Abstract
After an initial benefit, non‐small‐cell lung cancer (NSCLC) patients receiving therapy with tyrosine kinase inhibitors develop drug resistance through a variety of mechanisms. Among these, tumor histology changes are a mechanism of acquired resistance in epidermal growth factor receptor‐mutated and anaplastic lymphoma kinase‐rearranged NSCLC cases. The current availability of therapeutic approaches to overcome tyrosine kinase inhibitor resistance in oncogenic‐driven lung cancers justifies secondary tumor biopsy in these patients. On the other hand, little is known about the mechanism of disease progression in non‐oncogenic driven NSCLC. Nevertheless, NSCLC lacking “druggable” genetic alterations are not considered for secondary biopsy, as it is commonly believed that these tumors cannot develop histologic or molecular changes. Herein, we report two paradigmatic cases of wild‐type NSCLC showing histologic “change” on secondary biopsy, allowing for a successful switch in therapeutic strategy.
Keywords: CEA, histological change, lung cancer, NSE, wild‐type
Introduction
Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) are the targets of several tyrosine kinase inhibitors (TKIs), some of them approved for treatment and others currently in clinical development. Usually, TKI resistance develops after an initial benefit through a variety of mechanisms. Among these, tumor histology changes are a mechanism of acquired resistance in EGFR‐mutated and ALK‐rearranged NSCLC cases.1, 2, 3, 4, 5, 6, 7, 8, 9 The availability of therapeutic approaches to overcome TKI resistance in oncogenic‐driven lung cancers (LCs) justifies secondary tumor biopsy in these patients.1, 3, 4, 5, 9
However, about 35% of NSCLCs have no driver mutations showing a wild‐type genetic set up and as they are not considered for secondary biopsy (because it is commonly believed that they cannot develop molecular changes), little is known about the mechanism of disease progression in non‐oncogenic driven LC.10
Herein, we report two paradigmatic cases of NSCLC without driver mutations (wild‐type NSCLC) showing histologic “transformation” on secondary biopsy, allowing for a successful switch in therapeutic strategy. These observations could suggest the clinical benefit of secondary biopsy even in this subset of LC lacking “druggable” genetic alterations.
Patients
Case 1
In August 2012, a 64‐year‐old, male smoker presented with chest pain. A computed tomography (CT) scan showed a right lung nodule with rib metastasis (stage IV). His carcinoembryonic antigen (CEA) serum level was 12.5 ng/mL, while his neuron specific enolase (NSE) level was unremarkable. Bronchial biopsy revealed an adenocarcinoma. A multiplex analysis by MALDI‐TOF with LungCarta Panel (Agena Bioscience, Mountain View, CA, USA) yielded no mutations in EGFR, K‐RAS, BRAF and in the other tested genes (AKT1, ALK, BRAF, DDR2, EGFR, EPHA3, EPHA5, ERBB2, FGFR4, JAK2, KRAS, MAP2K1 , MET , NOTCH1 , NRAS, NRF2, NTRK1, NTRK2, NTRK3, PIK3CA, PTCH1, PTEN, PTPN11, PTPRD, STK11, TP53). ALK and ROS‐1 rearrangement by immunohistochemistry (IHC) and fluorescence in‐situ hybridization (FISH) were negative. The patient received cisplatin/pemetrexed chemotherapy for four cycles and achieved a partial response (PR). Afterward, the patient underwent an atypical lung resection with a concomitant biopsy of the rib lesion. The original histology was confirmed. Because the bone lesion was not radically resected, radiotherapy to the rib (45 Gy) was performed. Radiological follow‐up showed no clear signs of disease progression during the subsequent three years. In February 2015, a CT scan showed a significant increase in the lung lesion with concomitant enlargement of mediastinal lymph nodes. While CEA levels ranged from 2.5 to 5.6 ng/mL, a rapid increase of NSE (25.6 μg/L) was observed. As a part of re‐evaluation work‐up, a secondary bronchial biopsy revealed small cell lung cancer (SCLC). Based on this data, the patient was treated with four cycles of carboplatin/etoposide and once again achieved PR (Fig 1). The patient is still alive after 44 months of follow‐up.
Figure 1.
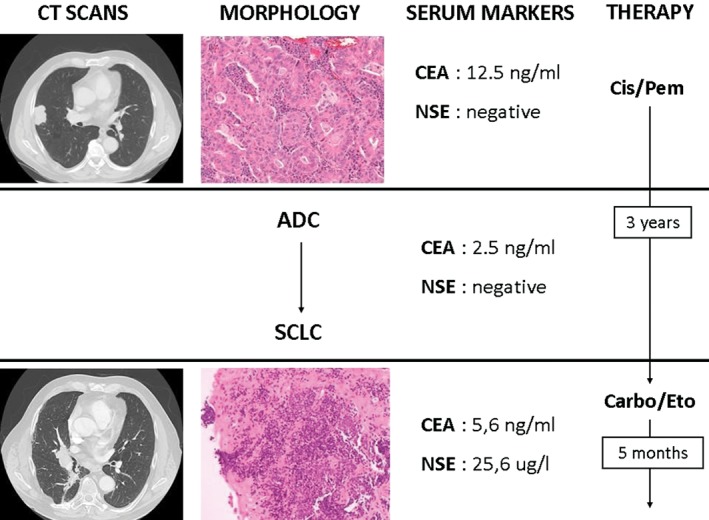
Case 1 clinical course including computed tomography (CT) scans, tumor histology on biopsy, serum tumor markers, and treatment history. The CT scan showed a peripheral lung nodule with irregular margins of the right upper lobe, consistent with an invasive adenocarcinoma (ADC) at histology. The carcinoembryonic antigen (CEA) serum level was 12.5 ng/mL (normal value <5 ng/mL), while the neuron specific enolase (NSE) level was unremarkable. Chemotherapy with cisplatinum (Cis) plus pemetrexed (Pem) was performed for four cycles. Three years later, a chest CT scan revealed a central lesion with enlargement of the mediastinal lymph nodes, corresponding to small‐cell lung cancer (SCLC) associated with an increased NSE level (25.6 μg/L; normal value <12 μg/L). The therapeutic strategy was altered to a chemotherapy regimen with carboplatinum (Carbo) plus etoposide (Eto).
Case 2
In May 2013, a 59‐year‐old, male smoker presented with cough and hemoptysis resulting from a mass located in the right upper lobe and involving hilar lymph nodes. NSE and CEA serum levels were 14 μg/L and 1.6 ng/mL, respectively. A bronchial biopsy revealed SCLC (stage T3N2M0). The patient achieved PR after four cycles of cisplatin/etoposide and sequential radiotherapy (57.2 Gy). Eight months later, ground‐glass opacity with http://radiopaedia.org/articles/interlobular-septal-thickening was detected during a CT scan of the right upper lobe. A slight increase in CEA (13.2 ng/mL) and a decrease in NSE (8 μg/L) levels were noted. The substantial modification of the radiological scenario combined with the fluctuation of serum markers led us to perform a secondary biopsy. A “wild‐type” adenocarcinoma with lymphangitic spread was diagnosed. Molecular and immunohistochemical analyses were completely negative for targetable mutations. The patient commenced a second‐line regimen with carboplatin/pemetrexed (six cycles) followed by maintenance with pemetrexed (three cycles, then halted as per the patient’s wishes), and achieved PR (Fig 2). No peculiar toxicity was reported from the second‐line treatment. The patient is alive and well after 36 months of follow‐up.
Figure 2.
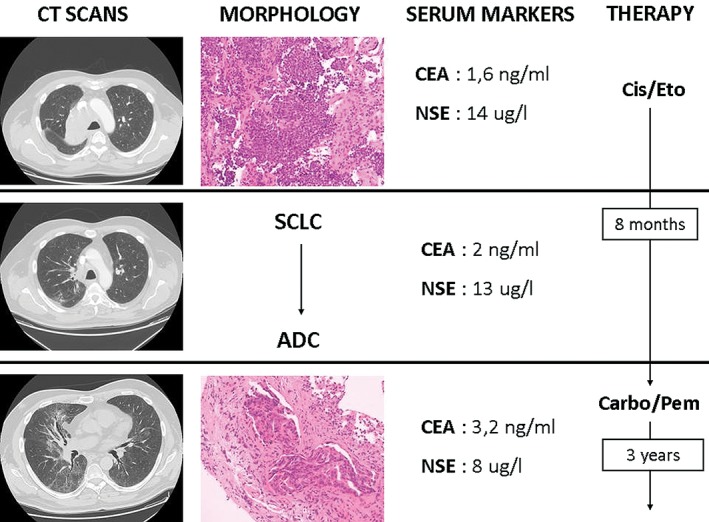
Case 2 clinical course including computed tomography (CT), tumor histology on biopsy, serum tumor markers, and treatment history. The CT scan disclosed a mass in the right upper lobe involving hilar structures consistent with a histologic diagnosis of small‐cell lung cancer (SCLC). The neuron specific enolase (NSE) serum level was slightly elevated (14 μg/L). Chemotherapy with cisplatinum (Cis) and etoposide (Eto) was started. After eight months, a chest CT scan revealed ground‐glass opacities with septal thickening, inconsistent with SCLC. A transbronchial biopsy was performed, yielding a diagnosis of adenocarcinoma (ADC). The carcinoembryonic antigen (CEA) serum level slightly increased, together with a decrease in NSE. The patient commenced alternative chemotherapy with carboplatinum (Carbo) plus pemetrexed (Pem), followed by maintenance with Pem.
Discussion
Histologic transformation is a well‐known phenomenon underlying acquired resistance to TKI in oncogenic‐driven LC.1 However, histologic transformation may be an acquired mechanism to chemotherapy resistance, even in non‐oncogenic driven LC. A possible explanation of this phenomenon is the transformation of NSCLC to SCLC, and vice versa. A second possibility is the presence of combined NSCLC and SCLC histology ab initio, also found in wild‐type carcinomas. This phenomenon is possibly underestimated because of a lack of robust data supporting a secondary biopsy practice at disease progression in wild‐type LC. In addition, pathological and molecular results from a secondary biopsy were obtained through a small fragment of tissue instead of surgical resection, which could introduce a sampling bias error because of the small amount of material. However, we hypothesized that a higher but still unknown percentage of advanced non‐operable LC cases were histologically heterogeneous, consisting of NSCLC associated with an SCLC component, in comparison with surgically resected cancers where the rate of major histologic heterogeneity is only 4% for NSCLC and 9–26% for SCLC. If the hypothesis is correct, the therapeutic strategy should be able to target one of the components, leading to the selective growth of the other.1, 4 Again, in the well‐known setting of oncogenic‐driven LC, several cases of mixed EGFR‐mutated NSCLC/SCLC have been reported, suggesting a degree of plasticity between the two histotypes in some cases, without the selective pressure of TKIs. One of the main molecular mechanisms necessary for transdifferentiation of adenocarcinoma cells to neuroendocrine cells is the loss of RB1, which always occurs during the transformation of EGFR‐mutated NSCLC to SCLC. However, further studies are necessary to identify the mechanisms of progression, histologic transformation, and molecular change in wild‐type LC, which have not yet been investigated.1, 8, 9, 10
Modifications of some ancillary findings during disease course may be a reliable predictor of histologic change: clinical course, serum tumor marker levels (CEA, NSE), and imaging pattern.5, 6 Although guidelines do not recommend the use of tumor markers in diagnosis or follow‐up, a careful examination of the fluctuation of NSE and CEA levels, coupled with modifications of the radiologic pattern of the tumor should be considered as possible predictors of histologic change in “non‐druggable” LC. Some authors have also suggested the potential clinical significance of pro‐gastrin‐releasing‐peptide as a tumor marker for the early prediction of disease transformation from adenocarcinoma to SCLC.9
Clinical course, fluctuation of serum tumor marker levels (CEA, NSE) and changes in imaging pattern represent key findings in suspected histologic transformation, even in wild‐type LC. Secondary tumor biopsy in these cases seems appropriate, as histologic transformation significantly alters treatment recommendations because patients may have a surprisingly prolonged course of disease as a result of the effective selection of histology‐driven chemotherapy.
Disclosure
No authors report any conflict of interest.
References
- 1. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol 2015; 16: e165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oxnard GR, Arcila ME, Sima CS et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011; 17: 1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cha YJ, Cho BC, Kim HR, Lee HJ, Shim HS. A case of ALK‐rearranged adenocarcinoma with small cell carcinoma‐like transformation and resistance to crizotinib. J Thorac Oncol 2016; 11: e55–8. [DOI] [PubMed] [Google Scholar]
- 4. Vatrano S, Righi L, Vavalá T et al. Molecular and histological changes in post‐treatment biopsies of non‐squamous non‐small cell lung cancer: A retrospective study. Target Oncol 2016; 11: 157–66. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Li XY, Tang Y et al. Rapid increase of serum neuron specific enolase level and tachyphylaxis of EGFR‐tyrosine kinase inhibitor indicate small cell lung cancer transformation from EGFR positive lung adenocarcinoma? Lung Cancer 2013; 81: 302–5. [DOI] [PubMed] [Google Scholar]
- 6. Facchinetti F, Tiseo M, Gnetti L, Silini EM, Ardizzoni A. NSE level in combined neuroendocrine and adenocarcinoma EGFR mutated lung cancer resistant to EGFR‐TKI. Lung Cancer 2013; 82: 177–8. [DOI] [PubMed] [Google Scholar]
- 7. Ahn S, Hwang SH, Han J et al. Transformation to small cell lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis of six cases. J Pathol Transl Med 2016; 50: 258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang SY, Zhao J, Wang MZ et al. Small‐cell lung cancer transformation in patients with pulmonary adenocarcinoma: A case report and review of literature. Medicine (Baltimore) 2016; 95: e2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norkowski E, Ghigna MR, Lacroix L et al. Small‐cell carcinoma in the setting of pulmonary adenocarcinoma: New insights in the era of molecular pathology. J Thorac Oncol 2013; 8: 1265–71. [DOI] [PubMed] [Google Scholar]
- 10. Yu HA, Arcila ME, Rekhtman N et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


