Abstract
The insulin/insulin-like growth factor-like signaling pathway, present in all multicellular organisms, regulates diverse functions including growth, development, fecundity, metabolic homeostasis, and lifespan. In flies, ligands of the insulin/insulin-like growth factor-like signaling pathway, the Drosophila insulin-like peptides, regulate growth and hemolymph carbohydrate homeostasis during development and are expressed in a stage- and tissue-specific manner. Here, we show that ablation of Drosophila insulin-like peptide-producing median neurosecretory cells in the brain leads to increased fasting glucose levels in the hemolymph of adults similar to that found in diabetic mammals. They also exhibit increased storage of lipid and carbohydrate, reduced fecundity, and reduced tolerance of heat and cold. However, the ablated flies show an extension of median and maximal lifespan and increased resistance to oxidative stress and starvation.
Keywords: aging
The insulin/insulin-like growth factor (IGF)-like signaling (IIS) pathway is present throughout multicellular animals (1). Its functions include regulation of growth (2–5), development (6), and metabolic homeostasis (7). Also, the pathway has been shown to regulate adult lifespan, resistance to stress (8–10), and fecundity in Caenorhabditis elegans, Drosophila, and mice (11–14).
In mice, both deletion of the insulin receptor in white-adipose tissue and ubiquitous heterozygosity for a null mutation in the IGF-I receptor increase longevity, implying that both insulin and IGF-I signaling may regulate lifespan (10, 15). In C. elegans and Drosophila, mutation of the single IIS receptor (6, 16, 17), the single insulin receptor substrate (9, 18), or the PI3 kinase (19–22), the protein kinases Akt (23) and Sgk-1 (24), or overexpression of the forkhead transcription factor (25–27), all increase lifespan and delay or reduce fecundity. Thus, the role of reduced insulin signaling in lifespan-extension appears to be conserved. In light of the different roles of insulin signaling in diverse tissues within an organism, the array of phenotypes that result from altered systemic signaling may be separable.
In the genomes of the invertebrates C. elegans and Drosophila, single genes encode the intracellular components of the signaling pathway, with the exception of the triplication of the protein kinase B, Akt, SGK-1 in C. elegans (23). In contrast, there are multiple invertebrate-genes for the insulin-like ligands, with seven in the Drosophila genome (3) and 38 in C. elegans (28). The genes encoding the seven Drosophila insulin-like peptides (DILPs) were originally identified by a similarity search that used the four conserved cysteine residues within the insulin A chain as bait (3). DILP1–DILP7 are predicted to resemble preproinsulin at the structural level, and therefore, they are considered orthologous to mammalian insulin, rather than IGF. The relative simplicity of the intracellular signaling-pathway, together with the genetic diversification of DILPs, implies that the multiple functions of IIS may be mediated in part by functional diversification of the ligands. In support of this notion, the genes encoding the DILPs are independently transcriptionally regulated in response to nutrition, as well as in a tissue- and stage-specific manner during development (3, 5). However, the distinct biochemical roles that the different ligands play in these contexts remain to be identified.
The mechanisms by which IIS regulates lifespan and fecundity and the nature of any causal relationships between these phenotypes and the other traits regulated by this pathway are not understood. We have investigated the roles of the insulin-like ligands in adult Drosophila. We have determined that, in adults, three of the dilp genes are expressed in brain neurosecretory cells, two of them exclusively so. We have investigated the consequences of ablation of these cells for adult lifespan, fecundity, stress resistance, and metabolic phenotypes.
Methods
Fly Stocks and Maintenance. The control whiteDahomey stock was derived by backcrossing w1118 into the outbred wild-type Dahomey background. Upstream activating sequence (UAS)-reaper (rpr) and dilp2-GAL (5) were backcrossed into wDah five times. UAS-GFP (1521) and UAS-LacZ (1776) were obtained from Bloomington Stock Center. Stocks were maintained and experiments were conducted at 25°C on a 12:12-h light/dark cycle at constant humidity by using standard sugar/yeast medium (29).
In Situ Hybridization. RNA in situ hybridizations on whole-mount adult central nervous systems, ovaries, and other abdominal organs by using digoxigenin (DIG)- and FITC-labeled dilp probes were performed as described in ref. 5.
Immunohistochemistry. DILP2 expression was determined by using rabbit anti-DILP2 diluted 1:500 according to the procedure described in ref. 30 and a secondary Texas red-conjugated donkey anti-rabbit IgG (Jackson ImmunoLabs).
Confocal Microscopy. Confocal imaging of UAS-GFP expression and Fast Red fluorescence was carried out on an LSM 510 microscope (Zeiss). The fluorophore Fast Red was excited at 488 nm and fluorescence measured beyond 545 nm. Confocal image stacks were converted to projections by using lsm 510 software (Zeiss). Cell counts were performed on 3D reconstructions of expression in the mNSCs of Drosophila brains by using axiovert software (Zeiss).
Quantitative RT-PCR. Total RNA was extracted by using TRIzol (GIBCO) according to the manufacturer's instructions. mRNA in total RNA was reverse transcribed by using oligo(dT) primer and the Superscript II system (Invitrogen). Quantitative PCR was performed by using the prism 7000 sequence-detection system (Applied Biosystems), SYBR Green (Molecular Probes), ROX ReferenceDye (Invitrogen), and Hot StarTaq (Qiagen, Valencia, CA) by following the manufacturers' instructions. For detailed information, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Lifespan. Procedures for lifespan studies were as described in refs. 9 and 31. For detailed information, see Supporting Materials and Methods.
Fecundity. The number of eggs laid per vial of females was counted, and the number of females per vial and the number of days (3 or 4) over which they had laid were noted. Data are reported as the mean number of eggs laid per day per female ± SEM over each 3- or 4-day period.
Stress Tests. UAS-rpr/+; dilp2-GAL/+ and UAS-rpr/+ mated females were generated as for lifespan. Survival was measured on (i) 20 mM paraquat in standard food (oxidative stress) or (ii) 1% agar (starvation). Time to knock-down was measured for flies exposed to 38.5°C (heat) and time to recovery from cold-shock was measured for flies exposed to 0°C for 4 h (32). Two experiments were performed for each treatment. For heat and paraquat, 6- and 10-day-old flies were tested in each experiment.
Trehalose and Glucose Measurement. Hemolymph was pooled from 8–10 females after a 5-h starvation on 1% agar. Flies were decapitated, and hemolymph was collected from the thorax by capillary action. Glucose amount was determined in ≈0.3 μl of pooled liquid by using Infinity Glucose Reagent (ThermoElectron). Whole-fly trehalose was measured as described in ref. 33, by using the glucose assay outlined above. For detailed information, see Supporting Materials and Methods.
Lipid and Glycogen Measurements. The glycogen and lipid content of individual, adult female flies were measured 3 days after eclosion (n = 20) according to the method described in ref. 34, which was modified to exclude protein separation. Data are expressed relative to the fresh body weight of each fly.
Statistical Analyses. All statistical analyses were performed by using jmp (version 4.0.5) software (SAS Institute, Cary, NC). Lifespan data were subjected to survival analysis (log-rank tests) and presented as survival curves. Mortality (μx) was estimated as μx = -ln (px), where px is the probability of an individual alive at age x - 1 surviving to age x days (35). Maximum-likelihood methods, executed in winmodest (36), were used to estimate mortality parameters of the Gompertz model: μx = aebx. Fecundity, cold recovery, glucose, trehalose, lipid, and glycogen data were tested for normality by using the Shapiro–Wilk W test on studentized residuals (37). One-way ANOVA was used, and means comparisons were made by using Student's t test. Data are presented as means ± SEM, and an asterisk indicates significant difference from control (P < 0.05).
Results
Characterization of dilp2, dilp3, and dilp5 Expression in Adult Flies. Previous work has characterized the expression and distribution of the DILPs in Drosophila larvae (3, 5). However, little is known about these molecules in adult flies. To detect the presence and location of RNA transcripts of dilp2, dilp3, and dilp5 in adults, RNA in situ hybridizations were performed, which revealed expression of all three genes in a cluster of cells in the adult brain that correspond to the median neurosecretary-cells (mNSC) of the pars intercerebralis (38) (Fig. 1A). Expression of the other four dilps was not detectable in these cells by in situ hybridization using probes complementary to dilp1, dilp4, dilp6, and dilp7 (data not shown). dilp5 expression was also detected in the ovary as noted in ref. 5, whereas dilp2 and dilp3 could not be detected anywhere else in the adult body (data not shown). These observations were supported by quantitative RT-PCR that revealed relatively strong expression of dilp2, dilp3, and dilp5 in head extracts but not dilp2 transcript, barely detectable levels of dilp3, and low levels of dilp5 in extracts from bodies (Fig. 1B).
Fig. 1.
Characterization of expression of dilp2, dilp3, and dilp5 in adult female Drosophila. Gene expression of dilp2, dilp3, and dilp5 was determined in 5-day-old adult female brains by RNA in situ hybridization and quantitative RT-PCR of the control genotype (UAS-rpr/+). (A) Exemplar in situ hybridization using a dilp2 DIG-labeled RNA probe and alkaline phosphatase-conjugated anti-DIG Ab. Cells containing the target gene constitute the median neurosecretory cells (mNSCs) in the pars intercerebralis of the adult brain. (Scale bars, 80 μm.) (B) Separate RNA extracts from heads and bodies of adult female flies were assayed for the presence of dilp2, dilp3, and dilp5 transcripts by RT-PCR. Data are shown as mean copy number ± SEM. Double in situ hybridizations were performed by using a FITC-labeled dilp5 probe and DIG-labeled dilp2 and 3 probes, followed by alkaline phosphatase-conjugated anti-DIG and anti-FITC Abs. (C) Double staining for dilp2 (visualized with fast red) and dilp5 probes (visualized with BCIP/NBT) revealed their colocalization. n = 7. (D) Likewise, double staining using DIG-labeled dilp3 (fast red) and FITC-labeled dilp5 (BCIP/NBT) probes revealed their colocalization (n = 8). (E) Fluorescent in situ hybridization was used to quantify dilp-expressing mNSCs. This example shows a fluorescent dilp3 probe localized to six pairs of mNSCs. (Scale bar, 10 μm.) Seven brains were examined by using the dilp2 probe, 12 brains were examined by using the dilp3 probe, and 11 brains were examined by using the dilp5 probe.
To determine whether the three dilp genes were all expressed in the same cells, double in situ hybridizations were performed. Combining the probes for dilp5 with dilp2 and dilp5 with dilp3 showed that all three genes were coexpressed in the same set of cells (Fig. 1 C and D). The number of these dilp-expressing mNSCs per individual was determined by fluorescent in situ hybridization using probes for each of the three genes separately (Fig. 1E). Within each brain, the number of cells expressing these dilps was bilaterally symmetrical, and it varied from five to seven pairs.
Targeted Disruption of dilp Expression by Cell Ablation. All studies on the role of IIS in adult flies have manipulated the pathway components in target tissues. We sought to investigate the effects of lowered IIS activity as a result of disruption of the insulin ligands. To achieve this aim, transcription of the proapoptotic gene rpr was driven by a GAL4 transgene whose expression was determined by a fragment of the dilp 2 promoter (dilp2GAL4). This construct has been shown to express exclusively in the mNSCs of larval brains, starting during late third-instar (5). Fig. 2 shows expression in adult brains of UAS-GFP driven by this dilp2GAL4 construct in the absence (A) and presence (B) of UAS-rpr. dilp2-driven rpr expression clearly resulted in a complete ablation of the cells in the adult brain in which it was expressed, as shown by the absence of any fluorescence in the ablated flies.
Fig. 2.
The mNSC-specific expression of the dilp2-GAL driver in the adult brain and the effect of mNSC ablation on dilp expression. (A and B) Confocal image (z-stack projection) of dilp2-GAL-driven expression of UAS-GFP in control (UAS-GFP/+;dilp2GAL/+) (A) and ablated (UAS-rpr/+;UAS-GFP/+;dilp2GAL/+)(B) adult female brains. (C–E) RNA in situ hybridizations were performed by using DIG-labeled probes and an alkaline phosphatase-conjugated anti-DIG Ab to UAS-rpr/+; dilp2GAL/+ adult female brains. (C) dilp2. (D) dilp3. (E) dilp5. (F) The effect of mNSC ablation on dilp expression measured by RT-PCR. Data are mean fold differences in expression between the ablated genotype (UAS-rpr/+; dilp2GAL/+) and the control (UAS-rpr/+) ± SEM. (G–I) Adult mNSCs immunostained with an Ab against DILP2 in a control brain (UAS-rpr/+; n = 5) (G), and ablated brains (UAS-rpr/+; dilp2-GAL/+) (H and I). For sample sizes and residual mNSC numbers, see Table 2, which is published as supporting information on the PNAS web site.
However, in situ hybridizations using brains from ablated flies revealed some expression of each of the dilp genes in a variable number of residual mNSCs (Fig. 2 C–E). This expression was confirmed by RT-PCR data that showed that expression of each of the dilps was reduced to 30–40% of the levels in wild-type brains (Fig. 2F). Because only a fragment of the dilp2 promoter was used to drive rpr expression, the expression pattern of the transgene may not always have fully replicated that found for the full-length promoter. This supposition was supported by immunostaining brains from control (Fig. 2G) and ablated (Fig. 2 H and I) animals by using a DILP2 Ab. Although in some brains there was no detectable protein (Fig. 2H), DILP-producing cells were still detectable in others (Fig. 2I). Attempts to quantify DILP2 levels in whole-body protein extracts by Western blot analysis failed because of the low abundance of the ligand (data not shown).
IIS activity is required for larval growth, and reduced activity in components of the signaling pathway have been shown to result in flies that are dramatically smaller and have delayed development (3, 39, 40). In contrast, the mNSC-ablated flies reported here were only slightly smaller than controls, and they showed no developmental delay, presumably because of the late developmental onset of expression of the GAL4 driver transgene used in these experiments (5).
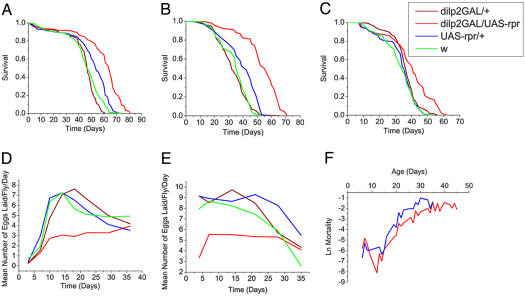
mNSC-Ablated Flies Have Longer Lifespan and Reduced Fecundity. Reduced IIS activity has a conserved role in extending adult lifespan. To investigate the effects of mNSC ablation and the resulting reduced levels of dilp2, dilp3, and dilp5 on lifespan, we assayed the longevity of female (mated and virgin) and male flies. The ablated flies exhibited a significant increase in median and maximum lifespan over that of control flies (Fig. 3 A–C). Median lifespan was increased by 10.5% in males and 18.5% and 33.5% in virgin and mated females, respectively. These increases in lifespan were accompanied by decreases in age-specific egg laying for virgin and mated females (Fig. 3 D and E).
Fig. 3.
mNSC-ablated flies are long-lived and females show reduced fecundity. (A) Survival of UAS-rpr/+; dilp2-GAL/+ virgin females (red), and dilp2-GAL/+ (brown), UAS-rpr/+ (blue), and w (green) controls. Median lifespans are as follows: UAS-rpr/+; dilp2-GAL/+, 64 days (18.5% increase over UAS-rpr/+ control, P < 0.0001; n = 173); dilp2GAL/+, 47 days (n = 187); UAS-rpr/+, 54 days (n = 232); and w, 47 days (n = 202). (B) Survival of UAS-rpr/+; dilp2-GAL/+ mated females (red), and dilp2-GAL/+ (brown), UAS-rpr/+ (blue), and w (green) controls. Median lifespans are as follows: UAS-rpr/+; dilp2-GAL/+, 56 days (33.5% increase over UAS-rpr control, P < 0.0001; n = 93); dilp2-GAL/+, 32 days (n = 115); UAS-rpr/+, 42 days (n = 88); and w, 35 days (n = 112). (C) Survival of UAS-rpr/+; dilp2-GAL/+ males (red), and dilp2-GAL/+ (brown), UAS-rpr/+ (blue), and w (green) controls. Median lifespans are as follows: UAS-rpr/+; dilp2-GAL/+, 41 days (10.5% increase over dilp2-GAL/+ control, P < 0.0001; n = 140); dilp2-GAL/+, 37 days (n = 150); UAS-rpr/+, 35 days (n = 128); and w, 34 days (n = 195). (D and E) Fecundity of females from experiment shown in A (D) and B (E). Data are given as mean number of eggs laid per female per day ± SEM. *, P < 0.05, compared with controls. (F) Age-specific mortality analysis of UAS-rpr/+ (n = 1,186) and dilp2-GAL4/UAS-rpr (n = 1,143) mated females. Natural log of the mortality rate (μx) is plotted.
Age-specific mortality analysis of lifespan data from mated female flies was performed (Fig. 3F). Over the linear portion of the mortality increase, fitting of a Gompertz model revealed a significant difference in the initial rate of mortality but no change in the slope of the mortality trajectory (Table 1). Thus, detectable aging-related mortality started later in the ablated flies but then proceeded at the same rate as in controls.
Table 1. Estimates of the parameters of the Gompertz mortality model with 95% lower and upper confidence intervals.
| Genotype | a* | LCI | UCI | b† | LCI | UCI |
|---|---|---|---|---|---|---|
| dilp2GAL/rpr | 0.00015 | 0.00010 | 0.00023 | 0.21128 | 0.19835 | 0.22505 |
| UAS-rpr/+ | 0.00037 | 0.00028 | 0.00049 | 0.22061 | 0.21012 | 0.23162 |
Mortality models and maximum-likelihood analysis were executed by using winmodest (36). LCI, lower confidence interval; UCI, upper confidence interval.
Initial mortality rate
Rate of exponential increase in mortality with age early in life
Physiological Changes in mNSC-Ablated Flies: Altered Carbohydrate and Lipid Metabolism. Insulin signaling is known to be involved in the regulation of energy homeostasis in mammals. One manifestation of this is that mammals with reduced systemic insulin signaling (such as that in diabetes) exhibit fasting hyperglycemia because of inappropriate regulation of glucose uptake from circulation and release from energy stores in the liver. To test whether reduction of insulin signaling has similar effects in Drosophila, we measured the levels of several molecules that are resources for energy generation.
Insects contain two types of sugar in circulation: glucose and trehalose. Glucose is obtained from the diet, and trehalose is used as a homeostatic molecule that originates from the fat body and is used to distribute sugar to peripheral tissues (41). Hemolymph was extracted from female flies that had been starved for 5 h, and the levels of these two sugars was measured. Glucose was elevated 2-fold above control values in mNSC-ablated flies (Fig. 4A), whereas trehalose was slightly (15%) but significantly lowered in ablated flies versus controls (Fig. 4B).
Fig. 4.
The effect of mNSC ablation on hemolymph glucose and trehalose levels, and whole-body trehalose, glycogen, and lipid content. (A) Hemolymph glucose concentration in UAS-rpr/+ and UAS-rpr/+; dilp2-GAL/+ flies. (B) Hemolymph trehalose concentration in UAS-rpr/+ and UAS-rpr/+; dilp2-GAL/+ flies (n = 8). (C) Whole-fly trehalose content in UAS-rpr/+ and UAS-rpr/+; dilp2-GAL/+ flies per mg of fly (fresh weight) (n = 10). (D) Glycogen content of UAS-rpr/+ and UAS-rpr/+; dilp2-GAL/+ flies per mg of fly (fresh weight) (n = 21). (E) Lipid content of UAS-rpr/+ and UAS-rpr/+; dilp2-GAL/+ flies per mg of fly (fresh weight) (n = 17). Data are shown as means ± SEM. *, P < 0.05, compared with control.
Examination of stored energy pools by using whole-body extracts revealed that mNSC-ablated flies contained significantly higher levels of trehalose (64% higher), glycogen (44% higher), and lipids (10% higher) relative to body mass when compared with controls (Fig. 4 C–E). Under normal conditions, these three storage molecules represent the largest readily accessible energy resource available to the fly. Thus, mNSC-ablated flies exhibit generally higher levels of stored energy and an altered profile of circulating carbohydrates compared with controls.
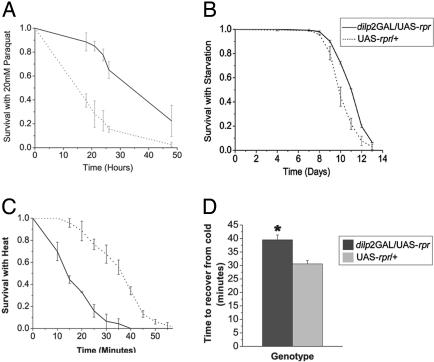
Altered Resistance to Stress. Enhanced resistance to various stresses is often associated with interventions that extend lifespan. We found that mNSC-ablated flies survived longer than controls when fed paraquat in their food. Assuming equal rates of paraquat ingestion, this indicates enhanced resistance to oxidative stress (Fig. 5A). Furthermore, the ablated flies were moderately starvation resistant when compared with controls (Fig. 5B). In contrast, the ablated flies showed greater sensitivity to heat shock (Fig. 5C) and took more time to recover from cold shock (Fig. 5D).
Fig. 5.
Long-lived mNSC-ablated adults are resistant to oxidative stress and starvation but show reduced thermotolerance. (A) Survival of 10-day-old adult female flies on 20 mM paraquat added to standard food. Sample sizes were n = 100 for each genotype in the first experiment and n = 85 for each genotype in second. (B) Survival of females during starvation (1% agar). n = 144 for dilp2GAL/UAS-rpr; n = 150 for UAS-rpr/+ in experiment 1; n = 182 for dilp2GAL/UAS-rpr; and n = 154 for UAS-rpr/+ in experiment 2. For both experiments, the median lifespans were as follows: dilp2GAL/UAS-rpr, 12 days; and UAS-rpr/+, 10 days. (C) Survival of 6-day-old females with 38.5°C heat. n = 20 for each genotype/experiment. (A–C) Data are shown as mean survival for each time point over two experiments ± SEM. P < 0.05, compared with control. (D) Mean time to recover from cold treatment of 9-day-old females ± SEM (n = 50 for each genotype).
Discussion
Several genetic interventions that have been shown to extend lifespan in worms (6, 16, 21), fruit flies (9, 17, 26, 27), and mice (10, 15) reduce the activity of the insulin-like signaling pathway. Here, we report extension of Drosophila lifespan by ablating cells in the adult brain that produce three of the seven DILPs.
During the preadult period, these peptides activate the IIS pathway in tissues expressing the insulin receptor to promote growth (4, 5, 42). During adulthood, flies and worms with reduced IIS activity exhibit higher lipid levels, increased oxidative stress resistance, and longer lifespan (6, 9, 16, 17, 21, 26, 27, 43). Each of these phenotypes is shared by the mNSC-ablated flies reported here, supporting the idea that the cell ablation is acting by reducing one or more of DILP2, DILP3, and DILP5, leading to lowered systemic insulin signaling. These data are supported by lowered levels of the dilp transcripts in the ablated flies. Although it remains possible that other unknown effects of the cell ablation are responsible for the observed lifespan extension, data from C. elegans further support IIS involvement, because RNA interference to specifically reduce ins-7 (1 of 38 genes encoding IIS ligands) has been shown to increase adult lifespan (44). These data support a model in which specific insulin-like ligands are involved in shortening adult lifespan, because their reduction is sufficient for lifespan extension despite the persistence of the remaining ligands.
Reduced insulin signaling in mammals is associated with the disease-state diabetes, characterized by high blood glucose, because of its decreased uptake from circulation into tissues such as muscle and liver, as well as increased glucose release into the blood from catabolism of glycogen and protein by the liver (45). Like diabetic mammals, mNSC-ablated flies showed higher starving levels of circulating glucose, indicating that one or more of DILP2, DILP3, and DILP5 may be required to stimulate glucose uptake in adult flies in a manner similar to mammalian insulin. Such a homeostatic control of blood sugar has been demonstrated in Drosophila, in which the early larval ablation of the DILP-producing mNSCs results in increased hemolymph sugar levels during development (4).
In flies and mammals, certain components of the insulin signaling pathway are represented by several genes. In Drosophila, the insulin-like ligands are encoded by seven genes, whereas in mammals the intracellular signaling components are multiply encoded. For some of these genes, it is also known that paralogues have different spatiotemporal expression patterns, indicating that they have functionally distinct roles (46). In general, functional diversity among IIS pathway paralogues raises the intriguing possibility that the lifespan-extension phenotype associated with lowered IIS activity may be separable from the various accompanying metabolic defects.
In stark contrast to reduction of systemic insulin-signaling in mammals, which results in diabetes, whose associated pathologies shorten lifespan, targeted ablation of the insulin receptor in white adipose-tissue in the mouse results in lifespan extension (15). Interestingly, reduced IIS activity in fat body (analogous to the mammalian adipose-tissue and liver) alone is also sufficient to elicit lifespan extension in flies (26, 27) and worms (47). However, in contrast to mammals, reduction of insulin ligands as a result of the cell ablation reported here or by RNAi for ins-7 in C. elegans (44) resulted in animals that were longer lived than controls. Although the exact relevance of the invertebrate models to the mammalian disease state is uncertain, a possible explanation is that the mortality caused by diabetes in mammals may be due to physiological damage that neither Drosophila nor C. elegans are at risk of contracting, such as the microvascular and macrovascular damage that results from hyperglycemia (45).
A feature that has been shared by all long-lived IIS mutant flies, including the mNSC-ablated flies described here, is increased lipid content (17, 39). Although also true of long-lived IIS-mutant worms (6), this elevation of lipid content may not be essential for the longevity phenotype (48). In mammals, reduced activity of the insulin pathway can also be associated with dyslipidemia. Neuron-specific disruption of the insulin-receptor gene in mice (NIRKO mice) resulted in decreased fertility and diet-dependent increased adiposity (49). In Drosophila, fat storage is mainly in the fat body. Thus, the role of IIS in generation and storage of fat in this tissue may be evolutionarily conserved. In addition, mNSC-ablated flies possessed increased relative levels of storage carbohydrates that could also be expected of NIRKO mice. The increased fat, trehalose, and glycogen stores seen in the mNSC-ablated flies are also unlikely to be causal in the extension of lifespan, because long-lived chico (Drosophila IRS) flies show no increase in glycogen content (39). Interestingly, studies of long-lived insulin-receptor mutant mice indicate that decreased fat levels are associated with longevity (15).
IIS is required for normal growth in Drosophila (50), and lifespan extension by this pathway has been associated with reduced body size and delayed development time, reminiscent of the constellation of phenotypes seen in long-lived dwarf mice (51). However, the reduction in body size caused by reduction of IIS signaling in development is not necessary for extension of lifespan. chico heterozygotes are wild type in body size and yet show extended lifespan (9), as do flies with adult-specific overexpression of the IIS transcription factor dFOXO (26, 27).
Increased resistance to various forms of physiological stress is often correlated with life-extending genetic alterations and has been proposed to be causal (52). The increased thermotolerance and accumulation of heat-shock proteins observed in IIS-mutant worms is consistent with this hypothesis (53, 54). However, we have found that long-lived mNSC-ablated flies are more sensitive to hot and cold treatments than controls, indicating that enhanced resistance to thermal stresses is not necessary for lifespan extension. One possible cause for the ablated flies reduced thermotolerance is their lowered circulating trehalose concentrations. Trehalose has been proposed to play a protective role against anoxia in Drosophila by preventing protein aggregation (55) and is a requirement for the stress-resistance of stationary-phase yeast cells (56, 57). However, this attractive hypothesis remains questionable because the ablated flies only contain lowered trehalose in circulation, while possessing higher levels of whole-body trehalose. Thus, any role for this disaccharide in thermal tolerance would be limited to its levels in the hemolymph. Another aspect of stress resistance that apparently correlates with longevity in mNSC-ablated flies and other longlived mutant IIS flies is enhanced resistance to oxidative stress (9, 26, 27). However, note that in each case the prooxidant paraquat was administered by feeding. Therefore, it is possible that unquantified feeding-rate differences between genotypes alter the dose received by each treatment group. Notwithstanding this possibility, increased oxidative stress resistance has been found for long-lived IIS-mutant worms (43) and mice (10), and in each case, it is associated with increased activity of the antioxidant enzyme superoxide dismutase (SOD). Also, increased activity of the JNK signaling pathway in Drosophila results in both increased resistance to paraquat and extended lifespan (58). Although some conflicting results exist in the literature regarding the sufficiency of overexpression of these systems to extend the lifespan of Drosophila (59–62), recent data support the hypothesis that SOD activity is indeed limiting for lifespan (63). Therefore, these studies agree that enhanced oxidative stress resistance correlates with and may be both necessary and sufficient for retarded aging.
Reduced female fecundity is also often correlated with lifespan extension in IIS-mutant animals (for review, see ref. 64). The mNSC-ablated flies presented are not sterile but have reduced fertility that could possibly contribute to the long lifespan of these flies. In worms and flies, reduced fertility has been uncoupled from longevity caused by reduced IIS activity (9, 16). Furthermore, control of fertility can be effected by only a subset of insulin signaling functions in mammals because knockouts of different IRS proteins in mice yield different effects on fertility (65, 66). In worms, reduced IIS activity limited to larval stages was shown to reduce fecundity without increasing lifespan, whereas lowering IIS during the adult period only extended lifespan without lowering fecundity (67). It is possible that a similar effect is also at work in Drosophila because our mNSC ablation occurred during the final larval stage (5), resulting in lowered IIS activity during the late larval and pupal periods as well as during adulthood. However, in Drosophila, IIS acts at least in part in the adult to determine fecundity, because lifespan extension by overexpression of dFOXO that was confined to the fat body of adult flies led to reduced fecundity (26). It would be interesting to characterize the relationship between this intervention and mNSC-ablation, because their phenotypes are consistent with a role for the DILPs signaling to the fat body to elicit alterations in lifespan and fecundity.
Together, the data presented here demonstrate that lowered levels of a subset of the ligands that are responsible for systemic insulin signaling in flies results in lifespan extension. Therefore, only a subset of the roles of IIS needs to be lowered to effect longevity. This extension of lifespan occurred despite a doubling of blood glucose levels, an interesting phenomenon when viewed in light of the pathological effects of diabetes in mammals. Further characterization of the mechanisms by which this intervention works is well suited to the omic techniques because at least some of the observable physiological correlates of longevity apparently do not indicate causation.
Acknowledgments
We thank Priyanka Belawat (University of Zurich–Irchel) for kindly providing us with the DILP2 Ab and Claire Milton and Martin Sikora for technical assistance. This work was supported by the Wellcome Trust and the Biotechnology and Biological Sciences Research Council.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: mNSC, median neurosecretary cells; IGF, insulin-like growth factor; DILP, Drosophila insulin-like peptide; IIS, insulin/IGF-like signaling; UAS, upstream activating sequence; DIG, digoxigenin; rpr, reaper.
References
- 1.Skorokhod, A., Gamulin, V., Gundacker, D., Kavsan, V., Muller, I. M. & Muller, W. E. (1999) Biol. Bull. (Woods Hole, Mass.) 197, 198-206. [DOI] [PubMed] [Google Scholar]
- 2.Butler, A. A. & Le Roith, D. (2001) Annu. Rev. Physiol. 63, 141-164. [DOI] [PubMed] [Google Scholar]
- 3.Brogiolo, W., Stocker, H., Ikeya, T., Rintelen, F., Fernandez, R. & Hafen, E. (2001) Curr. Biol. 11, 213-221. [DOI] [PubMed] [Google Scholar]
- 4.Rulifson, E. J., Kim, S. K. & Nusse, R. (2002) Science 296, 1118-1120. [DOI] [PubMed] [Google Scholar]
- 5.Ikeya, T., Galic, M., Belawat, P., Nairz, K. & Hafen, E. (2002) Curr. Biol. 12, 1293-1300. [DOI] [PubMed] [Google Scholar]
- 6.Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. (1997) Science 277, 942-946. [DOI] [PubMed] [Google Scholar]
- 7.Saltiel, A. R. & Kahn, C. R. (2001) Nature 414, 799-806. [DOI] [PubMed] [Google Scholar]
- 8.Lithgow, G. J., White, T. M., Melov, S. & Johnson, T. E. (1995) Proc. Natl. Acad. Sci. USA 92, 7540-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy, D. J., Gems, D., Harshman, L. G., Oldham, S., Stocker, H., Hafen, E., Leevers, S. J. & Partridge, L. (2001) Science 292, 104-106. [DOI] [PubMed] [Google Scholar]
- 10.Holzenberger, M., Dupont, J., Ducos, B., Leneuve, P., Geloen, A., Even, P. C., Cervera, P. & Le Bouc, Y. (2003) Nature 421, 182-187. [DOI] [PubMed] [Google Scholar]
- 11.Liang, H., Masoro, E., Nelson, J. F., Strong, R., McMahan, C. A. & Richardson, A. (2003) Exp. Gerontol. 38, 1353-1364. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, D. W. & Padgett, R. W. (2003) Genes Dev. 17, 813-818. [DOI] [PubMed] [Google Scholar]
- 13.Tatar, M., Bartke, A. & Antebi, A. (2003) Science 299, 1346-1351. [DOI] [PubMed] [Google Scholar]
- 14.Partridge, L. & Gems, D. (2002) Nat. Rev. Genet. 3, 165-175. [DOI] [PubMed] [Google Scholar]
- 15.Bluher, M., Kahn, B. B. & Kahn, C. R. (2003) Science 299, 572-574. [DOI] [PubMed] [Google Scholar]
- 16.Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. (1993) Nature 366, 461-464. [DOI] [PubMed] [Google Scholar]
- 17.Tatar, M., Kopelman, A., Epstein, D., Tu, M. P., Yin, C. M. & Garofalo, R. S. (2001) Science 292, 107-110. [DOI] [PubMed] [Google Scholar]
- 18.Wolkow, C. A., Munoz, M. J., Riddle, D. L. & Ruvkun, G. (2002) J. Biol. Chem. 20, 49591-49597. [DOI] [PubMed] [Google Scholar]
- 19.Klass, M. R. (1983) Mech. Ageing Dev. 22, 279-286. [DOI] [PubMed] [Google Scholar]
- 20.Morris, J. Z., Tissenbaum, H. A. & Ruvkun, G. (1996) Nature 382, 536-539. [DOI] [PubMed] [Google Scholar]
- 21.Friedman, D. B. & Johnson, T. E. (1988) J. Gerontol. 43, B102-B109. [DOI] [PubMed] [Google Scholar]
- 22.Dorman, J. B., Albinder, B., Shroyer, T. & Kenyon, C. (1995) Genetics 141, 1399-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradis, S. & Ruvkun, G. (1998) Genes Dev. 12, 2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hertweck, M., Gobel, C. & Baumeister, R. (2004) Dev. Cell 6, 577-588. [DOI] [PubMed] [Google Scholar]
- 25.Henderson, S. T. & Johnson, T. E. (2001) Curr. Biol. 11, 1975-1980. [DOI] [PubMed] [Google Scholar]
- 26.Giannakou, M. E., Goss, M., Junger, M. A., Hafen, E., Leevers, S. J. & Partridge, L. (2004) Science 305, 361. [DOI] [PubMed] [Google Scholar]
- 27.Hwangbo, D. S., Gersham, B., Tu, M. P., Palmer, M. & Tatar, M. (2004) Nature 429, 562-566. [DOI] [PubMed] [Google Scholar]
- 28.Pierce, S. B., Costa, M., Wisotzkey, R., Devadhar, S., Homburger, S. A., Buchman, A. R., Ferguson, K. C., Heller, J., Platt, D. M., Pasquinelli, A. A., et al. (2001) Genes Dev. 15, 672-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman, T. & Partridge, L. (1996) Proc. R. Soc. London Ser. B 263, 755-759. [DOI] [PubMed] [Google Scholar]
- 30.Lee, G., Foss, M., Goodwin, S. F., Carlo, T., Taylor, B. J. & Hall, J. C. (2000) J. Neurobiol. 43, 404-426. [DOI] [PubMed] [Google Scholar]
- 31.Mair, W., Goymer, P., Pletcher, S. D. & Partridge, L. (2003) Science 301, 1731-1733. [DOI] [PubMed] [Google Scholar]
- 32.Gibert, P. & Huey, R. B. (2001) Physiol. Biochem. Zool. 74, 429-434. [DOI] [PubMed] [Google Scholar]
- 33.Parrou, J. L. & Francois, J. (1997) Anal. Biochem. 248, 186-188. [DOI] [PubMed] [Google Scholar]
- 34.van Handel, E. (1965) Anal. Biochem. 11, 266-271. [DOI] [PubMed] [Google Scholar]
- 35.Lee, E. T. (1992) Statistical Methods for Survival Data Analysis (Wiley, New York).
- 36.Pletcher, S. D. (1999) J. Evol. Biol. 12, 430-439. [Google Scholar]
- 37.Sokal, R. R. & Rohlf, F. J. (1998) Biometry (Freeman, New York).
- 38.Cao, C. & Brown, M. R. (2001) Cell Tissue Res. 304, 317-321. [DOI] [PubMed] [Google Scholar]
- 39.Bohni, R., Riesgo-Escovar, J., Oldham, S., Brogiolo, W., Stocker, H., Andruss, B. F., Beckingham, K. & Hafen, E. (1999) Cell 97, 865-875. [DOI] [PubMed] [Google Scholar]
- 40.Rintelen, F., Stocker, H., Thomas, G. & Hafen, E. (2001) Proc. Natl. Acad. Sci. USA 98, 15020-15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klowden, M. J. (2002) in Physiologicla Systems in Insects (Academic, London), pp. 163-203.
- 42.Junger, M. A., Rintelen, F., Stocker, H., Wasserman, J. D., Vegh, M., Radimerski, T., Greenberg, M. E. & Hafen, E. (2003) J. Biol. 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarente, L. & Kenyon, C. (2000) Nature 408, 255-262. [DOI] [PubMed] [Google Scholar]
- 44.Murphy, C. T., McCarroll, S. A., Bargmann, C. I., Fraser, A., Kamath, R. S., Ahringer, J., Li, H. & Kenyon, C. (2003) Nature 424, 277-283. [DOI] [PubMed] [Google Scholar]
- 45.DeFronzo, R.A. (1997) Diabetes Rev. 5, 177-243. [Google Scholar]
- 46.Rhodes, C. J. & White, M. F. (2002) Eur. J. Clin. Invest. 32, Suppl. 3, 3-13. [DOI] [PubMed] [Google Scholar]
- 47.Libina, N., Berman, J. R. & Kenyon, C. (2003) Cell 115, 489-502. [DOI] [PubMed] [Google Scholar]
- 48.Wolkow, C.A., Kimura, K. D., Lee, M. S. & Ruvkun, G. (2000) Science 290, 147-150. [DOI] [PubMed] [Google Scholar]
- 49.Bruning, J. C., Gautam, D., Burks, D. J., Gillette, J., Schubert, M., Orban, P. C., Klein, R., Krone, W., Muller-Wieland, D. & Kahn, C. R. (2000) Science 289, 2122-2125. [DOI] [PubMed] [Google Scholar]
- 50.Oldham, S., Bohni, R., Stocker, H., Brogiolo, W. & Hafen, E. (2000) Philos. Trans. R. Soc. London B 355, 945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartke, A., Brown-Borg, H., Mattison, J., Kinney, B., Hauck, S. & Wright, C. (2001) Exp. Gerontol. 36, 21-28. [DOI] [PubMed] [Google Scholar]
- 52.Johnson, T. E., Lithgow, G. J. & Murakami, S. (1996) J. Gerontol. A Biol. Sci. Med. Sci. 51, B392-B395. [DOI] [PubMed] [Google Scholar]
- 53.Walker, G. A., White, T. M., McColl, G., Jenkins, N. L., Babich, S., Candido, E. P., Johnson, T. E. & Lithgow, G. J. (2001) J. Gerontol. A Biol. Sci. Med. Sci. 56, B281-B287. [DOI] [PubMed] [Google Scholar]
- 54.Walker, G. A. & Lithgow, G. J. (2003) Aging Cell 2, 131-139. [DOI] [PubMed] [Google Scholar]
- 55.Chen, Q., Ma, E., Behar, K. L., Xu, T. & Haddad, G. G. (2002) J. Biol. Chem. 277, 3274-3279. [DOI] [PubMed] [Google Scholar]
- 56.Elbein, A. D., Pan, Y. T., Pastuszak, I. & Carroll, D. (2003) Glycobiology 13, 17R-27R. [DOI] [PubMed] [Google Scholar]
- 57.Singer, M. A. & Lindquist, S. (1998) Trends Biotechnol. 16, 460-468. [DOI] [PubMed] [Google Scholar]
- 58.Wang, M. C., Bohmann, D. & Jasper, H. (2003) Dev. Cell 5, 811-816. [DOI] [PubMed] [Google Scholar]
- 59.Sun, J. & Tower, J. (1999) Mol. Cell. Biol. 19, 216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orr, W. C. & Sohal, R. S. (1994) Science 263, 1128-1130. [DOI] [PubMed] [Google Scholar]
- 61.Orr, W. C., Mockett, R. J., Benes, J. J. & Sohal, R. S. (2003) J. Biol. Chem. 278, 26418-26422. [DOI] [PubMed] [Google Scholar]
- 62.Spencer, C. C., Howell, C. E., Wright, A. R. & Promislow, D. E. (2003) Aging Cell 2, 123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun, J., Molitor, J. & Tower, J. (2004) Mech. Ageing Dev. 125, 341-349. [DOI] [PubMed] [Google Scholar]
- 64.Partridge, L. & Withers, D. J. (2005) Cell, in press. [DOI] [PubMed]
- 65.Burks, D. J., de Mora, J. F., Schubert, M., Withers, D. J., Myers, M. G., Towery, H. H., Altamuro, S. L., Flint, C. L. & White, M. F. (2000) Nature 407, 377-382. [DOI] [PubMed] [Google Scholar]
- 66.Fantin, V. R., Wang, Q., Lienhard, G. E. & Keller, S. R. (2000) Am. J. Physiol. 278, E127-E133. [DOI] [PubMed] [Google Scholar]
- 67.Dillin, A., Crawford, D. K. & Kenyon, C. (2002) Science 298, 830-834. [DOI] [PubMed] [Google Scholar]