Abstract
Background
The study was conducted to examine esophageal and gastric cardia precursor progression.
Methods
After population‐based baseline screening, 145 precursor and 335 chronic inflammation cases were endoscopically surveyed for six years.
Results
Surveillance of interval and baseline diagnoses for 18 severe dysplasia (SD) cases later detected were: 13, 23, 39, and 44 months since a diagnosis of chronic inflammation in four cases; 6, 6, 6, 11, 13, 16, 16, and 23 months since mild dysplasia (mD) diagnoses in eight; and 6, 9, 10, 13, 18, and 48 months since moderate dysplasia (MD) diagnoses in six. Rates for 11 carcinoma in situ (Cis) cases later detected were: 7 and 18 months since basal cell hyperplasia (Bch) diagnoses in two; and 6, 6, 9, 13, 13, 18, 35, 44, and 50 months since MD diagnoses in nine. In 10 cancer cases later detected, rates were: 6, 6, 7, 18, 19, 34, 36, and 48 months since SD diagnoses in eight cases with submucosal carcinoma; 46 months since MD diagnosis in a T 2 N 0 M 0 carcinoma case; and 52 months since Bch diagnosis in another T 2 N 0 M 0 case.
Conclusion
Esophageal and gastric cardia precursors are heterogeneous. Male gender, advanced age, family history of upper gastrointestinal cancer, and multifocal dysplasia are significant independent predictors for progression, and Bch/mD, MD, and SD constitute three distinctive entities regarding the risk of cancer.
Keywords: Endoscopic screening, endoscopic surveillance, esophageal precursor, heterogeneity, precursor progression
Introduction
According to GLOBOCAN 2012, esophageal cancer is the eighth most common cancer with an estimated 456 300 new cases worldwide in 2012, half of which were diagnosed in China, mostly in rural high‐risk areas.1 Because of the lack of early symptoms, most patients are diagnosed at advanced stage and are thus ineligible for surgery. With a five‐year survival rate below 30%, esophageal cancer is ranked first or second in terms of cancer‐related death in endemic Chinese locations.2 To improve the situation, mass endoscopic screening programs have been established in high‐risk areas since 2000 to accommodate early diagnosis and treatment.2, 3 Population‐based endoscopy is performed in the population aged 40–69, with the aim of detecting severe dysplasia (SD), carcinoma in situ (Cis), and intramucosal carcinoma cases and to treat these lesions with endoscopic mucosa resection (EMR).2, 3, 4 Because the Chinese Cancer Foundation only recommends EMR for SD/Cis/intramucosal carcinoma and not for mild dysplasia (mD) or moderate dysplasia (MD), vast numbers of mD/MD cases are left untreated. In 2005, the Chinese Cancer Foundation suggested that these low‐grade lesions be periodically surveyed every five years for base cell hyperplasia (Bch)/mD, and every three years for MD.2 Despite this recommendation, invasive cancers are still discovered at screening intervals, registered by a population‐based tumor registry.3
This paper examines the heterogeneity in precursor progression/development by repetitive endoscopy among 145 precursor and 335 chronic inflammatory cases for six years after a population‐based baseline endoscopic screening of the population aged 40–69, in a high‐risk area.
Methods
Population screening in a high‐risk area
The study was performed in Shexian County, which is located next to Linxian in the south and Cixian in the southeast. These three counties have very high incidence rates of esophageal and gastric cardia cancer. The world age‐standardized incidence rates of these cancers ranged from 140.3 to 214.0/100 000 in men and from 85.2 to 118.6/100 000 in women in the three counties from 1998 to 2002, accounting for over 50% of overall malignancies.5 To establish early diagnosis and treatment, a series of endoscopic screenings were offered to the population aged 40–69 years in 10 villages in Shexian County from March 2001 to February 2007. As described in an earlier report,3 in the 10 villages, there were 2145 residents aged 40–69‐years at the commencement of screening. Among them, 18 who had been diagnosed with upper gastrointestinal carcinomas (UGICs), and 24 who had been suffering from diseases in contraindication to endoscopy were excluded. The remaining 2103 candidates were invited to participate in baseline screening.
The Institutional Ethics Review Board of the Hebei Cancer Institute of Hebei Medical University approved the study. Informed consent was obtained at each screening and surveillance session.
Endoscopy, pathology, and surveillance
After providing informed consent, the screening recipient was interviewed by an epidemiologist who completed a questionnaire. Individuals who formerly or currently smoked five cigarettes per day for at least two years were defined as smokers, individuals who formerly or currently habitually drank alcohol over six months were defined as alcoholic drinkers, and individuals with at least one first‐degree or at least two second‐degree relatives with esophageal/cardiac/gastric cancer were defined as having a family history of UGIC. After the interview, an endoscopy oncologist from the Fourth Hospital of Hebei Medical University performed an endoscopy to examine the esophagus (with iodine staining), gastric cardia, and the distant stomach until the duodenum was reached. To control inter‐observer variation, the same endoscopist performed all endoscopies over the six‐year study period.
All focal lesions or lugol‐void locations in the esophagus were biopsied. If no focal lesion or lugol‐void locations were found, biopsies were taken at two standard sites, one at the mid‐esophagus and the other at the lesser curve near the posterior wall of the gastric cardia. Biopsy specimens were fixed in 95% ethanol, embedded in paraffin, cut in 5 5 μm sections, and stained with hematoxylin and eosin. Two pathologists separately read the biopsy slides without knowledge of the focal appearance. Histological diagnoses of precursors were made according to a three tier system of mD/Bch, MD, and SD, as reported by Dawsey et al.4, 6 If no satisfactory slides were obtained, the two pathologists strongly disagreed, or the histological diagnosis strongly contradicted the endoscopic appearance, a repeat endoscopy was performed to verify the results.
Middle or late stage UGICs detected were referred to Hebei Provincial Tumor Hospital for treatment. SD/Cis/intramucosal carcinoma cases were advised to undergo endoscopic mucosal resection or argon plasma coagulation. MD, mD or Bch cases were endoscopically surveyed.
To investigate the rate of precursor development or progression, 26.1% (335/1284) of the chronic inflammation subjects and 90.6% of the precursor cases confirmed at baseline (145/160, excluding 25 SD cases who undergone surgery) were endoscopically surveyed for six years according to a program recommended by the Chinese Cancer Foundation in 2005.2 In the program, a five‐year interval is recommended for the general population aged 40–69 years with Bch or mD, and a three‐year interval for MD.2 however, surveillance is voluntary. Because many factors affect participation, the screening intervals varied considerably (range 6–52 months).
At a follow‐up endoscopy, the site where early lesions had been found was re‐Lugol‐stained and biopsied to investigate any changes to the precursor over time. If results of consecutive endoscopies at least six months apart suggested that a precursor lesion had developed (by a formerly normal or chronic inflammatory mucosa) or progressed (by a former precursor), a progressive event was recorded. The six month interval was adopted as a cut‐off to account for biopsy sampling errors.7, 8
To encourage repeated endoscopy, local endoscopy stations were set up periodically and the screening team visited study participants’ homes to persuade them to undergo re‐examination.
Statistical analyses
A Mann–Whitney test was used to determine the difference in detection rates of precursor or cancer between men and women, participants aged < 50 and ≥ 50 years old, with or without a family history, smokers or non‐smokers, and alcoholic drinkers or non‐drinker groups. Comparisons were made between the initial and successive screening results for those repetitively screened to investigate the development or progression of precursors. If the result suggested that a lesion had progressed, the time between the two screenings was recorded as the interval. The time to precursor development/progression was estimated by Kaplan–Meier survival analysis according to baseline histology, and risk factors associated with precursor development/progression were analyzed with Cox proportional hazard regression models. We initially analyzed time to and risk factors for precursor development in the 335 normal/chronic inflammation subjects and for precursor progression in the 145 dysplastic cases separately. After similar results were observed, we combined the analyses. The factors examined as significant predictors in univariable analysis included gender, age, family history of UGIC, smoking, drinking, annual family income, presence of multiple dysplastic lesions, and presence of multiple lugol‐void lesions (scattered vs. uniform type). Factors significant at P < 0.10 in univariable analysis and common demographic variables such as gender, age, family history of UGIC, smoking, drinking, and family income were included in multiple variable analysis models. All analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline screening
As reported in our earlier study, a total of 1514 participants aged 40–69 underwent baseline screening, for a screening rate of 71.99% (1514/2103).3 No significant difference in the distribution of gender, age, family history of UGIC, smoking, or alcohol intake was observed between the screened sample (n =1514) and the local population aged 40–69 (n = 2145) (Table 1). The number and detection rates of mD, MD, SD, Cis/intramucosal carcinoma, and invasive cancer on the esophagus were 67 (4.4%), 39 (2.6%), 16 (1.1%), 32 (2.1%), and 6 (0.4%), respectively. On the gastric cardia the corresponding rates were 10 (0.7%), 7 (0.5%), 5 (0.3%), 9 (0.6%), and 25 (1.7%), respectively. The detection rates of cancer or precursors were significantly higher on the esophagus, for men than women, subjects aged > 50, participants with a positive family history of UGIC, and smokers. On the gastric cardia men, participants aged ≥ 50 years, and smokers had significantly higher detection rates (Table 2).
Table 1.
Comparison of demographic distribution between the screened group (n = 1514) and the 40–69 year old overall population (n = 2145) in a target population in Shexian County
| Topography and demographics | Overall population aged 40–69 | No. screened (%) | P † |
|---|---|---|---|
| Total | 2145 | 1514 | |
| Gender | |||
| Male | 1070 (49.9) | 763 (50.4) | 0.96 |
| Female | 1075 (50.1) | 751 (49.6) | |
| Age | |||
| < 50 | 1008 (47.0) | 708 (46.8) | 0.95 |
| ≥ 50 | 1137 (53.0) | 806 (53.2) | |
| Family history of UGIC | |||
| Positive | 858 (40.0) | 616 (40.7) | 0.94 |
| Negative | 1287 (60.0) | 898 (59.3) | |
| Tobacco | |||
| Smoker | 860 (40.1) | 642 (42.4) | 0.85 |
| Non‐smoker | 1285 (59.9) | 872 (57.6) | |
| Alcohol | |||
| Drinker | 601 (28.0) | 418 (27.6) | 0.88 |
| Non‐drinker | 1544 (72.0) | 1096 (72.4) | |
Comparison of demographic distribution between overall versus screened population.
UGIC, upper gastrointestinal cancer.
Table 2.
Baseline endoscopic screening of 1514 40–69 year old subjects in Shexian County for UGIC and precancerous lesions by demographics
| Topography and demographics | No. screened (%) | No. of cancerous and precancerous cases detected (prevalence %) | P Mann–Whitney test | ||||
|---|---|---|---|---|---|---|---|
| Invasive cancer | Cis/intramucosal carcinoma | SD | MD | mD/Bch | |||
| ESOPHAGUS | |||||||
| Total | 1514 | 6 (0.4) | 32 (2.1) | 16 (1.1) | 39 (2.6) | 67 (4.4) | |
| Gender | |||||||
| Male | 763 (50.4) | 5 (0.7) | 21 (2.8) | 11 (1.4) | 24 (3.1) | 33 (4.3) | 0.018* |
| Female | 751 (49.6) | 1 (0.1) | 11 (1.5) | 5 (0.7) | 15 (2.0) | 34 (4.5) | |
| Age | |||||||
| < 50 | 708 (46.8) | 3 (0.4) | 6 (0.8) | 5 (0.7) | 5 (0.7) | 26 (3.7) | 0.000** |
| ≥ 50 | 806 (53.2) | 3 (0.4) | 26 (3.2) | 11 (1.4) | 34 (4.2) | 41 (5.1) | |
| Family history of UGIC | |||||||
| Positive | 616 (40.7) | 4 (0.6) | 14 (2.3) | 9 (1.5) | 25 (4.1) | 44 (7.1) | 0.000** |
| Negative | 898 (59.3) | 2 (0.2) | 18 (2.0) | 7 (0.8) | 14 (1.6) | 23 (2.6) | |
| Tobacco | |||||||
| Smoker | 642 (42.4) | 6 (0.9) | 19 (3.0) | 9 (1.4) | 18 (2.8) | 30 (4.7) | 0.012* |
| Non‐smoker | 872 (57.6) | 0 (0.0) | 13 (1.5) | 7 (0.8) | 21 (2.4) | 37 (4.2) | |
| Alcohol | |||||||
| Drinker | 418 (27.6) | 4 (1.0) | 12 (2.9) | 7 (1.7) | 11 (2.6) | 19 (4.5) | 0.08 |
| Non‐drinker | 1096 (72.4) | 2 (0.2) | 20 (1.8) | 9 (0.8) | 28 (2.6) | 48 (4.4) | |
| GASTRIC CARDIA | |||||||
| Total | 1514 | 25 (1.7) | 9 (0.6) | 5(0.3) | 7(0.5) | 10(0.7) | |
| Gender | |||||||
| Male | 763 (50.4) | 23 (3.0) | 6 (0.8) | 3 (0.4) | 5 (0.7) | 6 (0.8) | 0.000** |
| Female | 751 (49.6) | 2 (0.3) | 3 (0.4) | 2 (0.3) | 2 (0.3) | 4 (0.5) | |
| Age | |||||||
| < 50 | 708 (46.8) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 4 (0.6) | 0.000** |
| ≥ 50 | 806 (53.2) | 25 (3.1) | 8 (1.0) | 4 (0.5) | 7 (0.9) | 6 (0.7) | |
| Family history of UGIC | |||||||
| Positive | 616 (40.7) | 8 (1.3) | 4 (0.6) | 3 (0.5) | 4 (0.6) | 2 (0.3) | 0.618 |
| Negative | 898 (59.3) | 17 (1.9) | 5 (0.6) | 2 (0.2) | 3 (0.3) | 8 (0.9) | |
| Tobacco | |||||||
| Smoker | 642 (42.4) | 19 (3.0) | 6 (0.9) | 2 (0.3) | 4 (0.6) | 5 (0.8) | 0.001** |
| Non‐smoker | 872 (57.6) | 6 (0.7) | 3 (0.3) | 3 (0.3) | 3 (0.3) | 5 (0.6) | |
| Alcohol | |||||||
| Drinker | 418 (27.6) | 6 (1.4) | 2 (0.5) | 3 (0.7) | 3 (0.7) | 3 (0.7) | 0.653 |
| Non‐drinker | 1096 (72.4) | 19 (1.7) | 7 (0.6) | 2 (0.2) | 4 (0.4) | 7 (0.6) | |
P < 0.010;
P < 0.050. Bch, basal cell hyperplasia; Cis, carcinoma in situ; mD, mild dysplasia; MD, moderate dysplasia; SD, severe dysplasia; UGIC, upper gastrointestinal cancer.
Of the 185 esophageal or gastric cardia precursor cases detected at baseline, there were 62 SD/Cis/intramucosal carcinoma, 46 MD, 67 mD, and 10 Bch cases. Of the 62 SD/Cis/intramucosal carcinoma cases, 25 underwent surgery, 29 underwent EMR treatment, and eight cases refused either treatment modality.
Endoscopic mucosa resection was not performed in any of the MD/mD/Bch (46 MD and 77 mD/Bch) cases detected at baseline. Consensus was reached on diagnosis for 46 MD and 67 mD cases, but the pathologists disagreed on the remaining 10 cases. Because Bch has been consistently reported to be a relevant precursor entity for esophageal squamous cancer and has shown a similar risk as mD in previous studies in the region,4, 6 the 10 cases were classified as Bch in survival analysis to examine the risk of progression independently.
Endoscopic mucosa resection was not performed in any of the cases detected at baseline (67 mD, 46 MD, 10 Bch). The expert panel decided that many of the precursors may regress and as an intervention policy, EMR was only recommended in SD/Cis cases.2 By February 2007, 108 cases (61 mD, 37MD, and 10 Bch) had undergone repeat endoscopy (range 6–52 months). The rate of endoscopic surveillance was 87.80% (108/123).
Surveillance of precursor and chronic inflammation cases
During the six year surveillance, 31.70% (480/1514) of the initially screened members underwent repetitive screening, including 145 precursor and 335 chronic inflammation cases (Table 3). The 145 precursor cases included 29 endoscopically treated and 116 untreated precursor cases. Surveillance was conducted in the endoscopically treated cases to investigate the rate of precursor recurrence, and surveillance of the untreated precursor cases (including 10 Bch, 61 mD, 37 MD, and 8 SD) to examine the rate of precursor progression, persistence, or regression.
Table 3.
Surveillance results by baseline diagnosis of normal/inflammation, precursor, and after EMR
| Groups according to the baseline diagnosis and intervention | N | Percent | Interval (months) |
|---|---|---|---|
| 335 initially normal/inflammatory subjects | |||
| Unchanged | 287 | 85.4 | 23.0(6.0 ~ 51.7) |
| Precursor development | 48 | 14.6 | 24.0(6.0 ~ 51.7) |
| 116 initial precursors | |||
| Progressed | 41 | 34.5 | 19.0(6.0 ~ 51.7) |
| Persistent | 24 | 21.6 | 12.0(6.0 ~ 51.7) |
| Regressed | 51 | 44.0 | 21.0(6.0 ~ 51.7) |
| 29 subjects endoscopically surveyed for six years after EMR | |||
| No recurrence after EMR | 17 | 58.6 | 49.0(48.0 ~ 49.6) |
| Recurred | 12 | 41.4 | 13.0(6.0 ~ 48.0) |
EMR, endoscopic mucosal resection.
At the end of the surveillance period, 35.34% (41/116) of the untreated precursor cases had progressed, 20.69% (24/116) persisted, and 43.97% (51/116) regressed (Table 3). During the six‐year surveillance period, 335 subjects were confirmed with chronic inflammation at baseline, which aims to investigate the rate of precursor development, and 14.33% (48/335) developed a precursor (Table 3). The precursor development rate was significantly lower than the progression rate in the 116 precursor cases (14.33% vs. 35.34%; P = 0.000).
In total, 89 precursor progression/development events occurred in the 480 subjects under surveillance. The 41 progression events in the 116 precursors comprised development of 6 MD, 14 SD, 11 Cis, and 10 cancer cases (8 submucosal and 2 T0N0M0 cancer cases); and the 48 precursor development events in the 335 inflammatory subjects comprised 4 Bch, 32 mD, 8 MD, and 4 SD. The screening history and intervals are summarized in Table 4.
Table 4.
Histology of precursor development or progression
|
Development history (n = 89) |
Screening intervals (months) | Median (months) |
|---|---|---|
| Chronic inflammation (4) → Bch (4) | 7, 8, 16, 24 | 8 |
| Chronic inflammation (32) → mD (32) | 6†4, 7†2, 8†4, 12†2, | 21 |
|
13, 17, 19, 21†2, 22, 23, 24, 26, 29, 31, 43†4, 48, 26,30,32,36 |
||
| Chronic inflammation (8) → MD (8) | 6†3, 28†2, 29, 43†2 | 28 |
| Chronic inflammation (4) → SD (4) | 13, 23, 44, 39 | 33.5 |
| Bch (2) → Cis (2) | 7, 18 | 12.5 |
| Bch (1) → cancer (T2N0M0) (1) | 52 | |
| mD (6) → MD (6) | 6, 8†2, 13, 16, 25 | 10.5 |
| mD (8) → SD (8) | 6†3, 10.5, 13, 16†2, 23 | 11.8 |
| MD (6) → SD (6) | 6, 9, 10, 13, 18, 48 | 10 |
| MD (9) → Cis (9) | 6, 6, 9, 13†2, 18, 35, 44, 50 | 13 |
| MD (1) → cancer (T2N0M0) (1) | 46 | |
| SD (8) → submucosal carcinoma (8) | 6†2, 7, 18, 19, 34, 36, 48 | 12 |
Patient developed mD from chronic inflammation in six months.
Bch, basal cell hyperplasia; Cis, carcinoma in situ; mD, mild dysplasia; MD, moderate dysplasia; SD, severe dysplasia; UGIC, upper gastrointestinal cancer.
Risk of precursor progression according to baseline histology
The 48 cases of precursor development, 41 of precursor progression (Table 4), and 12 cases of local recurrence in the 29 EMR‐treated patients (Table 5) were combined and treated as events in Kaplan–Meier analysis to estimate the time to and risk of precursor progression according to the baseline diagnoses of normal, mD, MD, and SD (Fig 1). Compared to the reference for normal, in which 7.7% developed a precursor lesion in 1.5 years and 21.2% in four years, precursor progression occurred significantly earlier and more frequently with increasing histology: mD, 22.5% progressed in 1.5 years and 28.4% in four years; MD, 36.60% progressed in 1.5 years and 62.40% in four years; and SD, 50.00% progressed in 1.5 years and 100.00% in four years. Overall, the trend (P = 0.00 based on log rank test) and the difference between each are significant.
Table 5.
Multivariable Cox proportional regression analyses for risk factors associated with esophageal and gastric cardia precursor progression
| Significant variables | Wald value | P | Hazard ratio | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender (male vs. female) | 14.45 | 0.00** | 2.74 | 1.63 | 4.60 |
| Age (< 50 vs. ≥ 50 years) | 8.86 | 0.00** | 2.31 | 1.33 | 4.02 |
| Family history of UGIC | 3.73 | 0.04* | 1.56 | 0.99 | 2.45 |
| Multifocal dysplasia | 36.60 | 0.00** | 5.11 | 3.01 | 8.68 |
| Baseline diagnosis over all | 10.03 | 0.02* | |||
| mD/Bch (vs. normal) | 0.45 | 0.08 | 1.40 | 0.81 | 2.02 |
| MD/marginal lugol‐stained mucosa at EMR (vs. normal) | 0.38 | 0.06 | 1.70 | 0.88 | 2.68 |
| SD (vs. normal) | 8.17 | 0.00** | 4.00 | 2.51 | 9.19 |
P < 0.01;
P < 0.05. Bch, basal cell hyperplasia; EMR, endoscopic mucosal resection; GIC, upper gastrointestinal cancer; mD, mild dysplasia; MD, moderate dysplasia; SD, severe dysplasia.
Figure 1.
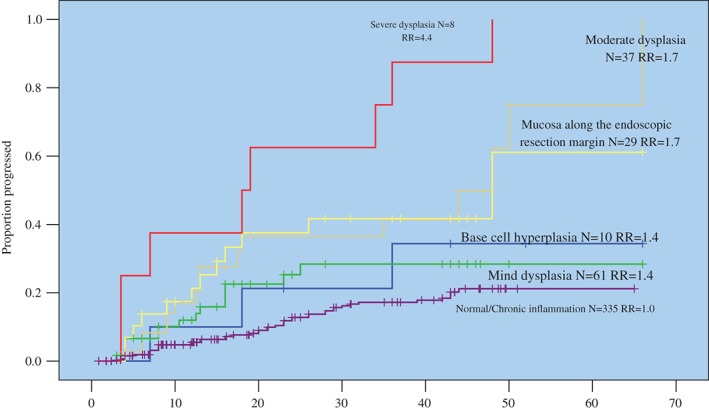
Time to and risk of esophageal and gastric cardia precursor progression according to baseline histology.
Of note, the Lugol‐stained mucosa along the margin of EMR in the 29 endoscopically treated patients had 1.5 and 4 year progression rates of 37.5% and 61.1%, respectively, very similar to that of 36.6% and 62.4% associated with MD (P = 0.816 by log rank test). Finally, Bch had 1.5 and 4 year progression rates of 21.2% and 34.4%, respectively, which is not significantly different from the 22.5% and 28.4% associated with mD (P = 0.768 by log rank test).
Compared to the reference, the relative risks (95% confidence interval [CI]) associated with SD, MD, mD/Bch were 4.4 (1.7–11.4), 1.7 (1.0–2.9), and 1.4 (0.8–2.5), respectively (Fig 1). These relative risks were adjusted by gender, age, family history of UGIC, smoking, alcohol drinking, and other confounding factors in Cox proportional hazard regression.
Cox regression analyses of significant predictors of precursor progression
Gender, age, family history of UGIC, multifocal dysplasia, and baseline histology was found to be significant, whereas smoking and alcoholic drinking were non‐significant predictors in univariate Cox regression analyses. In multivariate analyses, gender, age, family history of UGIC, multifocal dysplasia, and baseline histology were significant independent predictors. The hazard ratios (95% CI) associated were 2.7 (1.6–4.6) for men versus women, 2.3 (1.3–4.0) for ≥ 50 versus < 50 years, 1.6 (1.0–2.5) for positive versus negative family history of UGIC, 5.1 (3.0–8.7) for multifocal dysplasia versus none, 1.4 (0.8–2.0) for mD/Bch versus normal, 1.7 (0.9–2.7) for MD versus normal, and 4.0 (2.5–9.2) for SD versus normal (Table 5).
Severe dysplasia and carcinoma in situ/intramucosal carcinoma screening history
During surveillance, 18 SD and 11 Cis cases were identified by late screening. The interval and baseline diagnosis of the 18 SD cases were as follows: 13, 23, 39, 44 months since baseline diagnosis of chronic inflammation in four cases (median interval from chronic inflammation to SD 33.5 months); 6, 6, 6, 10.5, 13, 16, 16, and 23 months after baseline diagnosis of mD in eight cases (median interval from mD to SD 11.75 months); and 6, 9, 10, 13, 18 and 48 months since baseline diagnosis of MD in six cases (median interval from MD to SD: 11.5 months). The interval and baseline diagnosis of the 11 Cis cases were: 7 and 18 months since a diagnosis of Bch in two cases (median interval from BCH to Cis 12.5 months); and 6, 6, 9, 13, 13, 18, 35, 44, 50 months after a diagnosis of MD in nine cases (median interval from MD to Cis 13 months). As development in all 29 SD/Cis cases occurred within 50 months, these cases might be regarded as having rapidly developing lesions opposed to indolent lesions that persisted or regressed during surveillance (Table 1).
Screening history of invasive cancer cases
Ten cases of invasive cancer were identified by late round screening (8 submucosal carcinomas and 2 T2N0M0 carcinomas). The eight submucosal carcinomas developed in the eight SD cases that were identified at the baseline, but the patients refused any treatment modality. They were closely surveyed and re‐diagnosed with submucosal carcinoma at intervals of 6, 6, 7, 18, 19, 34, 36, and 48 months later; in one case, T2N0M0 carcinoma developed from MD 46 months later and in the other, T2N0M0 was diagnosed 52 months after the initial diagnosis of Bch. The 10 invasive cancer cases were referred to the Fourth Hospital of Hebei Medical University for surgery after diagnosis during surveillance (Table 4).
Recurrence after endoscopic mucosa resection
Of the 29 endoscopically treated patients, 41.38% (12/29) recurred, although Lugol‐stained mucosa was included at the time of EMR (Table 6). Histological examination based on the resected specimen found “normal mucosa” between the margin and the lesion in seven cases (complete resection achieved in 58.33%); degenerative tissue in only two cases (complete resection suspected in 16.67%); and in the remaining three cases, Bch, mD, and MD were found in the margin of the specimens, respectively (non‐complete resection in 25.00%). The histology of the recurring lesions and surveillance intervals for the 12 recurring cases are summarized in Table 3. Of note, nine of the 12 cases (75%) reported a positive family history of UGIC, and five of the nine had more than one first‐degree relative afflicted with UGIC.
Table 6.
Precursor recurrence by Lugol‐stained mucosa along the margin of endoscopic mucosa resection in 12 cases
| Development history (n) | Screening intervals (months) |
|---|---|
| Lugol‐stained mucosa surrounding EMR (3) → mF (3) | 9, 13, 48 |
| Lugol‐stained mucosa surrounding EMR (3) → MD (3) | 6, 15, 16 |
| Lugol‐stained mucosa surrounding EMR (3) → SD (3) | 6, 6, 12 |
| Lugol‐stained mucosa surrounding EMR/MD (1) → intramucosal carcinoma (1) | 26 |
| Lugol‐stained mucosa surrounding EMR/Bch (1) → SD (1) | 8 |
| Lugol‐stained mucosa surrounding EMR/mD (1) → Cis (1) | 18 |
Bch, basal cell hyperplasia; Cis, carcinoma in situ; EMR, endoscopic mucosal resection; mD, mild dysplasia; MD, moderate dysplasia; SD, severe dysplasia.
Discussion
Cancer is heterogeneous. The heterogeneity may manifest in etiology, behavior, treatment, and prognosis. Heterogeneity in cancer precursor progression makes cancer screening a subject of debate, because while indolent lesions tend to be arrested by screening tests and lead to overdiagnosis and treatment, rapidly progressing and clinically more relevant precursors are difficult to arrest and tend to develop as invasive cancer between screening intervals. This situation is well reflected in the endoscopic surveillance of lower grade esophageal precursors, such as BCH, mD, or MD in Chinese high‐risk areas.3
Heterogeneity of esophageal precursor progression may be related to two factors. First, the behavior of an esophageal precursor is determined by a pattern of molecular alterations or passing ways, different alterations having different progression potential. Second, squamous cell carcinomas of the esophagus are noted for field carcinogenesis or occurrence at multiple primary sites.
In our experience, rapidly progressing precursors do exist in esophageal cancer. For example, a 60‐year‐old man identified as normal at baseline developed SD 13 months later; two Bch subjects, a 68‐year‐old man and a 41‐year‐old woman, developed SD and Cis seven and 18 months later, respectively; a 60‐year‐old women initially diagnosed as mD was diagnosed with invasive cancer 13 months later; and another two MD subjects, a 49‐year‐old woman and a 52‐year‐old man, were diagnosed as having progressed into Cis and intramucosal cancer six and 13 months later, respectively. All of these cases had a positive family history of UGIC. Because they all developed a high‐grade precursor or cancer from a low grade within 18 months, their precursors have rapid progression potential.
Wang et al. endoscopically surveyed 578 non‐cancerous subjects for 11 years. They reported that the average time from entry into the study to cancer diagnosis was 5.0 ± 2.9 years in 18 cases of esophageal cancer in men, and 4.7 ± 3.2 years in seven cases in women. In the 25 esophageal carcinomas, 11 developed from normal epithelium defined at baseline.6 The lower interval in their observations is consistent with our results.
Regarding field carcinogenesis with esophageal cancer in Chinese high‐risk areas, Wang et al. reported that 4% (2/55) of dysplasia and 47% (26/55) of Bch cases had precursors originating from more than one primary site.9 Song examined 100 surgically resected specimens and found that 94% had unconnected Bch or Cis.10 When endoscopic mucosal resection is performed, 0.5 cm wide Lugol‐stained mucosa beyond the lesion is included. However, because molecular alterations, such as TP53 mutation, may be involved, whether this Lugol‐stained mucosa at the margin is biologically normal or not is disputed.9, 11, 12 In the present study, 41.38% (12/29) of EMR treated cases recurred although complete resection had been achieved in 58.33% (7/12) of them.
Recent studies have suggested that genetic predisposition may be implicated in the “field carcinogenesis” of esophageal cancer. Many studies of esophageal squamous cancer in the high‐risk region in northern central China have found that a positive family history of UGIC carries an over twofold risk.13, 14 Our results indicate that a positive family history of UGIC is associated with a significantly younger onset age, multiple primary lesions, and a poorer prognosis in surgically resected cases.15, 16 In population screening, the age when dysplasia is detectable was younger in participants with a positive compared to a negative family history of UGIC, and the former group are more likely to be associated with multiple Lugol‐void lesions and metachronous lesions after EMR.17 In studies focused on molecular aspects of genetic predisposition, some scholars have referred to the accumulation of mutant TP53 or loss of heterozygosity as the molecular base.9, 18 It is possible that with such an inherited background of molecular alteration, further genetic events may accumulate rapidly and at more sites, thus establishing a possible association between genetic predisposition, multifocal carcinogenesis, and rapid precursor progression. In this study, nine of the 12 cases that recurred after EMR had a positive family history of UGIC, five of which had more than one first‐degree relative implicated.
Although smoking and alcohol consumption are major risk factors for esophageal squamous cell carcinoma in Western populations,1 previous studies of the Taihang Mountain high‐risk area of the Linxian‐Cixian‐Shexian population did not find that smoking or alcohol consumption had a significant effect.19 In this population, about half of the esophageal squamous cell carcinoma cases occur in women, but few women smoke or drink alcohol.19 About 50–60% of men smoke, but even among men, smoking is only a mild risk factor (relative risk 1.33), and alcohol was not significantly associated with the risk of esophageal or gastric cardia cancer.19 Our analysis also failed to identify smoking or drinking as significant predictors for precursor progression. However, the small sample of precursors may have limited statistical efficiency. We intend to provide further updated analysis as the sample size increases.
As a population‐based and prospective study, our results have merit. The first round of endoscopy covered 71.99% (1514/2103) of the local population aged 40–69 years. After the first‐round screening, 90.63% (145/160) of the precursors identified at baseline, excluding the 25 surgically treated early cancer cases, and 25.87% (335/1295) of the chronic inflammatory cases confirmed at baseline were re‐screened within 6–52 months later. These 480 subjects were followed for six years to monitor cancer recurrence.
In summary, esophageal and gastric cardia precursors are heterogeneous. For risk stratification in screening and surveillance, male gender, advanced age, a family history of UGIC, and multifocal dysplasia are significant independent predictors for neoplastic progression. Bch/mD, MD, and SD constitute three distinctive entities regarding the risk of cancer.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by Key Medical Research Subjects in Hebei Province [2012] No 2056, headed by Prof Baoen Shan, Fourth Hospital of Hebei Medical University.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Wang GQ, Wei WQ. Esophagus cancer In: Dong ZW. (ed.). Direction for the Screening and Early Treatment of Cancer in China. Medical Press of Peking University, Beijing: 2005; 47–50 (In Chinese.) [Google Scholar]
- 3. Wen DG, Wang SJ, Zhang LW, Zhou W, Yu WF, Wang XL. Natural history of esophageal and gastric cardia precursor by repetitive endoscope screening with 425 adults in a high‐risk area in China. Cancer Epidemiol 2009; 33: 108–12. [DOI] [PubMed] [Google Scholar]
- 4. Dawsey SM, Lewin KJ, Liu FS, Wang GQ, Shen Q. Esophageal morphology from Linxian, China: Squamous histologic findings in 754 subjects. Cancer 1994; 73: 2027–37. [DOI] [PubMed] [Google Scholar]
- 5. Wen DG, Zhang N, Shan B, Wang S. Helicobacter pylori infection may be implicated in the topography and geographic variation of upper gastrointestinal cancers in the Taihang Mountain high‐risk region in northern China. Helicobacter 2010; 15: 416–21. [DOI] [PubMed] [Google Scholar]
- 6. Wang LD, Zhou Q, Feng CW et al. Intervention and follow‐up on human esophageal precancerous lesions in Henan, northern China, a high‐incidence area for esophageal cancer. Gan To Kagaku Ryoho 2002; 29 (Suppl. 1): 159–72. [PubMed] [Google Scholar]
- 7. Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 321–9. [DOI] [PubMed] [Google Scholar]
- 8. Warnakulasuriya S, Kovacevic T, Madden P et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10‐year period in south East England. J Oral Pathol Med 2011; 40: 677–83. [DOI] [PubMed] [Google Scholar]
- 9. Wang LD, Zhou Q, Hong JY, Qiu SL, Yang CS. p53 protein accumulation and gene mutations in multifocal esophageal precancerous lesions from symptom free subjects in a high incidence area for esophageal carcinoma in Henan, China. Cancer 1996; 77: 1244–9. [PubMed] [Google Scholar]
- 10. Song SQ. Histological genesis of esophageal cancer In: Lin PZ, Liu FS. (eds). Pathology and Prevention of Esophageal Cancer. Geographical Press, Beijing: 1994; 42 (In Chinese.) [Google Scholar]
- 11. Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato O, Asaka M. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc 2001; 54: 190–4. [DOI] [PubMed] [Google Scholar]
- 12. Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: Tumor properties and therapeutic implications. Biochim Biophys Acta 2005; 1756: 25–52. [DOI] [PubMed] [Google Scholar]
- 13. Hu N, Dawsey SM, Wu M et al. Familial aggregation of esophageal cancer in Yangcheng County, Shanxi Province, China. Int J Epidemiol 1992; 21: 877–82. [DOI] [PubMed] [Google Scholar]
- 14. Chang‐Claude J, Becher H, Blettner M, Qiu S, Yang G, Wahrendorf J. Familial aggregation of oesophageal cancer in a higher incidence area in China. Int J Epidemiol 1997; 26: 1159–65. [DOI] [PubMed] [Google Scholar]
- 15. Wen DG, Wang SJ, Zhang LW, Zhang JH, Wei LZ, Zhao XQ. Difference of onset age and survival rates in esophageal cancer patients with and without a family history of upper gastrointestinal cancer from a high‐incidence area in North China. Fam Cancer 2006; 5: 343–52. [DOI] [PubMed] [Google Scholar]
- 16. Wen DG, Wang SJ, Zhang LW, Wei LZ, Zhou WD, Peng Q. Early onset, multiple primary malignancies, and poor prognosis are indicative of an inherited predisposition to esophageal squamous cell carcinoma for the familial as opposed to the sporadic cases – An update on over 14‐year survival. Eur J Med Genet 2009; 52: 381–5. [DOI] [PubMed] [Google Scholar]
- 17. Wen DG, Shan B, Wang SJ et al. A positive family history of esophageal/gastric cardia cancer with gastric cardia adenocarcinoma is associated with a younger age at onset and more likely with another synchronous esophageal/gastric cardia cancer in a Chinese high‐risk area. Eur J Med Genet 2010; 53: 250–5. [DOI] [PubMed] [Google Scholar]
- 18. Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res 2000; 462: 335–42. [DOI] [PubMed] [Google Scholar]
- 19. Tran GD, Sun XD, Abnet CC et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005; 113: 456–63. [DOI] [PubMed] [Google Scholar]


