Abstract
Cholangiocytes, a small population of cells within the normal liver, have been the focus of a significant amount of research over the past two decades because of their involvement in cholangiopathies such as primary sclerosing cholangitis and primary biliary cholangitis. This article summarizes landmark studies in the field of cholangiocyte physiology and aims to provide an updated review of biliary pathogenesis. The historical approach of rodent extrahepatic bile duct ligation and the relatively recent utilization of transgenic mice have led to significant discoveries in cholangiocyte pathophysiology. Cholangiocyte physiology is a complex system based on heterogeneity within the biliary tree and a number of signaling pathways that serve to regulate bile composition. Studies have expanded the list of neuropeptides, neurotransmitters, and hormones that have been shown to be key regulators of proliferation and biliary damage. The peptide histamine and hormones, such as melatonin and angiotensin, angiotensin, as well as numerous sex hormones, have been implicated in cholangiocyte proliferation during cholestasis. Numerous pathways promote cholangiocyte proliferation during cholestasis, and there is growing evidence to suggest that cholangiocyte proliferation may promote hepatic fibrosis. These pathways may represent significant therapeutic potential for a subset of cholestatic liver diseases that currently lack effective therapies.
Key words: Biliary epithelium, Cholangiopathies, Cholestasis, Liver damage
ANATOMY/PHYSIOLOGY
The liver is primarily composed of two types of epithelial cells: hepatocytes and cholangiocytes1. The liver is primarily responsible for the bile acid production, serum detoxification, serum protein and cholesterol production, coagulation cascade maintenance, and glucose storage1. Hepatocytes make up 70% of the liver’s parenchyma and perform the majority of these functions2. Cholangiocytes account for 3%–5% of the endogenous liver cell population and form the biliary tree that begins as small intrahepatic ductules and terminates as the larger extrahepatic bile ducts3. Bile is secreted across the apical membrane of hepatocytes into canaliculi formed between adjacent cells4. Bile flows through the canaliculi and the canals of Hering into the small biliary ductules that ultimately results in bile secretion into the small intestine through the ampulla of Vater5. Despite providing only a fraction of the total liver cell population, cholangiocytes play an important physiologic role in altering ductal bile composition as it travels through the biliary system6,7. This process involves the secretion and absorption of water, electrolytes, and other organic solutes from hepatocellular bile6–8. This process is mediated by a series of hormone-regulated Ca2+-dependent or cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent resorptive and secretory events6,7,9–13.
Heterogeneity of the Biliary Tree
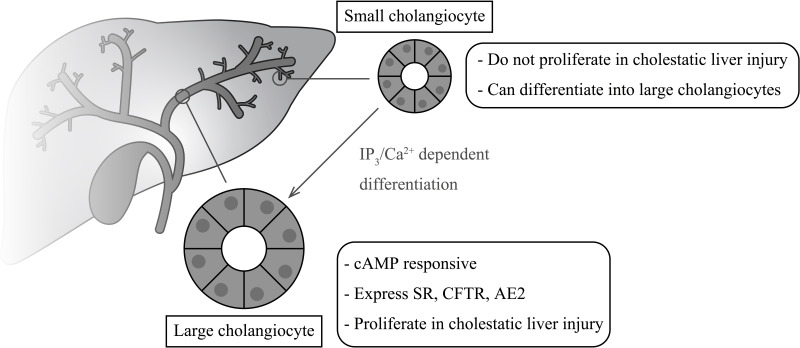
Heterogeneity in cholangiocyte structure and function has been defined throughout the biliary tree of rodents and humans3,9,13. Structurally, cholangiocytes take on the shape and size necessary to maintain the integrity of the biliary epithelium. In humans, intrahepatic bile ducts range in size from small ductules (<15 μm) to large hepatic ducts (>800 μm)3. Cholangiocytes in larger ducts tend to be larger and more cuboidal in shape than cholangiocytes of small intrahepatic bile ducts14–16. Only large cholangiocytes from rodents are cAMP responsive and express secretin receptors (SRs), cystic fibrosis transmembrane conductance regulator (CFTR), and the chloride bicarbonate anion exchanger 2 (Cl−/HCO3 − AE2)3,9,13,17,18. Activation of AE2 leads to changes in ductal secretion of water and electrolytes including HCO3 − ions5. Expression of the SR is a key factor regulating the secretory and proliferative activities of cholangiocytes and heterogeneity of the biliary tree. Cholangiocytes proliferate in response to biliary injury, such as extrahepatic obstruction and cholestatic liver diseases, and in response to alcohol, toxins, or drugs19. Using a rat model of extrahepatic cholestasis induced by bile duct ligation (BDL), studies have shown that cholangiocyte proliferation is limited to large bile ducts and is closely associated with SR gene expression and secretin-stimulated cAMP levels11,12. Figure 1 highlights the heterogeneity within the biliary tree and the response of small and large cholangiocytes during cholestasis.
Figure 1.
Heterogeneity within the biliary tree. Large cholangiocytes are cAMP responsive and express SR, CFTR, and AE2 that regulate bile homeostasis. Large cholangiocytes proliferate in response to cholestatic liver injury. Small cholangiocytes do not proliferate during cholestasis but may act as a progenitor cell population and differentiate into large cholangiocytes via the IP3/Ca2+ signaling pathways.
Electron microscopic studies have shown that small cholangiocytes display a larger nucleus: cytosplasm ratio compared to large cholangiocytes, suggesting that small cholangiocytes represent a hepatic progenitor cell population20. Small cholangiocytes do not express the SR and do not actively proliferate following cholestatic injury (BDL), but they are capable of proliferating [by amplification of inositol 1,4,5-triphosphate (IP3)/Ca2+-dependent signaling]20,21 and differentiating into a large cholangiocyte phenotype under specific pathological conditions associated with damage of large cAMP-responsive bile ducts, such as γ-aminobutyric acid-induced liver injury10,21–23. Additionally, small cholangiocytes have been shown to express a senescent phenotype in humans with primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC)24,25. Despite this understanding of biliary heterogeneity, the correlation of cholangiocyte phenotype to disease progression remains an area of investigation.
Bicarbonate Secretion
The excretion of bicarbonate into ductal bile remains one of the most important and well-studied functions of cholangiocytes. This process is stimulated by activation of the SR by its ligand, secretin, a 27-amino acid gastrointestinal peptide hormone. SRs are expressed only on the basolateral membrane of large cholangiocytes26,27. The SR is a G protein-coupled receptor (GPCR) that promotes bicarbonate excretion through a series of events activating adenylyl cyclase 8 and protein kinase A (PKA)28,29. Activated PKA promotes the phosphorylation of CFTR, which triggers opening of the Cl− channel and leads to secretion of the chloride ion at the apical membrane of cholangiocytes, depolarizing the cell membrane30,31. This ion gradient promotes activation of the Cl−/HCO3 − exchanger, resulting in secretin-stimulated bicarbonate-enriched bile7. The secretion of HCO3 − creates an AE2-dependent bicarbonate-rich umbrella and serves as a protective mechanism against the cytotoxicity of hydrophobic bile salts32. Not only does the SR regulate bicarbonate secretion but also is a key regulator in cholangiocyte proliferation.
MECHANISMS AND REGULATORS OF CHOLANGIOCYTE PROLIFERATION
Intracellular cAMP signaling plays an important role in cholangiocyte proliferation. Chronic administration of forskolin (an adenylate cyclase activator) to normal rats increased intrahepatic bile duct mass, cAMP levels, and secretin-induced choleresis following BDL33. Cholangiocyte proliferation was associated with increased activity of the PKA–Src–MEK–ERK1/2 pathway33. Ablation of this pathway using specific inhibitors resulted in decreased cholangiocyte proliferation33.
Additionally, bile acid-induced activation of receptor tyrosine kinases, specifically the epidermal growth factor receptor (EGFR), has been shown to stimulate human cholangiocyte proliferation via transforming growth factor-α (TGF-α)-dependent mechanisms34. Tyrosine kinase signaling via EGFR has also been demonstrated in cholangiocarcinoma cells35, and the development of ROS kinase fusions may contribute to cholangiocarcinoma oncogenesis36. The Janus kinase/signal transducer activator of transcription (JAK/STAT) pathway has also been implicated in cholangiocarcinoma growth, and inhibition of this pathway with sorafenib has been shown to accelerate dephosphorylation of STAT337.
Recent studies have expanded our knowledge of biliary secretion and the role of cilia in this process. Cholangiocytes possess cilia on their apical surface that act as mechanoreceptors, chemosensors, and osmosensors38. Shear stress along the apical membrane of cholangiocytes is detected by cilia, resulting in upregulation of Cl− currents via TMEM16A (aka anoctamin), a Ca2+-activated Cl− channel39. This process is dependent on PKC-α, extracellular ATP, purinergic P2 receptors, and increases in intracellular Ca2+ concentration39. Inhibition of CFTR did not change flow-stimulated currents in their studies, suggesting a different mechanism of Cl− transport when cholangiocytes are exposed to shear forces39. The key proteins involved in mechanosensation are polycystin-1 and polycystin-2. These proteins form a functional complex allowing Ca2+ influx, influencing secretory and proliferative functions. Cholangiocytes also function as chemosensors, capable of detecting changes in concentrations of molecules within the bile. The P2Y receptors, including P2Y12, activate intracellular calcium and cAMP signaling cascades when activated by nucleotides present in large concentrations in the bile38,40.
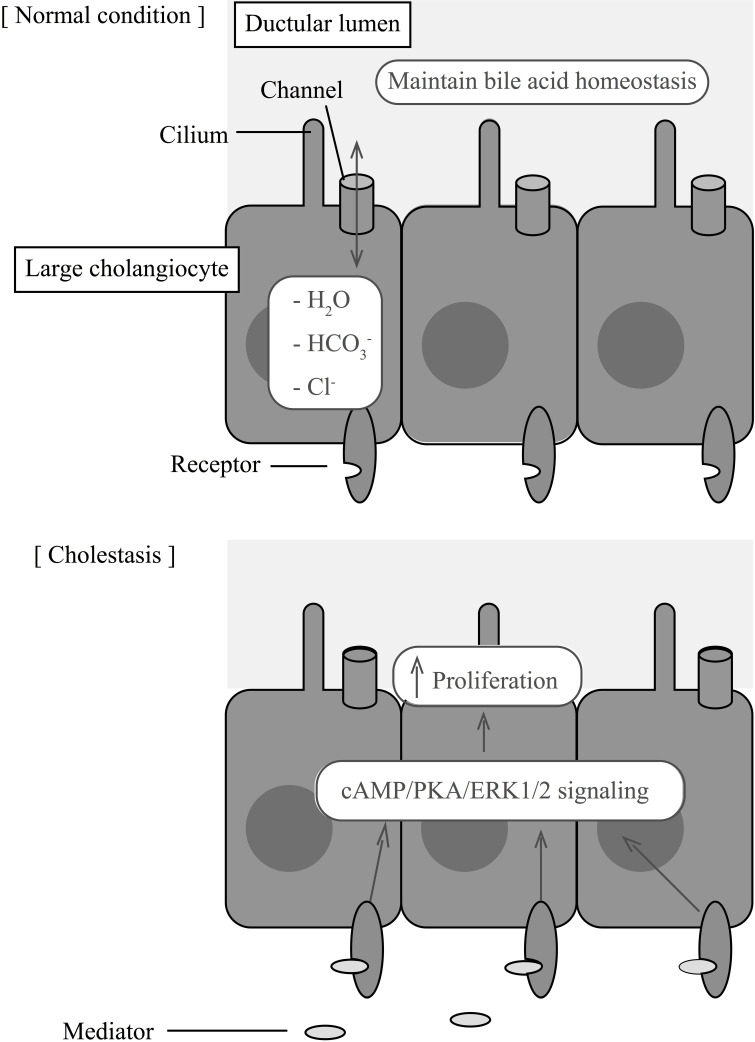
Numerous animal models of biliary hyperplasia, such as BDL, partial hepatectomy, chronic bile acid feeding (e.g., taurocholic acid), and cirrhosis induced by carbon tetrachloride (CCl4), have enabled researchers to greatly expand our knowledge of biliary proliferation and the response of cholangiocytes to biliary injury7,41–44. The use of transgenic mice, such as the Mdr2−/−, has also provided insight into the mechanisms of cholestatic liver injury. These mice serve as an animal model of sclerosing cholangitis because their loss of Mdr2 (a flippase) significantly reduces biliary phospholipid secretion and increases levels of nonmicellar bile acids that subsequently cause biliary injury with proliferation, pericholangitis, and fibrosis5,45. These models have contributed to several studies demonstrating that cholangiocytes display a neuroendocrine phenotype in response to biliary injury46. This phenotype allows cholangiocytes to secrete and respond to a number of hormones, neuropeptides, and neurotransmitters that regulate cholangiocyte proliferation. A list of the neuroendocrine modulators that regulate cholangiocyte proliferation is shown in Table 1. Figure 2 highlights how these modulators interact with cholangiocyte receptors to initiate proliferation during cholestasis. These pathways may provide novel therapeutic strategies to chronic cholestatic liver diseases, such as PBC and PSC47. In the remaining sections, we will review the neuroendocrine properties of cholangiocytes and discuss their involvement in cholangiocyte proliferation, cholestatic liver disease, and hepatic fibrosis.
Table 1.
Neuroendocrine Factors Affecting Cholangiocyte Proliferation
| Class/Modulator | Receptor | Effect on Proliferation | References |
|---|---|---|---|
| Gastrointestinal hormones | |||
| Secretin | SR | Maintains bile duct mass; promotes large cholangiocyte proliferation during cholestasis | 48–50 |
| Somatostatin | SSTR2 | Inhibits proliferation; counteracts secretin during cholestasis | 52–56 |
| Gastrin | CCK-B | Inhibits proliferation; reverses cholangiocyte response to BDL | 53,57 |
| GLP-1 | GLP-1R | Increases proliferation | 59–61 |
| Bile acids | |||
| Taurocholate | Increased proliferation, SR expression, and bile flow | 43,62 | |
| Taurolithocholate | Increased proliferation, SR expression, and bile flow | 43,62 | |
| Ursodeoxycholic acid | Decreased proliferation and secretion after BDL | 65,66 | |
| Tauroursodeoxycholic acid | Decreased proliferation and secretion after BDL | 65,66 | |
| Angiogenic factors | |||
| VEGF-A and VEGF-C | VEGFR-2, VEGFR-3 | Increase proliferation and fibrosis following BDL | 69–72 |
| Ang-1 | Tie-2 | Synergic with VEGF to induce proliferation | 73 |
| PDGF | PDGFR | Unknown effect on proliferation; promotes stellate cell activation after BDL | 82,83 |
| Neurotransmitters | |||
| Acetylcholine | M3 | Increases proliferation and secretin-induced secretion following BDL | 86,87 |
| Adrenergic stimulators (forskolin, clenbuterol, and dobutamine) | β1 and β2 | Increases proliferation | 33,87,89 |
| Phenylephrine | α1 | Increases small cholangiocyte proliferation | 88 |
| Dopamine | D2 | Inhibits secretin effects after BDL | 90 |
| Serotonin | 1A and 1B | Inhibits proliferation, fibrogenic effects on stellate cells | 92,93 |
| Histamine | HRH1, HRH2, HRH3,HRH4 | Decreases proliferation after BDL; small cholangiocytes proliferation with HRH1 agonist | 95–98 |
| αCGRP | CLR, RAMP1, RCP | Increases proliferation following BDL | 100 |
| Substance P | NK-1R | Increases proliferation | 101 |
| NGF | TkrA | Sustains proliferation following BDL | 102 |
| Melatonin | MT1, MT2 | Decreases bile duct mass and proliferation following BDL | 103–105 |
| Sex hormones | |||
| Estrogen | ER-α, ER-β | Stimulates proliferation | 107–109 |
| Progesterone | PRGMC1, PRGMC2, mPRα | Increase bile duct mass and proliferation following BDL | 112 |
| FSH | FSHR | Increase bile duct mass and proliferation following BDL | 113,114 |
| GnRH | GnRHR1, GnRHR2 | Stimulates proliferation | 116 |
| Prolactin | Long, short | Stimulates proliferation | 117 |
| Other hormones | |||
| Iodothyronine (T3) | α1-, α2-, β1-, and β2-THR | Decreased proliferation after BDL | 118 |
| Angiotensin II | Angiotensin receptor type 1 | Increases proliferation and fibrosis following BDL | 119 |
Figure 2.
Large cholangiocytes maintain bile homeostasis under normal conditions via the Cl−/HCO3 − exchanger. During cholestasis, many peptides, hormones, and neurotransmitters act on cholangiocyte receptors to modulate cell proliferation via cAMP/PKA/ERK1/2 signaling pathways.
Gastrointestinal Hormones
Secretin
As previously mentioned, the role of secretin in response to biliary injury has been widely studied. The SR is upregulated in cholangiocytes in response to biliary injury; however, little was known about secretin’s role in biliary proliferation until the development of a SR knockout (SR−/−) transgenic mouse. Glaser et al. demonstrated that knockout of the SR significantly reduced large intrahepatic bile duct mass in mice following BDL48. This was associated with increased cholangiocyte apoptosis and decreased markers of proliferation, as well as decreased ERK1/2 phosphorylation48. Furthermore, chronic treatment of normal rats with secretin significantly increased bile duct mass and cholangiocyte proliferation, a process that was inhibited with PKA and MAPK inhibitors49. These findings suggest that the SR has an important role in maintaining cholangiocyte growth. In a more recent study, we have demonstrated that secretin secretion from cholangiocytes and duodenal S cells is capable of decreasing miR-125 and let-7 levels, promoting an increase in vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) causing enhanced biliary hyperplasia18. Additionally, we have recently presented our data that showed treatment of Mdr2−/− mice with an SR antagonist significantly decreased biliary hyperplasia, bile duct mass, liver fibrosis, and expression of profibrotic genes50. We have also shown that the SR axis contributes to cholestatic-induced liver fibrosis through TGF-β1 signaling51.
Somatostatin
Other gastrointestinal hormones, such as somatostatin and gastrin, inhibit the response of cholangiocytes to secretin by inhibiting secretin-induced intracellular cAMP levels and bicarbonate excretion6,52,53. Somatostatin also decreases cholangiocyte proliferation via interaction with somatostatin receptor subtype 2 (SSTR2)12,54. The somatostatin analog octreotide has also been shown to decrease biliary fibrosis following extrahepatic biliary obstruction54. Studies with octreotide have also been performed in a murine model of polycystic liver disease. Administration of octreotide in these animals decreased cAMP content, limited cyst growth, and inhibited hepatic disease progression55. This parallels results of a double-blinded, randomized controlled trial in humans with liver manifestations of autosomal dominant polycystic kidney disease (ADPKD). Patients treated with octreotide for 6 months showed significant reductions in liver volumes after 6 months of therapy compared to placebo treatment56.
Gastrin
The primary role of gastrin is to stimulate acid secretion from gastric parietal cells and stimulate acid-secreting mucosal growth in the stomach; however, trophic effects of gastrin have also been reported in the colon, duodenum, and pancreas57. Similar to somatostatin in the liver, gastrin has been shown to inhibit secretin-inducted bicarbonate secretion and reduce secretin-induced cAMP levels by interacting with cholecystokinin-B (CCK-B) receptors53. Chronic administration of gastrin has also been shown to inhibit cholangiocyte proliferation, and administration of gastrin following BDL resulted in a reversal of cholangiocyte proliferation in rats53,58. The conclusions from these early studies suggest that gastrin may be beneficial to cholangiocytes during cholestasis; however, more studies are needed to determine if gastrin can limit the ductular reaction associated with cholestasis and provide a therapeutic benefit.
Glucagon-Like Peptide 1
Glucagon-like peptide 1 (GLP-1) is a peptide hormone released from neuroendocrine cells that stimulate a variety of neuroendocrine mechanisms, such as pancreatic β-cell proliferation, islet cell neurogenesis, and the neuroendocrine transdifferentiation of pancreatic ductal cells59,60. Studies have shown that cholangiocytes express the GLP-1 receptor (GLP-1R), and expression of the receptor is upregulated during the progression of cholestasis. Cholangiocytes also synthesize and release GLP-1 during times of proliferation, which acts locally in the cholangiocyte microenvironment61. Activation of GLP-1R via GLP-1 or a selective receptor agonist, exendin-4, stimulates biliary growth, which is mediated through the phosphatidylinositol 3-kinase (PI3K), cAMP/PKA, and Ca2+-CamKIIα pathway60. Administration of a GLP-1R antagonist demonstrates a marked reduction in bile duct mass in rats following BDL61.
Bile Acids
Bile acids accumulate during cholestasis and are capable of producing physiologic effects in cholangiocytes related to proliferation, secretion, and cell signaling. Administration of taurocholate (TC) and taurolithocholate (TLC) stimulated large cholangiocyte proliferation in vitro and in vivo43,62. This was associated with increased SR gene expression, secretin-induced cAMP levels and secretin-stimulated bile flow, and bicarbonate secretion43. Further studies have also shown that bile acids stimulate expression of the Na+-dependent apical bile acid transporter (ASBT) in small and large rat cholangiocytes, which was dependent on PKC activation63. Cholangiocyte proliferation and secretion in response to bile acids during cholestasis are promoted through a PI3K-dependent pathway64. Furthermore, secretin has been shown to promote cholehepatic shunting of conjugated bile acids through increased expression of ASBT17. Treatment of rats with ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) following BDL significantly reduced cholangiocyte proliferation and secretion through activation of Ca2+-dependent PKC-α65. Furthermore, UDCA and TUDCA were shown to be cytoprotective in rats following BDL + vagotomy, a model that induces cholestasis and bile duct loss through apoptosis of cholangiocytes66. Treatment of these rats with UDCA and TUDCA prevented cholangiocyte apoptosis by calcium-dependent MAPK and PI3K pathways66.
Many studies have contributed to our knowledge of bile acid physiology through other cholestatic models. For instance, Fickert et al. showed that 24-norUrsodeoxycholic (norUDCA), a C(23) homolog of UDCA, is capable of ameliorating sclerosing cholangitis in Mdr2−/− mice45. norUDCA is relatively resistant to conjugation and participates in a cholehepatic shunting process that sets up a bile acid-dependent, bicarbonate-rich choleresis67. Randomized controlled trials are currently underway using norUDCA for the management of PSC67. UDCA has remained the only approved pharmacotherapy for PBC for nearly two decades; however, many new therapies are currently being developed based on UDCA combination therapies68.
Angiogenic Factors
Vascular Endothelial Growth Factor
Cholangiocytes receive their blood supply from a complex microvascular network termed the peribiliary plexus, which stems from branches of hepatic arteries69. A key study has shown that this network enlarges following BDL, suggesting that biliary vascular supply contributes partly to cholangiocyte proliferation and response to biliary injury. They further demonstrated that secretion of VEGF-A and VEGF-C, and expression of their corresponding receptors are upregulated following BDL69. VEGF-A and VEGF-C induce cholangiocyte proliferation via activation of IP3/Ca2+/PKC-α and phosphorylation of Src/ERK1/2 pathways70. Administration of anti-VEGF-A and/or anti-VEGF-C and hepatic artery ligation inhibited the ductular reaction following BDL69. Administration of r-VEGF-A to hepatic artery-ligated rats induced ductular reaction following BDL71. Studies have also identified a link between bile acid signaling and VEGF expression in cholangiocytes. Mancinelli et al. showed that TC feeding to BDL rats prevented bile duct damage induced by caffeic acid, which is known to inhibit growth of many cell types72. The protective effect of TC was associated with increased expression of VEGF-A, VEGF-C, VEGFR-2, and VEGFR-372.
This work has also been replicated in other forms of human biliary disease, such as polycystic liver disease. Cholangiocyte epithelium from humans with polycystic liver disease strongly expresses VEGF and their corresponding receptors. VEGF not only induced cholangiocyte proliferation and cyst growth but also increased the density of the portal vasculature73. Furthermore, treatment of polycystic liver disease mice (WS25/−) with a potent VEGF receptor inhibitor (MU-5416) significantly decreased cyst growth and liver density74. Studies in PBC have also demonstrated strong upregulation of VEGF-A, as well as other angiogenic factors, which likely induce angiogenesis near injured bile ducts, contributing to inflammatory cell recruitment and disease progression75. These findings indicate that the autocrine/paracrine mechanisms of VEGF affect a variety of biliary pathologies.
Additionally, authors have demonstrated that VEGF plays an important role in hepatic regeneration following partial hepatectomy. Wang et al. demonstrated that liver sinusoidal endothelial progenitor cells (LSECs) are responsible for promoting liver regeneration in rats following partial hepatectomy76. These progenitor cells are recruited from the bone marrow as bone marrow-derived liver sinusoidal endothelial cell progenitor cells (BM SPCs) via a recruitment system that is regulated by hepatic VEGF77. Knockout of hepatic VEGF with oligonucleotides inhibited proliferation and recruitment of BM SPCs to the liver parenchyma77. DeLeve et al. have recently demonstrated that VEGF acts through stromal cell-derived factor-1 (SDF-1) to mediate recruitment of bone marrow-derived progenitor cells to the liver sinusoids following partial hepatectomy78. It appears that VEGF not only regulates cholangiocyte proliferation but also is vital for liver regeneration and reestablishing normal endothelial architecture following partial hepatectomy.
Angiopoietins
Another class of angiogenic factors, the angiopoietins, also plays a role in cholestatic liver injury. Angiopoietin-1 and -2 (Ang-1 and Ang-2) act through the tyrosine kinase receptor Tie-2 but have opposite effects. Ang-1 promotes tyrosine phosphorylation, and Ang-2 is a receptor antagonist. Whereas VEGF has been implicated in vasculogenesis, studies have demonstrated that Ang-1 is responsible for maturation of newly formed vessels by recruitment of mural pericytes79. Similar to VEGF, the angiopoietins are also upregulated during hepatic disease states. Cholangiocytes isolated from patients with ADPKD express Ang-1 and Ang-2 as well as Tie-273. Fabris et al. demonstrated that Ang-1 alone did not promote cholangiocyte proliferation but was synergic to VEGF73. PBC patients express high levels of VEGF-1, Ang-1, Ang-2, and Tie-2 on endothelial cells and periportal hepatocytes, which promotes angiogenesis near damaged bile ducts80.
Platelet-Derived Growth Factor
Platelet-derived growth factor (PDGF) is a polypeptide growth factor involved in numerous biological processes including the activity of mesenchymal cells to regulate matrix metabolism, chemotactic and vasoactive properties, as well as moderation of the inflammatory response to cellular injury81. Pinzani et al. demonstrated that PDGF and its receptor, PDGF-R, are overexpressed in human liver fibrogenesis81. Using BDL rats as a model of cholestasis, Grappone et al. further characterized PDGF expression and demonstrated that the β-chain is synthesized by cholangiocytes during cholestasis, and its receptor is primarily located on hepatic stellate cells82. These findings signified a cross-talking mechanism between cholangiocytes and hepatic stellate cells during cholestasis that may contribute to liver fibrosis. Furthermore, Kinnman et al. showed that PDGF initiates chemotaxis of hepatic stellate cells toward bile duct structures during cholestasis83. Another study by Kinnman et al. showed that peribiliary cells undergo a PDGF-mediated conversion into myofibroblasts that contribute to cholestatic liver fibrosis84. In a more recent study, the β-PDGFR expressed on hepatic stellate cells has been shown to be a key regulator of hepatic injury and fibrosis in a CCl4 model of cholestatic liver injury85.
Neuropeptides and Neurotransmitters
Acetylcholine
Nearly two decades ago, Alvaro et al. demonstrated that cholangiocytes express the M3 acetylcholine receptor, and acetylcholine works through this receptor to enhance the activity of the secretin-induced Cl−/HCO3 − ion exchanger by a Ca2+-dependent PKC-insensitive pathway that potentiates the stimulation of adenylyl cyclase86. Furthermore, vagotomy impairs cholangiocyte proliferation, enhances apoptosis, and limits ductular reaction following BDL in rats87. Vagotomy was also associated with decreased cAMP levels and secretin-induced choleresis following BDL. Forskolin treatment following vagotomy and BDL prevented the decrease in cAMP levels and maintained cholangiocyte proliferation87.
Adrenergic Fibers
Previous studies have demonstrated that sympathetic nerve activity is required for liver and cholangiocyte regeneration after partial hepatectomy88. Additionally, invoking adrenergic denervation to BDL rats using 6-hydroxydopamine decreased cholangiocyte proliferation and secretin-dependent secretion while increasing cholangiocyte apoptosis via decreasing cAMP levels89. Administration of an adenylyl cyclase activator or β1 and β2 agonists (forskolin, clenbuterol, and dobutamine, respectively) maintained normal cholangiocyte secretory and proliferative responses to BDL via phosphorylation of Akt89. Taurocholic acid prevented the induction of apoptosis in rats subjected to BDL plus autonomic denervation by preventing Akt dephosphorylation. Additionally, Alpini et al. demonstrated heterogeneity within the biliary tree in response to adrenergic stimulation. Small, but not large, cholangiocytes proliferate in response to phenylephrine via IP3/Ca2+-dependent signaling pathways and activation of nuclear factor of activated T cells 2 (NFAT2) and specificity protein 1 (SP1)10.
Dopamine
Cholangiocytes also express the D2 dopamine receptor (but not D1 or D3) on their basolateral surface90. Selective stimulation of this receptor increased intracellular IP3 and Ca2+ levels, inhibited secretin-stimulated bicarbonate-rich choleresis in BDL rats, and inhibited secretin-induced cAMP levels and PKA activity in purified cholangiocytes90. Additional studies demonstrated that dopamine works through the D2 receptor to inhibit secretin’s effects by increasing PKC-γ expression90. It is reasonable to hypothesize from these studies that dopamine may also regulate cholangiocyte proliferation in response to cholestasis; however, this has not been reported in a cholestasis model. Studies have shown that dopamine is secreted from cultured cholangiocarcinoma cells, and these cells proliferate in response to D2 and D4 receptor agonists91. More studies are needed to determine dopamine’s role in cholestasis-induced cholangiocyte proliferation and hepatic fibrosis.
Serotonin
Serotonin is a neuroendocrine hormone secreted from enterochromaffin cells throughout the gastrointestinal tract. Marzioni et al. demonstrated that cholangiocytes secrete serotonin and express the serotonin 1A and serotonin 1B receptors92. Activation of these receptors significantly inhibits cholangiocyte proliferation and choleresis in BDL rats, which is mediated by enhanced D-myo-IP3/Ca2+/PKC signaling and the consequent inhibition of the cAMP/PKA/Src/ERK1/2 signaling cascade92. This suggests that a serotonin-mediated autocrine loop is capable of limiting biliary growth and subsequent ductular reaction during cholestasis. A recent study using tryptophan hydroxylase-2 knock-in (TPH2KI) mice demonstrated that serotonin is vital for remodeling injured bile ducts, and mice with reduced biliary serotonin levels exhibit excessive cholangiocyte proliferation, duct accumulation, and liver fibrosis following BDL93. The same study also demonstrated that serotonin stimulates myofibroblast production of TGF-β1, which decreases tryptophan hydroxylase-2 (TPH2) expression and subsequently decreases serotonin levels and its effects on cholangiocyte proliferation93. A recent review by Mann et al. highlights these findings and further emphasizes the point that serotonin is involved in a complex network of pathways between cholangiocytes, myofibroblasts, and hepatocytes that regulates liver fibrosis through TGF-β1 signaling94.
Histamine
Histamine is an aminergic peptide and neurotransmitter that has also been identified to regulate cholangiocyte proliferation in response to biliary injury. Histamine acts through a variety of G protein-coupled histamine receptor subtypes (HRH1, HRH2, HRH3, and HRH4) that are all expressed in normal and BDL cholangiocytes95. Francis et al. demonstrated that expression of HRH3 is significantly upregulated in proliferating cholangiocytes following BDL and chronic stimulation of this receptor with a selective agonist decreased biliary growth following BDL, whereas a selective HRH3 antagonist treatment increased cholangiocyte hyperplasia95. Activation of HRH3 was associated with increased cAMP levels and PKA/ERK1/2/ELK-1 phosphorylation95. Furthermore, performing BDL in histidine decarboxylase knockout mice (HDC−/−) demonstrated that inhibiting the pathway for histamine production decreased cholangiocyte proliferation and VEGF/HIF-1α expression via decreasing PKA/ERK1/2 activation96. Kennedy et al. also demonstrated that histamine derived from mast cells within the liver also contribute to biliary proliferation following BDL. Cromolyn-treated BDL mice displayed decreased bile duct mass and biliary proliferation97. Also, exposure of cholangiocytes with supernatants from cromolyn-treated mast cells significantly reduced cholangiocyte proliferation in vitro97.
Francis et al. also demonstrated that heterogeneity exists between large and small cholangiocytes in relation to histamine’s effects on cholangiocyte proliferation. This study showed that small, but not large, cholangiocytes proliferate in response to an HRH1 agonist via an IP3/Ca2+-dependent signaling pathway98. Treatment of small cholangiocytes with a selective HRH1 antagonist successfully reduced proliferation98. Furthermore, another study demonstrated that histamine stimulates proliferation of large rat cholangiocytes, but not small cholangiocytes, via activation of HRH2 through a cAMP-dependent signaling mechanism23. In another study, Kennedy et al. demonstrated that administration of histamine following BDL + CCl4 treatment successfully restored large cholangiocyte proliferative capacity, further demonstrating histamine’s effects on cholangiocyte proliferation and homeostasis99.
α-Type Calcitonin Gene-Related Peptide
In addition to parasympathetic and sympathetic regulators of biliary proliferation, the sensory neuropeptide α-type calcitonin gene-related peptide (αCGRP) has also been shown to regulate cholangiocyte proliferation during cholestasis. In a αCGRP knockout mouse model, Glaser et al. demonstrated that biliary proliferation is inhibited via decreased levels of cAMP and PKA following BDL100. In vitro, αCGRP and βCGRP induced cholangiocyte proliferation and increased cAMP/PKA levels100.
Substance P
Substance P (SP) is a member of the tachykinin peptide family that preferentially binds to the neurokinin-1 receptor (NK-1R), another GPCR that is expressed and upregulated in cholangiocytes following BDL101. In a NK-1R knockout mouse model (NK-1R−/−), Glaser et al. demonstrated that loss of the NK-1R receptor was associated with increased biliary apoptosis, decreased serum transaminases, and decreased expression of fibrotic genes following BDL101. Targeting SP or NK-1R may lead to treatment strategies for cholangiopathies and other chronic inflammatory liver conditions.
Nerve Growth Factor
Similar to other hormones, NGF has been shown to enhance biliary proliferation during cholestatic liver disease. Gigliozzi et al. showed that NGF is secreted from cholangiocytes, and expression of the peptide and its receptor (TkrA) is upregulated following BDL102. NGF stimulated cholangiocyte proliferation in vitro through AKT and ERK1/2 pathways, whereas neutralizing NGF following BDL decreased biliary proliferation102.
Melatonin
Melatonin, primarily secreted from the pineal gland, the synthesis of which is regulated by the rate-limiting enzyme arylalkylamine N-acetyltransferase (AANAT), has also been shown to regulate cholangiocyte proliferation during cholestasis103. Large cholangiocytes express melatonin receptors (MT1 and MT2) as well as circadian locomotor output cycles kaput (Clock) genes104. These receptors and Clock genes are upregulated following BDL. Administration of melatonin to BDL rats significantly decreased bile duct mass in vivo and cholangiocyte proliferation in vitro by activating the MT1 receptor, decreasing cAMP levels and PKA phosphorylation104. These effects were inhibited in the presence of a melatonin receptor antagonist104. An additional study using BDL rats showed that melatonin decreases biliary fibrosis by decreasing oxidative stress during cholestasis105.
In a more recent study, Renzi et al. further characterized melatonin’s role in cholangiocyte proliferation during cholestasis. This study demonstrated that cholangiocytes express AANAT and secrete melatonin, both of which are increased following BDL106. Inhibition of AANAT expression using Vivo-Morpholinos increased biliary proliferation and expression of SR, CFTR, and Cl−/HCO3 − AE2106. A recent review by Glaser et al. provides a comprehensive evaluation of melatonin’s protective effects against liver injury in BDL, as well as other models, such as acetaminophen toxicity, nonalcoholic fatty liver disease (NAFLD), and ischemia/reperfusion injury103. It is clear that melatonin and the regulation of Clock genes play significant roles in liver injury; however, more studies are needed to develop appropriate therapeutic strategies for liver disease.
Sex Hormones
Many sex hormones, specifically estrogen and progesterone, stimulate a variety of cells to proliferate. The effects of sex hormones and their role in cholangiocyte proliferation have also been studied in the biliary epithelium. Many studies have evaluated the mechanisms by which estrogen and progesterone regulate cholangiocyte proliferation, but more recently, other sex hormones such as follicle-stimulating hormone (FSH) and gonadotropin-releasing hormone (GnRH) have become known for their involvement in cholangiocyte proliferation during cholestasis. The mechanisms by which these hormones regulate cholangiocyte proliferation will be reviewed in the following sections.
Estrogens
Alvaro et al. first demonstrated that cholangiocytes express the estrogen receptor (ER) α and β subtypes107. Immunoreactivity and receptor expression of the ER-β subtypes were significantly upregulated in BDL rats, more so than the ER-α subtype107. Treatment of BDL rats with tamoxifen inhibited cholangiocyte proliferation and increased cholangiocyte apoptosis, which was also replicated in vitro107. Treatment of cholangiocytes with 17-β-estradiol stimulates cholangiocyte proliferation by activation of the Src/Shc/ERK pathway, a response that is diminished by cotreatment with inhibitors of this pathway108. Furthermore, Alvaro et al. demonstrated that rats that underwent bilateral oophorectomy + BDL showed decreased cholangiocyte expression of ER-α and ER-β receptors, markedly reduced cholangiocyte proliferation, and increased cholangiocyte apoptosis compared to BDL-only rats109. Treatment of the oophorectomy rats with 17-β-estradiol restored normal biliary response to BDL109. These studies led to an interest in using tamoxifen for the treatment of PBC, and in a small retrospective review, patients with PBC treated with tamoxifen for breast cancer showed improved liver enzymes compared to other PBC patients110. Cholangiocytes from PBC patients express both ER subtypes, and their expression becomes markedly reduced in advanced-stage PBC, which correlates with the degree of ductopenia102. These findings, however, have not led to a recent advance in PBC treatment, and research for utilizing sex hormones for treating cholangiopathies is lacking.
Progesterone
In addition to estrogen, progesterone has also been shown to regulate cholangiocyte proliferation during cholestasis. Cholangiocytes express the nuclear progesterone receptor (PR-B) and several membrane receptors (PRGMC1, PRGMC2, and mPRα)111. Treatment of rats with progesterone increased the number of bile ducts, and treatment of BDL rats with anti-progesterone antibodies inhibited cholangiocyte proliferation111. The study also demonstrated that cholangiocytes express the progesterone biosynthetic pathway and secrete progesterone111.
Follicle-Stimulating Hormone
FSH is a sex hormone primarily secreted from the anterior pituitary gland that increases cAMP/adenylyl cyclase/PKA phosphorylation through interactions with a GPCR to stimulate estrogen secretion from the ovaries112. Mancinelli et al. demonstrated that cholangiocytes express the FSH receptor (FSHR) and secrete FSH, which stimulates cholangiocyte growth by increasing cAMP and ERK1/2 and Elk-1 phosphorylation113. Administration of anti-FSH antibodies to BDL rats significantly reduced bile duct mass, cholangiocyte proliferation, secretin-induced cAMP levels, and ERK1/2 and Elk-1 phosphorylation113.
Gonadotropin-Releasing Hormone
Additionally, GnRH is another sex hormone that has been shown to regulate cholangiocyte proliferation via autocrine/paracrine mechanisms. Traditionally, GnRH is secreted from the hypothalamus and stimulates FSH/luteinizing hormone (LH) from the anterior pituitary gland114. GnRH interacts with two receptor subtypes: GnRHR1 and GnRHR2 114. Ray et al. demonstrated that these receptors are expressed by cholangiocytes, and GnRH is secreted from cholangiocytes, which is increased in response to BDL115. Disruption of the GnRH/GnRHR interaction decreased cholangiocyte proliferation, bile duct mass, and liver fibrosis following BDL115. These recent findings make additional contributions to the growing field of sex hormones and their impacts on cell proliferation outside of the reproductive system. All of these findings suggest that sex hormones are important for cholangiocyte proliferation during cholestasis; however, widespread utilization of these pathways for cholangiopathy treatment is not established.
Prolactin
Prolactin is also a hormone secreted from the anterior pituitary gland that promotes proliferation of several cell types by interacting with two receptor subtypes, a long form and a short form116. Taffetani et al. showed that normal cholangiocytes express both receptor subtypes, which are upregulated following BDL116. Stimulation of these receptors promotes cholangiocyte proliferation via increased intracellular Ca2+ and PKC-β1 phosphorylation and decreased PKC-α phosphorylation116. Administration of anti-prolactin antibodies decreased cholangiocyte proliferation following BDL.
Other Moderators of Cholangiocyte Proliferation
Many other hormones have demonstrated effects on cholangiocyte proliferation during cholestasis, further expanding our knowledge about their neuroendocrine phenotypes. Fava et al. demonstrated that cholangiocytes express the thyroid hormone receptor (THR)117. These receptors were located in the cytoplasm of normal cholangiocytes, but only the α1 and α2 subunits were found in the nucleus following BDL117. Administration of 3,3′,5-l-tri-iodothyronine (T3) decreased cholangiocyte proliferation following BDL by PLC/IP3/Ca2+-dependent decreased phosphorylation of Src/ERK1/2117. This study indicates that THR expression and location may be implicated in the pathogenesis of cholestatic liver disease.
The renin–angiotensin system (RAS) is another neuroendocrine pathway that has profound effects on cholangiocyte proliferation and cholestatic liver injury. In a recent study, Afroze et al. discovered that renin, angiotensin-converting enzyme, angiotensinogen, and angiotensin receptor type 1 are expressed by cholangiocytes and upregulated following BDL in rats118. Treatment of cholangiocytes in vitro with angiotensin II increased proliferation, intracellular cAMP, and activation of PKA/ERK/CREB signaling pathway118. In vivo angiotensin II treatment following BDL also promoted expression of genes corresponding with hepatic fibrosis [fibronectin 1, collagen 1A1, and interleukin-6 (IL-6)]118. Treatment of BDL rats with losartan attenuated biliary proliferation and expression of these fibrotic markers118. These findings indicate that cholangiocytes express a local RAS pathway that promotes cholangiocyte proliferation and fibrosis from cholestatic liver injury.
Furthermore, environmental exposures, such as nicotine, have also been implicated in cholangiocyte proliferation. In a recent study, Jensen et al. demonstrated that cholangiocytes express the α7 nicotinic acetylcholine receptor (α7-nAChR)119. Chronic stimulation of this receptor with nicotine in rats using osmotic minipumps for 2 weeks stimulated ERK1/2-dependent cholangiocyte proliferation and expression of fibrotic genes, such as fibronectin-1 and α-smooth muscle actin119. Further studies are needed to determine how chronic nicotine exposure correlates with cholestatic liver injury and cholangiopathies, but these results demonstrate that environmental exposures may also contribute to cholangiocyte proliferation and fibrosis.
LINKING CHOLANGIOCYTE PROLIFERATION WITH PORTAL FIBROSIS
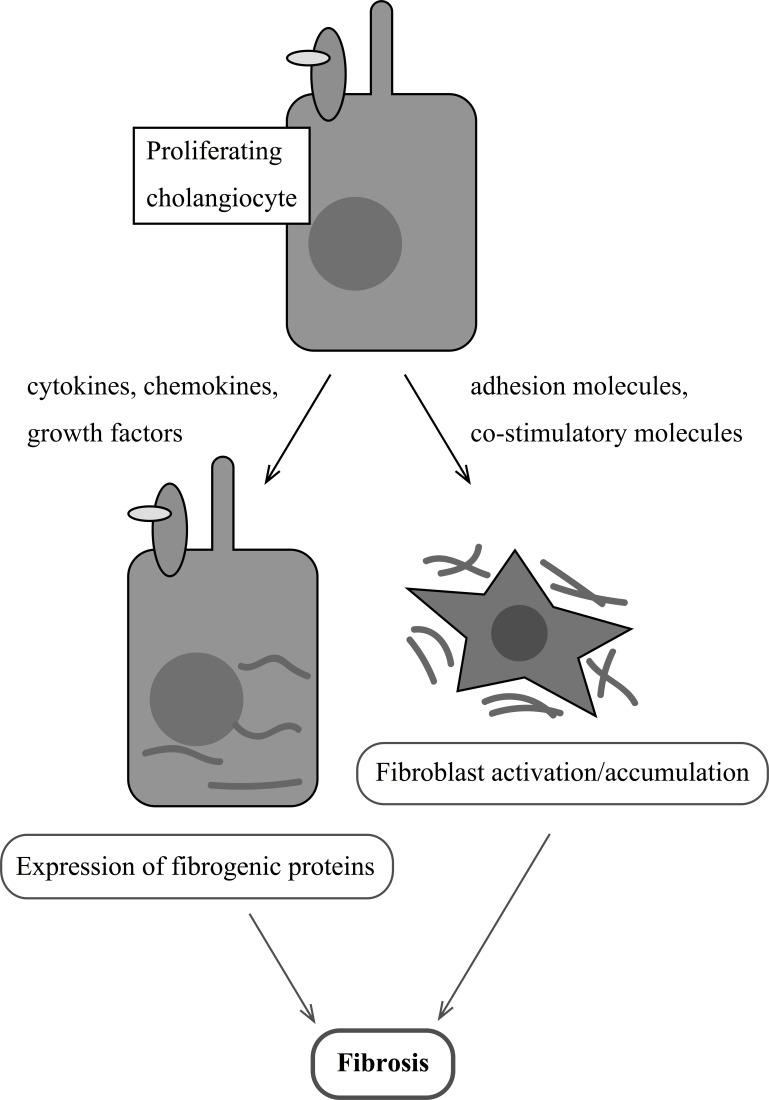
Ductular reaction is a common term in literature that refers to the classical findings of cholestatic liver injury: increased number of biliary ductules accompanied by polymorphonuclear leukocytes and extracellular matrix that results in portal fibrosis and biliary cirrhosis120. Proliferating cholangiocytes respond to a number of local and systemic moderators during cholestasis. Additional studies have demonstrated that proliferating cholangiocytes express many antiapoptotic genes, adhesion molecules, costimulatory molecules, cytokines, chemokines, growth factors, and profibrogenic stimuli121. These factors have autocrine and paracrine effects on myofibroblast activation, migration, and proliferation121. Figure 3 provides a working model of the interactions between cholangiocytes and fibroblasts during cholestasis.
Figure 3.
Proliferating cholangiocytes secrete cytokines, chemokines, growth factors, and costimulatory molecules that act via autocrine/paracrine mechanisms to interact with other cholangiocytes and activate fibroblasts, which initiates fibrogenesis.
Targeting the avβ6 integrin that is secreted from proliferating cholangiocytes has led to promising antifibrotic effects during cholestasis. It has been widely described that avβ6 binds to and activates TGF-β1 during cholestasis, which promotes activation of myofibroblasts and ductular reaction122. Genetically altered β6-null mice display decreased hepatic fibrosis when subjected to cholestasis122. A recent study from Pi et al. demonstrated that avβ6 is closely associated with connective tissue growth factor (CTGF) during cholestasis and may serve as therapeutic targets to control ductular reaction and fibrosis since they interact with common matrix and signaling partners, such as fibronectin and TGF-β1123. Targeting specific growth factors and integrins that are secreted from proliferating cholangiocytes and promote activation of myofibroblasts may lead to promising therapies to reduce ductular reaction during cholestasis.
Additional Pathways of Proliferation and Fibrosis
In addition to numerous growth factors and neuroendocrine molecules, recent studies have identified other pathways that contribute to biliary proliferation and the development of fibrosis during cholestasis. Omenetti et al. first described that the hedgehog (Hh) pathway promotes cross-talk between myofibroblastic cells and cholangiocytes in BDL mice. They demonstrated increased expression of Hh ligands and target genes in hepatic stellate cells and cholangiocytes following BDL124. Coculture of these cells resulted in increased Hh ligand expression, cell viability, and proliferation. Administration of Hh ligand-neutralizing antibodies reversed these effects124. Furthermore, activation of the Hh pathway following BDL-induced cholestatic liver injury promotes cholangiocyte secretion of cytokines, specifically chemokine motif ligand 16 (Cxcl16), which recruits natural killer cells to portal tracts and further propagates the inflammatory process125. Additionally, the Hh pathway has been shown to activate osteopontin, a profibrogenic extracellular matrix protein, in a murine model of nonalcoholic steatohepatitis (NASH)126. Recent studies have also shown that osteopontin mediates the inflammatory processes during cholestasis by initiating neutrophil-mediated injury following BDL in mice127. All of these studies suggest that the Hh pathway mediates the proliferative, inflammatory, and fibrogenic response to cholestatic liver injury.
Additionally, Cheng et al. demonstrated that Wnt signaling promotes activation of hepatic stellate cells and liver fibrosis. Inhibition of Wnt signaling with Dickkopf-1, a Wnt coreceptor antagonist, restores stellate cells to a quiescent state in vitro and ameliorates liver fibrosis in vivo128. Furthermore, Wnt signaling has been shown to regulate proliferation of cholangiocarcinoma, a malignancy of the biliary epithelium, and antagonizing this pathway decreases cholangiocarcinoma proliferation and tumor growth129,130. More studies are needed to determine the links between Wnt signaling, cholangiocyte proliferation, and portal fibrosis during cholestasis; however, it does appear that this pathway is capable of propagating liver injury.
Recently Zhang et al. has shown that Notch signaling contributes to the differentiation of hepatic progenitor cells into cholangiocytes following BDL in rats. Inhibition of Notch signaling with DAPT, a γ-secretase inhibitor, blocked expression of cholangiocyte-specific markers, cholangiocyte proliferation, and cholestatic liver fibrosis131. These recent insights into Hh, Wnt, and Notch signaling pathways represent potential novel therapeutic targets into the management of cholestatic liver disease, but more research is needed to determine the feasibility of targeting these pathways for future clinical trials.
Inflammation as a Contributor to Hepatic Fibrosis
Recent studies have shown that inflammatory cells also contribute to the pathogenesis of liver fibrosis. Österreicher et al. characterized human and animal liver cells positive for fibroblast-specific protein 1 (FSP1), a marker of fibroblasts. They identified a subset of FSP1+ inflammatory macrophages that were present in liver injury, fibrosis, and cancer132. These cells also expressed COX2 and osteopontin, as well as inflammatory chemokines and cytokines132. Furthermore, the interplay between hepatic stellate cells and macrophages has also been linked to Notch signaling. Bansal et al. demonstrated that inhibition of Notch signaling with a γ-secretase inhibitor significantly attenuated CCl4-induced liver fibrosis by decreasing stellate cell activation, inhibiting M1 inflammatory macrophages, and increasing M2-suppressive macrophages133. Furthermore, Locatelli et al. used a mouse model of congenital hepatic fibrosis to demonstrate that a genetically induced biliary epithelial dysfunction promotes the secretion of chemokines able to recruit macrophages that generate a profibrotic tissue response in an effort to create a reparative phenotype134.
CONCLUSIONS AND FUTURE DIRECTIONS
In this review, we have highlighted cholangiocyte anatomy, physiology, and the heterogeneity that exists within the biliary tree. Using multiple mechanisms of cholestasis, researchers over the past two decades have broadly described multiple mechanisms and pathways that stimulate cholangiocyte proliferation and ductular reactions during cholestasis. These models are pivotal to understand the pathogenesis of cholangiopathies, such as PBC and PSC, as well as primary cilia disorders, such as polycystic liver disease. There is growing evidence that proliferating cholangiocytes secrete a number of factors, including hormones, neuropeptides, and growth factors, that act via autocrine/paracrine mechanisms to activate fibroblasts and promote liver fibrosis in response to cholestasis. Additional studies are needed to target these mechanisms of cholangiocyte proliferation and secretion to develop pharmacologic therapies against these conditions. Based on the research discussed in this review, it may be possible to antagonize the pathways that promote cholangiocyte proliferation with receptor-specific antagonists. Our current understanding of cholangiocyte proliferation and fibrosis suggests that treatments to decrease cholangiocyte proliferation may also decrease the secretion of cytokines, chemokines, and other factors that drive hepatic fibrosis in cholestatic liver injury.
ACKNOWLEDGMENTS
This work was supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng from the US Department of Veterans Affairs Biomedical Laboratory Research, and National Institutes of Health grants DK058411, DK076898, DK107310, and DK062975 to Drs. Alpini, Meng, and Glaser. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
REFERENCES
- 1. Juza RM, Pauli EM. Clinical and surgical anatomy of the liver: A review for clinicians. Clin Anat. 2014;27(5):764–9. [DOI] [PubMed] [Google Scholar]
- 2. Malarkey DE, Johnson K, Ryan L, Boorman G, Maronpot RR. New insights into functional aspects of liver morphology. Toxicol Pathol. 2005;33(1):27–34. [DOI] [PubMed] [Google Scholar]
- 3. Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 2000;31(3):555–61. [DOI] [PubMed] [Google Scholar]
- 4. Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology 1991;14(3):551–66. [PubMed] [Google Scholar]
- 5. Kanz M. Anatomy and physiology of the biliary epithelium. In: McQueen CA, editor. Comprehensive toxicology, 2nd ed. New York (NY): Elsevier; 2010. p. 43–108. [Google Scholar]
- 6. Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G612–25. [DOI] [PubMed] [Google Scholar]
- 7. Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81(2):569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glaser S, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glaser S, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva SE, Savage JM, Alpini GD. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89(4):456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alpini G, Franchitto A, Demorrow S, Onori P, Gaudio E, Wise C, Francis H, Venter J, Kopriva S, Mancinelli R and others. Activation of alpha(1)-adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology 2011;53(2):628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272(5 Pt 1):G1064–74. [DOI] [PubMed] [Google Scholar]
- 12. Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274(4 Pt 1):G767–75. [DOI] [PubMed] [Google Scholar]
- 13. Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110(5):1636–43. [DOI] [PubMed] [Google Scholar]
- 14. Schaffner F, Popper H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- 15. Steiner JW, Carruthers JS. Studies on the fine structure of the terminal branches of the biliary tree: I. The morphology of normal bile canaliculi, bile pre-ductules (ducts of Hering) and bile ductules. Am J Pathol. 1961;38(6):639–61. [PMC free article] [PubMed] [Google Scholar]
- 16. Benedetti A, Bassotti C, Rapino K, Marucci L, Jezequel AM. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24(3):335–42. [DOI] [PubMed] [Google Scholar]
- 17. Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, LeSage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology 2005;41(5):1037–45. [DOI] [PubMed] [Google Scholar]
- 18. Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K, Marzioni M, Invernizzi P, Ueno Y, Lai JM, Huang L, Standeford H, Alvaro D, Gaudio E, Franchitto A, Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology 2014;146(7):1795–808 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. The liver: Biology & pathobiology, 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2001. p. 421–35. [Google Scholar]
- 20. Han Y, Glaser S, Meng F, Francis H, Marzioni M, McDaniel K, Alvaro D, Venter J, Carpino G, Onori P, Gaudio E, Alpini G, Franchitto A. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp Biol Med. (Maywood) 2013;238(5):549–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, Demorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpni G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176(4):1790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mancinelli R, Franchitto A, Glaser S, Meng F, Onori P, DeMorrow S, Francis H, Venter J, Carpino G, Baker K, Han Y, Ueno Y, Gaudio E, Alpini G. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+)/CaMK I-dependent adenylyl cyclase 8. Hepatology 2013;58(1):251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Francis HL, DeMorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92(2):282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014;59(6):2263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sasaki M, Ikeda H, Yamaguchi J, Nakada S, Nakanuma Y. Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence. Hepatology 2008;48(1):186–95. [DOI] [PubMed] [Google Scholar]
- 26. Farouk M, Vigna SR, Haebig JE, Gettys TW, McVey DC, Chari R, Pruthi RS, Meyers WC. Secretin receptors in a new preparation of plasma membranes from intrahepatic biliary epithelium. J Surg Res. 1993;54(1):1–6. [DOI] [PubMed] [Google Scholar]
- 27. Alpini G, Ulrich CD 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266(5 Pt 1):G922–8. [DOI] [PubMed] [Google Scholar]
- 28. Alvaro D, Mennone A, Boyer JL. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3- exchange in cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273(2 Pt 1):G303–13. [DOI] [PubMed] [Google Scholar]
- 29. Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, Alpini G. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology 2009;50(1):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGill JM, Basavappa S, Gettys TW, Fitz JG. Secretin activates Cl− channels in bile duct epithelial cells through a cAMP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 1994;266(4 Pt 1):G731–6. [DOI] [PubMed] [Google Scholar]
- 31. Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology 1994;19(6):1400–6. [PubMed] [Google Scholar]
- 32. Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, Beuers U. A biliary HCO3 − umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 2012;55(1):173–83. [DOI] [PubMed] [Google Scholar]
- 33. Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J and others. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41(4):528–37. [DOI] [PubMed] [Google Scholar]
- 34. Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G31–6. [DOI] [PubMed] [Google Scholar]
- 35. Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41(5):808–14. [DOI] [PubMed] [Google Scholar]
- 36. Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, Wang Y, Deng G, Zhu L, Tan Z and others. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One 2011;6(1):e15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology 2009;50(6):1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larusso NF, Masyuk TV. The role of cilia in the regulation of bile flow. Dig Dis. 2011;29(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dutta AK, Woo K, Khimji AK, Kresge C, Feranchak AP. Mechanosensitive Cl- secretion in biliary epithelium mediated through TMEM16A. Am J Physiol Gastrointest Liver Physiol. 2013;304(1):G87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LeSage G, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, Phinizy JL, Baiocchi L, Francis H, Lasater J, Ugili L, Alpini G. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology 1999;29(2):307–19. [DOI] [PubMed] [Google Scholar]
- 42. Alpini G, Elias I, Glaser S, Rodgers RE, Phinizy JL, Robertson WE, Francis H, Lasater J, Richards M, LeSage G. Gamma-Interferon inhibits secretin-induced choleresis and cholangiocyte proliferation in a murine model of cirrhosis. J Hepatol. 1997;27(2):371–80. [DOI] [PubMed] [Google Scholar]
- 43. Alpini G, Glaser S, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage G. Bile acid feeding induces cholangiocyte proliferation and secretion: Evidence for bile acid-regulated ductal secretion. Gastroenterology 1999;116(1):179–86. [DOI] [PubMed] [Google Scholar]
- 44. LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 1996;111(6):1633–44. [DOI] [PubMed] [Google Scholar]
- 45. Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, Denk H, Hofmann AF, Jaeschke H, Trauner M. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2006;130(2):465–81. [DOI] [PubMed] [Google Scholar]
- 46. Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: A neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132(1):415–31. [DOI] [PubMed] [Google Scholar]
- 47. Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004;127(5):1565–77. [DOI] [PubMed] [Google Scholar]
- 48. Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010;52(1):204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guerrier M, Attili F, Alpini G, Glaser S. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg Nutr. 2014;3(3):118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glaser S, Meng F, Wu N, Venter J, Kyritsi K, Alpini G. The secretin receptor antagonist (SCT 5-27) reduces biliary hyperplasia and liver fibrosis in an animal model of primary sclerosing cholangitis. FASEB J. 2016;30(1 Suppl):56.7. [Google Scholar]
- 51. Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, Marzioni M, Alvaro D, Franchitto A, Gaudio E, Glaser S, Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in TGF-beta1 biliary secretion. Hepatology 2016;64(3):865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tietz PS, Alpini G, Pham LD, LaRusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1995;269(1 Pt 1):G110–8. [DOI] [PubMed] [Google Scholar]
- 53. Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 2000;32(1):17–25. [DOI] [PubMed] [Google Scholar]
- 54. Tracy TF Jr, Tector AJ, Goerke ME, Kitchen S, Lagunoff D. Somatostatin analogue (octreotide) inhibits bile duct epithelial cell proliferation and fibrosis after extrahepatic biliary obstruction. Am J Pathol. 1993;143(6):1574–8. [PMC free article] [PubMed] [Google Scholar]
- 55. Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology 2007;132(3):1104–16. [DOI] [PubMed] [Google Scholar]
- 56. Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, Ruggenenti P. Reducing polycystic liver volume in ADPKD: Effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5(5):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walsh JH. Role of gastrin as a trophic hormone. Digestion 1990;47(Suppl 1):11–6; discussion 49–52. [DOI] [PubMed] [Google Scholar]
- 58. Glaser S, Alvaro D, Ueno Y, Francis H, Marzioni M, Phinizy JL, Baumann B, Mancino MG, Venter J, LeSage G, Alpini G. Gastrin reverses established cholangiocyte proliferation and enhanced secretin-stimulated ductal secretion of BDL rats by activation of apoptosis through increased expression of Ca2+- dependent PKC isoforms. Liver Int. 2003;23(2):78–88. [DOI] [PubMed] [Google Scholar]
- 59. Drucker DJ. Glucagon-like peptides: Regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17(2):161–71. [DOI] [PubMed] [Google Scholar]
- 60. Marzioni M, Alpini G, Saccomanno S, Candelaresi C, Venter J, Rychlicki C, Fava G, Francis H, Trozzi L, Glaser S, Benedetti A. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 2007;133(1):244–55. [DOI] [PubMed] [Google Scholar]
- 61. Marzioni M, Fava G, Alvaro D, Alpini G, Benedetti A. Control of cholangiocyte adaptive responses by visceral hormones and neuropeptides. Clin Rev Allergy Immunol. 2009;36(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers RE, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273(2 Pt 1):G518–29. [DOI] [PubMed] [Google Scholar]
- 63. Alpini G, Ueno Y, Glaser S, Marzioni M, Phinizy JL, Francis H, LeSage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology 2001;34(5):868–76. [DOI] [PubMed] [Google Scholar]
- 64. Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL, Mauldin J, LeSage G. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology 2002;123(4):1226–37. [DOI] [PubMed] [Google Scholar]
- 65. Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, LeSage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology 2002;35(5):1041–52. [DOI] [PubMed] [Google Scholar]
- 66. Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G, Glaser S. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168(2):398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Williamson KD, Chapman RW. New therapeutic strategies for primary sclerosing cholangitis. Semin Liver Dis. 2016;36(1):5–14. [DOI] [PubMed] [Google Scholar]
- 68. Corpechot C. Primary biliary cirrhosis beyond ursodeoxycholic acid. Semin Liver Dis. 2016;36(1):15–26. [DOI] [PubMed] [Google Scholar]
- 69. Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, Glaser S, Alvaro D, Onori P. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12(22):3546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130(4):1270–82. [DOI] [PubMed] [Google Scholar]
- 71. Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, Onori P, Ueno Y, Marzioni M, Fava G, Venter J, Reichenbach R, Summers R, Alpini G. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G307–17. [DOI] [PubMed] [Google Scholar]
- 72. Mancinelli R, Onori P, Gaudio E, Franchitto A, Carpino G, Ueno Y, Alvaro D, Annarale LP, Demorrow S, Francis H. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med. (Maywood) 2009;234(4):462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 2006;43(5):1001–12. [DOI] [PubMed] [Google Scholar]
- 74. Amura CR, Brodsky KS, Groff R, Gattone VH, Voelkel NF, Doctor RB. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/-) mice. Am J Physiol Cell Physiol. 2007;293(1):C419–28. [DOI] [PubMed] [Google Scholar]
- 75. Morell CM, Fabris L, Strazzabosco M. Vascular biology of the biliary epithelium. J Gastroenterol Hepatol. 2013;28(Suppl 1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122(4):1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, Deleve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology 2012;143(6):1555–1563 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DeLeve LD, Wang X, Wang L. VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2016:ajpgi 00056 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fabris L, Cadamuro M, Libbrecht L, Raynaud P, Spirli C, Fiorotto R, Okolicsanyi L, Lemaigre F, Strazzabosco M, Roskams T. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology 2008;47(2):719–28. [DOI] [PubMed] [Google Scholar]
- 80. Medina J, Sanz-Cameno P, Garcia-Buey L, Martin-Vilchez S, Lopez-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: An immunohistochemical descriptive study. J Hepatol. 2005;42(1):124–31. [DOI] [PubMed] [Google Scholar]
- 81. Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148(3):785–800. [PMC free article] [PubMed] [Google Scholar]
- 82. Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31(1):100–9. [DOI] [PubMed] [Google Scholar]
- 83. Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80(5):697–707. [DOI] [PubMed] [Google Scholar]
- 84. Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83(2):163–73. [DOI] [PubMed] [Google Scholar]
- 85. Kocabayoglu P, Lade A, Lee YA, Dragomir AC, Sun X, Fiel MI, Thung S, Aloman C, Soriano P, Hoshida Y, Friedman SL. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J Hepatol. 2015;63(1):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser S, Benedetti A. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100(6):1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. LeSage E, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999;117(1):191–9. [DOI] [PubMed] [Google Scholar]
- 88. Iwai M, Shimazu T. Alteration in sympathetic nerve activity during liver regeneration in rats after partial hepatectomy. J Auton Nerv Syst. 1992;41(3):209–14. [DOI] [PubMed] [Google Scholar]
- 89. Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, Marzioni M, Mancino MG, Phinizy JL, Reichenbach R, Fava G, Summers R, Venter J, Alpini G. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G813–26. [DOI] [PubMed] [Google Scholar]
- 90. Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284(4):G683–94. [DOI] [PubMed] [Google Scholar]
- 91. Coufal M, Invernizzi P, Gaudio E, Bernuzzi F, Frampton GA, Onori P, Franchitto A, Carpino G, Ramirez JC, Alvaro D, Marzioni M, Battisti G, Benedetti A, DeMorrow S. Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma. Int J Cancer 2010;126(9):2112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, LeSage GD, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128(1):121–37. [DOI] [PubMed] [Google Scholar]
- 93. Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, Caron MG, Diehl AM. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mann DA, Oakley F. Serotonin paracrine signaling in tissue fibrosis. Biochim Biophys Acta 2013;1832(7):905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, Alvaro D, Fava G, Marzioni M, Vaculin B, Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87(5):473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Graf A, Meng F, Hargrove L, Kennedy L, Han Y, Francis T, Hodges K, Ueno Y, Nguyen Q, Greene JF, Francis H. Knockout of histidine decarboxylase decreases bile duct ligation-induced biliary hyperplasia via downregulation of the histidine decarboxylase/VEGF axis through PKA-ERK1/2 signaling. Am J Physiol Gastrointest Liver Physiol. 2014;307(8):G813–23. [DOI] [PubMed] [Google Scholar]
- 97. Kennedy LL, Hargrove LA, Graf AB, Francis TC, Hodges KM, Nguyen QP, Ueno Y, Greene JF, Meng F, Huynh VD, Francis HL. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest. 2014;94(12):1406–18. [DOI] [PubMed] [Google Scholar]
- 98. Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295(2):C499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Johnson C, Hargrove L, Graf A, Kennedy L, Hodges K, Harris R, Francis T, Ueno Y, Francis H. Histamine restores biliary mass following carbon tetrachloride-induced damage in a cholestatic rat model. Dig Liver Dis. 2015;47(3):211–7. [DOI] [PubMed] [Google Scholar]
- 100. Glaser S, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis HL, Dickerson IM, DiPette DJ, Supowit SC, Alpini GD. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest. 2007;87(9):914–26. [DOI] [PubMed] [Google Scholar]
- 101. Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, Kopriva S, Chiasson V, DeMorrow S, Francis H, Meng F, Marzioni M, Franchitto A, Alvaro D, Supowit S, DiPete DJ, Onori P, Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser S, Francis H, Mancino MG, Ueno Y, Barbaro B, Benedetti A, Attili AF, Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 2004;127(4):1198–209. [DOI] [PubMed] [Google Scholar]
- 103. Glaser S, Han Y, Francis H, Alpini G. Melatonin regulation of biliary functions. Hepatobiliary Surg Nutr. 2014;3(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Renzi A, Glaser S, DeMorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, White M, Francis H, Han Y, Alvaro D, Gaudio E, Carpino G, Ueno Y, Onori P, Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aktas C, Kanter M, Erboga M, Mete R, Oran M. Melatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in rats. Toxicol Ind Health 2014;30(9):835–44. [DOI] [PubMed] [Google Scholar]
- 106. Renzi A, DeMorrow S, Onori P, Carpino G, Mancinelli R, Meng F, Venter J, White M, Franchitto A, Francis H, Han Y, Ueno Y, Dusio G, Jensen KJ, Greene JJ Jr, Glaser S, Gaudio E, Alpini G. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology 2013;57(3):1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F, Gaudio E. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 2000;119(6):1681–91. [DOI] [PubMed] [Google Scholar]
- 108. Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology 2002;36(2):297–304. [DOI] [PubMed] [Google Scholar]
- 109. Alvaro D, Gigliozzi A, Attili AF, Onori P, Franchitto A, Vetuschi A, Glaser S, Le Sage G, Alpini G, Morini S, Gaudio E. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology 2002;123(1):336–44. [DOI] [PubMed] [Google Scholar]
- 110. Invernizzi P, Alvaro D, Crosignani A, Gaudio E, Podda M. Tamoxifen in treatment of primary biliary cirrhosis. Hepatology 2004;39(4):1175–6. [DOI] [PubMed] [Google Scholar]
- 111. Glaser S, DeMorrow S, Francis H, Ueno Y, Gaudio E, Vaculin S, Venter J, Franchitto A, Onori P, Vaculin B, Marzioni M, Wise C, Pilanthananond M, Savage J, Pierce L, Mancinelli R, Alpini G. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G124–G136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ulloa-Aguirre A, Zarinan T, Pasapera AM, Casas-Gonzalez P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine 2007;32(3):251–63. [DOI] [PubMed] [Google Scholar]
- 113. Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer 2004;11(4):725–48. [DOI] [PubMed] [Google Scholar]
- 115. Ray D, Han Y, Franchitto A, DeMorrow S, Meng F, Venter J, McMillin M, Kennedy L, Francis H, Onori P, Mancinelli R, Gaudio E, Alpini G, Glaser SS. Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms. Am J Pathol. 2015;185(4):1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Taffetani S, Glaser S, Francis H, DeMorrow S, Ueno Y, Alvaro D, Marucci L, Marzioni M, Fava G, Venter J, Vaculin S, Vaculin B, Lam IP, Lee VH, Gaudio E, Carpino G, Benedetti A, Alpini G. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol. 2007;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fava G, Ueno Y, Glaser S, Francis H, DeMorrow S, Marucci L, Marzioni M, Benedetti A, Venter J, Vaculin B, Vaculin S, Alpini G. Thyroid hormone inhibits biliary growth in bile duct-ligated rats by PLC/IP(3)/Ca(2+)-dependent downregulation of SRC/ERK1/2. Am J Physiol Cell Physiol. 2007;292(4):C1467–75. [DOI] [PubMed] [Google Scholar]
- 118. Afroze SH, Munshi MK, Martinez AK, Uddin M, Gergely M, Szynkarski C, Guerrier M, Nizamutdinov D, Dostal D, Glaser S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 2015;308(8):G691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jensen K, Afroze S, Ueno Y, Rahal K, Frenzel A, Sterling M, Guerrier M, Nizamutdinov D, Dostal DE, Meng F, Glasser SS. Chronic nicotine exposure stimulates biliary growth and fibrosis in normal rats. Dig Liver Dis. 2013;45(9):754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15(4):259–69. [PubMed] [Google Scholar]
- 121. Penz-Osterreicher M, Osterreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25(2):245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 2007;46(5):1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, Trinh TL, Yianni P, Liu C, Leask A, Violette SM, Scott EW, Schultz GS, Petersen BE. Connective tissue growth factor and integrin αvβ6: A new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology 2015;61(2):678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87(5):499–514. [DOI] [PubMed] [Google Scholar]
- 125. Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology 2009;50(2):518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenentti A, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 2011;53(1):106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224(2):186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G39–49. [DOI] [PubMed] [Google Scholar]
- 129. Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST, Wigmore SJ, Sansom OJ, Forbes SJ. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125(3):1269–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang KS, Zhou Q, Wang YF, Liang LJ. Inhibition of Wnt signaling induces cell apoptosis and suppresses cell proliferation in cholangiocarcinoma cells. Oncol Rep. 2013;30(3):1430–8. [DOI] [PubMed] [Google Scholar]
- 131. Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, Chen G, Liu C, Zern MA, Mu Y, Liu P. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96(3):350–60. [DOI] [PubMed] [Google Scholar]
- 132. Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA. 2011;108(1):308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bansal R, van Baarlen J, Storm G, Prakash J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci Rep. 2015;5:18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Locatelli L, Cadamuro M, Spirli C, Fiorotto R, Lecchi S, Morell CM, Popov Y, Scirpo R, De Matteis M, Amenduni M, Pietrobattista A, Torre G, Schuppan D, Fabris L, Strazzabosco M. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 2016;63(3):965–82. [DOI] [PMC free article] [PubMed] [Google Scholar]