Healthy aging is a crucial area of research. This article details how differences in age and cardiorespiratory fitness level affect lung diffusing capacity, particularly during heavy exercise. We conclude that highly fit older adults do not experience a limit in lung diffusing capacity during heavy exercise. Interestingly, however, we found that highly fit older individuals demonstrate greater values of lung diffusing capacity during heavy exercise than their less fit age-matched counterparts.
Keywords: maximal aerobic capacity, lung diffusing capacity, pulmonary circulation, alveolar-capillary membrane conductance, pulmonary-capillary blood volume
Abstract
Aging is associated with deterioration in the structure and function of the pulmonary circulation. We characterized the lung diffusing capacity for carbon monoxide (DLCO), alveolar-capillary membrane conductance (DmCO), and pulmonary-capillary blood volume (Vc) response to discontinuous incremental exercise at 25, 50, 75, and 90% of peak work (Wpeak) in four groups: 1) Young [27 ± 3 yr, maximal oxygen consumption (V̇o2max): 110 ± 18% age predicted]; 2) Young Highly Fit (27 ± 3 yr, V̇o2max: 147 ± 8% age predicted); 3) Old (69 ± 5 yr, V̇o2max: 116 ± 13% age predicted); and 4) Old Highly Fit (65 ± 5 yr, V̇o2max: 162 ± 18% age predicted). At rest and at 90% Wpeak, DLCO, DmCO, and Vc were decreased with age. At 90% Wpeak, DLCO, DmCO, and Vc were greater in Old Highly Fit vs. Old adults. The slope of the DLCO-cardiac output (Q̇) relationship from rest to end exercise at 90% Wpeak was not different between Young, Young Highly Fit, Old, and Old Highly Fit (1.35 vs. 1.44 vs. 1.10 vs. 1.35 mlCO·mmHg−1·liter blood−1, P = 0.388), with no evidence of a plateau in this relationship during exercise; this was also true for DmCO-Q̇ and Vc-Q̇. V̇o2max was positively correlated with 1) DLCO, DmCO, and Vc at rest; and 2) the rest to end exercise change in DLCO, DmCO, and Vc. In conclusion, these data suggest that despite the age-associated deterioration in the structure and function of the pulmonary circulation, expansion of the pulmonary capillary network does not become limited during exercise in healthy individuals regardless of age or cardiorespiratory fitness level.
NEW & NOTEWORTHY Healthy aging is a crucial area of research. This article details how differences in age and cardiorespiratory fitness level affect lung diffusing capacity, particularly during high-intensity exercise. We conclude that highly fit older adults do not experience a limit in lung diffusing capacity during high-intensity exercise. Interestingly, however, we found that highly fit older individuals demonstrate greater values of lung diffusing capacity during high-intensity exercise than their less fit age-matched counterparts.
maximal aerobic capacity (V̇o2max) has been shown to, at least in part, be determined by the structure and function of the pulmonary vasculature in health and chronic disease (1, 10, 22, 23, 25). For example, it has been shown that V̇o2max is positively correlated with resting pulmonary capillary blood volume (Vc) and pulmonary vascular distensibility and inversely related to pulmonary vascular resistance at maximal exercise in healthy individuals (23). This suggests that a larger, more distensible pulmonary vascular network is associated with greater aerobic exercise capacity in humans.
Measures of lung diffusing capacity for carbon monoxide (DLCO) and nitric oxide (DLNO), alveolar-capillary membrane conductance (DmCO), and Vc are considered to reflect the pulmonary vascular response to whole body exercise. Indeed, increased cardiac output and pulmonary perfusion pressure during exercise cause a marked expansion of the highly compliant pulmonary capillary network that is associated with an increase DLCO, DLNO, DmCO, and Vc (17, 27, 34). Additionally, it is thought that the DLNO/DLCO ratio provides insight into the mechanism by which expansion of the pulmonary capillary network during exercise occurs, with an increase in the ratio indicating a thinning of the pulmonary capillary sheet (i.e., predominant vessel recruitment) and a decrease in the ratio indicating a thickening of the blood sheet (i.e., predominant vessel distension) (13, 23).
Healthy aging is associated with a progressive deterioration in the structure and function of the pulmonary circulation that is characterized by an increase in pulmonary vascular stiffness, pulmonary vascular pressures, and pulmonary vascular resistance (20, 24, 28). Additionally, from maturity to senescence, there is a decrease in resting Vc and DmCO that is consistent with a reduction in alveolar-capillary surface area (1, 15). These age-related changes in the pulmonary vasculature may impair recruitment and/or distension of the pulmonary capillaries during exercise in healthy older adults, subsequently impairing the increase in alveolar-capillary surface area needed for effective gas exchange and resulting in an excessive rise in pulmonary vascular pressures relative to the metabolic demand of exercise. However, it has been shown that DLCO and Vc increase linearly relative to exercise intensity in old as well as young healthy adults (34), indicating that expansion of the pulmonary capillaries does not become limited during exercise in these individuals. This finding implies that the changes in the pulmonary circulation that occur with healthy aging are somewhat mild and not sufficient to affect pulmonary vascular expansion and the recruitment of effective alveolar-capillary surface area during exercise in healthy older adults.
The pulmonary vascular response to exercise in aged adults who have maintained a high cardiorespiratory fitness is, however, currently less well characterized. Theoretically, better maintenance of V̇o2max through conditioning may cause the demand for Q̇ and pulmonary blood flow during exercise to remain elevated in endurance-trained highly fit older subjects compared with their younger counterparts. This, in the face of age-related alterations in the structure and function of the pulmonary circulation, may predispose highly fit older adults to impairments in pulmonary vascular expansion and pulmonary gas exchange relative to the metabolic demand during exercise. Accordingly, the aim of the present study was to characterize the DLCO, DmCO, and Vc response to incremental exhaustive exercise in healthy, aerobically trained older adults relative to their age-matched less aerobically fit counterparts, as well as younger adults of various cardiorespiratory fitness levels. We hypothesized that those older individuals who had maintained an elevated cardiorespiratory fitness would encroach upon their maximal ability to expand the pulmonary vascular network during high-intensity exercise, as evidenced by a plateau and/or decrease in the rate of rise in one or more of DLCO, DmCO, or Vc.
METHODS
Subjects.
Sixteen young adults (27 ± 3 yr) and 15 older adults (67 ± 6 yr) who had pulmonary function within normal limits participated in the study (Table 1). The subjects were subdivided into four groups according to age (≤30 yr = “Young”; ≥60 yr = “Old”) and cardiorespiratory fitness (V̇o2max ≥140% of age predicted = “Highly Fit”) as follows: group 1) Young: age: 27 ± 3 yr (range: 22–29), V̇o2max: 110 ± 18% predicted (range: 85–133) (n = 9); group 2) Young Highly Fit: age: 27 ± 3 yr (range: 23–30), V̇o2max: 147 ± 8% predicted (range: 140–163) (n = 7); group 3) Old: age: 69 ± 5 yr (range: 60–76), V̇o2max: 116 ± 13% predicted (range: 100–132) (n = 7, 1 female); and group 4) Old Highly Fit: age: 65 ± 5 yr (range: 60–74), V̇o2max: 162 ± 18% predicted (range: 140–198) (n = 8, 1 female) (Table 1). All subjects were healthy and had no history of respiratory, cardiovascular, or metabolic disease. Each participant gave written informed consent after being provided a detailed description of the study requirements. The experimental procedures were approved by the Mayo Clinic Institutional Review Board and were performed in accordance with the ethical standards of the Declaration of Helsinki.
Table 1.
Subject characteristics
| Young | Young Highly Fit | Old | Old Highly Fit | |
|---|---|---|---|---|
| Age, yr | 27 ± 3 | 27 ± 3 | 69 ± 5*† | 65 ± 5*† |
| n (female) | 9 (0) | 7 (0) | 7 (1) | 8 (1) |
| Height, cm | 178 ± 4 | 178 ± 4 | 173 ± 4 | 176 ± 7 |
| Mass, kg | 78.0 ± 5.8 | 72.5 ± 6.8 | 73.6 ± 8.8 | 75.4 ± 9.0 |
| BMI, kg/m2 | 24.7 ± 2.0 | 22.8 ± 2.1 | 24.8 ± 3.4 | 24.5 ± 2.2 |
| Wpeak, W | 269 ± 43 | 338 ± 28* | 157 ± 26*† | 239 ± 29†# |
| V̇o2max, ml/min | 3,519 ± 449 | 4,577 ± 419* | 2,050 ± 381*† | 3,140 ± 368†# |
| V̇o2max, ml·kg−1·min−1 | 45.3 ± 6.5 | 63.4 ± 6.0* | 28.2 ± 6.4*† | 41.8 ± 3.8†# |
| %Pred. V̇o2max | 110 ± 18 | 147 ± 8* | 116 ± 13† | 162 ± 18*# |
| FEV1/FVC | 82 ± 6 | 80 ± 4 | 78 ± 5 | 74 ± 5* |
| %Pred. | 99 ± 8 | 96 ± 5 | 105 ± 7 | 98 ± 5 |
| FEV1, liter | 4.8 ± 0.4 | 5.0 ± 0.8 | 3.2 ± 0.3*† | 3.7 ± 0.9*† |
| %Pred. | 106 ± 9 | 110 ± 13 | 110 ± 12 | 112 ± 21 |
| FVC, liter | 5.9 ± 0.7 | 6.3 ± 0.9 | 4.2 ± 0.4*† | 5.0 ± 1.2† |
| %Pred. | 106 ± 10 | 114 ± 12 | 104 ± 11 | 114 ± 21 |
| FEF25–75, liter | 4.6 ± 0.7 | 4.5 ± 1.1 | 2.9 ± 0.7*† | 2.8 ± 1.0*† |
| %Pred. | 100 ± 19 | 97 ± 21 | 126 ± 32 | 104 ± 30 |
| TLC, liter | 7.7 ± 0.9 | 8.0 ± 1.3 | 6.9 ± 1.0*† | 8.2 ± 1.2*† |
| %Pred. | 112 ± 10 | 116 ± 16 | 106 ± 12 | 121 ± 11 |
Values are reported as means ± SD. BMI, body mass index; Wpeak, peak power output during maximal exercise test; V̇o2max, maximal oxygen consumption; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25–75, average forced expiratory volume during middle portion of FVC; TLC, total lung capacity; %Pred., percent predicted. See methods for group definitions. Significance set at P < 0.05:
significantly different vs. Young;
significantly different vs. Young Highly Fit; and
significantly different vs. Old.
Experimental procedures.
The experimental procedures were conducted during two laboratory visits separated by at least 2 but no more than 14 days. The subjects abstained from caffeine for 12 h and exercise for 24 h before each visit. During visit 1, pulmonary function was assessed via full-body plethysmography (MedGraphics Elite Series Plethysmograph; Medical Graphics, St. Paul, MN) according to standard procedures (26). Next, subjects performed a maximal incremental exercise test on an electromagnetically braked cycle ergometer (Lode Corival; Lode Medical Technology, Groningen, The Netherlands) for determination of peak work rate (Wpeak) and maximal oxygen consumption (V̇o2max). Exercise was initiated at 60, 80, or 100 W, depending on self-reported fitness, and work rate was increased by 20 W every 2 min until volitional exhaustion. Wpeak was calculated as the sum of the final work rate completed plus the fraction of the partially completed work rate before exhaustion. V̇o2max was the highest mean value recorded over 30 s.
During visit 2, subjects performed discontinuous graded cycle exercise. Following 5 min of quiet rest, participants cycled for 6 min at 25, 50, and 75% of Wpeak before cycling at 90% of Wpeak to volitional exhaustion. Between exercise bouts, subjects recovered quietly until heart rate returned to within 10 beats/min of resting values (at least 4 min). Additionally, a 1-min “warm-up” at 40% of the workload about to be completed was allowed before each exercise bout. Lung diffusing capacity for carbon monoxide (DLCO) and nitric oxide (DLNO) and cardiac output (Q̇) was measured in duplicate at rest and during the final 90 s of each exercise bout via a rebreathe technique.
Lung diffusing capacity and cardiac output.
DLCO, DLNO, and Q̇ were assessed using a rebreathe technique as we have described previously (5, 36). With the use of this technique, DLCO, DLNO, and Q̇ are determined via the rate of disappearance of CO, NO, and acetylene (C2H2), respectively. Briefly, subjects sat upright on a cycle ergometer and breathed through a two-way switching valve (Hans Rudolph 4285 series; Hans Rudolph, Kansas City, MO) connected to a pneumotachometer (MedGraphics PreVent Pneumotach; Medical Graphics), mass spectrometer (Marquette 1100 Medical Gas Analyzer; Perkin-Elmer, St. Louis, MO), and NO analyzer (Sievers 280i NOA; Sievers, Boulder, COA). The inspiratory port of the switching valve was open to room air or a 6-liter anesthesia bag filled with 0.3% CO (C18O), 40 ppm NO, 9% He, 0.6% C2H2, and 35% O2 and N2 balance. The total volume of gas added to the rebreathe bag was determined as the average tidal volume of the subject during the 20–30 s immediately before each measurement. To ensure the volume of the test gas was consistent across multiple rebreathe maneuvers, the bag was filled using a timed switching circuit that, given a constant flow rate from the gas tank, resulted in the desired volume. The test gas volume given by the switching circuit was verified before exercise using a 3-liter syringe. Following a normal expiration, subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for 8–10 consecutive breaths. A respiratory frequency of 32 breaths/min was maintained by following a metronome with inspiratory and expiratory tones; this respiratory rate was necessary to collect enough data to correctly trace NO decay. If the subject’s respiratory frequency was above 32 breaths/min during exercise, the subject was allowed to breathe at this higher rate. This maneuver was performed in duplicate at rest and during the final 90 s of each exercise bout.
From the measurements of DLCO and DLNO, alveolar-capillary membrane conductance (DmCO) and pulmonary capillary blood volume (Vc) were calculated (5, 32). The coefficient relating DLNO to DmCO (α-ratio) was set at 2.26 (5) such that DmCO was calculated as the DLNO/α-ratio. Next, the reaction rate of CO with hemoglobin (θCO) was calculated using the equation derived by Ceridon et al. (5) and Reeves and Park (29) in which θCO is dependent on the capillary partial pressure of oxygen (PcapO2):
| (1) |
where PcapO2 is estimated as alveolar Po2 – V̇o2/(DLCO × 1.23) with partial pressures in mmHg and V̇o2 in ml/min. Finally, the values of DmCO and θCO were used to solve for Vc according to the following equation derived by Roughton and Forster (31):
| (2) |
Capillary blood was sampled from an earlobe and measured for hemoglobin (Hb) concentration via centrifugal hematology (QBC Autoreader; Bector Dickinson, Port Matilda, PA). Vc was then corrected for standard concentrations of Hb in men (14.6 g/dl) and women (13.4 g/dl) as calculated Vc × (standard Hb concentration/measured Hb concentration).
Lung diffusing capacity-cardiac output slope.
We calculated the slope of the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ relationship from rest to end exercise in each experimental group by plotting DLCO, DmCO, or Vc as a function of Q̇. These slopes represent unit DLCO, DmCO, and Vc changes per unit change in pulmonary vascular blood flow (Q̇) and provide an indirect measure of the hemodynamic response of the pulmonary circulation to exercise.
Statistical analysis.
One-way ANOVA with Tukey-Kramer post hoc analysis (two-tailed) was used to compare 1) subject characteristics, 2) pulmonary function, and 3) measures of DLCO, DmCO, and Vc at rest and at 90% of Wpeak. The effect of exercise on DLCO, DmCO, Vc, and DLNO/DLCO was tested using a linear mixed effects model. In this model, the dependent variable was DLCO, DmCO, Vc, or DLNO/DLCO, and the five exercise levels (rest, 25, 50, 75, and 90% Wpeak) were treated as repeated measures with Q̇ as the continuous variable. Age (Young or Old) and cardiorespiratory fitness (normal or Highly Fit) were included as independent predictor variables. That is, with the use of this model, the response of a given variable to exercise as well as the offset in baseline values was assessed as a function of age and/or cardiorespiratory fitness. Coefficient of determination (r2) was computed to assess the proportion of V̇o2max that was predicted by 1) resting DLCO, resting DmCO, and resting Vc; 2) the change (Δ) in DLCO, DmCO, and Vc from rest to end exercise; and 3) the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ slope in response to exercise. In all analyses, the acceptable type I error was set at P < 0.05. Results are expressed as means ± SD. The linear mixed effects model was performed in Matlab (version R2016a, MathWorks, Natick, MA); all other statistical analyses were performed using IBM SPSS Statistics 20 for Windows (IBM, Armonk, NY).
RESULTS
Subjects.
Subject characteristics and pulmonary function are shown in Table 1. Group mean age was not different in Young vs. Young Highly Fit or in Old vs. Old Highly Fit. In addition, all subject groups were well matched for height, body mass, and body mass index. Absolute and relative (to body mass) V̇o2max and absolute Wpeak were greater in Young Highly Fit compared with Young, Old, and Old Highly Fit (all P < 0.01) (Table 1). In addition, V̇o2max (absolute and relative) and absolute Wpeak were lower in Old vs. Young, Young Highly Fit, and Old Highly Fit (all P < 0.01, Table 1). Interestingly, however, neither V̇o2max nor Wpeak was different in Young vs. Old Highly Fit (Table 1).
Lung diffusing capacity at rest and at 90% Wpeak.
At rest, group mean DLCO, DmCO, and Vc were lower in Old vs. Young (P ≤ 0.003) and Young Highly Fit (P < 0.001) (Table 2 and Fig. 1). In addition, group mean resting DLCO, DmCO, and Vc were lower in Old Highly Fit compared with Young Highly Fit (P < 0.001, 0.016, and 0.021, respectively) (Table 2 and Fig. 1). No other differences in resting measures of DLCO, DmCO, and Vc were observed between the four experimental groups (Table 2).
Table 2.
Lung diffusing capacity and cardiac output
| Rest |
Exercise at 90% of Wpeak |
|||||||
|---|---|---|---|---|---|---|---|---|
| Young | Young Highly Fit | Old | Old Highly Fit | Young | Young Highly Fit | Old | Old Highly Fit | |
| DLCO, ml·min−1·mmHg | 28 ± 4 | 33 ± 4 | 17 ± 3*† | 23 ± 5† | 47 ± 6 | 53 ± 7 | 25 ± 3*† | 37 ± 10*†# |
| DLNO, ml·min−1·mmHg | 101 ± 22 | 112 ± 16 | 67 ± 10*† | 85 ± 18† | 153 ± 16 | 171 ± 18 | 87 ± 7*† | 122 ± 30*†# |
| DLNO/DLCO, unitless | 3.6 ± 0.4 | 3.4 ± 0.4 | 4.0 ± 0.6 | 3.8 ± 0.4 | 3.3 ± 0.3 | 3.2 ± 0.2 | 3.6 ± 0.2 | 3.3 ± 0.2 |
| DmCO, ml·min−1·mmHg | 45 ± 10 | 50 ± 7 | 30 ± 4*† | 37 ± 8† | 71 ± 12 | 77 ± 10 | 39 ± 3*† | 54 ± 13*†# |
| Vc, ml | 82 ± 20 | 95 ± 14 | 44 ± 9*† | 68 ± 20† | 127 ± 30 | 149 ± 15 | 66 ± 10*† | 110 ± 29†# |
| Q̇, l/min | 5.6 ± 2.0 | 6.2 ± 1.1 | 3.5 ± 0.6*† | 5.1 ± 1.0 | 17.3 ± 3.1 | 20.3 ± 2.2 | 10.3 ± 1.6*† | 14.7 ± 3.5†# |
Values are reported as means ± SD. DLCO, lung diffusing capacity for carbon monoxide; DLNO, lung diffusing capacity for nitric oxide; DmCO, alveolar-capillary membrane conductance; VC, pulmonary-capillary blood volume; Q̇, cardiac output. Significance set at P < 0.05:
significantly different vs. Young;
significantly different vs. Young Highly Fit; and
significantly different vs. Old.
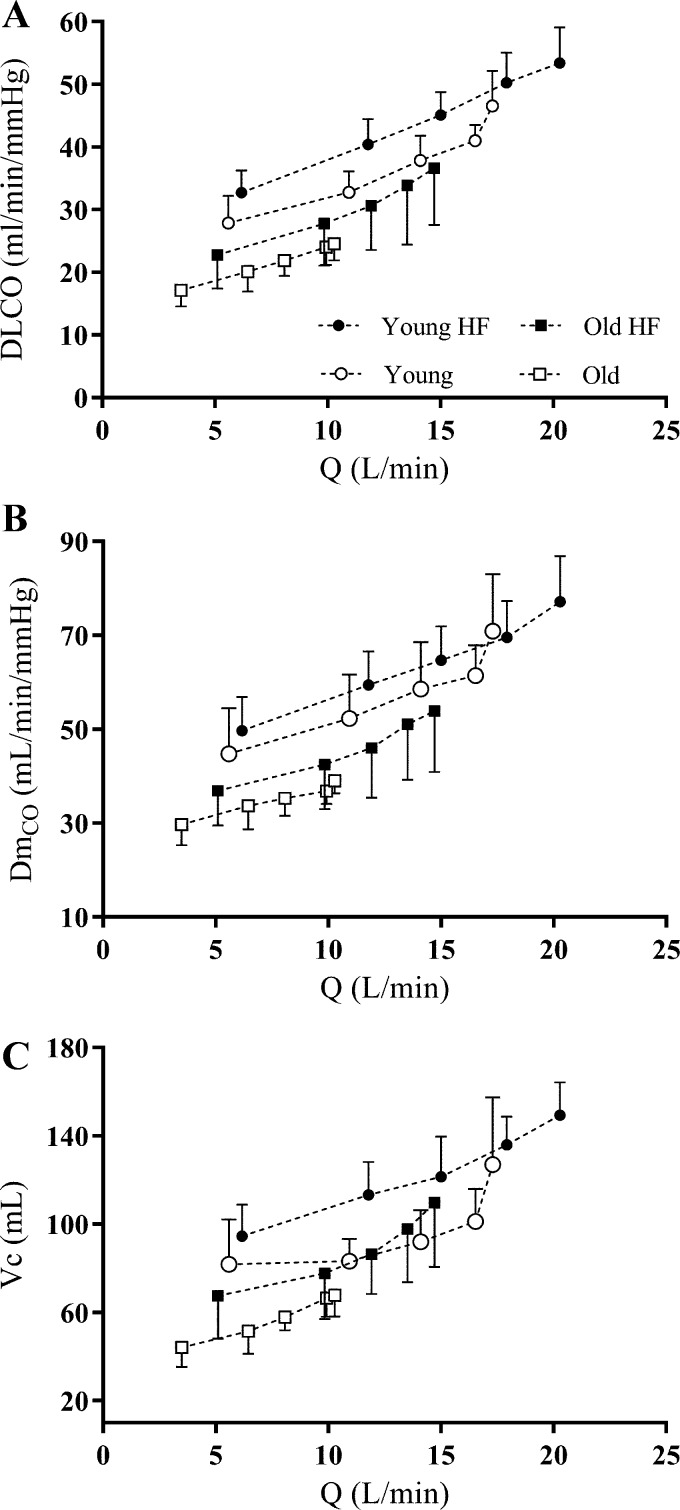
Fig. 1.
Group mean values of lung diffusing capacity for carbon monoxide (DLCO; A), alveolar-capillary membrane conductance (DmCO; B), and pulmonary-capillary blood volume (Vc; C) as a function of cardiac output (Q̇) during exercise. DLCO, DmCO, and Vc increase relatively linearly from rest to exercise at 90% Wpeak in all groups. Error bars denote means ± SD. See methods for group definitions. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
At 90% of Wpeak, group mean DLCO, DmCO, and Vc were lower in Old vs. Young (P < 0.001), Young Highly Fit (P < 0.001), and Old Highly Fit (P ≤ 0.050) (Table 2 and Fig. 1). Also, group mean DLCO and DmCO at 90% of Wpeak were lower in Old Highly Fit compared with Young (P ≤ 0.019) and Young Highly Fit (P ≤ 0.001). Moreover, Vc was lower in Old Highly Fit vs. Young Highly Fit at 90% of Wpeak (P = 0.016) (Table 2 and Fig. 1). These data suggest that at rest and during exercise, lung diffusing capacity and its component parts (i.e., DLCO, DmCO, and Vc) are decreased with age. Additionally, lung diffusing capacity and its component parts are greater during maximal exercise in Highly Fit older adults compared with their age-matched less fit counterparts.
Lung diffusing capacity response to exercise: effect of age and cardiorespiratory fitness.
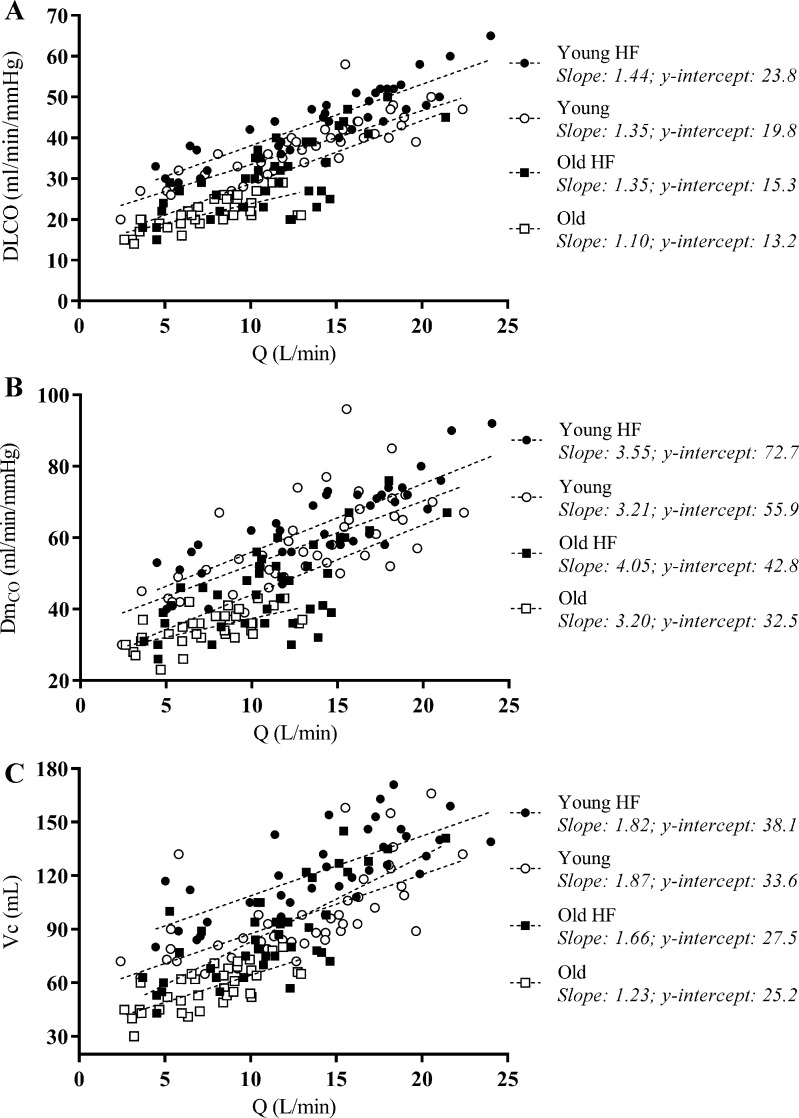
DLCO, DmCO, and Vc rose steadily with increasing Q̇ during exercise, with no evidence of a plateau and/or decrease in the rate of rise in these variables from rest to end exercise in any of the experimental groups (Fig. 1). These data suggest that no group encroached upon their maximal ability to expand the pulmonary vascular network and increase lung surface area for gas exchange during exercise. Throughout exercise, DLCO was significantly lower with greater age (P < 0.001) but significantly higher with greater fitness (P = 0.016) (Fig. 2). DmCO and Vc were significantly lower with greater age (P < 0.001) throughout exercise; the effect of fitness was not significant (Fig. 2). The relationships between lung diffusing capacity and its component variables (i.e., DLCO, DmCO, and Vc) and Q̇ from rest to end exercise are shown in Fig. 2. The rate of rise in DLCO, DmCO, and Vc relative to Q̇ during exercise was remarkably similar among the four experimental groups. Indeed, there was no significant effect of age or fitness on the slope of the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ response to exercise (Fig. 2). These data suggest that all groups experienced a similar pulmonary vascular response to exercise.
Fig. 2.
Individual values of lung diffusing capacity for carbon monoxide (DLCO; A), alveolar-capillary membrane conductance (DmCO; B), and pulmonary-capillary blood volume (Vc; C) as a function of cardiac output (Q̇) during exercise. The means ± SD of the slope of the lung diffusing capacity-cardiac output relationship is given for each group; there was no significant difference in slope between groups for any measure. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
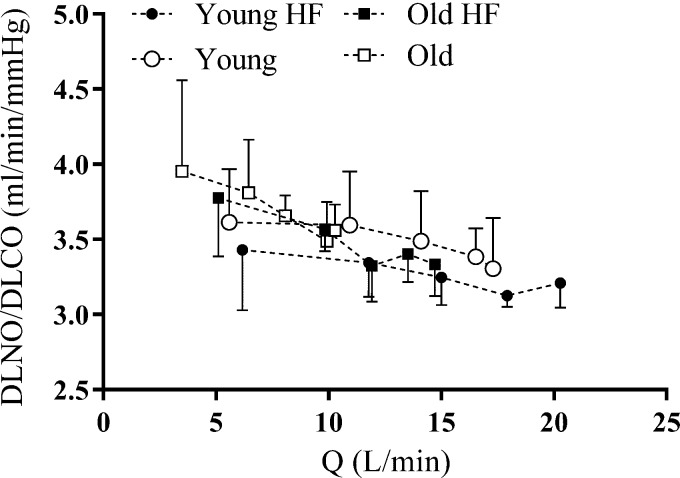
The DLNO/DLCO ratio decreased from rest to throughout exercise in Young (P = 0.033), Old (P = 0.028), and Old Highly Fit (P = 0.004), indicating that pulmonary capillary expansion during exercise was at least partially achieved via vessel distension; this fall was not significant in the Young Highly Fit individuals (P = 0.051; Fig. 3). Furthermore, there was no significant effect of age (P = 0.055) or fitness on the rate of fall in DLNO/DLCO from rest to end exercise (Fig. 3).
Fig. 3.
Group mean values of the ratio of lung diffusing capacity for nitric oxide to carbon monoxide (DLNO/DLCO) as a function of cardiac output (Q̇) during exercise. DLNO/DLCO falls from rest to exercise at 90% Wpeak in Young, Old, and Old Highly Fit. Error bars denote means ± SD. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
Relationship between lung diffusing capacity and V̇o2max.
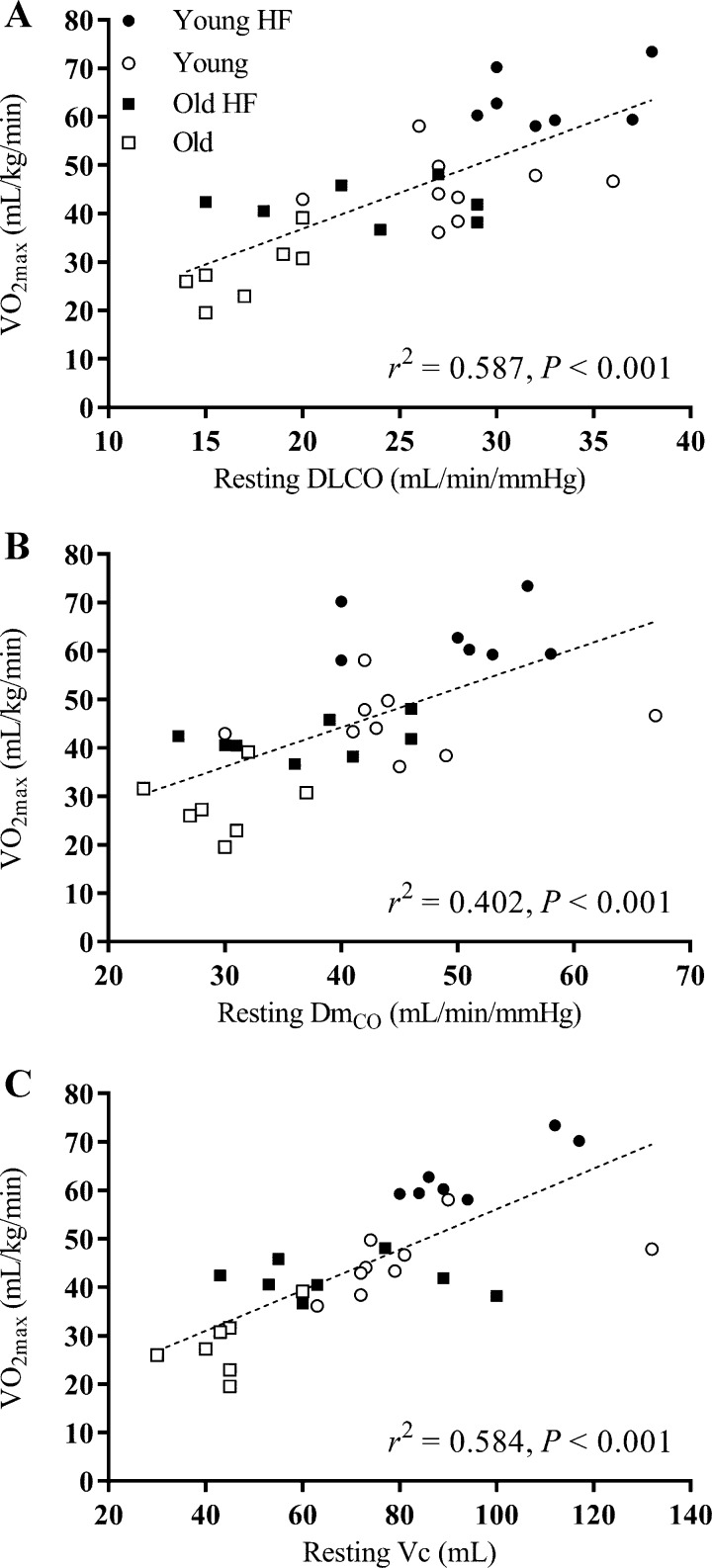
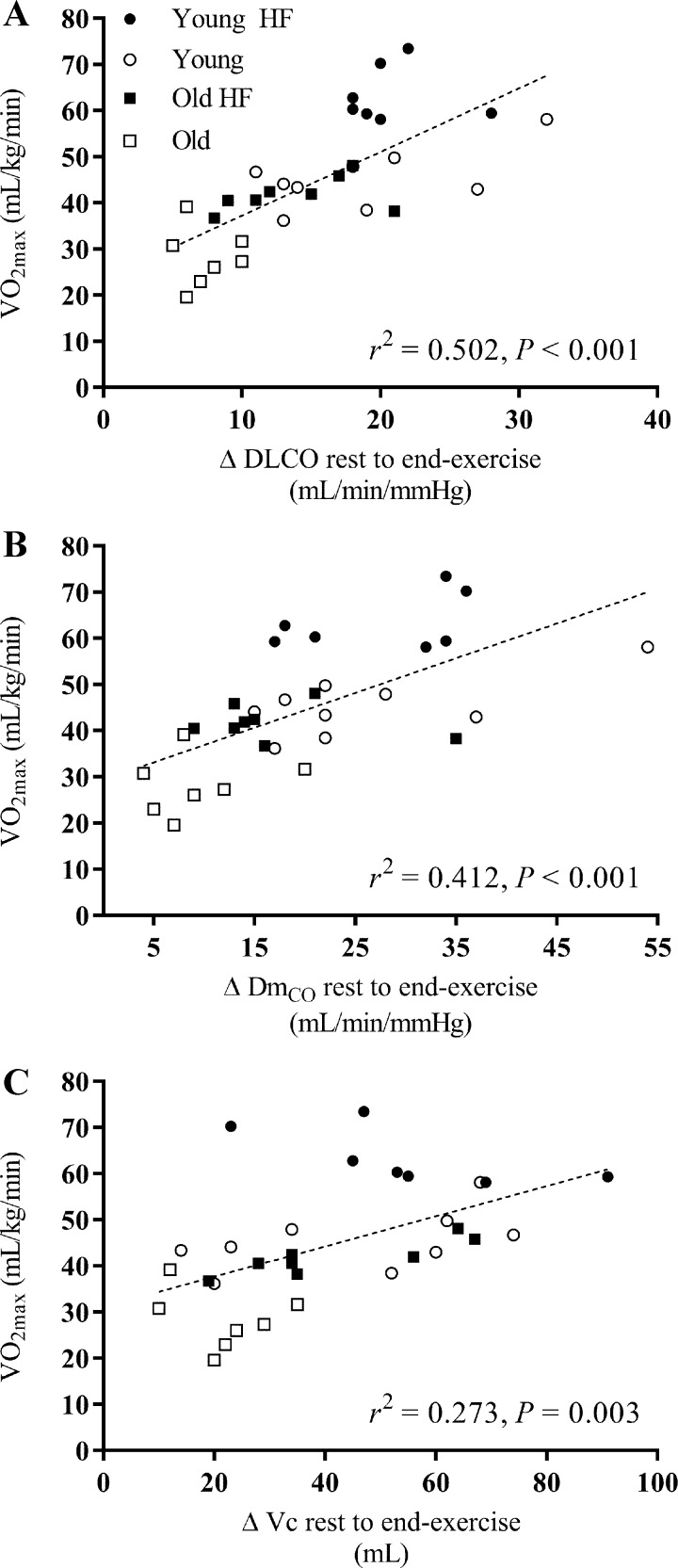
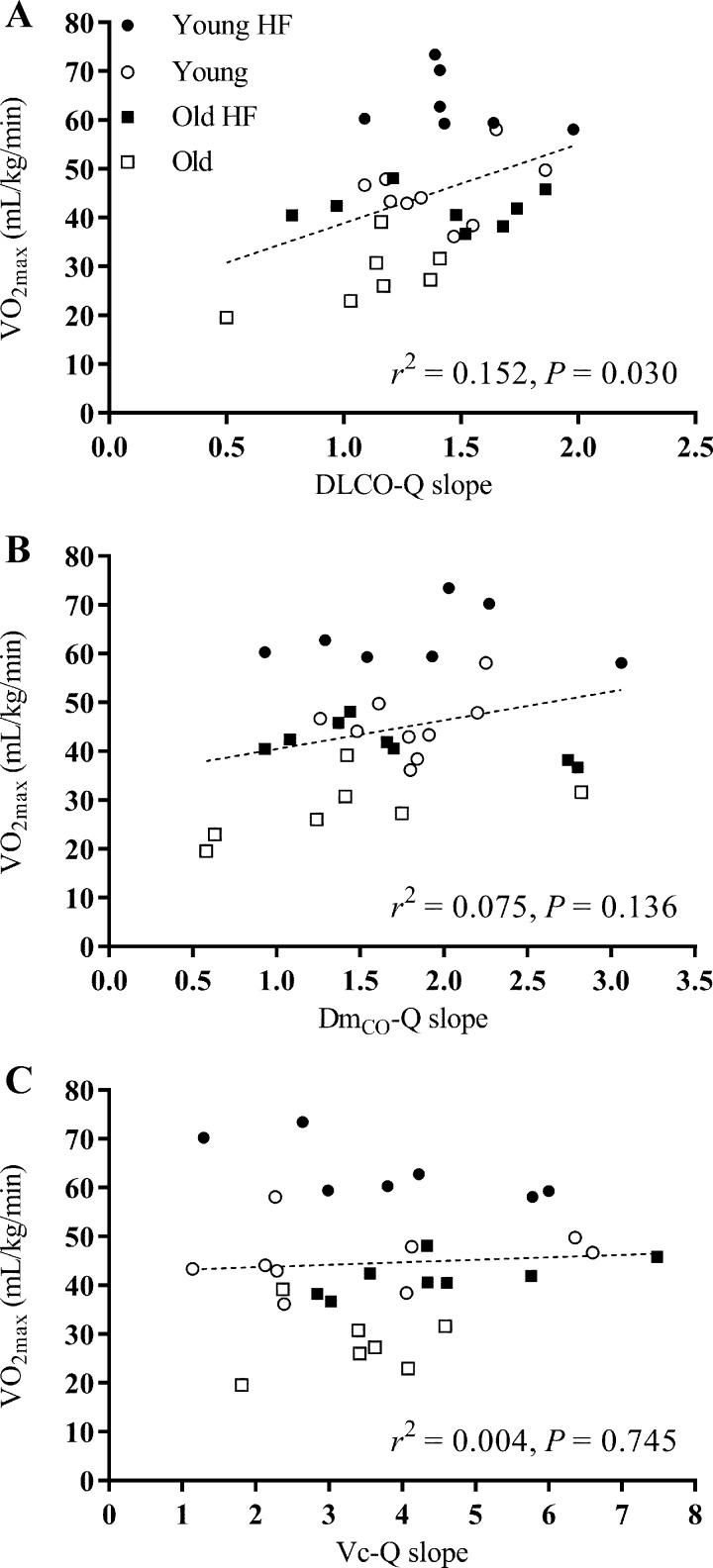
The relationships between V̇o2max and 1) resting measures of DLCO, DmCO, and Vc; 2) the change (Δ) in DLCO, DmCO, and Vc from rest to end exercise; and 3) the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ slope in response to exercise for all 31 subjects are shown in Figs. 4, 5, and 6, respectively. A significant positive correlation was found between V̇o2max and resting measures of DLCO (r2 = 0.587, P < 0.001), DmCO (r2 = 0.402, P < 0.001), and Vc (r2 = 0.584, P < 0.001) (Fig. 4). Similarly, there was a significant positive relationship between V̇o2max and the rest to end exercise change (Δ) in DLCO (r2 = 0.502, P < 0.001), DmCO (r2 = 0.412, P < 0.001), and Vc (r2 = 0.273, P = 0.003) (Fig. 5). Finally, there was a positive relationship between V̇o2max and the DLCO-Q̇ slope in response to exercise (r2 = 0.152, P = 0.030); the relationship between V̇o2max and the DmCO-Q̇ and Vc-Q̇ slope in response to exercise was not statistically significant (Fig. 6). Together, these data suggest that a higher baseline DLCO, DmCO, and Vc, as well as a larger increase these values in response to exercise are associated with greater maximal aerobic capacity in young and older adults regardless of cardiopulmonary fitness level.
Fig. 4.
Individual values of V̇o2max as a function of resting values of lung diffusing capacity for carbon monoxide (DLCO; A), alveolar-capillary membrane conductance (DmCO; B), and pulmonary-capillary blood volume (Vc; C). A linear regression was fit to all data points for each measure. V̇o2max is positively correlated with resting values of DLCO, DmCO, and Vc. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
Fig. 5.
Individual values of V̇o2max as a function of the absolute change (Δ) in values of lung diffusing capacity for carbon monoxide (DLCO; A), alveolar-capillary membrane conductance (DmCO; B), and pulmonary-capillary blood volume (Vc; C) from rest to exercise at 90% of Wpeak. A linear regression was fit to all data points for each measure. V̇o2max is positively correlated with the absolute change in values of DLCO, DmCO, and Vc from rest to exercise at 90% of Wpeak. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
Fig. 6.
V̇o2max as a function of the lung diffusing capacity-cardiac output slope for each individual subject (from Fig. 2). A linear regression was fit to all data points for each measure. V̇o2max is positively correlated with the DLCO-Q̇ slope (A); V̇o2max is not significantly correlated with the DmCO-Q̇ or Vc-Q̇ slopes(B and C). DLCO, lung diffusing capacity for carbon monoxide; DmCO, alveolar-capillary membrane conductance; Vc, pulmonary-capillary blood volume. ●: Young Highly Fit (HF); ○: Young; ■: Old Highly Fit (HF); □: Old.
DISCUSSION
Major findings: comparison to previous studies.
In the present study, we characterized the lung diffusing capacity for carbon monoxide (DLCO), alveolar-capillary membrane conductance (DmCO), and pulmonary-capillary blood volume (Vc) response to discontinuous incremental exercise in healthy, aerobically-trained older adults relative to their age-matched less fit counterparts as well as younger adults. We hypothesized that healthy older adults (~65 yr old) with a high cardiorespiratory fitness level (V̇o2max: ~162% of age predicted) would encroach upon their maximal ability to expand the pulmonary capillary network during high-intensity cycle exercise, as evidenced by a limit in DLCO, DmCO, and Vc near maximal end exercise. The main findings were as follows: 1) healthy aging was associated with a decrease in DLCO, DmCO, and Vc at rest and during near maximal exercise (Table 2); 2) better maintained cardiorespiratory fitness was associated with greater DLCO, DmCO, and VC during near-maximal exercise in older adults (Table 2); 3) there was no plateau (i.e., a limitation) in the DLCO, Vc, and DmCO response to exercise in any subject group regardless of age or cardiorespiratory fitness level (Fig. 1); 4) throughout exercise, DLCO, DmCO, and Vc were systematically lower in older individuals regardless of fitness, and DLCO was systematically higher with maintained cardiorespiratory fitness regardless of age; and 5) the slope, or rate of rise, of the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ relationship from rest to end exercise was not different between subjects regardless of age or cardiorespiratory fitness level (Fig. 2).
Our findings are confirmatory that healthy aging is associated with a progressive decline in resting DLCO (1, 6, 9, 11, 15, 33, 34). For example, Guénard and Marthan (15) demonstrated that both DLCO and DLCO relative to minute ventilation (DLCO/V̇e) are negatively correlated with age according to the equations DLCO = 126 – 0.90 × age and DLCO/V̇e = 13.5 – 0.85 × age, respectively. Likely contributors to this age-related reduction in DLCO are a decrease in the number of capillaries perfusing the lungs with a reduction in Vc (6, 7, 12), as well as a decrease in alveolar surface area with a consequent reduction in membrane diffusing capacity (6, 12). Our findings are also in agreement with previous reports that exercise is associated with a marked, mostly linear, increase in DLCO, Vc, and DmCO in healthy adults (16, 18, 27, 34). This increase, at least in part, reflects expansion of the highly compliant pulmonary capillary network secondary to the elevation in Q̇ and pulmonary perfusion pressure that occurs with exercise.
To date, however, there is a relative paucity of data regarding the effect of healthy aging on the lung diffusing capacity response to exercise. In a limited number of subjects (n = 12) of a broad age range (23 to 79 yr), Tamhane et al. (34) reported that DLCO, DmCO, and lung diffusing capacity for nitric oxide (DLNO) increased linearly with Q̇ from rest to exercise regardless of age. However, while the authors found that age was a significant determinant of resting DLNO, no such analysis was done examining the influence of age on the lung diffusing capacity response to exercise. Additionally, we are unaware of any previous study that has examined the influence of healthy aging plus maintained cardiorespiratory fitness on lung diffusing capacity during exercise. In combination, the present findings suggest that despite the age-associated deterioration in the structure and function of the pulmonary circulation, expansion of the pulmonary capillary network does not become limited during high-intensity exercise (i.e., there is still a reserve to recruit the pulmonary vasculature) in healthy individuals regardless of age or cardiorespiratory fitness level. In addition, we suggest that maximal oxygen consumption (V̇o2max) is positively related DLCO, DmCO, and Vc, both at rest and in response to exercise, across all ages and cardiorespiratory fitness levels.
Lung diffusing capacity response to exercise: effect of age and cardiorespiratory fitness.
Exercise is associated with an increase in cardiac output and pulmonary perfusion pressure that causes both recruitment of underperfused pulmonary capillaries and distension of already perfused pulmonary blood vessels, as evidenced by an increase in DLCO, DmCO, and Vc (16, 18, 22, 23, 36). The resulting increase in pulmonary blood flow and marked expansion of the highly compliant pulmonary vasculature acts to increase the alveolar-capillary surface area available for effective gas exchange.
Healthy aging is associated with a deterioration in the structure and function of the pulmonary vasculature that is characterized by an increase in pulmonary vascular stiffness, pulmonary vascular pressures, and pulmonary vascular resistance (20, 24, 28), as well as reductions in resting Vc and DmCO (1, 9, 12, 15). It has been shown, however, that despite these age-related changes DLCO does not become limited during maximal exercise in the older adult of average cardiorespiratory fitness (34). This is likely because the age-associated decline in the maximal metabolic demand of exercise occurs at rate equal to or greater than the deleterious changes in the pulmonary circulation (14, 19, 37). However, it is conceivable that healthy older individuals who have maintained cardiorespiratory fitness, and thus metabolic demand, at an exceedingly high level may experience a limit in the capacity of the pulmonary vasculature to expand relative to the demand for pulmonary blood flow during exercise.
In the present study, we found that DLCO, DmCO, and Vc increased steadily with increasing cardiac output (Q̇), with no evidence of a plateau in these variables in both Young and Old subjects, regardless of cardiorespiratory fitness level (Fig. 1). Moreover, the slope of the DLCO-Q̇, DmCO-Q̇, and Vc-Q̇ relationship from rest to end exercise was not different among Young, Young Highly Fit, Old, and Old Highly Fit subjects (Fig. 2). This suggests that the recruitment and/or distension of the pulmonary capillaries and thus expansion of alveolar-capillary surface area remain adequate for the metabolic demand of exercise regardless of age and cardiorespiratory fitness level. In agreement with previous findings, DLCO, DmCO, and Vc at rest and during exercise were decreased with advanced age (1, 6, 11, 12, 15, 33, 34). Interestingly, however, we also found that DLCO, DmCO, and Vc were greater during maximal exercise in highly fit older individuals compared with their less fit age-matched counterparts (Fig. 1). Additionally, regardless of age, maintained cardiorespiratory fitness was associated with a significantly greater DLCO from rest through to maximal exercise.
It has been suggested previously that exercise training has no effect on DLCO and its components parts (8, 30). For example, Reuschlein et al. (30) reported no change in DLCO or Vc at rest and during submaximal exercise from before to after 5 mo of combined strength and endurance training. By contrast, it has been shown that cardiac and great vessel function is better in older, habitually active, fit adults relative to their more sedentary counterparts. Indeed, Arbab-Zadeh et al. (2) reported that prolonged, sustained endurance training improves stroke volume for a giving filling pressure and preserves left ventricular compliance in aged adults such that the capillary wedge pressure-left ventricular end diastolic volume curve in Masters athletes was indistinguishable from that of young, sedentary control subjects. In addition, central arterial compliance is 20–35% greater in endurance-trained middle-aged and older men compared with their less active age-matched counterparts (35). Furthermore, 3 mo of aerobic exercise training increases central arterial compliance (~25%) in middle-aged men (~53 yr) to a level similar to that observed in older endurance-trained men (35), possibly due to modified cross linking of “stretched” collagen fibers and/or a reduction in the chronic suppressive influence exerted by sympathetic adrenergic tone (4, 35). Theoretically, it is entirely possible that habitual physical activity may also better preserve the function of the pulmonary circulation, attenuating the age-related decline in pulmonary vascular distensibility (28) and compliance. This would allow for greater expansion of the pulmonary capillary network with increasing cardiac output, thus facilitating a greater DLCO, DmCO, and Vc response to exercise in highly fit older individuals compared with their less fit counterparts. However, whether preservation of cardiorespiratory fitness does indeed “protect” against the age-related deterioration of the pulmonary circulation cannot be deduced from the present findings and as such remains purely speculative.
Expansion of the pulmonary capillary network during exercise: recruitment vs. distension.
The mechanism by which the pulmonary vasculature expands to accept increased blood flow and increase effective alveolar-capillary surface area during exercise is also of interest. In the healthy pulmonary circulation, vessel distensibility is largely independent of vessel size, location, and animal species (21) but appears to be lower in older adults relative to their younger counterparts (28). Assessing the change in the ratio between DLNO and DLCO during exercise may allow insight into whether pulmonary vascular volume increases due to recruitment or distension of the pulmonary capillaries (13, 23, 27). Specifically, a fall in the DLNO/DLCO ratio with exercise is thought to indicate a disproportionate rise in Vc relative to DmCO, which in turn suggests predominant pulmonary capillary distension over recruitment (13). In the present study, all groups with the exception of the Young Highly Fit (P = 0.051) demonstrated a significant reduction in the DLNO/DLCO ratio from rest to end exercise (Fig. 3). This finding suggests that despite the previously reported decay in pulmonary vascular distensibility associated with healthy aging, increased blood flow through the pulmonary circulation during exercise is at least partially achieved via distension of the pulmonary capillaries in old as well as young individuals.
Relationship between lung diffusing capacity response to exercise and V̇o2max.
The importance of pulmonary vascular hemodynamics in determining V̇o2max in both health and disease is well known (10, 23). Fujii et al. (10) reported that the slope of the mean pulmonary artery pressure-to-cardiac output relationship was negatively correlated with V̇o2max in chronic obstructive pulmonary disease patients. That is, a more compliant pulmonary vasculature, or one that experiences lower vascular pressures, allows for greater maximal aerobic capacity in healthy individuals as well as diseased patients.
In this study, we did not examine pulmonary vascular pressures in response to exercise. Conceptually, however, a greater increase lung diffusing capacity relative to cardiac output in response to exercise could be indicative of a greater ability of the pulmonary vasculature to expand and accept the increase in pulmonary blood flow while minimizing the increase in pulmonary vascular pressure. That is, it is conceivable that a steeper DLCO-Q̇ slope during exercise is reflective of a more compliant pulmonary vascular network. In support of this notion, we found that DLCO-Q̇ was positively correlated to V̇o2max across all subjects (Fig. 6). In addition, resting values of DLCO, DmCO, and Vc, as well as the absolute change in these variables from rest to maximal exercise, are positively correlated with V̇o2max across all subjects (Figs. 4 and 5). That expansion of the pulmonary vasculature appears not to reach a maximum during exercise (Fig. 1) likely serves to allow an increase in the alveolar-capillary surface area for effective gas exchange while minimizing the exercise-induced increase in pulmonary arterial pressure, pulmonary vascular resistance and right-ventricular afterload, and thus allowing a greater maximum cardiac output and maximum oxygen consumption in healthy adults regardless of age and cardiorespiratory fitness (22, 23, 27).
Technical considerations: effect of acetylene solubility upon calculation of cardiac output.
A concern with the use of acetylene uptake in the noninvasive determination of Q̇ is that an assumption, rather than direct assessment, of acetylene solubility (λ) in individual subjects can result in considerable error in the measurement of Q̇. For example, Barker et al. (3) suggested that failure to account for intersubject variability in λ can lead to substantial underestimation (up to 27%) or overestimation (up to 13%) of Q̇ in young healthy adults. In the present study, we did not measure, and subsequently account for, between-subject differences in λ upon the calculation of Q̇. However, while any such error may have negatively impacted the accuracy of our measure of Q̇, we are able to demonstrate good within-session within-subject reliability of our acetylene derived Q̇ measure at rest [coefficient of variation (CV) = 8.5%]. This is comparable with the CV reported previously by our group for open-circuit acetylene wash-in estimated Q̇ (17). In addition, although not assessed in the present study, it is likely that the variability in our measure of Q̇ improved to ~4% during exercise (17). As such, based on the relatively low CV (i.e., good reproducibility) along with the repeated-measures design of our study, we are confident that our findings are not greatly affected by any underlying variability in λ between individual subjects.
In conclusion, DLCO, DmCO, and Vc are decreased with age and increased with greater cardiorespiratory fitness in older individuals during maximal exercise. Interestingly, there is a systematic increase in DLCO throughout exercise with maintained cardiorespiratory fitness, regardless of age. Older highly fit individuals do not appear to encroach upon a pulmonary vascular limit, and expansion of the pulmonary capillary network is able to adequately increase DLCO, DmCO, and Vc, even during high-intensity exercise. Furthermore, the response (i.e., rate of increase) of DLCO, DmCO, and Vc to exercise is not altered by age and/or cardiorespiratory fitness level. Future studies should incorporate measures of pulmonary vascular pressures to elucidate the relationship between increases in lung diffusing capacity and the pulmonary vascular response to exercise.
GRANTS
K. E. Coffman is supported by Mayo Graduate School and National Heart, Lung, and Blood Institute (NHLBI) Grant F31-HL-131076. B. J. Taylor is supported by American Heart Association Grant AHA12POST12070084. This study was funded by NHLBI Grant HL-71478.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.C., B.D.J., and B.J.T. conceived and designed the research; K.E.C., A.R.C., A.D.M., and B.J.T. performed experiments; K.E.C., A.R.C., A.D.M., and B.J.T. analyzed data; K.E.C., B.D.J., and B.J.T. interpreted results of experiments; K.E.C. and B.J.T. prepared figures; K.E.C. and B.J.T. drafted manuscript; K.E.C., B.D.J., and B.J.T. edited and revised manuscript; K.E.C., A.R.C., A.D.M., B.D.J., and B.J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Josh O’Malley for work as a research technologist during these studies.
REFERENCES
- 1.Al Dandachi G, Londner C, Caumont-Prim A, Plantier L, Chevalier-Bidaud B, Toussaint JF, Desgorces FD, Delclaux C. Ageing and endurance training effects on quantity and quality of pulmonary vascular bed in healthy men. Respir Res 15: 8, 2014. doi: 10.1186/1465-9921-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 3.Barker RC, Hopkins SR, Kellogg N, Olfert IM, Brutsaert TD, Gavin TP, Entin PL, Rice AJ, Wagner PD. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol (1985) 87: 1506–1512, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Brüel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140: 135–145, 1998. doi: 10.1016/S0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 5.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol (1985) 109: 643–653, 2010. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SC, Chang HI, Liu SY, Shiao GM, Perng RP. Effects of body position and age on membrane diffusing capacity and pulmonary capillary blood volume. Chest 102: 139–142, 1992. doi: 10.1378/chest.102.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Crapo RO, Morris AH, Gardner RM. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopathol Respir 18: 893–899, 1982. [PubMed] [Google Scholar]
- 9.Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci (Lond) 65: 653–660, 1983. doi: 10.1042/cs0650653. [DOI] [PubMed] [Google Scholar]
- 10.Fujii T, Kurihara N, Fujimoto S, Hirata K, Yoshikawa J. Role of pulmonary vascular disorder in determining exercise capacity in patients with severe chronic obstructive pulmonary disease. Clin Physiol 16: 521–533, 1996. doi: 10.1111/j.1475-097X.1996.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Rio F, Dorgham A, Galera R, Casitas R, Martinez E, Alvarez-Sala R, Pino JM. Prediction equations for single-breath diffusing capacity in subjects aged 65 to 85 years. Chest 142: 175–184, 2012. doi: 10.1378/chest.11-2021. [DOI] [PubMed] [Google Scholar]
- 12.Georges R, Saumon G, Loiseau A. The relationship of age to pulmonary membrane conductance and capillary blood volume. Am Rev Respir Dis 117: 1069–1078, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Glénet SN, De Bisschop C, Vargas F, Guénard HJ. Deciphering the nitric oxide to carbon monoxide lung transfer ratio: physiological implications. J Physiol 582: 767–775, 2007. doi: 10.1113/jphysiol.2007.133405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldspink DF. Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics 48: 1334–1351, 2005. doi: 10.1080/00140130500101247. [DOI] [PubMed] [Google Scholar]
- 15.Guénard H, Marthan R. Pulmonary gas exchange in elderly subjects. Eur Respir J 9: 2573–2577, 1996. doi: 10.1183/09031936.96.09122573. [DOI] [PubMed] [Google Scholar]
- 16.Hsia CC, McBrayer DG, Ramanathan M. Reference values of pulmonary diffusing capacity during exercise by a rebreathing technique. Am J Respir Crit Care Med 152: 658–665, 1995. doi: 10.1164/ajrccm.152.2.7633723. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol (1985) 88: 1650–1658, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RL Jr, Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol 15: 893–902, 1960. [DOI] [PubMed] [Google Scholar]
- 19.Kasch FW, Boyer JL, Van Camp S, Nettl F, Verity LS, Wallace JP. Cardiovascular changes with age and exercise. A 28-year longitudinal study. Scand J Med Sci Sports 5: 147–151, 1995. doi: 10.1111/j.1600-0838.1995.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 34: 888–894, 2009. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 21.Krenz GS, Dawson CA. Flow and pressure distributions in vascular networks consisting of distensible vessels. Am J Physiol Heart Circ Physiol 284: H2192–H2203, 2003. doi: 10.1152/ajpheart.00762.2002. [DOI] [PubMed] [Google Scholar]
- 22.La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbüchel H, Prior DL. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol (1985) 109: 1307–1317, 2010. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- 23.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 590: 4279–4288, 2012. doi: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119: 2663–2670, 2009. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 128: 1470–1479, 2013. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Pavelescu A, Faoro V, Guenard H, de Bisschop C, Martinot JB, Mélot C, Naeije R. Pulmonary vascular reserve and exercise capacity at sea level and at high altitude. High Alt Med Biol 14: 19–26, 2013. doi: 10.1089/ham.2012.1073. [DOI] [PubMed] [Google Scholar]
- 28.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 288: L419–L425, 2005. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 29.Reeves RB, Park HK. CO uptake kinetics of red cells and CO diffusing capacity. Respir Physiol 88: 1–21, 1992. doi: 10.1016/0034-5687(92)90025-R. [DOI] [PubMed] [Google Scholar]
- 30.Reuschlein PS, Reddan WG, Burpee J, Gee JB, Rankin J. Effect of physical training on the pulmonary diffusing capacity during submaximal work. J Appl Physiol 24: 152–158, 1968. [DOI] [PubMed] [Google Scholar]
- 31.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11: 290–302, 1957. [DOI] [PubMed] [Google Scholar]
- 32.Sackner MA, Greeneltch D, Heiman MS, Epstein S, Atkins N. Diffusing capacity, membrane diffusing capacity, capillary blood volume, pulmonary tissue volume, and cardiac output measured by a rebreathing technique. Am Rev Respir Dis 111: 157–165, 1975. [DOI] [PubMed] [Google Scholar]
- 33.Stam H, Hrachovina V, Stijnen T, Versprille A. Diffusing capacity dependent on lung volume and age in normal subjects. J Appl Physiol (1985) 76: 2356–2363, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Tamhane RM, Johnson RL Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest 120: 1850–1856, 2001. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 36.Taylor BJ, Carlson AR, Miller AD, Johnson BD. Exercise-induced interstitial pulmonary edema at sea-level in young and old healthy humans. Respir Physiol Neurobiol 191: 17–25, 2014. doi: 10.1016/j.resp.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trappe SW, Costill DL, Vukovich MD, Jones J, Melham T. Aging among elite distance runners: a 22-yr longitudinal study. J Appl Physiol (1985) 80: 285–290, 1996. [DOI] [PubMed] [Google Scholar]