptor autoradiography. In sum, melatonin most likely acts directly on GnIH neurons through Mel1c-mediated mechanisms to induce GnIH expression. Nevertheless, we cannot preclude an indirect effect of melatonin on GnIH neurons via synaptic connections, because melatonin receptor was apparently also expressed in cells in the PVN, which did not contain GnIH.
Previously, we demonstrated developmental changes in GnIH expression in the quail hypothalamo-hypophysial system (3). According to Ubuka et al. (3), GnIH begins its function around the time of hatching and acts as a hypothalamic factor to regulate gonadotropin release in quail. It has also been reported that melatonin synthesis and secretion begin during late-embryonic life in the chicken (30–32). From the present and previous studies, we suspect that the action of melatonin on GnIH expression begins around the time of hatching, and remains during development and in adulthood.
To give our findings a broader perspective, we recently cloned a homolog of GnIH from the brain of a Siberian hamster, a photoperiodic mammal (K. Inoue, T.U., K.U., L. Kriegsfeld, and K.T., unpublished data). Interestingly, the expression of the GnIH homolog in hamster hypothalamus was also controlled by melatonin (K. Inoue, T.U., K.U., L. Kriegsfeld, and K.T., unpublished observation). It is likely that the mammalian homolog of GnIH transduces photoperiodic information by means of changes in the melatonin signal, and thus influences the reproductive axis of hamsters as in birds.
The neuropeptide control of gonadotropin secretion is primarily through the stimulatory action of the hypothalamic decapeptide GnRH (33–35) in birds. Although studies of the different forms of GnRH and its receptors have revealed a wealth of information as to their function, an inhibitory hypothalamic neuropeptide for gonadotropin release has, until recently, remained unknown. The form of GnRH that controls reproductive function in birds is considered to be GnRH-1 (36), but gonadal regression caused by a decrease in photoperiod is not associated with a decrease in hypothalamic GnRH-1 in quails (37, 38). In this study, the expression of GnIH in the diencephalon increased in quail exposed to SD, which was associated with gonadal regression. Therefore, the inhibitory action of GnIH on gonadotropin secretion may be one of the main causes for gonadal regression in birds under SD photoperiods. A gonadotropin inhibitory system is an intriguing concept and provides us with an opportunity to study the regulation of avian reproduction from an alternative standpoint.
Supplementary Material
Acknowledgments
We thank the Institute for Molecular and Cellular Regulation for the supply of the antiserum cross-reacting with melatonin. We also thank Drs. A. Hattori, T. Oishi, Y. Haida, M. Hau, and M. Wikelski for valuable discussions. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (Tokyo) and the National Institutes of Health.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GnIH, gonadotropin-inhibitory hormone; GnRH, gonadotropin-releasing hormone; PVN, paraventricular nucleus; ME, median eminence; LD, long-day; SD, short-day; Px, pinealectomy, Ex, orbital enucleation; SH, Sham-operated; IMEL, [125I]iodo-melatonin.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB195674).
References
- 1.Tsutsui, K., Saigoh, E., Ukena, K., Teranishi, H., Fujisawa, Y., Kikuchi, M., Ishii, S. & Sharp, P. J. (2000) Biochem. Biophys. Res. Commun. 275, 661-667. [DOI] [PubMed] [Google Scholar]
- 2.Ukena, K., Ubuka, T. & Tsutsui, K. (2003) Cell Tissue Res. 312, 73-79. [DOI] [PubMed] [Google Scholar]
- 3.Ubuka, T., Ueno, M., Ukena, K. & Tsutsui, K. (2003) J. Endocrinol. 178, 311-318. [DOI] [PubMed] [Google Scholar]
- 4.Satake, H., Hisada., M., Kawada, T., Minakata, H., Ukena, K. & Tsutsui, K. (2001) Biochem. J. 354, 379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osugi, T., Ukena, K., Bentley, G. E., O'Brien, S., Moore, I. T., Wingfield, J. C. & Tsutsui, K. (2004) J. Endocrinol. 182, 33-42. [DOI] [PubMed] [Google Scholar]
- 6.Bronson, F. H. (1989) Mammalian Reproductive Biology (Univ. of Chicago Press, Chicago).
- 7.Wilson, F. E. (1991) J. Exp. Zool. 259, 117-127. [Google Scholar]
- 8.Juss, T. S., Meddle, S. L., Servant, R. S. & King, V. M. (1993) Proc. R. Soc. London Ser. B 254, 21-28. [DOI] [PubMed] [Google Scholar]
- 9.Ohta, M., Kadota, C. & Konishi, H. (1989) Biol. Reprod. 40, 935-941. [DOI] [PubMed] [Google Scholar]
- 10.Bentley, G. E., Demas, G. E., Nelson, R. J. & Ball, G. F. (1998) Proc. R. Soc. London Ser. B 265, 1191-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley, G. E., Van't Hof, T. J. & Ball, G. F. (1999) Proc. Natl. Acad. Sci. USA 96, 4674-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentley, G. E. & Ball, G. F. (2000) J. Neuroendocrinol. 12, 745-752. [DOI] [PubMed] [Google Scholar]
- 13.Bentley, G. E. (2001) Microsc. Res. Tech. 53, 63-71. [DOI] [PubMed] [Google Scholar]
- 14.Guyomarc'h, C., Lumineau, S., Vivien-Roels, B., Richard, J.-P. & Deregnaucourt, S. (2001) Behav. Processes 53, 121-130. [DOI] [PubMed] [Google Scholar]
- 15.Rozenboim, I., Aharony, T. & Yahav, S. (2002) Poult. Sci. 81, 1354-1359. [DOI] [PubMed] [Google Scholar]
- 16.Underwood, H., Binkley, S., Siopes, T. & Mosher, K. (1984) Gen. Comp. Endocrinol. 56, 70-81. [DOI] [PubMed] [Google Scholar]
- 17.Cockrem, J. F. & Follett, B. K. (1985) J. Endocrinol. 107, 317-324. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, V. & Follett, B. K. (1993) J. Pineal Res. 14, 192-200. [DOI] [PubMed] [Google Scholar]
- 19.Reppert, S. M., Weaver, D. R., Cassone, V. M., Godson, C. & Kolakowski, L. F., Jr. (1995) Neuron 15, 1003-1015. [DOI] [PubMed] [Google Scholar]
- 20.Oishi, T. & Lauber, J. K. (1974) Endocrinology 94, 1731-1734. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui, K., Li, D., Ukena, K., Kikuchi, M. & Ishii, S. (1998) Endocrinology 139, 4230-4236. [DOI] [PubMed] [Google Scholar]
- 22.Ubuka, T., Sakamoto, H., Li, D., Ukena, K. & Tsutsui, K. (2001) J. Endocrinol. 170, 357-368. [DOI] [PubMed] [Google Scholar]
- 23.Li, D., Tsutsui, K., Muneoka, Y., Minakata, H. & Nomoto, K. (1996) Endocrinology 137, 1618-1626. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto, H., Ubuka, T., Kohchi, C., Li, D., Ukena, K. & Tsutsui, K. (2000) Endocrinology 141, 4402-4412. [DOI] [PubMed] [Google Scholar]
- 25.Vakkuri, O., Leppaluoto, J. & Vuolteenaho, O. (1984) Acta Endocrinol. 106, 152-157. [DOI] [PubMed] [Google Scholar]
- 26.Ukena, K., Kohchi, C. & Tsutsui, K. (1999) Endocrinology 140, 805-813. [DOI] [PubMed] [Google Scholar]
- 27.Sawada, K., Ukena, K., Kikuyama, S. & Tsutsui, K. (2002) J. Endocrinol. 174, 395-402. [DOI] [PubMed] [Google Scholar]
- 28.Sawada, K., Ukena, K., Satake, H., Iwakoshi, E., Minakata, H. & Tsutsui, K. (2002) Eur. J. Biochem. 269, 6000-6008. [DOI] [PubMed] [Google Scholar]
- 29.Liu, F., Yuan, H., Sugamori, K. S., Hamadanizadeh, A., Lee, F. J., Pang, S. F., Brown, G. M., Pristupa, Z. B. & Niznik, H. B. (1995) FEBS Lett. 374, 273-278. [DOI] [PubMed] [Google Scholar]
- 30.Zeman, M. & Illnerova, H. (1990) Comp. Biochem. Physiol. A. Physiol. 97, 175-178. [DOI] [PubMed] [Google Scholar]
- 31.Zeman, M., Gwinner, E. & Somogyiova, E. (1992) Experientia 48, 765-768. [DOI] [PubMed] [Google Scholar]
- 32.Zeman, M., Gwinner, E., Herichova, I., Lamosova, D. & Kost'al, L. (1999) J. Pineal Res. 26, 28-34. [DOI] [PubMed] [Google Scholar]
- 33.King, J. A. & Millar, R. P. (1982) J. Biol. Chem. 257, 10722-10728. [PubMed] [Google Scholar]
- 34.Miyamoto, K., Hasegawa, Y., Minegishi, T., Nomura, M., Takahashi, Y., Igarashi, M., Kangawa, K. & Matsuo, H. (1982) Biochem. Biophys. Res. Commun. 107, 820-827. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto, K., Hasegawa, Y., Nomura, M., Igarashi, M., Kangawa, K. & Matsuo, H. (1984) Proc. Natl. Acad. Sci. USA 81, 3874-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp, P. J., Talbot, R. T., Main, G. M., Dunn, I. C., Fraser, H. M. & Huskisson, N. S. (1990) J. Endocrinol. 124, 291-299. [DOI] [PubMed] [Google Scholar]
- 37.Foster, R. G., Panzica, G. C., Parry, D. M. & Viglietti-Panzica, C. (1988) Cell Tissue Res. 253, 327-335. [DOI] [PubMed] [Google Scholar]
- 38.Dawson, A., King, V. M., Bentley, G. E. & Ball, G. F. (2001) J. Biol. Rhythms 16, 365-380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.