Our observations suggest that dynamic changes in the heterogeneity of microvascular blood flow at the gut mucosa are closely related to mesenteric O2 extraction, thus supporting the role of decreasing functional capillary density and increased intercapillary distances on alterations of O2 uptake during development and resuscitation from septic shock. Addition of a low-fixed dose of dobutamine might reverse such flow heterogeneity, improving microcirculatory flow distribution and tissue O2 consumption.
Keywords: microcirculation, microcirculatory blood flow, villi perfusion, oxygen extraction ratio, oxygen consumption, gut mucosal perfusion
Abstract
Derangements of microvascular blood flow distribution might contribute to disturbing O2 extraction by peripheral tissues. We evaluated the dynamic relationships between the mesenteric O2 extraction ratio () and the heterogeneity of microvascular blood flow at the gut and sublingual mucosa during the development and resuscitation of septic shock in a swine model of fecal peritonitis. Jejunal-villi and sublingual microcirculation were evaluated using a portable intravital-microscopy technique. Simultaneously, we obtained arterial, mixed-venous, and mesenteric blood gases, and jejunal-tonometric measurements. During resuscitation, pigs were randomly allocated to a fixed dose of dobutamine (5 µg·kg−1·min−1) or placebo while three sham models with identical monitoring served as controls. At the time of shock, we observed a significant decreased proportion of perfused intestinal-villi (villi-PPV) and sublingual percentage of perfused small vessels (SL-PPV), paralleling an increase in in both dobutamine and placebo groups. After starting resuscitation, villi-PPV and SL-PPV significantly increased in the dobutamine group with subsequent improvement of functional capillary density, whereas exhibited a corresponding significant decrease (repeated-measures ANOVA, P = 0.02 and P = 0.04 for time × group interactions and intergroup differences for villi-PPV and , respectively). Variations in villi-PPV were paralleled by variations in (R2 = 0.88, P < 0.001) and these, in turn, by mesenteric lactate changes (R2 = 0.86, P < 0.001). There were no significant differences in cardiac output and systemic O2 delivery throughout the experiment. In conclusion, dynamic changes in microvascular blood flow heterogeneity at jejunal mucosa are closely related to the mesenteric O2 extraction ratio, suggesting a crucial role for microvascular blood flow distribution on O2 uptake during development and resuscitation from septic shock.
NEW & NOTEWORTHY Our observations suggest that dynamic changes in the heterogeneity of microvascular blood flow at the gut mucosa are closely related to mesenteric O2 extraction, thus supporting the role of decreasing functional capillary density and increased intercapillary distances on alterations of O2 uptake during development and resuscitation from septic shock. Addition of a low-fixed dose of dobutamine might reverse such flow heterogeneity, improving microcirculatory flow distribution and tissue O2 consumption.
one of the most notable characteristics of the inflammatory response during sepsis in both humans and animal experimental models is its deleterious effect on microcirculation. These microcirculatory blood flow alterations are mainly characterized by decreased functional capillary density and increased heterogeneity leading to blood flow misdistribution (1, 10, 18, 23, 34), tissue hypoperfusion, and the subsequent development of multiple organ dysfunction (48). It was initially proposed that a mismatch of O2 demand to supply could impair O2 utilization by tissues (26, 40, 41). This notion was reinforced by mathematical models suggesting that heterogeneity of O2 delivery could decrease tissue O2 extraction (49). In fact, evidence coming from ex vivo intestinal tissue in endotoxemic pigs revealed the importance of increased heterogeneity in microcirculatory transit times on the impairment of O2 extraction (21). The nature of such experiments, however, hindered the temporal variations of heterogeneity of flow during the development of, and resuscitation from, septic shock. Other experimental data suggested that the misdistribution of microvascular blood flow leads to O2 capillary extraction derangements during sepsis (11) such that heterogeneous flow cessation of individual capillaries could determine O2 supply dependence during the most severe cases of septic shock (16).
The gastrointestinal tract is particularly prone to being affected during low flow states (9, 44), and it has been hypothesized that improvement in splanchnic perfusion might prevent the progression of shock. Thus, some synthetic catecholamines capable of increasing cardiac output and O2 delivery have been used aiming at reverting tissue hypoperfusion. Its impact on splanchnic circulation remains controversial, however, with apparent favorable (3, 33, 37, 39, 47), unchanged (35), or even negative effects (2, 13) on both splanchnic arterial flow and total microcirculatory blood flow at the intestinal mucosa. Despite these different effects on macro and total microvascular blood flow, some vasoactive amines and inodilators have been shown to promote favorable effects on intestinal oxygenation (14), although mucosal oxygenation might, in turn, be dissociated from splanchnic O2 delivery when some of them are infused (33, 43), which suggests the predominance of distributive alterations over total reductions in microcirculatory blood flow. Notably, microvascular blood flow distribution could potentially be modified by inodilators during septic shock (7, 42), although its effect during human septic shock remains controversial (12, 18).
We therefore proposed to evaluate the dynamic variations of the heterogeneity of blood flow distribution at the intestinal mucosa and its relation with the regional O2 extraction ratio during the development and resuscitation of septic shock in a model of fecal peritonitis subjected to hemodynamic goal-directed fluid resuscitation and randomly assigned to receive, or not, a low-fixed dose of dobutamine, hypothesizing that variations in microcirculatory heterogeneity are closely related to changes in regional O2 extraction independent of macrohemodynamic changes.
METHODS
Animal preparation.
Our institutional Animal Research Committee approved the present study (Res. 001–12). Fifteen female Landrace pigs (35–42 kg) were kept fasting for a 12-h period, with free access to water. After this preconditioning period, they were initially sedated with intramuscular injections of ketamine (5–10 mg/kg) and xylazine (0.1 mg/kg). Next, a venous access (Insyte Autoguard infusion therapy system) was inserted in the ear to facilitate the administration of medication and fluids. After an intravenous dose of propofol (2–4 mg/kg) and fentanyl (5 µg/kg), an endotracheal tube and mainstream volumetric capnography were placed (Infinity EtCO2 + respiratory mechanics module; Dräger Medical Systems). The animals were connected to mechanical ventilation (Servo 900C; Siemens, Solna, Sweden) in assist control mode, with a tidal volume of 12 ml/kg, and the volume per minute was adjusted to maintain arterial Pco2 at 38 ± 5 mmHg. Anesthesia was maintained throughout the experiment with midazolam (3–5 µg·kg−1·min−1), fentanyl (0.03–0.05 µg·kg−1·min−1), and propofol (50 µg·kg−1·min−1). Muscular paralysis was provided with pancuronium bromide (5 µg·kg−1·min−1) during the entire experiment. Neck vessels were accessed by direct dissection, and catheters were inserted in the carotid artery (Single lumen central venous 7-Fr catheter; Arrow International) to monitor arterial pressure and to enable blood sampling while the left internal jugular vein was used for resuscitation fluid infusion. We also placed a continuous-cardiac-output (CCO) pulmonary artery catheter (7.5-Fr, Edwards Swan-Ganz CCO; Baxter Edwards Critical Care, Irvine, CA) through the right internal jugular vein to measure pulmonary arterial pressures and continuous cardiac output and to enable the withdrawal of mixed-venous blood samples. Core temperature was continuously monitored using a thermistor in the pulmonary artery catheter, and external heating was used to maintain a central temperature of 36.5 ± 1°C. Continuous electrocardiographic, pulsioximetry, and invasive pressures were also monitored throughout the experiment (Drägger Infinity Vista XL; Drägger Medical System, Lübeck, Germany).
A midline laparotomy was performed, and a gas-tonometer catheter (TRIP Tonometry Catheter, 8-Fr; Tonometrics Division, Instrumentarium, Helsinki, Finland) was placed in the jejunal lumen at 50 cm beyond the Treitz angle for local tissue CO2 and pHi measurements. A double-lumen catheter (2-lumen central venous 7-Fr gauge catheter; Arrow International) was inserted in the superior mesenteric vein through the splenic vein after splenectomy and local constriction with epinephrine, ensuring its position under echographic guide. An infusion with 0.9% saline solution at 5 ml/h through this catheter was provided during the experiment to ensure its permeability. Surgical cystostomy was also undertaken to monitor urinary output. A jejunum loop was exteriorized through the midline incision, and a small segment was opened along its antimesenteric border using an electrocautery. After baseline measurements, cecal ligation and puncture with 16-gauge needle followed by peritoneal contamination (1.5 g/kg of feces) were performed in experimental models (not for sham animals). After careful hemostasis, the abdominal contents were returned to the cavity, and the abdomen was closed, leaving out the jejunostomy loop, which was then covered with moistened compresses and an anti-adherent bag to avoid heat and fluid loss.
General monitoring.
Mean arterial pressure was monitored throughout the experiment, and pulse pressure variations (dPP) were calculated during the respiratory cycle as: PPinsp – PPexp/(PPinsp + PPexp/2) (Drägger Infinity Vista XL; Drägger Medical System). Mean pulmonary artery, central venous, and pulmonary arterial occlusion pressures were measured at the end of expiration and referenced to the midchest level. Cardiac output was continuously measured using the thermodilution principle with a thermal filament on the pulmonary artery catheter (Vigilance; Baxter Edwards Critical Care). A mainstream capnography and respiratory module (Dräger Medical Systems) was used to measure airway and alveolar dead-space fraction, end-tidal CO2, and complete respiratory mechanics.
Calculation of CO2 and O2 variables.
Simultaneous arterial (a), mixed-venous (), and mesenteric-venous (mes) blood samples were withdrawn at each measurement time point to determine blood gases, hemoglobin, and lactate concentrations (The Alere Epoc blood analysis system; Alere, Waltham, MA). Concurrent mucosal jejunal CO2 was measured by gas tonometry (Tonocap, Datex-Ohmeda; Tonometrics Division, Instrumentarium) as described elsewhere (4). O2 and CO2 parameters were calculated according to the following formulas:
where and are the arterial and venous O2 content, respectively; and represent the arterial and venous partial pressures, respectively, and CO represents cardiac output. and represent the total and mesenteric O2 extraction ratio, respectively.
Microcirculatory measurements.
We used the Sidestream dark-field (SDF) technique (Micro Scan; MicroVision Medical, Amsterdam, The Netherlands) to explore microcirculation at each measurement time point (Fig. 1). In all cases, images were acquired by the same operator (Ospina-Tascón) who remained blinded as to dobutamine or placebo use both during acquisition and the semiquantitative analysis of the video sequences. After careful removal of intestinal secretions by warm water and gentle aspiration, the SDF device was directly applied through the surgically prepared jejunostomy and on the antimesenteric opposite serosa surface to evaluate jejunal villi and serosa microcirculation, respectively, covering an intestinal segment of at least 15 cm. Meanwhile, sublingual microcirculation was assessed by SDF soft application to the lateral side of the tongue covering an area approximately of 4–6 cm from the tip of tongue after gentle removal of secretions with gauze. At each measurement time point (Fig. 1), five video sequences of 20 s each were acquired at five different points from the respective mucosa or adjacent serosa areas using a videocard (MicroVideo; Pinnacle System, Mountain Views, CA). These sequences of video were stored under a random number and later evaluated by two investigators blind to the origin of such sequences (Quiñones and Ospina-Tascón). For this semiquantitative analysis, we counted the number of villi in each image, and individual villi microcirculation was classified according to its predominant blood flow as either normal perfused (continuous blood flow), hypoperfused (intermittent or sluggish blood flow), or nonperfused (stopped blood flow). Thus, we calculated the percentage of villi with normal-perfused capillaries in each video sequence to finally report the mean of the five video sequences acquired at each time point. Meanwhile, for serosa and sublingual microcirculation evaluation, we used a cutoff value of 20 μm to classify the vessels as large or small. Microvessels with continuous flow were considered as normal, whereas sluggish, intermittent, and stopped flows were considered as abnormal. Serosa and sublingual microcirculatory blood flow were evaluated according to the consensus for the evaluation of microcirculation (8), and we also calculated the proportion of small-perfused vessels (<20 μm diameter), the heterogeneity index (HI), the total vascular density (all vessels), and the functional capillary density (i.e., the number of vessels <20 μm of diameter adequately perfused, per area unit). The intra- and interobserver variabilities were determined by using five sequences analyzed five times at 8-wk intervals by two observers (Quiñones and Ospina-Tascón). We calculated the intraobserver and interobserver coefficient of variability for both the total number of vessels and the proportion of perfused vessels.
Fig. 1.
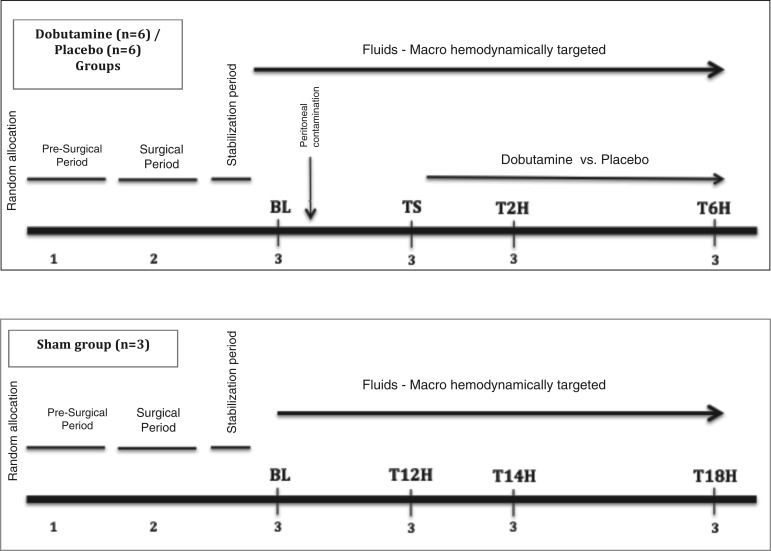
Experimental protocol. 1, Presurgical period (acclimatization-adaptation period, initial sedation, muscular paralysis, endotracheal intubation); 2, surgical period (catheters insertion: carotid and pulmonary artery, internal jugular vein, mesenteric vein; laparotomy: jejunal tonometer insertion; cystostomy; jejunal ostomy preparation); 3, serial measurements [arterial, mixed-venous, and mesenteric-venous blood sampling; jejunal tonometric measurements; acquisition of microcirculation images (jejunal mucosa, jejunal serosa, sublingual mucosa)]. BL, baseline; TS, time of shock; T2H, 2 h after starting resuscitation; T6H, 6 h after starting resuscitation.
Experimental protocol.
The experimental protocol is outlined in Fig. 1. After the initial preparation, a stabilization period of 30 min was ensured, and then baseline measurements (BL) were performed. Animals were subsequently randomly allocated to the dobutamine (n = 6), placebo (n = 6), or sham (n = 3) group. An independent laboratory staff member (not participating as an author in the present manuscript) was in charge of the randomization and preparation of these experimental infusions. Cecal ligation and puncture, followed by peritoneal contamination, was performed only in the dobutamine and placebo models. All animals received intravenous lactate Ringer fixed infusion at 3 ml/kg throughout the experiment. When hypotension was developed and it was not corrected by successive crystalloid boluses (at least 30 ml/kg), norepinephrine (NE) infusion was started and titrated to maintain mean arterial pressure (MAP) >65 mmHg. Once the NE dose remained stable for at least 30 min, time shock (TS) was declared, and a new set of measurements were obtained. After this point, resuscitation was conducted using successive fluid loads of crystalloid (at 10 ml/kg) guided by dynamic predictors of fluid responsiveness to optimize cardiac output. Concomitantly, fixed doses of dobutamine (5 µg·kg−1·min−1) or placebo (0.9% saline solution at isovolumetric dose) were infused throughout the experiment. A new set of measurements was performed at 2 (T2H) and 6 (T6H) h afterward. Sham animals were subjected to identical monitoring as the experimental groups. The timing used for measurements in the sham group was referenced to the median time required from peritonitis induction to fulfilling shock definition during the preexperimental standardization phase. Thus, T12H in the sham group was selected as the matched time for TS, so new measurements were performed 2 and 6 h later, thus constituting T14H and T18H, i.e., the equivalent to T2H and T6H in the experimental models (Fig. 1). Euthanasia was performed at the end of the experiment using Euthanex according to the local regulations for animal research.
Statistical analysis.
Data are reported as medians and interquartile ranges. After testing the sphericity assumptions, time, time-group interactions, and intergroup differences for the experimental groups were evaluated using the repeated-measures analysis of variance, with a subsequent Student-Newman-Keuls (SNK) test for multiple comparisons. The relationship between microcirculatory blood flow variables, lactate levels, and mesenteric O2 extraction ratio was tested using the Spearman Rho test, and the coefficient of determination (R2) was calculated to establish the strength of such associations. A P value ≤ 0.05 (2-tailed) was considered significant.
RESULTS
Systemic hemodynamics and O2 transport.
General hemodynamics, blood gases, and fluids/vasopressor use are presented in Table 1. Hypodynamic shock was developed 12 (10–14) h after the induction of peritonitis in the dobutamine (D group) and placebo (P group) models, with similar falls in cardiac output [91.2 (84.8–94.1) vs. 75.0 (68.2–95.1) ml·kg−1·min−1 for P and D group, respectively; P = 0.21] and systemic O2 delivery [13.4 (12.5–15.1) vs. 13.9 (10.9–15.0) ml·kg−1·min−1 for P and D groups, respectively; P = 0.88] (Table 1). Subsequently, cardiac output and systemic O2 delivery exhibited a similar improvement in the D and P groups at T2H and T6H (repeated-measurements analysis, P = 0.86 and 0.84 for time × group interactions, and P = 0.51 and 0.56 for intergroup differences for CO and Do2, respectively) (Table 1).
Table 1.
Hemodynamics, blood gases, and O2/CO2 parameters
| Intragroup Difference |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | TS | T2H | T6H | Time effect | Time × group effect | Intergroup Difference | |
| Hemodynamic variables | |||||||
| HR, beats/min | |||||||
| Placebo | 90 (81–130) | 154 (131–180) | 155 (153–168) | 164 (132–174) | 0.01 | 0.99 | 0.34 |
| Dobutamine | 97 (87–128) | 187 (113–205) | 156 (142–189) | 172 (146–197) | |||
| Sham | 146 (140–151) | 108 (96–120) | 99 (98–100) | 70 (54–85) | |||
| CO, ml·kg−1·min−1 | |||||||
| Placebo | 122.9 (111.8–138.2) | 91.2 (84.8–94.1) | 125.7 (120.6–138.2) | 138.2 (128.6–145.5) | <0.001 | 0.86 | 0.51 |
| Dobutamine | 100.0 (90.9–120.0) | 75.0 (68.2–95.1) | 106.8 (90.9–144.1) | 123.2 (104.5–152.7) | |||
| Sham | 104.3 (100.0–108.6) | 108.7 (108.6–108.8) | 108.7 (105.7–111.8) | 113.0 (111.8–114.3) | |||
| MAP, mmHg | |||||||
| Placebo | 102 (92–114) | 70 (69–77) | 71 (69–82) | 65 (63–97) | 0.001 | 0.18 | 0.54 |
| Dobutamine | 100 (88–105) | 81 (77–87) | 67 (65–71) | 77 (60–82) | |||
| Sham | 97 (87–106) | 100 (81–118) | 102 (100–105) | 129 (123–135) | |||
| CVP, mmHg | |||||||
| Placebo | 12 (7–21)- | 13 (10–15) | 13 (11–18) | 14 (12–22) | 0.15 | 0.98 | 0.29 |
| Dobutamine | 10 (5–14) | 9 (8–10) | 11 (10–12) | 12 (10–15) | |||
| Sham | 14 (13–15) | 15 (13–16) | 13 (12–15) | 15 (11–18) | |||
| Temperature, °C | |||||||
| Placebo | 35.8 (34.3–36.4) | 38.1 (35.1–38.3) | 37.8 (34.3–38.1) | 36.8 (33.8–38.4) | 0.02 | 0.76 | 0.38 |
| Dobutamine | 35.9 (34.9–37.0) | 38.2 (37.1–39.8) | 37.0 (36.4–37.6) | 36.6 (36.3–37.3) | |||
| Sham | 34.7 (33.8–35.6) | 34.6 (33.5–35.7) | 35.1 (34.1–36) | 34.5 (33.1–35.6) | |||
| Blood gases, lactate, and O2 variables | |||||||
| pH (arterial) | |||||||
| Placebo | 7.38 (7.37–7.40) | 7.34 (7.28–7.36) | 7.31 (7.24–7.41) | 7.20 (7.10–7.42) | 0.002 | 0.11 | 0.55 |
| Dobutamine | 7.38 (7.38–7.39) | 7.27 (7.21–7.28) | 7.20 (7.14–7.26) | 7.29 (7.27–7.32) | |||
| Sham | 7.39 (7.38–7.40) | 7.42 (7.40–7.43) | 7.41 (7.39–7.42) | 7.42 (7.40–7.45) | |||
| , mmHg | |||||||
| Placebo | 34.6 (32.9–37.5) | 40.1 (35.0–40.2) | 37.6 (36.2–42.4) | 38.6 (37.2–40.3) | 0.01 | 0.15 | 0.83 |
| Dobutamine | 29.9 (29.5–33.2) | 41.7 (36.3–50.5) | 42.2 (41.6–46.3) | 39.1 (36.0–39.8) | |||
| Sham | 34.1 (33.6–34.5) | 34.7 (34.2–35.1) | 34.7 (34.2–35.1) | 35.0 (34.6–35.3) | |||
| , mmHg | |||||||
| Placebo | 3.0 (2.7–4.2) | 12.0 (11.0–15.5) | 11.7 (11.6–16.0) | 9.9 (9.3–14.1)* | <0.001 | 0.06 | 0.05 |
| Dobutamine | 4.9 (4.5–5.1) | 13.6 (12.1–16.6) | 9.2 (6.6–10.2) | 6.5 (4.7–7.5) | |||
| Sham | 3.5 (3.3–3.7) | 4.1 (3.7–4.4) | 4.0 (3.7–4.4) | 4.4 (4.0–4.7) | |||
| , mmHg | |||||||
| Placebo | 5.8 (3.7–6.8) | 18.4 (18.2–18.9) | 17.6 (11.0–22.4)* | 19.2 (9.3–19.2)* | <0.001 | 0.01 | 0.05 |
| Dobutamine | 5.8 (3.8–6.4) | 20.3 (17.5–23.4) | 7.0 (5.7–11.1) | 5.1 (3.7–5.9) | |||
| Sham | 6.3 (4.8–7.7) | 3.6 (3.5–3.7) | 3.6 (3.5–3.7) | 4.6 (2.7–6.5) | |||
| , mmHg | |||||||
| Placebo | 10.4 (6.4–11.1) | 32.0 (25.9–34.0) | 23.4 (23.3–30.6) | 33.4 (24.7–34.8)* | <0.001 | 0.01 | 0.01 |
| Dobutamine | 11.1 (4.5–14.0) | 30.7 (28.5–34.7) | 20.4 (19.4–24.3) | 16.0 (14.2–16.9) | |||
| Sham | 9.5 (8.5–10.4) | 6.4 (3.9–8.8) | 6.8 (5.2–8.4) | 13.1 (12.4–13.7) | |||
| Lactate-art, mmol/l | |||||||
| Placebo | 1.8 (1.7–2.3) | 7.4 (7.1–7.8) | 8.3 (6.7–9.9)* | 8.9 (8.4–9.3)* | <0.001 | 0.03 | 0.02 |
| Dobutamine | 1.9 (1.9–2.1) | 7.5 (7.5–8.1) | 4.4 (3.8–7.4) | 2.3 (2.1–3.9) | |||
| Sham | 1.4 (1.1–1.7) | 0.9 (0.8–1.0) | 1.1 (1.0–1.2) | 1.2 (0.7–1.8) | |||
| Lactate-mes, mmol/l | |||||||
| Placebo | 2.6 (2.0–2.6) | 8.2 (7.9–8.5) | 8.9 (5.6–12.2) | 7.3 (4.6–10.3)* | <0.001 | 0.02 | 0.03 |
| Dobutamine | 2.1 (1.8–2.2) | 7.6 (7.3–8.2) | 6.4 (3.8–7.1) | 4.2 (3.4–4.8) | |||
| Sham | 1.6 (1.2–2.0) | 1.0 (1.0–1.1) | 1.2 (1.1–1.3) | 1.1 (0.8–1.4) | |||
| , % | |||||||
| Placebo | 67.6 (61.4–70.3) | 68.8 (55.6–72.5) | 73.7 (73.4–74.8) | 77.5 (71.4–77.8) | 0.14 | 0.19 | 0.22 |
| Dobutamine | 62.5 (53.0–71.1) | 62.0 (61.2–71.7) | 66.1 (62.6–66.4) | 65.8 (64.5–66.0) | |||
| Sham | 71.4 (69.8–71.9) | 72.7 (68.3–77.1) | 65.9 (65.8–66.0) | 77.1 (74.5–79.6) | |||
| , % | |||||||
| Placebo | 74.4 (72.3–75.0) | 34.4 (32.5–36.0) | 38.4 (35.1–52.7)* | 52.2 (48.9–57.2)* | <0.001 | 0.08 | 0.03 |
| Dobutamine | 75.5 (74.9–78.0) | 32.0 (30.5–32.5) | 58.7 (45.0–60.5) | 67.5 (64.4–68.4) | |||
| Sham | 73.9 (72.3–75.6) | 67.5 (66.1–68.8) | 68.5 (67.0–70.0) | 70.8 (69.9–71.6) | |||
| Do2, ml·kg−1·min−1 | |||||||
| Placebo | 17.0 (14.1–18.9) | 13.4 (12.5–15.1) | 17.1 (15.8–21.9) | 18.4 (17.7–18.7) | 0.01 | 0.84 | 0.56 |
| Dobutamine | 14.5 (13.5–17.3) | 13.9 (10.9–15.0) | 18.6 (12.2–19.3) | 18.0 (15.4–20.0) | |||
| Sham | 12.5 (12.4–12.5) | 12.9 (12.3–13.5) | 13.0 (12.3–13.6) | 13.0 (12.1–13.8) | |||
| V̇o2, ml·kg−1·min−1 | |||||||
| Placebo | 5.9 (4.4–7.0) | 5.8 (4.9–6.2) | 8.2 (7.8–8.3) | 8.9 (7.1–8.9) | 0.003 | 0.38 | 0.18 |
| Dobutamine | 5.6 (4.7–5.7) | 6.0 (4.7–6.5) | 7.3 (6.3–7.9) | 6.2 (6.1–7.1) | |||
| Sham | 4.2 (4.0–4.4) | 4.7 (4.7–4.8) | 4.5 (4.2–4.8) | 4.7 (4.4–5.0) | |||
| Systemic | |||||||
| Placebo | 0.34 (0.30–0.40) | 0.42 (0.37–0.46) | 0.47 (0.41–0.48) | 0.48 (0.40–0.55) | 0.02 | 0.20 | 0.40 |
| Dobutamine | 0.36 (0.35–0.39) | 0.40 (0.39–0.47) | 0.41 (0.40–0.41) | 0.40 (0.36–0.40) | |||
| Sham | 0.33 (0.32–0.35) | 0.37 (0.35–0.38) | 0.35 (0.34–0.35) | 0.36 (0.36–0.37) | |||
| Mesenteric | |||||||
| Placebo | 0.27 (0.27–0.29) | 0.65 (0.64–0.65) | 0.62 (0.47–0.63)* | 0.44 (0.44–0.46)* | <0.001 | 0.04 | 0.02 |
| Dobutamine | 0.26 (0.23–0.26) | 0.68 (0.64–0.68) | 0.37 (0.37–0.49) | 0.32 (0.25–0.35) | |||
| Sham | 0.27 (0.25–0.28) | 0.33 (0.32–0.35) | 0.32 (0.30–0.34) | 0.31 (0.29–0.32) | |||
| Fluids/vasopressors | |||||||
| Cumulative fluids, ml | |||||||
| Placebo | 2,013 (1,977–2,237) | 2,810 (2,643–2,877) | 3,285 (3,218–3,339) | <0.001 | 0.90 | 0.85 | |
| Dobutamine | 2,190 (2,112–2,376) | 2,792 (2,790–3,116) | 3,248 (3,150–3,644) | ||||
| Sham | 1259 (1200–1290) | 1,559 (1,500–1,600) | 1,973 (1,950–1,998) | ||||
| Norepinephrine, µg·kg−1·min−1 | |||||||
| Placebo | 0.52 (0.42–0.53) | 0.28 (0.25–0.30) | 0.20 (0.18–0.24) | <0.001 | 0.94 | 0.44 | |
| Dobutamine | 0.49 (0.45–0.58) | 0.26 (0.24–0.32) | 0.20 (0.20–0.22) | ||||
| Sham | |||||||
All data are presented as medians (25–75 percentiles); n = 6 (dobutamine), 6 (placebo), and 3 (sham) animals in each group. TS, time shock; T2H, 2 h after starting resuscitation; T6H, 6 h after starting resuscitation; HR, heart rate; CO, cardiac output; MAP, mean arterial pressure; CVP, central venous pressure; pH, arterial pH; , arterial CO2 partial pressure; , mixed venous-to-arterial CO2 difference; , mesenteric venous-to-arterial CO2 difference; , tissue (jejunal mucosa)-to-arterial CO2 difference; Lactate art, arterial lactate; Lactate mes, mesenteric lactate; , mixed-venous O2 saturation; , mesenteric-venous O2 saturation; Do2, systemic O2 delivery; V̇o2, systemic O2 consumption; systemic , systemic O2 extraction ratio; mesenteric , mesenteric O2 extraction ratio.
SNK test, P < 0.05 for P vs. D group.
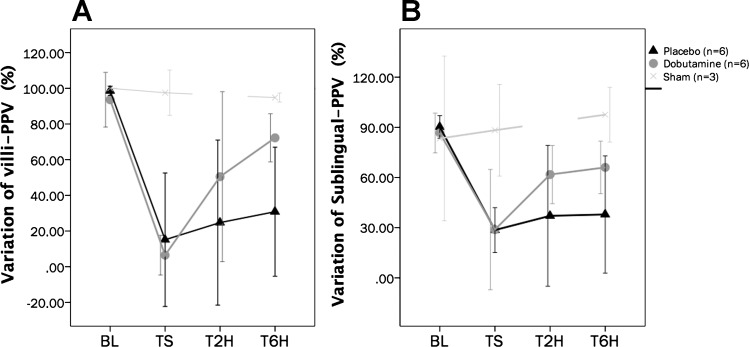
Microcirculatory blood flow.
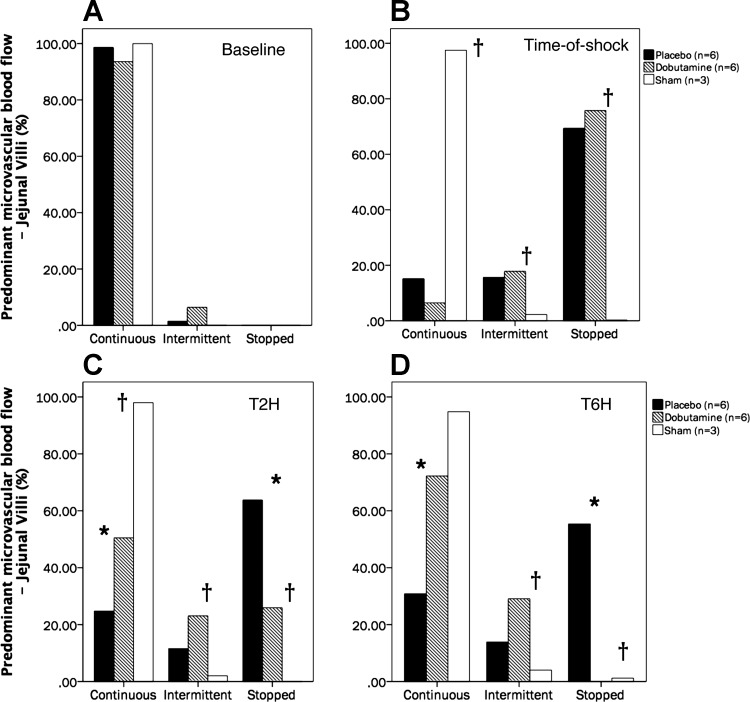
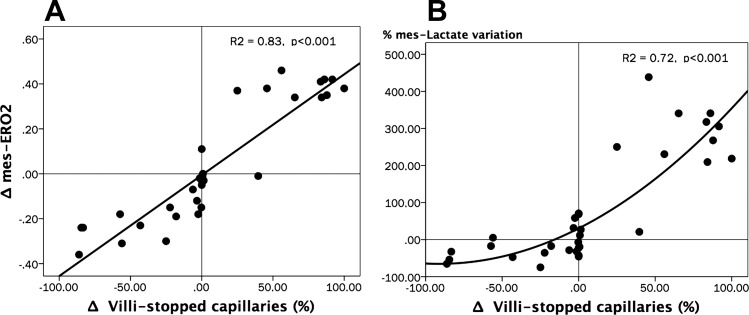
Complete intestinal and sublingual microcirculatory parameters are presented in Table 2. The time course of the proportion of jejunal villi with normal-perfused vessels (%Villi-PPV) was significantly different between groups (repeated-measures analysis, P = 0.02 for time × group interactions and P = 0.04 for intergroup differences; SNK test, P < 0.05 for P vs. D at T2H and T6H) (Fig. 2). The percentage of small vessels perfused at the sublingual mucosa (SL-PPV) exhibited similar variations to villi-PPV, with significant improvement in the D group, although without attaining complete normalization at the end of the experiment (Fig. 2). An excess of stopped vessels at the intestinal mucosa explained the decreasing %villi-PPV at TS in the P and D groups (Fig. 3). Thereafter, the D group exhibited significant and progressive improvement of microcirculation at the jejunal-villi, moving from stopped to intermittent and continuous flows, thus improving functional capillary density and decreasing microvascular blood flow heterogeneity (Fig. 3), although without attaining complete normalization at T6H. Dynamic changes in the proportion of capillaries with stopped flow were significantly related to variations in (Spearman Rho test, R2 = 0.83, P < 0.001) and lactate levels (Spearman Rho test, R2 = 0.72, P < 0.001) (Fig. 4). Meanwhile, jejunal serosa and sublingual mucosa exhibited similar variations to those observed at the jejunal-villi, with severely decreased PPV at TS, followed by significant recovery in the D group (Table 2). Such alterations were paralleled by decreased functional capillary density and increasing microvascular blood flow heterogeneity at both the jejunal serosa and sublingual mucosa (Table 2). Examples of the intestinal microcirculatory blood flow images captured by the SDF technique during the development and resuscitation phase of the experiment are presented as supplemental data [baseline (Supplemental Videos 1A–1B); TS (Supplemental Videos 2A–2B), and dobutamine at T6H (Supplemental Videos 3A–3B) (Supplemental videos for this article can be found on the journal website.)]. Our coefficient of variability of the determination of one video sequence ranged from 2.9 to 6.4% (intraobserver) and from 3.8 to 6.2% (interobserver) for the total number of vessels and from 1.9 to 4.5% (intraobserver) and from 3.4 to 6.8% (interobserver) for the proportion of perfused vessels (all sizes).
Table 2.
Microcirculatory blood flow parameters
| Intragroup Difference |
|||||||
|---|---|---|---|---|---|---|---|
| Baseline | TS | T2H | T6H | Time effect | Time × group effect | Intergroup Difference | |
| Villi-PPV, % | |||||||
| Placebo | 100.0 (97.5–100.0) | 0.0 (0.0–6.8) | 6.9 (0.0–28.9)* | 18.8 (17.0–31.8)* | <0.001 | 0.02 | 0.04 |
| Dobutamine | 98.5 (98.0–100.0) | 0.0 (0.0–13.9) | 48.9 (16.7–76.5) | 79.0 (67.4–79.6) | |||
| Sham | 97.5 (96.5–98.5) | 97.5 (96.5–98.5) | 94.8 (94.6–95.0) | ||||
| Ser-PPV, % | |||||||
| Placebo | 97.4 (90.1–99.7) | 31.2 (16.7–37.8) | 34.3 (22.6–49.2) | 32.7 (6.5–58.7)* | <0.001 | 0.01 | 0.04 |
| Dobutamine | 100.0 (87.9–100.0) | 16.7 (0.0–20.8) | 45.4 (7.4–45.7) | 85.7 (75.7–91.5) | |||
| Sham | 68.7 (67.8–69.6) | 89.0 (88.5–89.4) | 97.8 (95.6–100.0) | ||||
| Ser-FCD, vessels/mm2 | |||||||
| Placebo | 6.8 (5.6–7.9) | 1.3 (0.4–2.2) | 2.0 (1.1–2.3) | 2.5 (2.1–2.7)* | <0.001 | 0.12 | 0.03 |
| Dobutamine | 6.3 (5.9–8.8) | 0.9 (0.0–1.2) | 2.1 (0.4–2.4) | 5.2 (3.5–5.6) | |||
| Sham | 5.5 (5.3–5.6) | 6.8 (6.7–6.8) | 6.3 (5.2–7.4) | ||||
| Ser-HI | |||||||
| Placebo | 0.2 (0.0–0.2) | 1.5 (0.8–2.0) | 1.0 (1.0–1.5)* | 2.0 (1.5–2.6)* | 0.03 | 0.12 | 0.03 |
| Dobutamine | 0.0 (0.0–0.3) | 2.0 (0.0–2.8) | 0.5 (0.2–0.6) | 0.1 (0.0–0.3) | |||
| Sham | 0.7 (0.3–1.1) | 0.3 (0.2–0.4) | 0.3 (0.0–0.6) | ||||
| SL-PPV, % | |||||||
| Placebo | 87.0 (85.9–95.7) | 31.8 (21.2–32.1) | 34.5 (8.2–67.2)* | 22.8 (22.7–67.0)* | <0.001 | 0.02 | 0.01 |
| Dobutamine | 86.2 (81.0–95.1) | 20.7 (13.2–29.9) | 68.3 (47.5–71.1) | 69.1 (58.2–70.3) | |||
| Sham | 83.3 (79.5–87.2) | 88.3 (86.1–90.4) | 97.6 (96.3–98.9) | ||||
| SL-FCD, vessels/mm2 | |||||||
| Placebo | 7.0 (6.9–7.1) | 2.5 (1.6–3.0) | 2.8 (0.5–3.8)* | 2.1 (1.9–4.5)* | <0.001 | 0.11 | 0.04 |
| Dobutamine | 6.5 (6.0–6.9) | 1.7 (0.8–2.2) | 4.6 (4.0–5.7) | 4.6 (4.5–5.7) | |||
| Sham | 7.6 (7.4–7.8) | 9.1 (8.9–9.3) | 10.9 (10.9–11.0) | ||||
| SL-HI | |||||||
| Placebo | 0.3 (0.1–0.4) | 2.1 (2.0–3.1) | 1.5 (0.8–2.3) | 1.7 (1.1–2.5)* | <0.001 | 0.02 | 0.04 |
| Dobutamine | 0.8 (0.8–0.9) | 1.5 (1.5–2.6) | 0.9 (0.8–1.2) | 0.9 (0.4–1.0) | |||
| Sham | 0.4 (0.3–0.4) | 0.3 (0.2–0.3) | 0.1 (0.1–0.1) | ||||
All data are presented as medians (25–75 percentiles); n = 6 (dobutamine), 6 (placebo), and 3 (sham) animals in each group. Villi-PPV, proportion of jejunal-villi with well-perfused vessels; Ser-PPV, percentage of small vessels perfused at jejunal serosa; Ser-FCD, functional capillary density at jejunal serosa; Ser-HI, heterogeneity index at jejunal serosa; SL-PPV, percentage of small vessels perfused at sublingual mucosa; SL-FCD, functional capillary density at sublingual mucosa; SL-HI, heterogeneity index at sublingual mucosa.
SNK test, P < 0.05 for P vs. D group.
Fig. 2.
Time course of variations in the percentage of villi with predominantly well-perfused small vessels (%villi-PPV) and the percentage of small vessels perfused at sublingual mucosa (SL-PPV). A: %villi-PPV: repeated-measurements analysis, P < 0.001 for time effect; P = 0.02 for time-group interactions; P = 0.04 for intergroup differences. Student-Newman-Keuls (SNK) test, P < 0.05 for sham vs. P and sham vs. D groups at TS, T2H, and T6H; SNK test, P < 0.05 for P vs. D at T2H and T6H. B: SL-PPV: repeated-measures analysis, P < 0.001 for time effect; P = 0.02 for time-group interaction; P = 0.01 for intergroup differences. SNK test, P < 0.05 for sham vs. P and sham vs. D groups at TS, T2H, and T6H; P < 0.05 for P vs. D at T2H and T6H). No. of animals is as follows: dobutamine (n = 6), placebo (n = 6), and sham (n = 3).
Fig. 3.
Predominant type of microvascular flow in the jejunal villi at each measurement time. Percentage distribution of predominant microvascular blood flow in jejunal villi capillaries at baseline (A), TS (B), T2H (C), and T6H (D). †P < 0.05 between sham vs. dobutamine and placebo groups. *P < 0.05 between dobutamine vs. placebo groups. No. of animals is as follows: dobutamine (n = 6), placebo (n = 6), and sham (n = 3).
Fig. 4.
Relationships between the variations of the percentage of villi with stopped capillary flow (Δvilli-stopped capillaries), mesenteric O2 extraction ratio (Δ), and percentage changes of mesenteric lactate levels (%mes-Lactate variation). A: scatterplot depicting the relationships between Δvilli-stopped capillaries vs. Δ. Spearman Rho test, R2 = 0.83, P < 0.001. B: scatterplot depicting the relationships between Δvilli-stopped capillaries vs. %mes-Lactate variation. Spearman Rho test, R2 = 0.72, P < 0.001. All absolute or percentage variations (Δ) were calculated as the change between actual and its immediately preceding value (A and B).
and its relationships with microcirculatory blood flow.
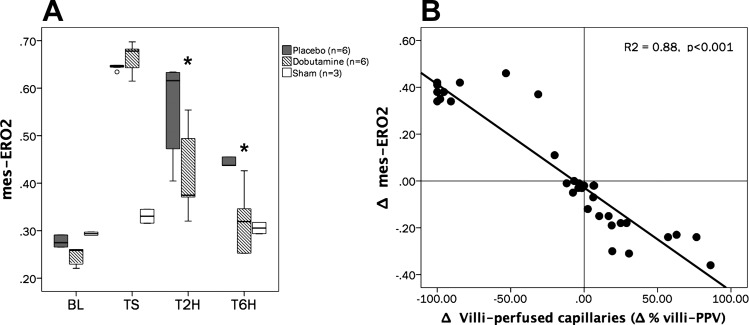
showed a significant increase in both P and D groups at TS [0.65 (0.64–0.65) vs. 0.68 (0.64–0.68) for P and D groups, respectively; Mann-Whitney Rank sum test, P > 0.05]. Despite significant improvement in after starting resuscitation in both experimental groups, the slope of recovery was significantly steeper in the D group (repeated-measures analysis, P = 0.04 for time × group interaction and P = 0.02 for intergroup differences. SNK test, P < 0.05 for sham vs. P and D at TS; P < 0.05 for P vs. D at T2H and T6H) (Fig. 5A). Variations of villi-PPV during shock development and resuscitation depicted a very good agreement with variations in (Spearman Rho test, R2 = 0.88, P < 0.001) (Fig. 5B).
Fig. 5.
Time course of mesenteric O2 extraction ratio () and its relationships with variations in villi-PPV. A: time course of . Repeated measurements analysis, P = 0.04 for time-group interactions; P = 0.02 for intergroup differences. *P < 0.05 for P vs. D groups. B: scatterplot for Δ% villi-PPV vs. Δ. (Spearman Rho test, R2 = 0.88, P < 0.001). Δ% villi-PPV and Δ were calculated as the variation between actual and its immediately preceding value (B). No. of animals is as follows: dobutamine (n = 6), placebo (n = 6), and sham (n = 3).
Systemic, regional, and local CO2 to arterial differences.
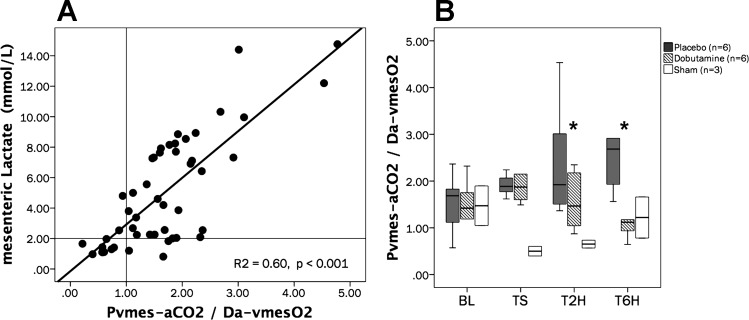
Variations in the tissue-to-arterial CO2 difference () agreed with regional changes () and these, in turn, with systemic venous-to-arterial CO2 differences () (Table 1). and exhibited a good agreement with villi-PPV (Spearman-Rho test, R2 = 0.69 and 0.63, respectively, P < 0.001), and showed a more discrete agreement with Ser-PPV (Spearman-Rho test, R2 = 0.48 and 0.47, respectively, P < 0.001). Meanwhile, mesenteric venous-arterial CO2 to arterial-venous O2 pressure differences ratio (/ ratio) was well correlated with mesenteric lactate levels (Spearman-Rho test, R2 = 0.60, P < 0.001), suggesting the appearance and reversal of anaerobic metabolism during shock development and the resuscitation phase (in the D group), respectively (Fig. 6A). Notably, the time course of the -to- ratio was significantly different in experimental groups, showing a significant decrease in the D group at T6H (Fig. 6B).
Fig. 6.
Relationships between / ratios vs. mesenteric-venous lactate levels and time course of / ratios. A: scatterplot depicting the relationships between the mesenteric venous-arterial CO2 to arterial-venous O2 pressure differences ratio (/ ratio) vs. mesenteric-venous lactate levels. Spearman Rho test, R2 = 0.60, P < 0.001. B: time course of the mesenteric venous-arterial CO2 to arterial-venous O2 pressure differences (/). Repeated-measures analysis, P = 0.32 for time effect; P = 0.06 for time-group interaction; P = 0.05 for intergroup differences. *P < 0.05 for D vs. P groups (B). No. of animals is as follows: dobutamine (n = 6), placebo (n = 6), and sham (n = 3).
Relationships between variations of lactate levels, regional O2 extraction ratio, and jejunal microcirculatory blood flow.
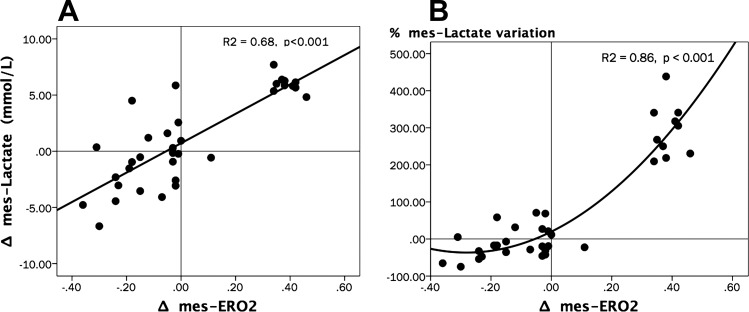
We observed significant increases in arterial and venous mesenteric lactate levels at TS. Models subjected to dobutamine infusion showed a significant decrease at T2H and T6H in both arterial lactate (repeated-measures analysis, P ≤ 0.001 for the time effect; P = 0.03 for time × group interaction; and P = 0.02 for intergroup differences; SNK test, P < 0.05 for P vs. D at T2H and T6H) and venous-mesenteric lactate (repeated-measures analysis, P ≤0.001 for the time effect, P = 0.02 for time × group interaction, and P = 0.03 for intergroup differences; SNK test, P < 0.05 for P vs. D at T2H and T6H) levels, whereas lactate levels remained high in the placebo group (Table 1). Variations in were well correlated with changes in both absolute venous lactate values and the percentage of variation of venous lactate (using as reference the immediately preceding value) (Fig. 7).
Fig. 7.
Relationships between variations of Δ, Δmes-Lactate, and %mes-Lactate variation. A: scatterplot depicting the relationships between Δ vs. Δmes-Lactate. Spearman Rho test, R2 = 0.68, P < 0.001. B: scatterplot depicting the relationships between Δ vs. %mes-Lactate variation. Spearman Rho test, R2 = 0.86, P < 0.001. All percentage or absolute variations (Δ) were calculated as the variation between actual and its immediately preceding value (A and B).
DISCUSSION
Our results suggest that variations in the distribution of intestinal microcirculatory blood flow at the jejunal-villi during the development of, and resuscitation from, septic shock are closely related to changes in regional O2 extraction ratios and these, in turn, to variations in mesenteric lactate levels. Although cardiac output and systemic O2 delivery evolved similarly in both the D and P groups, the heterogeneity of microcirculatory blood flow at the jejunal mucosa and serosa was significantly reversed after dobutamine infusion, which was accompanied by decreases in mesenteric O2 extraction ratio and mesenteric lactate levels.
Variations in denote adaptive cellular responses to O2 availability. Indeed, experimental models during early stages of sepsis demonstrated up to a threefold increase in the capillary in normal perfused muscle, which intuitively suggest that increases in should reflect the maximization of such adaptation to hypoxic tissue challenges (12). However, the assumption that tissue oxygenation can be preserved by maintaining its blood supply is derived from models that presume uniformly perfused capillaries. Conversely, septic shock is characterized by increased heterogeneity of microcirculatory blood flow, which implies the presence of zones of tissue receiving an adequate perfusion through capillaries with continuous flow in close proximity with zones with no microvascular perfusion due to capillaries with stopped flow (12), which leads to inhomogeneity of O2 distribution and, therefore, to abnormal cellular respiration, such as has been observed using in vivo NADH videofluorimetry techniques (22). We observed a severe decline in the proportion of small vessels perfused at both the jejunal and sublingual mucosa at the time of shock with a subsequent increase in blood flow heterogeneity and decrease in functional capillary density. Such as mathematical models have suggested, heterogeneity of O2 delivery should respond in part for tissue O2 derangements in peripheral tissues (49). Indeed, experimental evidence from ex vivo intestinal pieces after endotoxin infusion suggests that the heterogeneity of gut capillary transit times is related to impairment of gut O2 extraction (21). However, the static concept of such experiments (21) makes it difficult to understand the relationships between microvascular blood flow heterogeneity and O2 utilization during the development and resuscitation of septic shock. Conversely, the dynamic nature of our experiment reveals that variations in the percentage of well-perfused capillaries at jejunal villi (villi-PPV) are closely related with dynamic changes in mesenteric and these, in turn, with variations in mesenteric lactate values. Mathematical modeling of O2 demand (do2) to O2 supply (qo2) distributions (do2/qo2) demonstrates that increases in its relative dispersion lead to a nonlinear decrease in the critical O2 extraction ratio (49). Thus, deviations of do2 vs. do2/qo2 distribution “to the right” (i.e., >1.0) suggest the appearance of anaerobic metabolism even at the same total O2 transport (Do2) values, i.e., attainment of anaerobic thresholds at lower critical and Do2 values (49). In our experiment, rises in regional O2 extraction ratios were accompanied by increases in both total (crude) and percentage of variation in mesenteric lactate levels, which reinforces the notion that alterations in do2/qo2 distributions attained during our experiment were enough to overcome anaerobic metabolism thresholds previously reported in experimental conditions (21, 27, 28, 38).
We observed an increased number of stopped vessels at the time of shock coinciding with the higher and mesenteric lactate levels. We hypothesized that increases in the proportion of capillaries with stopped blood flow could overcome the capacity of O2 delivery from capillaries with normal flow, thus leading to tissue O2 supply limitation. Thus, mesenteric- was increased to the extent that functional capillary density declined with the subsequent widening of O2 diffusion distances. As a result, anaerobic metabolism was triggered, leading to increases of regional lactate levels, thus suggesting a key role for the heterogeneous stoppage of individual capillaries on abnormal O2 utilization during very early stages of septic shock. Previous mathematical modeling revealed that, for three forms of progressive hypoxia (anemic, stagnant, and hypoxic), critical total O2 transport is quite similar, as long as intercapillary distances are <80 µm (39). Nevertheless, at higher intercapillary distances (i.e., at lower functional capillary densities), O2 consumption (V̇o2) becomes dependent from total O2 transport (Do2) for a wide range of values (39), suggesting that increments in heterogeneity of microvascular blood flow lead to do2/qo2 mismatching (which is reflected by low critical values), thus indicating an impairment of O2 extraction ability (49). In our study, models receiving dobutamine showed a gradual replacement of stopped with intermittent and normal flows, which improved the functional capillary density and led to a parallel reduction in and regional lactate levels. This phenomenon suggests that, as microvascular blood flow became more homogeneous, tissues should exhibit better do2/qo2 distributions with the subsequent reversion of anaerobic metabolism. Some studies have proposed that the heterogeneous cessation of flow in individual capillaries could determine O2 supply dependence during septic shock (11), whereas mathematical modeling of this phenomenon suggests that oxygenation derangements are more severe when capillary blood flow is totally stopped than when it is intermittent (16), which also agrees with our results.
Dobutamine has shown contradictory results on macrovascular splanchnic (2, 3, 13, 33, 35, 37, 39, 47), total intestinal microvascular blood flow (19, 29), and sublingual microcirculation (7, 12, 18). Conversely, information about the effect of dobutamine on microcirculatory blood flow distribution at intestinal villi during sepsis or endotoxemia has been limited but favorable (42). Interestingly, mesenteric arterial blood flow can be relatively dissociated from microvascular blood flow (46), and misdistribution of blood flow within the intestinal wall might be observed at both hypodynamic and resuscitated phases of septic shock (20). We also observed this apparent dissociation between macro- and microhemodynamics during the resuscitation phase, as has been noted in previous studies (8, 30), but we did not observe discrepancies for serosal-to-mucosal microvascular blood flow distribution during development of shock nor during the resuscitation phase. Effects of dobutamine on villi microcirculation could be mediated by direct adrenergic stimulation (43), nitric oxide generation (24), decreased expression of endothelial E-selectin (45), or inhibition of nuclear factor-κB (25), although the exact mechanisms through which dobutamine exerted its effect on the microcirculatory blood flow go beyond the scope of our study.
The microcirculatory alterations observed in our experiment were accompanied by increases in jejunal-mucosa and regional mesenteric-to-arterial CO2 differences, and these were, in turn, reflected by the accumulation of systemic-venous CO2. Interestingly, the grade of recovery of microcirculation was mirrored by decreases in and , suggesting a link between microcirculatory blood flow and tissue or venous CO2 accumulation, respectively, as has recently been suggested by observations in human septic shock (31). Additionally, we observed an increased mesenteric-venous CO2-to-arterial venous O2 differences ratio (/ ratio) during the development of shock in both experimental groups, evolving in parallel with regional lactate accumulation. Such increased CO2-to-O2 relationship has been proposed as reflection of anaerobic metabolism, since under aerobic steady-state conditions, CO2 production (V̇co2) approximates O2 consumption (V̇o2), and, consequently, the mixed venous-to-arterial CO2 content difference () approximates the arterial-to-mixed-venous O2 content difference (). Accordingly, the V̇co2-to-V̇o2 ratio (i.e., the respiratory quotient) should not be higher than 1.0, whereby nonsymmetric decreases in V̇o2 and V̇co2 with subsequent rises in the respiratory quotient could reflect nonaerobic CO2 generation (30, 32). Importantly, models subjected to dobutamine infusion in our experiment showed a progressive improvement in microvascular blood flow distribution that was in turn related to decreases in the / ratio (a regional surrogate of the V̇co2/V̇o2 ratio), suggesting the reversal of anaerobic metabolism while mesenteric lactate levels also decreased.
It could be argued that the thermogenic effects induced by dobutamine could explain the increases in the O2 extraction ratio in our experiment (15, 17). V̇o2 responses to prostacyclin (6) and nitroprusside (5) are, however, quite similar to the responses to dobutamine in septic patients and healthy volunteers (36). Additionally, the thermogenic effects of catecholamines are related to progressively increased doses, a situation that is avoided when low fixed doses are used (as in our study).
Our model had limitations. First, we focused on microcirculatory blood flow alterations while resuscitation was guided by macrohemodynamic variations, as occurs during resuscitation from human septic shock. Nonetheless, the macrohemodynamics evolved similarly in the dobutamine and placebo groups, suggesting that the effects of dobutamine were mediated at the microcirculatory level. Second, our institutional Animal Research Committee suggested limiting the total number of models, including those used during the preexperimental standardization phase, with the result that we included only three animals in the sham group. Admittedly, some differences were observed in certain macrohemodynamic variables at baseline when comparing the sham animals with the two experimental groups. The inclusion of a higher number of sham models would not, however, have changed our final results, since microcirculatory flow variables were almost identical at baseline in the sham, dobutamine, and placebo groups, whereas macrohemodynamics evolved in parallel in the two experimental groups. Third, our experiment reproduces a severe hypodynamic septic shock, which could distance our results from usual clinical observations. Our model does, however, closely recreate the initial phases of nonresuscitated septic shock with a complete spectrum of enteric pathogens, with immunological and macrohemodynamic disturbances occurring gradually. Fourth, we did not measure variations of arterial mesenteric blood flow, which precludes the analysis of mesenteric-Do2. However, alterations in microvascular blood flow distribution cannot be predicted from macrohemodynamics or even from total microcirculatory flow (i.e., by laser Doppler techniques). Thus, measurements of total microvascular blood flow and its distribution (i.e., estimation of blood flow heterogeneity) could be more, or at least, as relevant as isolated mesenteric arterial blood flow measurements. Furthermore, although indirect, the proportional decrease in mesenteric lactate levels in our experiment provides a strong suggestion that the increase in microvascular perfusion was associated with an improved metabolism associated with better O2 utilization.
In conclusion, variations in the heterogeneity of microcirculatory blood flow at the jejunal mucosa are closely linked to regional splanchnic O2 extraction ratios and mesenteric lactate levels, thus suggesting a key role for microvascular blood flow distribution on O2 utilization in septic shock. Low fixed doses of dobutamine can decrease such flow heterogeneity, promoting O2 metabolism recovery.
GRANTS
This work was supported by Tecnoquímicas (Colombia)—Centro Investigaciones Clínicas, Fundación Valle del Lili (Colombia) (CIC 001) and Universidad ICESI (Colombia) (IP-FO-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A.O.-T. conceived and designed research; G.A.O.-T., A.F.G.M., G.J.E., W.F.B., H.J.M.N., J.D.V., E.Q., F.R., and A.M. performed experiments; G.A.O.-T., A.F.G.M., G.J.E., W.F.B., H.J.M.N., J.D.V., E.Q., F.R., A.M., C.A.A.D., A.B., G.H., and D.D.B. analyzed data; G.A.O.-T., A.F.G.M., G.J.E., E.Q., A.M., C.A.A.D., A.B., and G.H. interpreted results of experiments; G.A.O.-T. prepared figures; G.A.O.-T. and C.A.A.D. drafted manuscript; G.A.O.-T., A.F.G.M., G.J.E., W.F.B., H.J.M.N., J.D.V., E.Q., A.M., C.A.A.D., A.B., G.H., and D.D.B. edited and revised manuscript; G.A.O.-T., A.F.G.M., G.J.E., W.F.B., H.J.M.N., J.D.V., E.Q., F.R., A.M., C.A.A.D., A.B., G.H., and D.D.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Fernando Rosso and Marcela Granados (Fundación Valle del Lili- Cali, Colombia) and Drs. Yuri Takeuchi and Francisco Piedrahita (Universidad ICESI, Cali, Colombia) for unconditional support to this project. We also thank Andrés Hurtado, Edwin Rios, and Jackeline Vivas (Universidad ICESI) for support during the experimental phase.
Glossary
- CO
Cardiac output
- CVP
Central venous pressure
- Do2
Systemic O2 delivery
- FCD
Functional capillary density
- HI
Heterogeneity index
- Lactate-art
Arterial lactate
- Lactate-mes
Mesenteric lactate
- MAP
Mean arterial pressure
Mesenteric O2 extraction ratio
Arterial CO2 partial pressure
Arterial O2 partial pressure
- PAOP
Pulmonary artery occlusion pressure
- pH
Arterial pH
- PPV
Percentage of small vessels perfused
Tissue (jejunal mucosa)-to-arterial CO2 difference
Tissue (jejunal mucosa) CO2 pressure
Mixed venous-to-arterial CO2 difference
- /
Mixed venous-to-arterial CO2 to arterial-venous O2 pressure differences ratio
Mesenteric venous-to-arterial CO2 difference
- /
Mesenteric blood venous-to-arterial CO2 to arterial-venous O2 pressure difference ratio
Mesenteric venous CO2 partial pressure
Mesenteric venous O2 partial pressure
Mixed-venous O2 partial pressure
Mixed-venous CO2 partial pressure
- Ser-FCD
Functional capillary density at jejunal serosa
- Ser-HI
Heterogeneity index at jejunal serosa
- Ser-PPV
Percentage of small vessels perfused at jejunal serosa
- SL-FCD
Functional capillary density at sublingual mucosa
- SL-HI
Heterogeneity index at sublingual mucosa
- SL-PPV
Percentage of small vessels perfused at sublingual mucosa
Mixed-venous O2 saturation
Systemic O2 extraction ratio
- TCD
Total capillary density
- %Villi-PPV
Proportion of jejunal-villi with well-perfused vessels
- V̇o2
Systemic O2 consumption
REFERENCES
- 1.Bateman RM, Jagger JE, Sharpe MD, Ellsworth ML, Mehta S, Ellis CG. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am J Physiol Heart Circ Physiol 280: H2848–H2856, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bersten AD, Hersch M, Cheung H, Rutledge FS, Sibbald WJ. The effect of various sympathomimetics on the regional circulations in hyperdynamic sepsis. Surgery 112: 549–561, 1992. [PubMed] [Google Scholar]
- 3.Biro GP, Douglas JR, Keon WJ, Taichman GC. Changes in regional blood flow distribution induced by infusions of dopexamine hydrochloride or dobutamine in anesthetized dogs. Am J Cardiol 62: 30C–36C, 1988. doi: 10.1016/S0002-9149(88)80064-X. [DOI] [PubMed] [Google Scholar]
- 4.Creteur J, De Backer D, Vincent JL. Monitoring gastric mucosal carbon dioxide pressure using gas tonometry: in vitro and in vivo validation studies. Anesthesiology 87: 504–510, 1997. doi: 10.1097/00000542-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 5.De Backer D, Berre J, Moraine JJ, Melot C, Vanfraechem J, Vincent JL. Effects of dobutamine on the relationship between oxygen consumption and delivery in healthy volunteers: comparison with sodium nitroprusside. Clin Sci (Lond) 90: 105–111, 1996. doi: 10.1042/cs0900105. [DOI] [PubMed] [Google Scholar]
- 6.De Backer D, Berré J, Zhang H, Kahn RJ, Vincent JL. Relationship between oxygen uptake and oxygen delivery in septic patients: effects of prostacyclin versus dobutamine. Crit Care Med 21: 1658–1664, 1993. doi: 10.1097/00003246-199311000-00014. [DOI] [PubMed] [Google Scholar]
- 7.De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34: 403–408, 2006. doi: 10.1097/01.CCM.0000198107.61493.5A. [DOI] [PubMed] [Google Scholar]
- 8.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care 11: R101, 2007. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai VS, Weil MH, Tang W, Yang G, Bisera J. Gastric intramural PCO2 during peritonitis and shock. Chest 104: 1254–1258, 1993. doi: 10.1378/chest.104.4.1254. [DOI] [PubMed] [Google Scholar]
- 10.Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 40: 1443–1448, 2012. doi: 10.1097/CCM.0b013e31823dae59. [DOI] [PubMed] [Google Scholar]
- 11.Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am J Physiol Heart Circ Physiol 282: H156–H164, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Enrico C, Kanoore Edul VS, Vazquez AR, Pein MC, Pérez de la Hoz RA, Ince C, Dubin A. Systemic and microcirculatory effects of dobutamine in patients with septic shock. J Crit Care 27: 630–638, 2012. doi: 10.1016/j.jcrc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara JJ, Dyess DL, Peeples GL, Christenberry DP, Roberts WS, Tacchi EJ, Swafford AN, Ardell JL, Powell RW. Effects of dopamine and dobutamine on regional blood flow distribution in the neonatal piglet. Ann Surg 221: 531–540, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germann R, Haisjackl M, Schwarz B, Deusch E, Meusburger S, Gruber E, Pajk W, Hausdorfer H, Bonatti J, Furtner B, Ulmer H, Hasibeder W. Inotropic treatment and intestinal mucosal tissue oxygenation in a model of porcine endotoxemia. Crit Care Med 25: 1191–1197, 1997. doi: 10.1097/00003246-199707000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert EM, Haupt MT, Mandanas RY, Huaringa AJ, Carlson RW. The effect of fluid loading, blood transfusion, and catecholamine infusion on oxygen delivery and consumption in patients with sepsis. Am Rev Respir Dis 134: 873–878, 1986. doi: 10.1164/arrd.1986.134.5.873. [DOI] [PubMed] [Google Scholar]
- 16.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol 290: H2277–H2285, 2006. doi: 10.1152/ajpheart.00547.2005. [DOI] [PubMed] [Google Scholar]
- 17.Green CJ, Frazer RS, Underhill S, Maycock P, Fairhurst JA, Campbell IT. Metabolic effects of dobutamine in normal man. Clin Sci (Lond) 82: 77–83, 1992. doi: 10.1042/cs0820077. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, Florez J, Castro R, Aquevedo A, Pairumani R, McNab P, Ince C. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med 39: 1435–1443, 2013. doi: 10.1007/s00134-013-2982-0. [DOI] [PubMed] [Google Scholar]
- 19.Hiltebrand LB, Krejci V, Sigurdsson GH. Effects of dopamine, dobutamine, and dopexamine on microcirculatory blood flow in the gastrointestinal tract during sepsis and anesthesia. Anesthesiology 100: 1188–1197, 2004. doi: 10.1097/00000542-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Hiltebrand LB, Krejci V, tenHoevel ME, Banic A, Sigurdsson GH. Redistribution of microcirculatory blood flow within the intestinal wall during sepsis and general anesthesia. Anesthesiology 98: 658–669, 2003. doi: 10.1097/00000542-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Humer MF, Phang PT, Friesen BP, Allard MF, Goddard CM, Walley KR. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J Appl Physiol (1985) 81: 895–904, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Ince C, van der Sluijs JP, Sinaasappel M, Avontuur JA, Coremans JM, Bruining HA. Intestinal ischemia during hypoxia and experimental sepsis as observed by NADH videofluorimetry and quenching of Pd-porphine phosphorescence. Adv Exp Med Biol 361: 105–110, 1994. doi: 10.1007/978-1-4615-1875-4_14. [DOI] [PubMed] [Google Scholar]
- 23.Lam C, Tyml K, Martin C, Sibbald W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 94: 2077–2083, 1994. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobo SM, Soriano FG, Barbeiro DF, De Backer D, Sun Q, Tu Z, Dimopoulos G, Preiser JC, Vray B, Vercruysse V, Vincent JL. Effects of dobutamine on gut mucosal nitric oxide production during endotoxic shock in rabbits. Med Sci Monit 15: BR37–BR42, 2009. [PubMed] [Google Scholar]
- 25.Loop T, Bross T, Humar M, Hoetzel A, Schmidt R, Pahl HL, Geiger KK, Pannen BH. Dobutamine inhibits phorbol-myristate-acetate-induced activation of nuclear factor-kappaB in human T lymphocytes in vitro. Anesth Analg 99: 1508–1515, 2004. doi: 10.1213/01.ANE.0000132976.19021.1B. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DP, Beyer C, Samsel RW, Wood LD, Schumacker PT. Pathological supply dependence of O2 uptake during bacteremia in dogs. J Appl Physiol (1985) 63: 1487–1492, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DP, King CE, Dodd SL, Schumacker PT, Cain SM. Systemic and intestinal limits of O2 extraction in the dog. J Appl Physiol (1985) 63: 387–394, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DP, Samsel RW, Wood LD, Schumacker PT. Pathological supply dependence of systemic and intestinal O2 uptake during endotoxemia. J Appl Physiol (1985) 64: 2410–2419, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Nevière R, Chagnon JL, Vallet B, Lebleu N, Marechal X, Mathieu D, Wattel F, Dupuis B. Dobutamine improves gastrointestinal mucosal blood flow in a porcine model of endotoxic shock. Crit Care Med 25: 1371–1377, 1997. doi: 10.1097/00003246-199708000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Ospina-Tascón GA, Umaña M, Bermúdez W, Bautista-Rincón DF, Hernandez G, Bruhn A, Granados M, Salazar B, Arango-Dávila C, De Backer D. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med 41: 796–805, 2015. doi: 10.1007/s00134-015-3720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ospina-Tascón GA, Umaña M, Bermúdez WF, Bautista-Rincón DF, Valencia JD, Madriñán HJ, Hernandez G, Bruhn A, Arango-Dávila C, De Backer D. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 42: 211–221, 2016. doi: 10.1007/s00134-015-4133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ospina-Tascón GA, Hernández G, Cecconi M. Understanding the venous-arterial CO2 to arterial-venous O2 content difference ratio. Intensive Care Med 42: 1801–1804, 2016. doi: 10.1007/s00134-016-4233-7. [DOI] [PubMed] [Google Scholar]
- 33.Parviainen I, Ruokonen E, Takala J. Dobutamine-induced dissociation between changes in splanchnic blood flow and gastric intramucosal pH after cardiac surgery. Br J Anaesth 74: 277–282, 1995. doi: 10.1093/bja/74.3.277. [DOI] [PubMed] [Google Scholar]
- 34.Piper RD, Pitt-Hyde M, Li F, Sibbald WJ, Potter RF. Microcirculatory changes in rat skeletal muscle in sepsis. Am J Respir Crit Care Med 154: 931–937, 1996. doi: 10.1164/ajrccm.154.4.8887588. [DOI] [PubMed] [Google Scholar]
- 35.Priebe HJ, Nöldge GF, Armbruster K, Geiger K. Differential effects of dobutamine, dopamine, and noradrenaline on splanchnic haemodynamics and oxygenation in the pig. Acta Anaesthesiol Scand 39: 1088–1096, 1995. doi: 10.1111/j.1399-6576.1995.tb04236.x. [DOI] [PubMed] [Google Scholar]
- 36.Ronco JJ, Fenwick JC, Wiggs BR, Phang PT, Russell JA, Tweeddale MG. Oxygen consumption is independent of increases in oxygen delivery by dobutamine in septic patients who have normal or increased plasma lactate. Am Rev Respir Dis 147: 25–31, 1993. doi: 10.1164/ajrccm/147.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Ruokonen E, Takala J, Kari A. Regional blood flow and oxygen transport in patients with the low cardiac output syndrome after cardiac surgery. Crit Care Med 21: 1304–1311, 1993. doi: 10.1097/00003246-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Samsel RW, Nelson DP, Sanders WM, Wood LD, Schumacker PT. Effect of endotoxin on systemic and skeletal muscle O2 extraction. J Appl Physiol (1985) 65: 1377–1382, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Schneider AJ, Groeneveld AB, Teule GJ, Wesdorp RI, Thijs LG. Total body blood volume redistribution in porcine E. coli septic shock: effect of volume loading, dobutamine, and norepinephrine. Circ Shock 35: 215–222, 1991. [PubMed] [Google Scholar]
- 40.Schumacker PT, Cain SM. The concept of a critical oxygen delivery. Intensive Care Med 13: 223–229, 1987. doi: 10.1007/BF00265110. [DOI] [PubMed] [Google Scholar]
- 41.Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol Lung Cell Mol Physiol 265: L395–L402, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Secchi A, Wellmann R, Martin E, Schmidt H. Dobutamine maintains intestinal villus blood flow during normotensive endotoxemia: an intravital microscopic study in the rat. J Crit Care 12: 137–141, 1997. doi: 10.1016/S0883-9441(97)90043-5. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd AP, Kiel JW. A model of countercurrent shunting of oxygen in the intestinal villus. Am J Physiol Heart Circ Physiol 262: H1136–H1142, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Tang W, Weil MH, Sun S, Noc M, Gazmuri RJ, Bisera J. Gastric intramural PCO2 as monitor of perfusion failure during hemorrhagic and anaphylactic shock. J Appl Physiol (1985) 76: 572–577, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Trabold B, Lunz D, Gruber M, Fröhlich D, Graf B. Immunomodulation of neutrophil–endothelial interaction by inotropes. Injury 41: 1079–1083, 2010. doi: 10.1016/j.injury.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Tugtekin IF, Radermacher P, Theisen M, Matejovic M, Stehr A, Ploner F, Matura K, Ince C, Georgieff M, Träger K. Increased ileal-mucosal-arterial PCO2 gap is associated with impaired villus microcirculation in endotoxic pigs. Intensive Care Med 27: 757–766, 2001. doi: 10.1007/s001340100871. [DOI] [PubMed] [Google Scholar]
- 47.Uusaro A, Ruokonen E, Takala J. Splanchnic oxygen transport after cardiac surgery: evidence for inadequate tissue perfusion after stabilization of hemodynamics. Intensive Care Med 22: 26–33, 1996. doi: 10.1007/BF01728327. [DOI] [PubMed] [Google Scholar]
- 48.Vincent JL, De Backer D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care 9, Suppl 4: S9–S12, 2005. doi: 10.1186/cc3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walley KR. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: theory. J Appl Physiol (1985) 81: 885–894, 1996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.