Abstract
Background
The prognostic values of preoperative neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), and platelet/lymphocyte ratio (PLR) in non‐small cell lung cancer (NSCLC) have been previously described. This study assessed the prognostic values of other pretreatment complete blood cell parameters in Chinese patients with curatively resected NSCLC.
Methods
A total of 1466 consecutive NSCLC patients who received curative surgery from January 1, 2005 to December 31, 2009 with complete data from pretreatment blood tests were enrolled in this retrospective study. Correlations between each blood test parameter and overall survival were examined by Kaplan–Meier method or Cox proportional hazards regression, followed by a stratification analysis of significant variables.
Results
Optimal cut‐off values of 0.55 for neutrophil/white blood cell ratio (NWR), 0.28 for lymphocyte/white blood cell ratio (LWR), 0.09 for monocyte/white blood cell ratio (MWR), 2.06 for NLR, 0.35 for MLR, 204.00 for PLR, and 38.25 for platelet/white blood cell ratio (PWR) were identified using X‐tile software. Univariate analysis suggested that NWR ≥ 0.55, LWR < 0.28, MWR ≥ 0.09, NLR ≥ 2.06, MLR ≥ 0.35, and PLR ≥ 204.00 predicted a poor prognosis in NSCLC patients. However, only NWR and MLR were identified as independent significant prognostic factors in multivariable analysis, especially in tumor node metastasis stage I and I/II/III NSCLCs.
Conclusion
Pretreatment NWR, MWR, LWR, NLR, MLR, and PLR values are associated with poor overall survival for patients with curatively resected NSCLC. NWR and MLR are independent prognostic factors in curatively resected NSCLC.
Keywords: Blood test, non‐small cell lung cancer, prognosis, survival
Introduction
In China, lung cancer remains the leading cause of cancer mortality for both men and women, regardless of the fact that stable trends in incidence have been observed in recent years. Approximately 733 300 new cases and 610 200 deaths were estimated in China in 2015, of which non‐small cell lung cancer accounts for 80–85%.1 Despite rapid advances in surgical techniques and medical therapy, the prognosis for lung cancer is still poor, with a five‐year survival rate less than 15%.2 Thus, sensitive and specific factors for the prediction of prognosis are needed to guide clinical practice.
Inflammatory responses are now generally believed to play pivotal roles at different stages of tumor development by inhibiting apoptosis and promoting angiogenesis. Additionally, more studies have revealed that the inflammatory microenvironment is closely linked to immune surveillance and responses to therapy in tumor treatment.3 The neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), and platelet/lymphocyte ratio (PLR) based on complete blood cell (CBC) counts, which are simple parameters for evaluating systemic inflammatory responses, are prognostic factors for various malignancies, including gastric, colon, and lung cancers.4, 5, 6, 7, 8, 9 However, a comprehensive analysis of other CBC parameters is still lacking for lung cancer. We therefore examined the associations between various CBC parameters and prognosis in 1466 NSCLC patients.
Methods
Patient selection and data collection
This retrospective analysis included a cohort of 1466 consecutive patients who received curative resection for stage I–IIIA NSCLC at Sun Yat‐sen University Cancer Center (SYSUCC) from January 1, 2005 to December 31, 2009. All patients were restaged using the 7th International Classification System for Lung Cancer. The SYSUCC medical ethics and clinical trial review committees approved the study.
Information was collected from individual patient records and survival data was obtained from the SYSUCC follow‐up registry. The information collected included age, gender, smoking history, histology, pathological stage, pretreatment CBC counts, time of surgery, and death from any cause. All CBC counts were performed in the Department of Clinical Laboratory, SYSUCC.
Curatively resected pathologically confirmed stage I–IIIA NSCLC patients who had not received previous therapy other than surgical resection and neoadjuvant chemotherapy were included in the study. Exclusion criteria were previous malignancies, perioperative death, and insufficient data of survival or pretreatment hematology test data. To avoid the potential impact of surgery or chemotherapy on CBC counts, we ensured that each eligible patient had a pretreatment blood routine test within seven days before the first treatment. All parameters were calculated using the equations as follows: NWR = neutrophil/white blood cell, LWR = lymphocyte/white blood cell, MWR = monocyte/white blood cell, NLR = neutrophil/lymphocyte, MLR = monocyte/lymphocyte, PLR = platelet/lymphocyte, and PWR = platelet/white blood cell.
Statistical analysis
X‐tile software (Yale University, New Haven, CT, USA) was used to determine the optimal cut‐off values of NWR, MWR, LWR, NLR, MLR, PLR, and PWR for predicting lung cancer prognosis.10 The entire cohort was then respectively dichotomized into high and low groups according to each optimal cut‐off value. The chi‐square test was used to compare categorical variables.
Overall survival (OS), defined as the interval from the date of surgery to the date of death from any cause, was evaluated as the endpoint. Kaplan–Meier curves were drawn for OS, and differences were compared by log‐rank test. Variables reaching 0.1 significance in univariate analysis were included in the Cox proportional hazards model for multivariate analysis. Two‐tailed P values of <0.05 were considered statistically significant. All analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Patient population
A total of 1466 patients, including 1058 men and 408 women, who had undergone curative resection for primary stage I–IIIA NSCLC were included in this study (Fig 1). After a median follow‐up of 69.9 months (95% confidence interval 64.3–75.4), 732 of the patients had died, while 734 were still alive at the last follow‐up or censored, with a five‐year OS rate of 45.4% (Fig 2). Table 1 shows the baseline characteristics of the patient population. A high NWR was observed in 70.6% of the patient population, while a high MLR was detected in 71.0%. There were more male patients and more advanced tumors in the high‐NWR and high‐MLR groups, especially in the pathologic tumor (pT) category. Additionally, larger numbers of older patients, current smokers, squamous carcinomas, and pneumonectomy surgeries were observed in the high‐MLR than in the low‐MLR group (Table 1).
Figure 1.
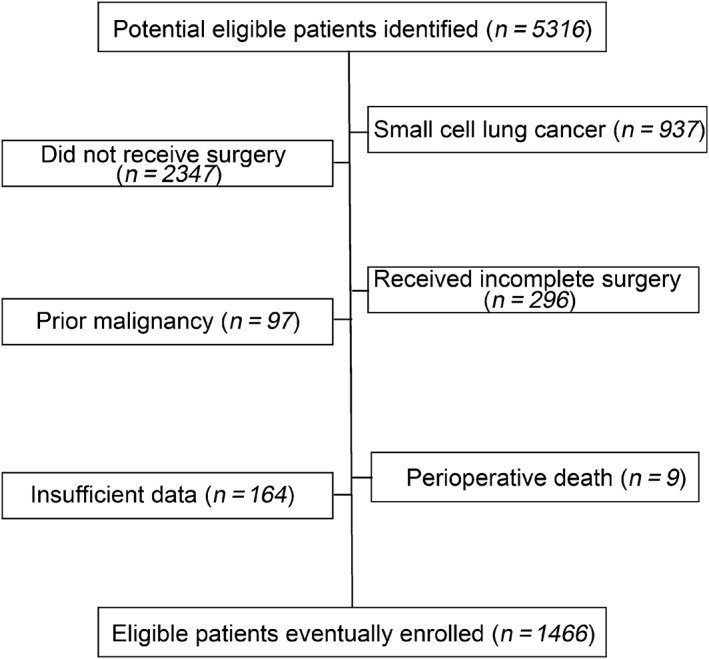
Patient selection process. A total of 1466 patients with non‐small cell lung cancer who underwent curative resection were included in this study.
Figure 2.
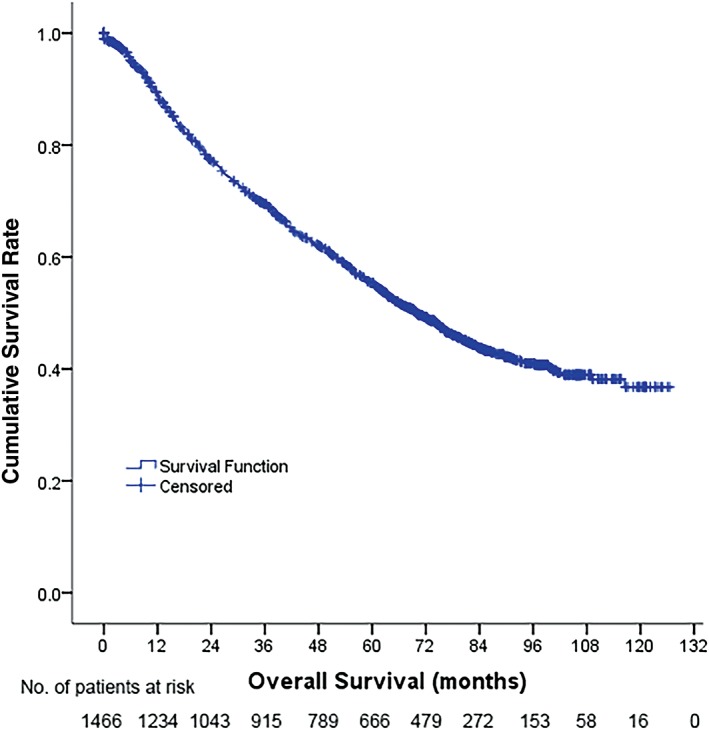
Overall survival of 1466 non‐small cell lung cancer (NSCLC) patients.
Table 1.
Baseline clinicopathological characteristics of patients in NWR and MLR groups
| NWR group | MLR group | |||||
|---|---|---|---|---|---|---|
| Characteristics | <0.55 | ≥0.55 | P † | <0.35 | ≥0.35 | P † |
| Age | 0.257 | 0.030 | ||||
| ≥55 years | 280 (65.0) | 704 (68.0) | 681 (65.4) | 303 (71.3) | ||
| <55 years | 151 (35.0) | 331 (32.0) | 360 (34.6) | 122 (28.7) | ||
| Gender | 0.010 | <0.001 | ||||
| Male | 291 (67.5) | 767 (74.1) | 687 (66.0) | 371 (87.3) | ||
| Female | 140 (32.5) | 268 (25.9) | 354 (34.0) | 54 (12.7) | ||
| Smoking status | 0.099 | <0.001 | ||||
| Never | 190 (44.1) | 407 (39.3) | 484 (46.5) | 113 (26.6) | ||
| Former | 51 (11.8) | 108 (10.4) | 110 (10.6) | 49 (11.5) | ||
| Current | 190 (44.1) | 520 (50.2) | 447 (42.9) | 263 (61.9) | ||
| Pathology | 0.320 | <0.001 | ||||
| Squamous carcinoma | 136 (31.6) | 379 (36.6) | 322 (30.9) | 193 (45.4) | ||
| Adenocarcinoma | 255 (59.2) | 564 (54.5) | 620 (59.6) | 199 (46.8) | ||
| Adenosquamous carcinoma | 26 (6.0) | 58 (5.6) | 65 (6.2) | 19 (4.5) | ||
| Others | 14 (3.2) | 34 (3.3) | 34 (3.3) | 14 (3.3) | ||
| pT category | <0.001 | <0.001 | ||||
| 1 | 107 (24.8) | 146 (14.1) | 209 (20.1) | 44 (10.4) | ||
| 2 | 267 (61.9) | 680 (65.7) | 663 (63.7) | 284 (66.8) | ||
| 3 | 35 (8.1) | 121 (11.7) | 99 (9.5) | 57 (13.4) | ||
| 4 | 22 (5.1) | 88 (8.5) | 70 (6.7) | 40 (9.4) | ||
| pN category | 0.341 | 0.606 | ||||
| N0 | 249 (57.8) | 586 (56.6) | 600 (57.6) | 235 (55.3) | ||
| N1 | 64 (14.8) | 132 (12.8) | 140 (13.4) | 56 (13.2) | ||
| N2 | 118 (27.4) | 317 (30.6) | 301 (28.9) | 134 (31.5) | ||
| pTNM stage | 0.099 | 0.073 | ||||
| I | 228 (52.9) | 487 (47.1) | 525 (50.4) | 190 (44.7) | ||
| II | 71 (16.5) | 177 (17.1) | 177 (17.0) | 71 (16.7) | ||
| III | 132 (30.6) | 371 (35.8) | 339 (32.6) | 164 (38.6) | ||
| Chemotherapy | 0.076 | 0.092 | ||||
| Neoadjuvant + adjuvant | 5 (1.2) | 29 (2.8) | 18 (1.7) | 16 (3.8) | ||
| Neoadjuvant only | 2 (0.5) | 16 (1.5) | 12 (1.2) | 6 (1.4) | ||
| Adjuvant only | 191 (44.3) | 459 (44.3) | 472 (45.3) | 178 (41.9) | ||
| No | 233 (54.1) | 531 (51.3) | 539 (51.8) | 225 (52.9) | ||
| Surgery type | 0.072 | 0.046 | ||||
| Pneumonectomy | 21 (4.9) | 84 (8.1) | 66 (6.3) | 39 (9.2) | ||
| Lobectomy | 410 (95.1) | 950 (91.8) | 975 (93.7) | 385 (90.6) | ||
| Others | 0 (0) | 1 (0.1) | 0 (0) | 1 (0.1) | ||
| Total | 431 (100) | 1035 (100) | 1041 (100) | 425 (100) | ||
χ2 test (multigroup comparison).
MLR, monocyte/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; pN, pathologic node; pT, pathologic tumor; pTNM, pathologic tumor node metastasis.
Optimal cut‐off values
Using X‐tile software, we defined the cut‐off values for predicting prognosis in NSCLC patients as 0.55 for NWR (P = 0.002), 0.28 for LWR (P < 0.001), 0.09 for MWR (P = 0.003), 2.06 for NLR (P < 0.001), 0.35 for MLR (P < 0.001), 204.00 for PLR (P = 0.002), and 38.25 for PWR (P = 0.121) (Fig 3).
Figure 3.
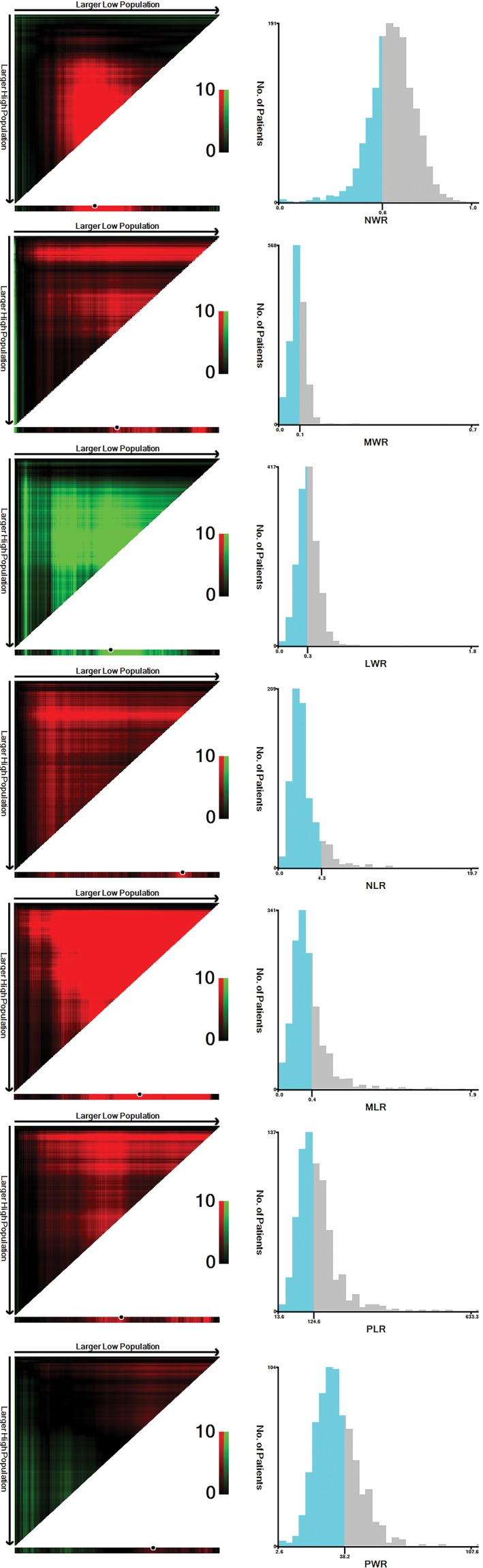
Cut‐off values of neutrophil/white blood cell ratio (NWR), monocyte/white blood cell ratio (MWR), lymphocyte/white blood cell ratio (LWR), neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), platelet/lymphocyte ratio (PLR), and platelet/white blood cell ratio (PWR) in 1466 non‐small cell lung cancer patients. The optimal cut‐off values were 0.55 for NWR, 0.28 for LWR, 0.09 for MWR, 2.06 for NLR, 0.35 for MLR, 204.00 for PLR, and 38.25 for PWR.
Univariate and multivariate analysis
NWR, MWR, LWR, NLR, MLR, PLR, and PWR were then examined in univariate and multivariate analyses to identify prognostic predictors in NSCLC patients. In univariate analyses, smoking status, pT category, pathologic node (pN) category, pathologic tumor node metastasis (pTNM) category, surgery type, chemotherapy, NWR, LWR, MWR, NLR, MLR, and PLR were identified as significant prognostic factors. In multivariate analyses, we found that pT category (P < 0.001), pN category (P < 0.001), pTNM stage (P < 0.001), chemotherapy (P = 0.002), NWR (P = 0.014), and MLR (P = 0.035) had significant hazard ratios, indicating that they were independent, significant predictors of OS (Table 2). Elevated NWR and MLR were independent predictive factors for poor prognosis.
Table 2.
Univariate and multivariate analysis for OS of NSCLC patients
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Median OS (months) | HR (95% CI) | P | β | HR (95% CI) | P | |
| Age | ||||||
| ≥55 years vs. <55 years | 67.1 vs. 74.1 | 1.118 (0.957,1.305) | 0.160 | |||
| Gender | ||||||
| Males vs. females | 67.8 vs 76.0. | 1.160 (0.985,1.365) | 0.075 | −0.048 | 0.953 (0.754,1.203) | 0.684 |
| Smoking status | ||||||
| Ever vs. never | 64.7 vs. 76.0 | 1.204 (1.038,1.397) | 0.014 | 0.191 | 1.211 (0.978,1.499) | 0.079 |
| Pathology | ||||||
| Squamous vs. non‐squamous | 72.4 vs. 69.2 | 1.001 (0.860,1.166) | 0.998 | |||
| pT category | ||||||
| 3/4 vs. 1/2 | 30.6 vs. 80.9 | 2.084 (1.761,2.466) | <0.001 | 0.415 | 1.514 (1.257,1.825) | <0.001 |
| pN category | ||||||
| ≥ N1 vs. N0 | 40.7 vs. NA | 2.899 (2.497,3.365) | <0.001 | 0.820 | 2.271 (1.804,2.858) | <0.001 |
| pTNM stage | ||||||
| III vs. I–II | 33.8 vs. 109.1 | 2.962 (2.559,3.428) | <0.001 | 0.439 | 1.551 (1.232,1.952) | <0.001 |
| Chemotherapy | ||||||
| No vs. yes | 85.0 vs. 58.5 | 1.385 (1.197,1.601) | <0.001 | −0.258 | 0.772 (0.654,0.912) | 0.002 |
| Surgery type | ||||||
| Pneumonectomy vs. others | 28.4 vs. 72.5 | 1.783 (1.389,2.289) | <0.001 | 0.150 | 1.162 (0.897,1.504) | 0.256 |
| NWR | ||||||
| High group vs. low group | 63.2 vs. 90.7 | 1.379 (1.168,1.629) | <0.001 | 0.277 | 1.319 (1.057,1.645) | 0.014 |
| LWR | ||||||
| High group vs. low group | 82.3 vs. 59.3 | 0.746 (0.644,0.863) | <0.001 | 0.039 | 1.040 (0.769,1.406) | 0.800 |
| MWR | ||||||
| High group vs. low group | 59.8 vs. 74.6 | 1.195 (1.022,1.398) | 0.026 | 0.112 | 1.118 (0.926,1.351) | 0.245 |
| NLR | ||||||
| High group vs. low group | 59.7 vs. 82.5 | 1.351 (1.166,1.566) | <0.001 | −0.048 | 0.954 (0.690,1.318) | 0.773 |
| MLR | ||||||
| High group vs. low group | 53.2 vs. 76.6 | 1.493 (1.281,1.740) | <0.001 | 0.224 | 1.252 (1.016,1.542) | 0.035 |
| PLR | ||||||
| High group vs. low group | 50.5 vs. 72.2 | 1.406 (1.126,1.755) | 0.003 | 0.053 | 1.055 (0.831,1.340) | 0.661 |
| PWR | ||||||
| High group vs. low group | 63.7 vs. 72.2 | 1.123 (0.962,1.312) | 0.141 | |||
β, partial regression coefficient; CI, confidence interval; HR, hazard ratio; LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; MWR, monocyte/white blood cell ratio; NA, not available; NSCLC, non‐small cell lung cancer; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; OS, overall survival; PLR, platelet/lymphocyte ratio; pN, pathologic node; pT, pathologic tumor; pTNM, pathologic tumor node metastasis; PWR, platelet/white blood cell ratio.
The OS rates of 1466 NSCLC cancer patients according to NWR and MLR are shown in Fig 3. The high NWR group had significantly poorer OS than the low NWR group, with a median OS of 63.2 versus 90.7 months (P < 0.001). Similarly, the high MLR group had significantly poorer OS than the low MLR group, at 53.2 versus 76.6 months (P < 0.001) (Fig 4).
Figure 4.

Overall survival of 1466 non‐small cell lung cancer patients according to neutrophil/white blood cell ratio (NWR), and monocyte/lymphocyte ratio (MLR), respectively. (a) The high NWR group has significantly poorer overall survival (OS) than the low NWR group. (b) The high MLR group has significantly poorer OS than the low MLR group.
The prognostic value of NWR and MLR for OS were then further analyzed with patients stratified by TNM stage. While NWR only predicted prognosis in stage I NSCLC patients (P = 0.001), MLR predicted prognosis in stage I (P = 0.006), stage II (P = 0.040), and stage III (P = 0.005) NSCLC patients (Fig 5).
Figure 5.
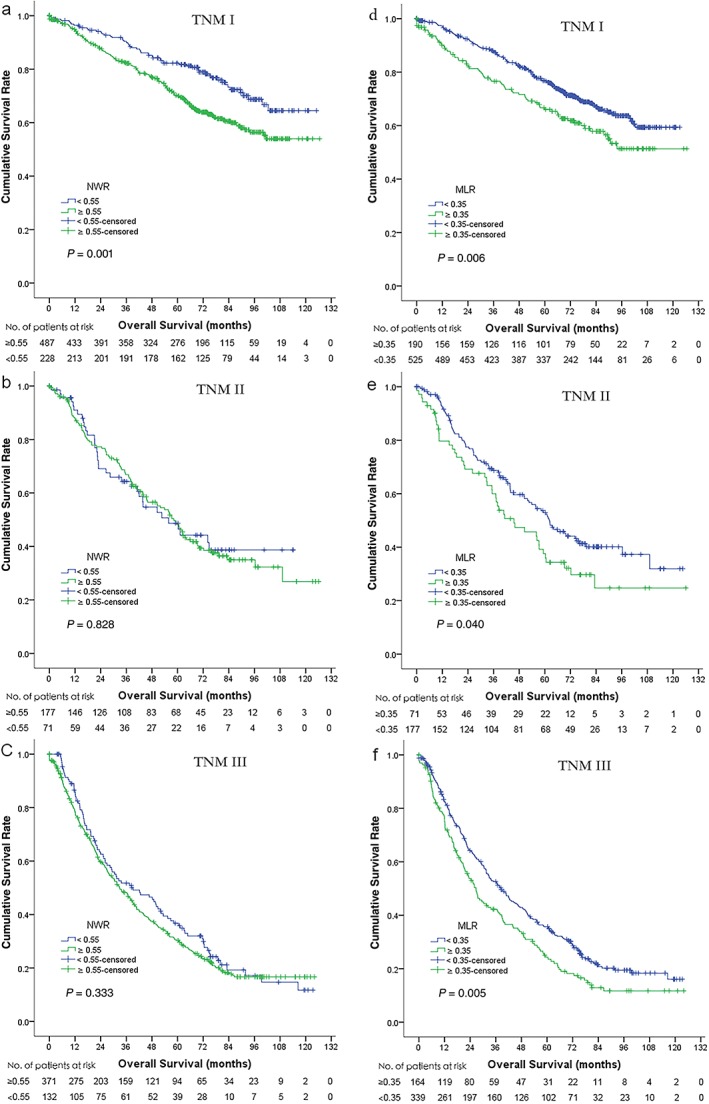
Overall survival (OS) of 1466 non‐small cell lung cancer patients according to neutrophil/white blood cell ratio (NWR) and monocyte/lymphocyte ratio (MLR), stratified by tumor node metastasis (TNM) stage. Significant differences in OS can be observed in (a) TNM stage I but not in (b) TNM II and (c) TNM III, according to NWR. OS rates were significantly different in (d) TNM stage I, (e) TNM II, and (f) TNM III according to MLR.
Discussion
With the development of precision medicine in recent years, various methods for cancer diagnosis and the evaluation of prognosis have been applied at the molecular level, such as in the Genome‐Wide Association Study. These new technologies have largely expanded the information we can collect from patients, which is used to easily evaluate prognosis and administer appropriate and individualized treatment. However, most of these methods are difficult to extend to much of the public because of limitations in funds and facilities. Thus, simple and cost‐efficient biomarkers for evaluating prognosis must be found.
The association between the inflammatory microenvironment and tumor prognosis has long been verified, and the prognostic value of the inflammatory parameters of NLR, PLR, and MLR has recently been revealed.11, 12, 13 To further investigate the prognostic value of various inflammatory parameters based on CBC, we performed this retrospective research in a single cancer center. The results support the findings of previous studies, indicating that elevated NLR, MLR, and PLR were linked to poor prognosis in lung cancer. Moreover, high NWR, MWR, and low LWR were revealed for the first time to predict poor prognosis in NSCLC. Furthermore, NWR and MLR were independent prognostic factors in NSCLC. Finally, NWR was only predictive of prognosis in stage I NSCLC patients.
Neutrophils and lung cancer
Neutrophils are usually recognized as short‐lived cells because of their antimicrobial functions. However, tumor‐associated neutrophils (TANs), derived from peripheral neutrophils, have been proposed as key mediators in tumor progression specifically because they induce genetic instability, promote tumor growth, stimulate angiogenesis, and favor cancer cells’ invasive behavior.14 NLR, a good index reflecting the balance between inflammation and immunoreaction in patients, has long been proven to be a biomarker for prognosis in various cancers, including lung cancer.9, 15, 16, 17, 18, 19, 20, 21 However, reports on the association between NWR and prognosis in lung cancer are still lacking. In the present study, we demonstrated for the first time that NWR is an independent prognostic factor of curative resected NSCLC, although the underlying mechanisms require further exploration. On the other hand, patients with high NLR were observed to have longer OS than those with low NLR, although NLR was not an independent prognostic factor for NSCLC in our study.
Monocytes and lung cancer
Tumor‐associated macrophages (TAMs), which are derived from circulating monocytic precursors, also play key roles in the inflammatory microenvironment of tumor progression.14 In parallel with TAN, TAM can stimulate tumor cell proliferation, promote angiogenesis, and favor invasion and metastasis by producing growth and angiogenic factors, as well as protease enzymes, which degrade the extracellular matrix.22 The prognostic value of MLR, which represents the relative levels of peripheral monocytes and lymphocytes, had been observed in numerous studies as a favorable predictor for prognosis in breast, colon, and lung cancers.5, 7, 23 In the 1466 cases we studied, elevated MLR was identified as an independent factor for poor prognosis in NSCLC, which was consistent with the results of previous studies. Nevertheless, MWR was only associated with OS in univariate analysis and not in multivariate analysis.
Lymphocytes and lung cancer
Lymphocytes, different from neutrophils and monocytes, play a crucial role in host cell‐mediated immunity regulation, which is important to destruct residual malignant cells and related micrometastases.19 It is now widely believed that tumor‐infiltrating lymphocytes (TILs) are associated with better clinical outcomes in cancers.24 Furthermore, the preoperative lymphocyte count was defined as a favorable prognostic factor for disease‐free survival in NSCLC.25 In accordance with a previous study, high NLR, MLR, and PLR all related to poor OS in NSCLC patients in our study. While high LWR predicts favorable prognosis in OS, it was not an independent factor when adjusted by other characteristics in multivariate analysis. To some degree, low levels of peripheral lymphocytes may explain these poor outcomes.
Platelets and lung cancer
The correlation between platelets and the prognosis of solid tumors has drawn much attention in recent years, although the underlying mechanisms remain unclear.6, 26, 27 It has been proposed that platelets are an important factor in tumor angiogenesis, as platelets may adhere to tumor vessels and release granules that contain potent stimulators of angiogenesis, such as vascular endothelial cell growth factor (VEGF) and platelet‐derived endothelial cell growth factor (PDEGF).28, 29 High PLR was suggested to be a predictive factor for poor prognosis in NSCLC,6 which was then verified in a meta‐analysis based on 12 independent studies.30 Similarly, we found that high PLR predicted adverse outcomes in the OS of patients with NSCLC. However, PLR was not a significant factor in subsequent multivariate analysis.
In summary, we propose that NWR and MLR are independent prognostic factors for NSCLC, which to some degree reflect the status of both inflammation and immunoreaction in patients with lung cancer. As highly reproducible, easily obtained, and reliable biomarkers for the evaluation of prognosis, NWR and MLR can be applied to distinguish high‐risk from low‐risk patients after curative surgery, thus helping to determine postoperative treatment.
Several limitations should be noted. First, as in other retrospective studies, we could not completely rule out the impact of selection bias. The patients were included over a relatively long period (2005–2009), and many patients were initially staged according the 6th International Classification System for Lung Cancer, whereas they were restaged according to the 7th Classification System during our analysis. Second, East Asians constituted most of our study population; therefore, our results may not apply to other ethnic populations. Third, we were unable to collect information on other potential significant prognostic factors for NSCLC, such as the mutational status of epidermal growth factor receptor and anaplastic lymphoma kinase rearrangement status.
In conclusion, high NWR, MWR, LWR, NLR, MLR, and PLR predict poor prognosis in curatively resected NSCLC. Furthermore, NWR and MLR are independent prognostic factors in curatively resected NSCLC, especially in stage I for NWR.
Ackowledgment
We would like to thank Prof. Qing Liu for his excellent statistical assistance.
Disclosure
No authors report any conflict of interest.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Li N, Xu M, Cai MY et al. Elevated serum bilirubin levels are associated with improved survival in patients with curatively resected non‐small‐cell lung cancer. Cancer Epidemiol 2015; 39: 763–8. [DOI] [PubMed] [Google Scholar]
- 3. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Templeton AJ, McNamara MG, Seruga B et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: A systematic review and meta‐analysis. J Natl Cancer Inst 2014; 106: dju124. [DOI] [PubMed] [Google Scholar]
- 5. Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation‐based lymphocyte‐ monocyte ratio in patients with lung cancer: Based on a large cohort study. PLoS ONE 2014; 9: e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu X, Sun S, Gao XS et al. Prognostic value of platelet to lymphocyte ratio in non‐small cell lung cancer: Evidence from 3,430 patients. Sci Rep 2016; 6: 23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stotz M, Pichler M, Absenger G et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014; 110: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stotz M, Szkandera J, Stojakovic T et al. The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer. Clin Chem Lab Med 2015; 53: 499–506. [DOI] [PubMed] [Google Scholar]
- 9. Käsmann L, Bolm L, Schild SE, Janssen S, Rades D. Neutrophil‐to‐lymphocyte ratio predicts outcome in limited disease small‐cell lung cancer. Lung 2017; 195: 217–24. [DOI] [PubMed] [Google Scholar]
- 10. Camp RL, Dolled‐Filhart M, Rimm DL. X‐tile: A new bio‐informatics tool for biomarker assessment and outcome‐based cut‐point optimization. Clin Cancer Res 2004; 10: 7252–9. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Zhang L, Zhu K et al. Prognostic significance of combination of preoperative platelet count and neutrophil‐lymphocyte ratio (COP‐NLR) in patients with non‐small cell lung cancer: Based on a large cohort study. PLoS ONE 2015; 10: e0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SH, Lee HW, Go SI, Lee SI, Lee GW. Clinical significance of the preoperative platelet count and platelet‐to‐lymphocyte ratio (PLT‐PLR) in patients with surgically resected non‐small cell lung cancer. Oncotarget 2016; 7: 36198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omar M, Tanriverdi O, Cokmert S et al. Role of increased mean platelet volume (MPV) and decreased MPV/platelet count ratio as poor prognostic factors in lung cancer. Clin Respir J 2016. https://doi.org/10.1111/crj.12605 [DOI] [PubMed] [Google Scholar]
- 14. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol 2013; 228: 1404–12. [DOI] [PubMed] [Google Scholar]
- 15. Byun SS, Hwang EC, Kang SH et al. Prognostic significance of preoperative neutrophil‐to‐lymphocyte ratio in nonmetastatic renal cell carcinoma: A large, multicenter cohort analysis. Biomed Res Int 2016; 2016: 5634148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allan RE, Luis RP, Juan P. Neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio as prognostic factors in non‐metastatic breast cancer patients from a Hispanic population. Breast Dis 2016. https://doi.org/10.3233/BD‐160251 [DOI] [PubMed] [Google Scholar]
- 17. Chen F, Lin L, Yan L, Qiu Y, Cai L, He B. Preoperative neutrophil‐to‐lymphocyte ratio predicts the prognosis of oral squamous cell carcinoma: A large‐sample prospective study. J Oral Maxillofac Surg 2016. pii:; S0278‐2391(16)31203‐4. [Google Scholar]
- 18. Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non‐small cell lung cancer: A systemic review and meta‐analysis. Int J Clin Exp Med 2015; 8: 3098–106. [PMC free article] [PubMed] [Google Scholar]
- 19. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non‐small cell lung cancer. J Thorac Cardiovasc Surg 2009; 137: 425–8. [DOI] [PubMed] [Google Scholar]
- 20. Cannon NA, Meyer J, Iyengar P et al. Neutrophil‐lymphocyte and platelet‐lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early‐stage non‐small‐cell lung cancer. J Thorac Oncol 2015; 10: 280–5. [DOI] [PubMed] [Google Scholar]
- 21. Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil‐to‐lymphocyte ratio in non‐small cell lung cancer: A meta‐analysis. Sci Rep 2015; 5: 12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet 2001; 357: 539–45. [DOI] [PubMed] [Google Scholar]
- 23. Ni XJ, Zhang XL, Ou‐Yang QW et al. An elevated peripheral blood lymphocyte‐to‐monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS ONE 2014; 9: e111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang L, Conejo‐Garcia JR, Katsaros D et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–13. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Huang SH, Li H et al. Preoperative lymphocyte count is a favorable prognostic factor of disease‐free survival in non‐small‐cell lung cancer. Med Oncol 2013; 30: Article: 352. [DOI] [PubMed] [Google Scholar]
- 26. Cox G, Walker RA, Andi A, Steward WP, O'Byrne KJ. Prognostic significance of platelet and microvessel counts in operable non‐small cell lung cancer. Lung Cancer 2000; 29: 169–77. [DOI] [PubMed] [Google Scholar]
- 27. Qiang G, Liang C, Xiao F et al. Prognostic significance of platelet‐to‐lymphocyte ratio in non‐small‐cell lung cancer: A meta‐analysis. Onco Targets Ther 2016; 9: 869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet 1998; 352: 1775–7. [DOI] [PubMed] [Google Scholar]
- 29. O'Byrne KJ, Koukourakis MI, Giatromanolaki A et al. Vascular endothelial growth factor, platelet‐derived endothelial cell growth factor and angiogenesis in non‐small‐cell lung cancer. Br J Cancer 2000; 82: 1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non‐small cell lung cancer: A systematic review and meta‐analysis. Sci Rep 2016; 6: 22618. [DOI] [PMC free article] [PubMed] [Google Scholar]


