Abstract
We report the first case of a 62‐year‐old man with a sclerosing pneumocytoma (SP) combined with a typical carcinoid (TC) and pulmonary adenocarcinoma in different lung lobes. Computed tomography revealed two nodules. The radiological diagnosis was primary lung cancer and a metastatic nodule; however, no enlarged lymph nodes were observed. Histological and immunohistochemical analyses defined the 1.7 cm nodule in the right upper lobe as adenocarcinoma and the 1.3 cm nodule in the left lower lobe as SP combined with TC. This case is noteworthy because of the rarity of SP combined with TC, the comprehensive examination of frozen and permanent sections, and the clinical implications of the differential diagnosis of lung nodules.
Keywords: Carcinoid tumor, neuroendocrine tumor, pulmonary adenocarcinoma, sclerosing pneumocytoma, sclerosing hemangioma
Introduction
Sclerosing pneumocytoma (SP) is a slow‐growing benign tumor that was previously known as “sclerosing hemangioma.”1 It typically occurs in non‐smoking middle‐aged Asian women and presents as a solitary well‐defined mass in the lung parenchyma.2 However, some SP cases have unusual clinical and pathological presentations, making diagnosis difficult.3 Herein, we report the first case of a synchronous occurrence of lung adenocarcinoma and a solitary typical carcinoid (TC) combined with SP in different lobes of the lung.
Case report
In 2014, a nodular lesion was incidentally detected in a 62‐year‐old man who had quit smoking in 1995. The patient initially refused to undergo chest computed tomography (CT) and further diagnostic tests and treatment, but 10 months later agreed to undergo a chest CT scan. The CT scan revealed a 1.7 cm lobulated enhancing nodule in the right upper lobe (RUL) and a 1.3 cm well‐defined, well‐enhanced nodule in the left lower lobe (LLL) of the lung (Fig 1). The radiological diagnosis was primary lung cancer in the RUL with lung‐to‐lung metastasis or benign nodule (e.g. granuloma) in the LLL. No enlarged lymph nodes were observed.
Figure 1.
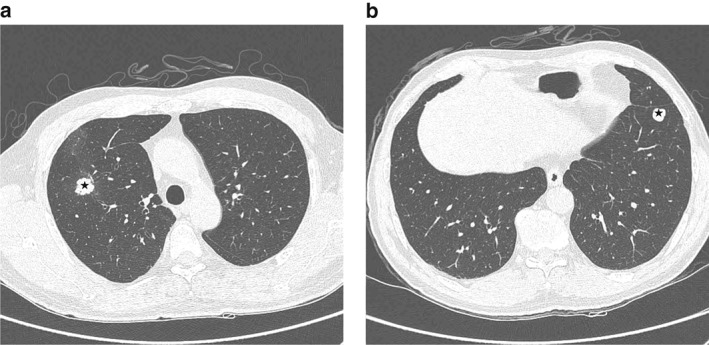
Preoperative chest computed tomography. (a) A lobulated enhancing nodule (*) was observed in the anterior segment of the right upper lobe. (b) Another lobulated well‐enhancing nodule (*) was observed in the anterior basal segment of the left lower lobe. Multiple tiny nodules were found in both lobes.
The patient underwent percutaneous needle biopsy on both nodules. The RUL nodule was diagnosed as adenocarcinoma. However, the biopsy of the LLL nodule was non‐diagnostic. A subsequent whole‐body positron emission tomography scan confirmed lung cancer with hypermetabolism and a nodule with mild hypermetabolism in the RUL and LLL, respectively. Thus, we could not rule out malignancy in the LLL nodule.
The surgeon decided to perform lobectomy of the RUL and wedge resection of the LLL in one procedure. During surgery, the LLL wedge resection specimen was sent for frozen diagnosis. The cut surface of the LLL nodule was well circumscribed with a focal light yellow‐colored area. Microscopically, one side of the tumor showed a papillary pattern with sclerotic features. The columnar cells enclosing the papillae had benign features (no pleomorphism and a low nuclear‐cytoplasmic ratio), supporting an SP diagnosis (Fig 2a). The other side showed a solid area composed of bland‐looking spindle cells (Fig 2b). The nuclear‐cytoplasmic ratio was low, and no pleomorphism or mitosis was observed. The frozen section diagnosis was atypical epithelial proliferation and spindle cell lesion of undetermined malignant potential. In permanent sections, most of the tumor had an SP‐like papillary pattern. The spindle cell lesion occupied about 40% of the tumor (Fig 3a,h).
Figure 2.
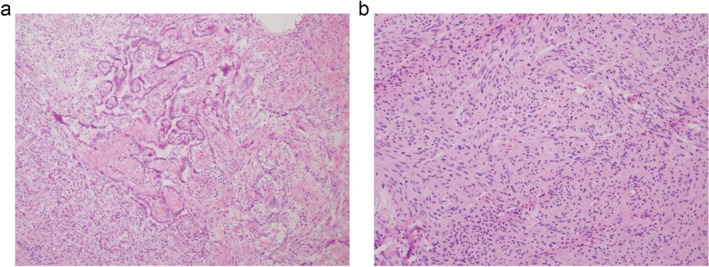
Microscopic findings of the intraoperative specimen submitted for frozen section diagnosis. The tumor showed two distinct histological features: (a) a papillary pattern with sclerotic foci, and (b) a spindle cell proliferative pattern with ill‐defined rosettoid foci.
Figure 3.
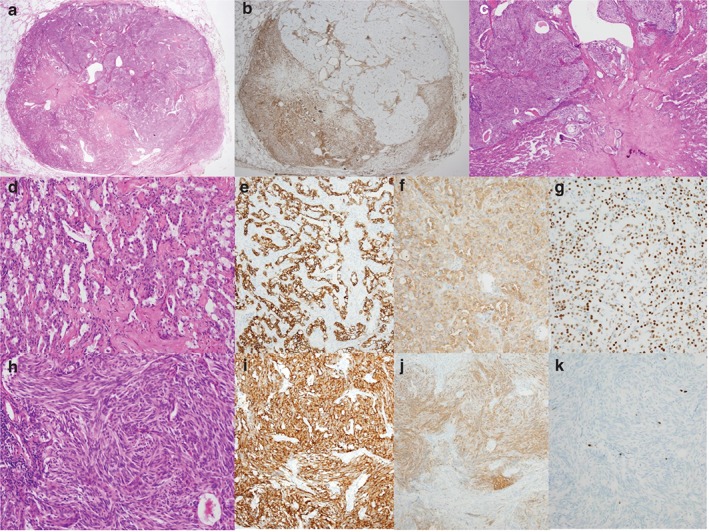
Microscopic findings of the postoperative specimen. (a) Hematoxylin and eosin (H&E) stain shows a well‐circumscribed non‐encapsulated nodule with a pattern of a typical carcinoid (TC) within a sclerosing pneumocytoma (SP). (b) The vimentin staining pattern revealed clear borders between the SP and TC. (c) The TC showed pushing rather than mixed or infiltrative borders. (d) Two cell types were observed in the SP: cuboidal surface and stromal round cells. (e) The SP surface cells were positive for pan‐cytokeratin, whereas the round cells were positive for both (f) epithelial membrane antigen (EMA) and (g) thyroid transcription factor 1 (TTF‐1). (h) The TC had a prominent spindle cell pattern, (i) strong cytoplasmic chromogranin staining, (j) patchy staining for EMA, and (k) a low Ki‐67 labeling index.
The tumor surrounding the spindle cell lesion was diagnosed as SP, after immunohistochemical confirmation. In the hematoxylin and eosin (H&E) stain, the SP showed a mixture of papillary and sclerotic patterns with two cell types: cuboidal surface and stromal round cells (Fig 3d). Occasionally, the cuboidal surface cells had clear, vacuolated foamy cytoplasms. Immunohistochemical staining revealed that the surface cells were positive for pan‐cytokeratin (Table 1,Fig 3e). Both cell types were positive for vimentin (Fig 3b), epithelial membrane antigen (EMA), and thyroid transcription factor 1 (TTF‐1) (Fig 3f,g).
Table 1.
Results of immunohistochemical analyses for SP combined with TC
| Antibody (dilution) | SP component | TC component |
|---|---|---|
| Pancytokeratin (×100)† | P in surface epithelial cells | N |
| N in round cells | ||
| EMA (×200)‡ | P in surface and round cells | Focally P |
| TTF‐1 (×200)† | P in surface and round cells | N |
| Vimentin (Ventana, R‐T‐U × 30) | P in surface and round cells | N |
| CD56 (Zymed, ×400)§ | N | Diffuse P |
| Chromogranin (×200)† | N | Diffuse P |
| Synaptophysin (×100)† | N | N |
| Ki‐67 (×200)† | Less than 4% | Up to 3% |
Manufactured by Dako (Carpinteria, CA, USA).
Manufactured by Leica (Richmond, IL, USA).
Manufactured by Zymed (San Francisco, CA, USA).
EMA, epithelial membrane antigen; N, negative; P, positive; SP, sclerosing pneumocytoma; TTF‐1, thyroid transcription factor 1; TC, typical carcinoid.
The spindle cell lesion seemed to be “within” the SP and had clear‐cut pushing borders in the H&E (Fig 3c) and immunohistochemical stains. Vimentin was negative in the spindle cell lesion (Fig 3b). Chromogranin staining revealed a diffuse pattern (Fig 3i), whereas the EMA pattern had a patchy appearance (Fig 3j). Therefore, the spindle cell lesion was consistent with a TC.
After surgery, the patient experienced neither complications nor atypical radiologic findings within 17 months of follow‐up.
Discussion
We present a rare case of synchronous adenocarcinoma and a TC combined with an SP. To the best of our knowledge, no cases of this tumor combination have been reported in the literature.
Microscopically, the TC was within or close to the SP and showed a pushing margin toward the SP. The finding of a TC combined with an SP raised two possibilities. First, the TC could have originated from the SP (“one tumor” theory).4 Second, the TC and SP could have coincidentally developed close to each other concurrently. SP‐induced epithelial and microenvironmental damage could have induced neuroendocrine cell (NC) proliferation (“collision tumor”).
Wang et al. suggested that the mixture of carcinoid tumors and multiple SPs in their case might be explained by the one tumor theory.4 In their case, the two morphologically different SP and TC components were so well mingled that they appeared as a single tumor; moreover, both components expressed TTF‐1. The one tumor theory is supported by previous studies on the differentiation status of the two types of SP cells by immunohistochemical stain and molecular studies.5, 6
The collision tumor theory is based on the functional role of NCs, which act as self‐renewing progenitors when the cells lining the lung parenchyma are injured.7, 8 During SP tumor cell proliferation and progression, NCs can be activated and grow in response to locally blocked airflow because they act as oxygen‐sensing chemoreceptors that proliferate in a hypoxic environment.9, 10
Previous immunohistochemical and electron microscopic analyses identified neuroendocrine markers and neurosecretory granules in the round cells of SP, indicating that SP might originate from neuroendocrine neoplasms.3 It has also been suggested that SP is a kind of benign neuroendocrine tumor of the lung.11 Recent studies found that both round and surface cells are derived from primitive respiratory epithelium, as TTF‐1 was identified in both cell types.2 These studies reported a gradual transition of atypical alveolar hyperplasia, alveolar adenoma‐like, and adenocarcinoma‐like areas to SP.12 Thus, we hypothesize that the proliferating surface epithelial cells in SP might lead to changes in the surrounding pneumocytes.
Considering the rarity of an SP combined with a TC, understanding this condition is important in the differential diagnosis of small solitary pulmonary nodules. When a benign‐looking solitary pulmonary nodule showing features of SP in the focal area with spindle cell proliferation in the other is submitted for frozen section diagnosis, the lesion could be an SP combined with a neuroendocrine tumor. The differential diagnosis of small pulmonary nodules should therefore include carcinoma (invasive or non‐invasive adenocarcinoma), carcinoid tumor, large cell carcinoma, SP, pulmonary blastoma, and solitary fibrous tumor.13
Acknowledgments
H.J.C. performed most of the experiments; J.H.L. assisted to get informations of clinical and pathologic data; G.K.L. provided the core discussion points of this case report; E.K.H. diagnosed the frozen slides and provided the discussion points of this case; and H.Y.K. diagnosed radiography of this patient and provided discussion points of this case. This case belongs to National Cancer Center, Korea.
Disclosure
No authors report any conflict of interest.
References
- 1. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956; 9: 53–75. [DOI] [PubMed] [Google Scholar]
- 2. Chen B, Gao J, Chen H et al. Pulmonary sclerosing hemangioma: A unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol 2013; 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devouassoux‐Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF‐1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000; 24: 906–16. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, He Q, Shi W, Wang J, Ji H. A mixture of carcinoid tumors, extensive neuroendocrine proliferation, and multiple pulmonary sclerosing hemangiomas. World J Surg Oncol 2014; 12: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gong L, Ren KX, Li YH et al. Determination of clonal status of pulmonary sclerosing hemangioma with X‐chromosome inactivation mosaicism and polymorphism of phosphoglycerate kinase and androgen receptor genes. Med Oncol 2011; 28: 913–8. [DOI] [PubMed] [Google Scholar]
- 6. Wang EH, Dai SD, Qi FJ, Hong‐Tao X, Wei Q. Gene expression and clonality analysis of the androgen receptor and phosphoglycerate kinase genes in polygonal cells and cuboidal cells in so‐called pulmonary sclerosing hemangioma. Mod Pathol 2007; 20: 1208–15. [DOI] [PubMed] [Google Scholar]
- 7. Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 2012; 109: 17531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynolds SD, Hong KU, Giangreco A et al. Conditional clara cell ablation reveals a self‐renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000; 278: L1256–63. [DOI] [PubMed] [Google Scholar]
- 9. Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature 1993; 365: 153–5. [DOI] [PubMed] [Google Scholar]
- 10. Nassar AA, Jaroszewski DE, Helmers RA, Colby TV, Patel BM, Mookadam F. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: A systematic overview. Am J Respir Crit Care Med 2011; 184: 8–16. [DOI] [PubMed] [Google Scholar]
- 11. Xu HM, Li WH, Hou N et al. Neuroendocrine differentiation in 32 cases of so‐called sclerosing hemangioma of the lung: Identified by immunohistochemical and ultrastructural study. Am J Surg Pathol 1997; 21: 1013–22. [DOI] [PubMed] [Google Scholar]
- 12. Liu W, Tian XY, Li Y, Zhao Y, Li B, Li Z. Coexistence of pulmonary sclerosing hemangioma and primary adenocarcinoma in the same nodule of lung. Diagn Pathol 2011; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchevsky AM, Changsri C, Gupta I, Fuller C, Houck W, RJ MK Jr. Frozen section diagnoses of small pulmonary nodules: Accuracy and clinical implications. Ann Thorac Surg 2004; 78: 1755–9. [DOI] [PubMed] [Google Scholar]


