Abstract
Phosphatidylinositol‐3,5‐bisphosphate (PI(3,5)P2) is a low‐abundance signaling lipid associated with endo‐lysosomal and vacuolar membranes in eukaryotic cells. Recent studies on Arabidopsis indicated a critical role of PI(3,5)P2 in vacuolar acidification and morphology during ABA‐induced stomatal closure, but the molecular targets in plant cells remained unknown. By using patch‐clamp recordings on Arabidopsis vacuoles, we show here that PI(3,5)P2 does not affect the activity of vacuolar H+‐pyrophosphatase or vacuolar H+‐ATPase. Instead, PI(3,5)P2 at low nanomolar concentrations inhibited an inwardly rectifying conductance, which appeared upon vacuolar acidification elicited by prolonged H+ pumping activity. We provide evidence that this novel conductance is mediated by chloride channel a (CLC‐a), a member of the anion/H+ exchanger family formerly implicated in stomatal movements in Arabidopsis. H+‐dependent currents were absent in clc‐a knock‐out vacuoles, and canonical CLC‐a‐dependent nitrate/H+ antiport was inhibited by low concentrations of PI(3,5)P2. Finally, using the pH indicator probe BCECF, we show that CLC‐a inhibition contributes to vacuolar acidification. These data provide a mechanistic explanation for the essential role of PI(3,5)P2 and advance our knowledge about the regulation of vacuolar ion transport.
Keywords: patch‐clamp, phosphoinositide, proton antiport, vacuolar acidification
Subject Categories: Membrane & Intracellular Transport, Plant Biology, Signal Transduction
Introduction
Phosphoinositides have multiple functions in eukaryotic cells including cellular signaling, membrane trafficking, cytoskeletal organization and the regulation of membrane proteins 1, 2, 3. They are derivatives of the phospholipid phosphatidylinositol, which is dynamically phosphorylated at defined positions of the inositol head group by organelle‐specific lipid kinases, which are counteracted by specific lipid phosphatases, creating distinct, spatially restricted phosphoinositide pools at different intracellular membranes 4. PI(3,5)P2 appears to perform similar functions in eukaryotic cells 5, 6, among which the fragmentation and acidification of the vacuolar compartment. PI(3,5)P2 deficiency causes enlarged vacuoles in yeast 7 and dilated endolysosomes in animal cells 8, and seems to affect luminal acidification in these organelles 7, 9. The levels of endogenous PI(3,5)P2 in plant cells are considered to be extremely low (< 0.1% of total phosphoinositides), close to the detection limit of analytical techniques 10. Its synthesis is stimulated in yeast and plant cells exposed to hyperosmotic stress 11, 12, 13, possibly as part of a cellular mechanism to counteract cytosolic water deficit by reducing the vacuolar volume. In Arabidopsis, a group of phosphoinositide phosphatases, designated suppressor of actin (SAC), has been identified at the vacuolar membrane, and consistent with a role in vacuolar morphology in other eukaryotes, enlarged vacuoles were observed in overexpressing plants, whereas many small vacuoles in multiple sac gene knock‐out mutants 10. Conditional knock‐down of two PI3P 5‐kinase genes, FAB1A and FAB1B, caused impaired vacuolar acidification of central vacuoles in Arabidopsis root epidermal cells 14. During rapid stomatal closure, guard cells go through massive morphological changes during which the large central vacuole converts to many vesicle‐like and tubular structures 15, 16. Interestingly, this so‐called convolution is accompanied by the acidification of the vacuolar lumen 17. Interference with PI(3,5)P2 production suppressed both acidification and convolution, ultimately leading to impaired ABA‐induced stomatal closure 17. The authors hypothesized that PI(3,5)P2 may stimulate H+‐pumping activity, supported by their own finding that PI(3,5)P2 bound to purified vacuolar H+‐pyrophosphatase and by more recent results showing that PI(3,5)P2 levels regulate the stability of yeast V‐ATPase 18. Here, we tested this hypothesis directly using the patch‐clamp technique on isolated Arabidopsis vacuoles. Our results suggest that PI(3,5)P2 may exert its positive effect on vacuolar acidification indirectly by inhibiting major anion/H+ antiport activity rather than by direct stimulation of H+ pump activity residing at the tonoplast.
Results and Discussion
The phosphoinositide PI(3,5)P2 does not affect vacuolar H+ pump activity
Vacuolar H+‐ATPase in isolated Arabidopsis vacuoles (ecotype Columbia‐0) was activated by application of 5 mM adenosine triphosphate (ATP) (Fig 1A), and vacuolar H+‐pyrophosphatase by application of 100 μM inorganic pyrophosphate (PPi) to the external solution bathing the cytosolic side of isolated vacuoles (Fig 1C). In both cases, substrate application elicited small outward currents, consistent with H+ pumping activity into the vacuolar lumen 19. In order to evaluate the effect of PI(3,5)P2, outward currents were recorded twice in the same experiment, first in control solution, then again in the presence of 400 nM PI(3,5)P2 (in the water‐soluble diC8 form) in the bath solution. Current amplitudes recorded in the presence of the phosphoinositide were almost identical to control values (Fig 1B and D) indicating that PI(3,5)P2 does not have any effect—neither inhibitory nor stimulatory—on the activity of the two major vacuolar H+ pumps.
Figure 1. PI(3,5)P2 does not affect the activity of major vacuolar H+ pumps.
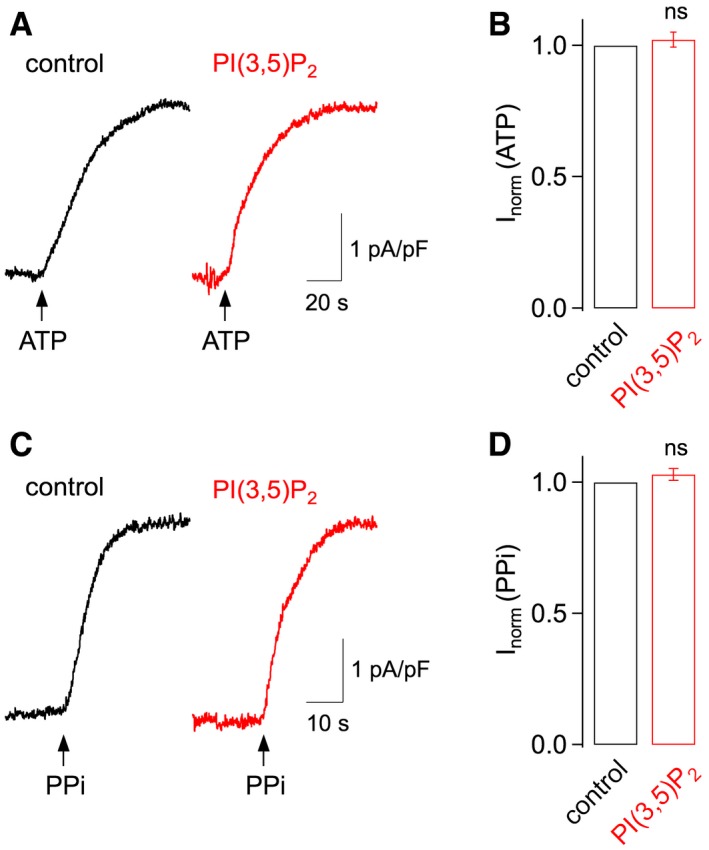
- H+ pump activity of vacuolar H+‐ATPase, elicited by application of 5 mM ATP (arrow) in control conditions (black trace) and in the presence of PI(3,5)P2 (400 nM) in gluconate‐based bath solution (red trace). Whole‐vacuolar membrane currents were recorded at a holding potential of 0 mV and normalized to the vacuolar membrane capacitance.
- ATP‐evoked membrane currents in the presence of PI(3,5)P2 (400 nM) were normalized to the amplitude recorded in control conditions (mean ± SEM of n = 5 vacuoles; Student's paired t‐test P = 0.88). ns, not significant.
- H+ pump activity of vacuolar H+‐pyrophosphatase, elicited by application of 100 μM PPi (arrow), otherwise as in (A).
- PPi‐evoked membrane currents in the presence of PI(3,5)P2 (400 nM) were normalized to the amplitude recorded in control conditions (mean ± SEM of n = 5 vacuoles; Student's paired t‐test P = 0.19).
Luminal acidification induces novel vacuolar inward currents
PPi application caused a uniform shift of the current–voltage relationships towards positive current values (compare the black to the grey trace in Fig 2A), suggesting that the activation of the vacuolar H+‐PPase does not present significant voltage dependence in the range between −80 to +80 mV. Furthermore, under these conditions, with gluconate (100 mM) as the major anion in the bath solution, currents were quasi‐stationary in the continuous presence of PPi (Fig 2A, right panel). When, instead of gluconate, equimolar chloride was present in the bath solution, prolonged PPi application lead to the appearance of a novel inward current at negative membrane potentials (compare the blue to the black trace in Fig 2B), which reached a steady‐state 2–3 min after PPi‐evoked outward currents had reached their peak (Fig 2B, right panel). A similar inward current activation was observed during prolonged ATP application (Fig 2C), indicating that trans‐tonoplast H+‐transport was the key step necessary for current activation. Theoretical considerations provided support to the view that typical vacuolar H+ pumping activities, when active over a time period of several minutes, can indeed drive a sufficiently high accumulation of luminal H+ to overcome the buffering capacity of 20 mM HEPES used in our experimental conditions. The novel current was named Ivac (current evoked by vacuolar acidification) and defined as the difference between the current level after prolonged PPi exposure and the positive peak current following PPi application at −80 mV (Fig 2B, right panel). In support of the prediction that higher H+ transport activity would lead to stronger Ivac activation, we found a good correlation between the H+‐PPase/H+‐ATPase current density and the Ivac density in individual vacuoles (Fig 2D).
Figure 2. Luminal acidification elicits novel vacuolar chloride currents.

- Current–voltage relationships (left panel) and time course of current amplitudes at 0 mV and −80 mV (right panel) in control conditions (gluconate‐based bath solution) and in response to exposure to 100 μM PPi (applied at t = 0). Current traces correspond to the indicated time points: grey control, black maximal PPi‐evoked current at 0 mV, blue steady‐state after continued PPi exposure. Whole‐vacuolar membrane currents in Col‐0 vacuoles were evoked by a 200‐ms voltage ramp from −80 to +80 mV and normalized to the vacuolar membrane capacitance. Pipette solution adjusted to pH 7.2.
- As in (A), but in chloride‐based bath solution. I (PPi) indicates the difference between black and grey data points at 0 mV. Ivac indicates the difference between blue and black data points at −80 mV.
- As in (B), but exposure to 5 mM ATP instead of PPi.
- Relationship between Ivac and I (PPi/ATP) amplitudes, as indicated in (B), for 17 individual vacuoles exposed to PPi and three vacuoles exposed to ATP. The continuous line represents the linear fit of PPi data points.
- Current recordings from a vacuole successively exposed to chloride‐based bath solution (upper traces) and to bath solution containing 100 μM PPi (lower traces). Pipette solution adjusted to pH 7.2. Currents were elicited in response to 1‐s voltage steps ranging from +60 to −100 mV in 20‐mV decrements. The zero current level is indicated by dashed lines.
- Current recordings from a vacuole successively exposed to chloride‐based (upper traces) and to gluconate‐based bath solution (lower traces), pipette solution adjusted to pH 5.5. Voltage protocol as in (E). The zero current level is indicated by dashed lines.
- Representative vacuolar membrane currents recorded at four different vacuolar pH values, obtained by subtracting currents recorded in gluconate‐based bath solution from those recorded in chloride‐based bath solution (see traces in (F)). Scale bars as in (F).
- Average current–voltage relationships of difference currents (see traces in (G)). Mean ± SEM of n = 5 vacuoles for pHvac 7.2, n = 5 for pHvac 6.5, n = 9 for pHvac 5.5 and n = 6 for pHvac 4.5.
Current traces recorded in steady‐state conditions in response to a 1‐s step protocol (Fig 2E) show the appearance of Ivac in the presence of 100 μM PPi, with slowly inactivating kinetics at negative membrane potentials as a distinguishing feature. Similar slowly inactivating current kinetics were detected, when currents were recorded at acidic vacuolar pH in the presence of high cytosolic Cl− (Fig 2F, upper traces). In order to isolate Ivac at different vacuolar pH values, we recorded whole‐vacuolar membrane currents in chloride‐based bath solution and subtracted the respective background currents recorded in gluconate‐based bath solution (see example traces at pHvac 5.5 in Fig 2F). Inward current densities were low at pHvac 7.2, but increased strongly at acidic pH values, with an apparent optimum at pHvac 5.5 (Fig 2G and H). In summary, we have identified a novel inward current in Arabidopsis mesophyll vacuoles, which depends both on cytosolic chloride and on acidic conditions on the luminal side of the tonoplast.
Ivac is highly sensitive to PI(3,5)P2
In contrast to vacuolar H+ pumps, Ivac elicited by prolonged PPi application was highly sensitive to PI(3,5)P2 (Fig 3A). Current amplitudes recorded at pHvac 5.5 were reduced in the presence of 10 nM PI(3,5)P2 and completely abolished by 300 nM PI(3,5)P2 applied to chloride‐based bath solution (Fig 3B and C). Current inhibition was fully reversible upon wash‐out (Fig 3B and C). Dose–response analyses revealed that half‐inhibition of Ivac at −80 mV occurred at 11.7 nM (Fig 3D). For comparison, reported values for the apparent affinity of lysosomal cation channels for PI(3,5)P2 were 48 nM for mouse TRPML1 20 and 390 nM for Drosophila TRPML1 21, 390 nM/160 nM for human TPC2 22, 23 and ~100 nM/15 nM for human TPC1 24, 25. It is important to emphasize that saturating PI(3,5)P2 concentrations left the vacuolar membrane with very small background currents, indicating that PI(3,5)P2‐sensitive currents strongly dominate the vacuolar conductance under these experimental conditions.
Figure 3. Inhibition of Ivac by nanomolar PI(3,5)P2 concentrations.
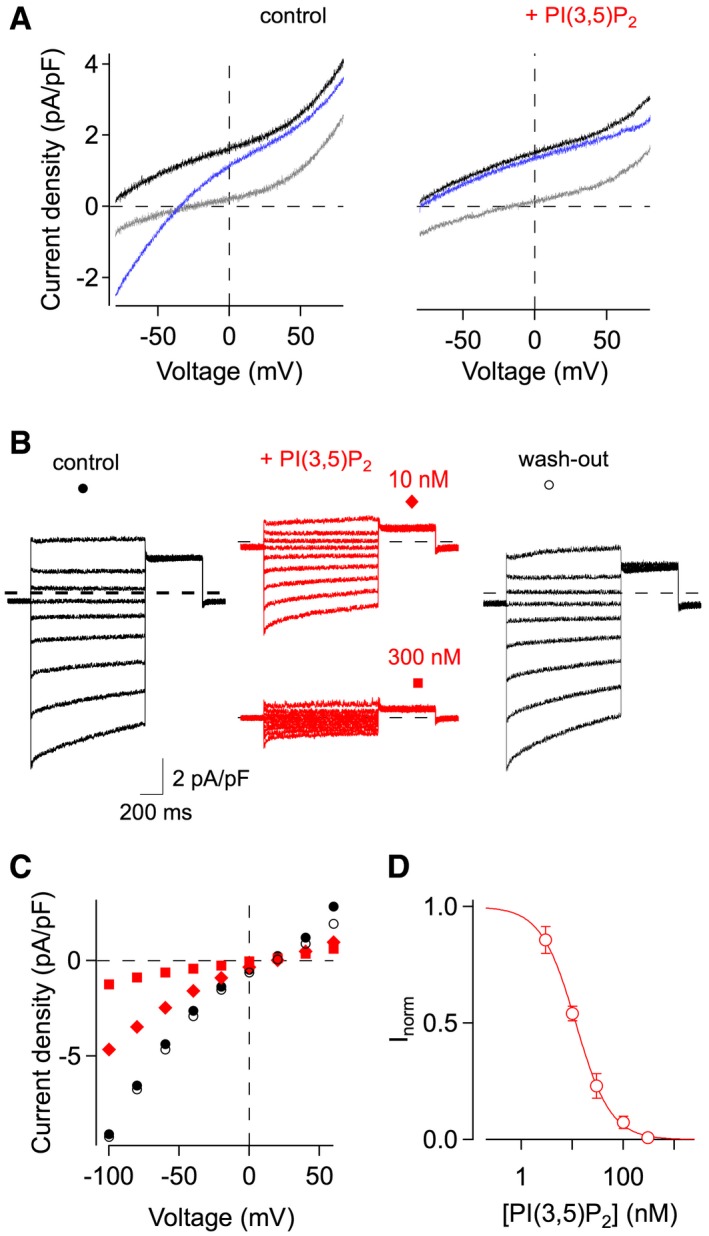
- Current–voltage relationships in response to 100 μM PPi exposure, in chloride‐based control solution (left panel), and successively in the presence of 400 nM PI(3,5)P2 (right panel). Current traces correspond to the following time points (see Fig 2): grey before PPi application, black maximal PPi‐evoked current at 0 mV, blue steady‐state after continued PPi exposure. Whole‐vacuolar membrane currents in Col‐0 vacuoles were evoked by a 200‐ms voltage ramp from −80 to +80 mV and normalized to the vacuolar membrane capacitance. Pipette solution adjusted to pH 7.2.
- Current recordings from a vacuole successively exposed to chloride‐based bath solution (black traces; left: control; right: wash‐out) and to bath solution containing PI(3,5)P2 (red traces), at concentrations of 10 and 300 nM, as indicated. Pipette solution adjusted to pH 5.5. Currents were elicited in response to 1‐s voltage steps ranging from +60 to −100 mV in 20‐mV decrements. The zero current level is indicated by dashed lines.
- Instantaneous I‐V curves of the current recordings shown in (B).
- Dose–response analysis of Ivac inhibition. Normalized residual currents (Inorm), determined at −80 mV, were plotted against the applied PI(3,5)P2 concentration. Data points represent mean ± SEM of n = 5–8 vacuoles. Data fitting with the Hill equation (continuous line) yielded a half‐inhibition concentration of 11.7 nM and a Hill coefficient of 1.3.
In order to determine the phosphoinositide specificity of current inhibition, we successively applied four structurally related variants at a concentration of 200 nM. Compared to PI(3,5)P2, PI(4,5)P2 produced 66 ± 6% inhibition of Ivac recorded at ‐80 mV, PI(3,4,5)P3 produced 33 ± 5% inhibition, and PI(3,4)P2 was the least effective with only 7 ± 2% inhibition (Fig EV1), suggesting that the number and position of the phosphate groups on the inositide backbone are important for high‐affinity inhibition of Ivac.
Figure EV1. Phosphoinositide specificity of Ivac inhibition.

- Current recordings from a vacuole successively exposed to chloride‐based bath solution (control; black traces) and to bath solution containing PI(3,4)P2 (grey traces), PI(3,4,5)P3 (green traces), PI(4,5)P2 (blue traces) and PI(3,5)P2 (red traces), each at a concentration of 200 nM. Pipette solution adjusted to pH 5.5. Currents were elicited by 1‐s voltage steps ranging from +60 to −100 mV in 20‐mV decrements. The zero current level is indicated by dashed lines.
- Average current inhibition (determined at −80 mV), normalized to the current inhibition by PI(3,5)P2, mean ± SEM of n = 4 vacuoles. Statistical significance versus PI(3,5)P2 (Tukey's test after ANOVA for repeated measurements): ***P = 2.4e−4 for PI(4,5)P2, ***P = 2.2e−7 for PI(3,4,5)P3, ***P = 5.7e−9 for PI(3,4)P2.
AtCLC‐a is the mediator of Ivac and the target of PI(3,5)P2
The exclusive activation of inward currents in the presence of cytosolic chloride and acidic conditions on the luminal side of the vacuolar membrane is compatible with an antiport mechanism coupling the vacuole‐directed movement of cytosolic chloride to the cytosol‐directed movement of luminal protons. The only known class of proteins operating with an anion/H+ antiport mechanism in plant vacuoles is constituted by the chloride channel (CLC) family 26. Its most prominent member in Arabidopsis thaliana is AtCLC‐a, which has been described as an anion/H+ exchanger in mesophyll vacuoles mediating the accumulation of nitrate 27. A further family member, AtCLC‐g, is expressed in mesophyll cells 28, but currently no ion transport activity is reported. Strikingly, in vacuoles isolated from Arabidopsis clc‐a knock‐out plants, prolonged PPi application did not lead to the appearance of Ivac (Fig 4A and B), while Ivac amplitudes in vacuoles derived from the respective wild‐type plants of the Wassilewskija (Ws) ecotype were comparable to those recorded in Col‐0 vacuoles (Fig 4B). Likewise, current recordings in steady‐state conditions in response to voltage steps revealed that clc‐a vacuoles in control solution had lower background currents than Ws vacuoles, even at positive membrane potentials, and in the presence of 100 μM PPi, they completely lacked the characteristic slowly inactivating currents at negative membrane potentials (Fig 4C and D). Difference currents obtained from the subtraction of background currents in gluconate‐based bath solution were virtually absent in clc‐a vacuoles at pHvac 7.2, and hardly responded to acidic luminal conditions at pHvac 5.5 (Fig 4E), whereas Ws amplitudes were highly similar to the values of Col‐0 vacuoles. Finally, reversal potential measurements for Ivac in Col‐0 vacuoles (after background current subtraction in the presence of saturating [PI(3,5)P2]) resulted in a mean value of +29.3 ± 5.9 mV (n = 8), which is in full agreement with the theoretical value (Vtheo = +33 mV) for a anion/H+ coupling ratio of 2:1 previously determined for nitrate/H+ antiport 27. Taken together, these data strongly indicate that CLC‐a is responsible for Ivac in Arabidopsis mesophyll vacuoles and imply that CLC‐a transport activity is highly sensitive to the presence of PI(3,5)P2. Therefore, we expected that PI(3,5)P2 would equally inhibit CLC‐a's canonical nitrate/H+ antiport activity 27. Under conditions of high luminal nitrate and low cytosolic chloride, Col‐0 mesophyll vacuoles showed the typical time‐dependent outward currents at positive membrane potentials (Fig EV2A, left traces). When vacuoles were exposed to the same PI(3,5)P2 concentrations as in Fig 3B, time‐dependent current amplitudes were gradually reduced (Fig EV2A), and fully recovered after wash‐out. Similarly to the inhibition of Ivac, dose–response analyses revealed a half‐inhibition concentration of PI(3,5)P2 in the low nanomolar range (8.5 nM at +63 mV; Fig EV2B). Inhibition at saturating PI(3,5)P2 concentrations, however, was incomplete, leaving about 14% of the time‐dependent currents. Taken together, these data firmly establish the CLC‐a protein (i) as the mediator of a novel anion current dominating the vacuolar conductance under conditions of symmetrical 100 mM KCl; and (ii) as the first transport protein identified as a PI(3,5)P2 target in the vacuolar membrane.
Figure 4. Ivac is mediated by the anion/H+ exchanger CLC‐a.
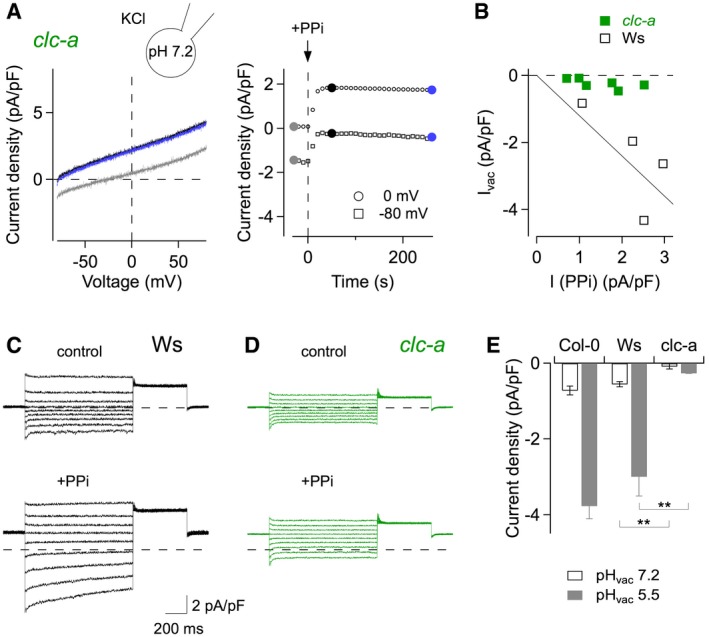
-
ACurrent–voltage relationships (left panel) and time course of current amplitudes at 0 mV and −80 mV (right panel) recorded from a clc‐a knock‐out vacuole, in control conditions (chloride‐based bath solution) and in response to exposure to 100 μM PPi (applied at t = 0). Current traces correspond to the indicated time points: grey control, black maximal PPi‐evoked current at 0 mV, blue steady‐state after continued PPi exposure. Whole‐vacuolar membrane currents were evoked by a 200‐ms voltage ramp from −80 to +80 mV and normalized to the vacuolar membrane capacitance. Pipette solution adjusted to pH 7.2.
- B
-
C, DCurrent recordings from a Ws wild‐type vacuole (C) and a clc‐a knock‐out vacuole (D) successively exposed to chloride‐based bath solution (upper traces) and to bath solution containing 100 μM PPi (lower traces). Currents were elicited by 1‐s voltage steps ranging from +60 to −100 mV in 20‐mV decrements. The zero current level is indicated by dashed lines.
-
ESummary plot showing the pHvac dependence of average difference currents recorded at ‐80 mV, obtained by subtracting currents recorded in gluconate‐based bath solution from those recorded in chloride‐based bath solution, for Col‐0 vacuoles (data taken from Fig 2H), Ws vacuoles (mean ± SEM; n = 4 for each pHvac) and clc‐a vacuoles (n = 4 for pHvac 7.2; n = 3 for pHvac 5.5). Student's unpaired t‐test between Ws and clc‐a: **P = 0.0034 for pHvac 7.2, **P = 0.0064 for pHvac 5.5.
Figure EV2. Inhibition of CLC‐a‐mediated nitrate/H+ antiport activity by PI(3,5)P2 .
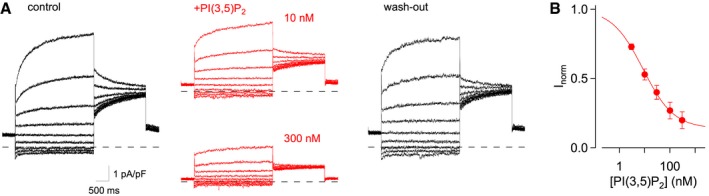
- Current recordings from a vacuole successively exposed to low‐chloride bath solution (black traces; left: control; right: wash‐out) and to bath solution containing PI(3,5)P2 (red traces), at concentrations of 10 and 300 nM, as indicated. Pipette solution contained 200 mM nitrate. Currents were elicited by 3‐s voltage steps ranging from −97 to +63 mV in 20‐mV increments. The zero current level is indicated by dashed lines.
- Dose–response analysis of nitrate/H+ current inhibition. Normalized residual time‐dependent currents (Inorm), determined at +63 mV, were plotted against the applied PI(3,5)P2 concentration. Data points represent mean ± SEM of n = 5 vacuoles. Data fitting with the Hill equation (continuous line) yielded a half‐inhibition concentration of 8.5 nM, a Hill coefficient of 0.71 and a residual current at saturating [PI(3,5)P2] of 0.14.
PI(3,5)P2‐mediated inhibition of CLC‐a boosts vacuolar acidification
Considering that PI(3,5)P2 does not directly stimulate vacuolar H+ pumping activity (Fig 1), it likely exerts its positive effect on vacuolar acidification indirectly, by inhibiting secondary active transport processes consuming the H+ gradient established by H+ pumps during their activity. CLC‐a activity is of this type, coupling the vacuolar accumulation of nitrate to the release of vacuolar H+ down their electrochemical gradient 27. Consistently, antiport activity of the yeast CLC homolog Gef1 has been shown to counteract vesicular acidification 29. We performed BCECF fluorescence experiments to directly monitor the effect of PI(3,5)P2 on luminal acidification in isolated vacuoles. Bath application of 100 μM PPi reliably elicited a strong decrease of the BCECF emission ratio, indicative of an increase of the vacuolar H+ concentration (Fig 5A). Under these conditions, additional application of 200 nM PI(3,5)P2 caused a significant further decrease of the emission ratio in Ws vacuoles (Fig 5A, black trace), but not in clc‐a vacuoles (Fig 5A, green trace). While in both genotypes a comparable minimum pH value was reached (Fig 5B), PI(3,5)P2 decreased the vacuolar pH, on average, by 0.33 units in Ws vacuoles, but only by 0.01 units in clc‐a vacuoles (Fig 5C), demonstrating that PI(3,5)P2‐dependent inhibition of CLC‐a can indeed significantly contribute to vacuolar acidification. In addition to the results from reversal potential measurements, these data provide further demonstration that CLC‐a mediates Cl−‐dependent Ivac by an H+ antiport mechanism.
Figure 5. Inhibition of CLC‐a by PI(3,5)P2 contributes to vacuolar acidification.

- BCECF fluorescence ratio recordings performed on a Ws wild‐type vacuole (black trace) and a clc‐a vacuole (green trace), in response to the bath application of 100 μM PPi (white bar) and 200 nM PI(3,5)P2 (red bar). Fluorescence ratio changes were converted into pH units.
- Summary plot showing the minimum pH value (pHmin) reached during the combined application of PPi and PI(3,5)P2, mean ± SEM of n = 5 vacuoles for both Ws and clc‐a. Student's unpaired t‐test P = 0.44. ns, not significant.
- Summary plot showing the pH change (ΔpH) evoked by PI(3,5)P2 application, number of vacuoles as in (B). Mann–Whitney U‐test **P = 0.0023.
Apart from its essential role for nitrate accumulation in mesophyll vacuoles 27, 30, CLC‐a expression in Arabidopsis guard cells contributes to stomatal opening and closing 31. CLC‐a function during ABA‐evoked stomatal closure is closely related to its phosphorylation by protein kinase OST1, a central player in ABA signal transduction in guard cells 31, 32. Here, we add new pieces to the picture by showing that CLC‐a activity is a target of the phosphoinositide PI(3,5)P2, an essential factor for vacuolar acidification and convolution during ABA‐induced stomatal closure 17. Our data provide a mechanistic explanation for the positive effect of PI(3,5)P2 on acidification: rather than by direct stimulation of H+ pump activity residing at the tonoplast, PI(3,5)P2 may act indirectly by inhibiting a major secondary active transport process consuming the trans‐tonoplast H+ gradient. This hypothesis is strongly supported by our results showing that CLC‐a inhibition can indeed contribute to vacuolar acidification. Future studies will show if PI(3,5)P2 inhibition of H+‐coupled transport processes is a common theme used by guard cells and other cell types for the accomplishment of vacuolar acidification and morphology changes. Finally, it will also be intriguing to see whether PI(3,5)P2 similarly targets CLC‐type anion exchangers operating in the endolysosomal compartment of animal cells.
Materials and Methods
Plant growth conditions and protoplast isolation
Plants of Arabidopsis thaliana Columbia‐0 (Col‐0), Wassilewskija (Ws) and clc‐a 27 were grown on soil in a growth chamber at 22°C and 8‐h light/16‐h dark regime. Mesophyll tissue of Arabidopsis plants was enzymatically digested for 45 min at 23°C. The enzyme solution contained 0.5% (w/v) cellulase R‐10, 0.05% (w/v) pectolyase Y‐23, 1 mM CaCl2, 500 mM sorbitol, 10 mM 2‐(N‐morpholino)ethanesulfonic acid (MES), pH 5.3. Protoplasts were washed twice and maintained in a solution containing 125 mM CaCl2, 154 mM NaCl, 5 mM KCl, 2 mM MES, pH 5.6 (KOH). Vacuoles were freshly released from mesophyll protoplasts by bath perfusion of VR solution 33 containing 100 mM malic acid, 155 mM 1,3‐bis(tris(hydroxymethyl)methylamino)propane (BTP), 5 mM ethylene glycol‐bis(2‐aminoethylether)‐N,N,N′,N′‐tetraacetic acid (EGTA), 3 mM MgCl2, pH 7.5, adjusted to 450 mOsm with D‐sorbitol.
Patch‐clamp recordings and data analysis
Patch‐clamp experiments were performed as described elsewhere 23, 33. Recordings were performed in the whole‐vacuole configuration using EPC‐7 (HEKA Elektronik, Lambrecht, Germany) or Axopatch 200A (Molecular Devices, Sunnyvale, USA) patch‐clamp amplifiers. Patch pipettes were pulled from thin‐walled borosilicate glass (Clark Electrochemical Instruments, Pangbourne, Reading, UK). The standard pipette solution contained (in mM): 100 KCl, 3 MgCl2, 20 HEPES, adjusted to pH 7.2 (with KOH) and 520 mOsm (with D‐sorbitol). In experiments performed at acidic vacuolar pH values, HEPES was replaced by MES (20 mM) in pipette solutions adjusted to pH 6.5 and 5.5, and by citrate (5 mM) in the pipette solution adjusted to pH 4.5. Gluconate‐based bath solution contained (in mM): 100 K‐gluconate, 2 EGTA, 3 MgCl2, 20 HEPES, adjusted to pH 7.2 (with KOH) and 540 mOsm (with D‐sorbitol). In chloride‐based bath solution, K‐gluconate was substituted with equimolar KCl. For recordings of vacuolar nitrate/H+ antiport activity 27, the pipette solution contained (in mM) 200 HNO3, 100 BTP, 1 CaCl2, 5 MgCl2, 5 MES, adjusted to pH 5.5 (with BTP) and 480 mOsm (with D‐sorbitol), and the bath solution contained 15 HCl, 18 BTP, 0.1 CaCl2, 2 MgCl2, 15 MES, adjusted to pH 7.0 (with BTP) and 500 mOsm (with D‐sorbitol).
Inorganic pyrophosphate (PPi, K+ salt) was prepared weekly as a 100‐mM aqueous stock solution. Adenosine triphosphate (ATP, Mg2+ salt) was dissolved freshly at a concentration of 5 mM in the respective bath solution, and the pH value was re‐adjusted by the addition of KOH. PI(3,5)P2, PI(4,5)P2, PI(3,4)P2 and PI(3,4,5)P3 were purchased as dioctanoyl esters (diC8) from Echelon Biosciences Inc (USA), prepared as 1‐mM aqueous stock solutions and stored at −20°C. Unless otherwise indicated, chemicals were purchased from Sigma‐Aldrich (Milan, Italy).
High‐resistance membrane seals were generally formed in VR solution. After the establishment of the whole‐vacuole configuration, the bath solution was exchanged and membrane currents were allowed to stabilize for at least 15 min, before current recordings were considered for data analysis. Bath solutions were exchanged by means of a gravity‐driven perfusion system coupled to a peristaltic pump.
During prolonged H+ pump activation, membrane currents were elicited by 200‐ms voltage ramps ranging from −80 to +80 mV, preceded by a 50‐ms voltage step to −80 mV. In steady‐state conditions, currents were elicited by 1‐s voltage steps ranging from +60 to −100 mV in 20‐mV decrements, followed by a 500‐ms voltage step to +50 mV. The holding potential was set to 0 mV in these protocols. Nitrate/H+ antiport activity was recorded in response to 3‐s voltage steps ranging from −97 to +63 mV in 20‐mV increments, followed by a 2‐s voltage step to +33 mV, from a holding potential of −17 mV.
H+ pump activity was quantified using the mean current amplitude determined within a 50‐ms interval at 0 mV. Ivac amplitude was quantified using the mean current amplitude determined within a 30‐ms interval at −80 mV, immediately before application of the voltage ramp. Current amplitudes of Cl−/H+ antiport were determined at the beginning of the voltage pulse. Time‐dependent activation of nitrate/H+ antiport was evaluated by subtracting the mean current at the beginning of the voltage pulse from the steady‐state current. Current amplitudes were normalized to the vacuolar membrane capacitance. The liquid junction potential between the pipette solution and VR bath solution was measured (+1.7 mV) and used to correct the reversal potential of PI(3,5)P2‐sensitive Ivac. Theoretical reversal potentials for chloride/H+ antiport (Etheo) were calculated using the antiporter equation proposed by Accardi and Miller 34. For recordings of vacuolar nitrate/H+ antiport activity, applied membrane potentials were corrected offline for the respective liquid junction potential (−17 mV) 27.
BCECF fluorescence recordings
The pH indicator dye BCECF was loaded into the vacuolar lumen in the whole‐vacuole patch configuration. Bath and pipette solutions contained (in mM): 100 KCl, 3 MgCl2, 0.1 HEPES, adjusted to pH 7.0 (with KOH) and 540 mOsm (with sorbitol). The pipette solution contained additionally 50 μM BCECF. The pH value of the solutions was checked daily and re‐adjusted if necessary.
Fluorescence signals were evoked using a fast CAIRN monochromator (Cairn Research, UK) and detected by a cooled CCD camera (Roper Scientific, Germany) mounted on an Axiovert 135 microscope (Zeiss, Germany). The system was controled by MetaFluor software (Molecular Devices, USA). The vacuole was viewed through a PlanApo 40× Zeiss silica objective (NA 1.4) and illuminated by a 75‐Watt Xenon short arc lamp (USHIO, Japan). Fluorescence was excited alternately at 490 and 440 nm in 100‐ms excitation cycles, detected using a 515‐nm bandpass emission filter and recorded every 300 ms. A region of interest (ROI) was placed inside the vacuole. The fluorescence ratio (490/440) was calculated online (background fluorescence in our experimental conditions was negligible). After reaching the whole‐vacuole configuration, we followed BCECF loading into the vacuole and stabilization of the fluorescence ratio, which took about 10–15 min (depending on the vacuole size). Baseline pH values recorded in individual vacuoles were subject to some variability, likely due to the low buffering capacity of HEPES at 0.1 mM. The vacuole was successively perfused with bath solution containing 100 μM PPi and bath solution containing 100 μM PPi plus 200 nM PI(3,5)P2. The vacuole was held at a membrane potential of −20 mV during the experiment.
For the calibration of fluorescence ratios, 50 μM BCECF was dissolved in pipette solutions, adjusted to pH 5.5, 6.5 (containing 20 mM MES) and 7.0 (containing 20 mM HEPES), respectively. Fluorescence ratios were determined in two different conditions: i) for the free BCECF solution, after placing a large droplet (500 μl) onto the bottom of the recording chamber, creating a flat surface (in order to avoid undesired light scattering and reflection); and ii) in the whole‐vacuole configuration, after loading the BCECF solution into the vacuolar lumen through a patch pipette. Values were almost identical for the two conditions (Fig EV3). Data were fitted with the polynomial function f(x) = k0 + k1 x + k2 x2, where x indicates the fluorescence ratio. The fit derived from whole‐vacuole calibration was used to convert experimentally determined fluorescence ratios into pH units.
Figure EV3. Calibration of BCECF fluorescence recordings.
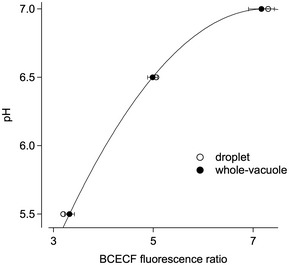
BCECF fluorescence ratios were determined in droplets (open circles) and in the whole‐vacuole configuration (closed circles), with BCECF solutions adjusted to pH 5.5, 6.5 and 7.0, respectively. Whole‐vacuole data points (mean ± SEM of n = 3 vacuoles for each pH) were fitted with the polynomial function described in Materials and Methods (continuous line; k0 = 1.91; k1 = 1.40; k2 = −9.66 10−2), and the data fit was used to convert experimentally determined fluorescence ratios into pH units.
Statistical analyses
All data are reported as mean values ± standard error of the mean (for n vacuoles). Statistical significance was determined using paired or unpaired Student's t‐tests, Mann–Whitney U‐test or ANOVA, as appropriate. When a statistically significant difference was determined with ANOVA, a post hoc Tukey's test was used to evaluate which data groups showed significant differences. P‐values < 0.05 were considered significant. Data analysis was done with IgorPro software (Wavemetrics, Lake Oswego, OR, USA).
Author contributions
JS‐S conceived and designed the experiments; JS‐S, AC, AB, LL and EDZ performed the experiments; JS‐S and AC analyzed the data; JS‐S wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
The technical assistance of Francesca Quartino is gratefully acknowledged. Research was supported by the Italian Ministry of Education, University and Research (grant 2015795S5W_003 to AC).
EMBO Reports (2017) 18: 1100–1107
References
- 1. Schink KO, Tan KW, Stenmark H (2016) Phosphoinositides in control of membrane dynamics. Annu Rev Cell Dev Biol 32: 143–171 [DOI] [PubMed] [Google Scholar]
- 2. Marat AL, Haucke V (2016) Phosphatidylinositol 3‐phosphates‐at the interface between cell signalling and membrane traffic. EMBO J 35: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heilmann I (2016) Plant phosphoinositide signaling – dynamics on demand. Biochim Biophys Acta 1861: 1345–1351 [DOI] [PubMed] [Google Scholar]
- 4. Balla T (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93: 1019–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ho CY, Alghamdi TA, Botelho RJ (2012) Phosphatidylinositol‐3,5‐bisphosphate: no longer the poor PIP2. Traffic 13: 1–8 [DOI] [PubMed] [Google Scholar]
- 6. Jin N, Lang MJ, Weisman LS (2016) Phosphatidylinositol 3,5‐bisphosphate: regulation of cellular events in space and time. Biochem Soc Trans 44: 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD (1998) Fab1p is essential for PtdIns(3)P 5‐kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143: 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikonomov OC, Sbrissa D, Shisheva A (2001) Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5‐kinase PIKfyve. J Biol Chem 276: 26141–26147 [DOI] [PubMed] [Google Scholar]
- 9. Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD et al (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9: 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novakova P, Hirsch S, Feraru E, Tejos R, van Wijk R, Viaene T, Heilmann M, Lerche J, De Rycke R, Feraru MI et al (2014) SAC phosphoinositide phosphatases at the tonoplast mediate vacuolar function in Arabidopsis . Proc Natl Acad Sci USA 111: 2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH (1997) Osmotic stress activates phosphatidylinositol‐3,5‐bisphosphate synthesis. Nature 390: 187–192 [DOI] [PubMed] [Google Scholar]
- 12. Meijer HJG, Divecha N, van den Ende H, Musgrave A, Munnik T (1999) Hyperosmotic stress induces rapid synthesis of phosphatidyl‐D‐inositol 3,5‐bisphosphate in plant cells. Planta 208: 294–298 [Google Scholar]
- 13. Zonia L, Munnik T (2004) Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol 134: 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirano T, Matsuzawa T, Takegawa K, Sato MH (2011) Loss‐of‐function and gain‐of‐function mutations in FAB1A/B impair endomembrane homeostasis, conferring pleiotropic developmental abnormalities in Arabidopsis . Plant Physiol 155: 797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC (2005) The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba . Plant Physiol 139: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka Y, Kutsuna N, Kanazawa Y, Kondo N, Hasezawa S, Sano T (2007) Intra‐vacuolar reserves of membranes during stomatal closure: the possible role of guard cell vacuoles estimated by 3‐D reconstruction. Plant Cell Physiol 48: 1159–1169 [DOI] [PubMed] [Google Scholar]
- 17. Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU (2013) Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5‐bisphosphate. Plant Cell 25: 2202–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li SC, Diakov TT, Xu T, Tarsio M, Zhu W, Couoh‐Cardel S, Weisman LS, Kane PM (2014) The signaling lipid PI(3,5)P(2) stabilizes V(1)‐V(o) sector interactions and activates the V‐ATPase. Mol Biol Cell 25: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedrich R, Kurkdjian A, Guern J, Flugge UI (1989) Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar‐lysosomal compartment. EMBO J 8: 2835–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M et al (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1: 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng X, Huang Y, Lu Y, Xiong J, Wong CO, Yang P, Xia J, Chen D, Du G, Venkatachalam K et al (2013) Drosophila TRPML forms PI(3,5)P2‐activated cation channels in both endolysosomes and plasma membrane. J Biol Chem 289: 4262–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J et al (2012) TPC proteins are phosphoinositide‐ activated sodium‐selective ion channels in endosomes and lysosomes. Cell 151: 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boccaccio A, Scholz‐Starke J, Hamamoto S, Larisch N, Festa M, Gutla PV, Costa A, Dietrich P, Uozumi N, Carpaneto A (2014) The phosphoinositide PI(3,5)P(2) mediates activation of mammalian but not plant TPC proteins: functional expression of endolysosomal channels in yeast and plant cells. Cell Mol Life Sci 71: 4275–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cang C, Bekele B, Ren D (2014) The voltage‐gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol 10: 463–469 [DOI] [PubMed] [Google Scholar]
- 25. Lagostena L, Festa M, Pusch M, Carpaneto A (2017) The human two‐pore channel 1 is modulated by cytosolic and luminal calcium. Sci Rep 7: 43900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinoia E, Meyer S, De Angeli A, Nagy R (2012) Vacuolar transporters in their physiological context. Annu Rev Plant Biol 63: 183–213 [DOI] [PubMed] [Google Scholar]
- 27. De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier‐Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442: 939–942 [DOI] [PubMed] [Google Scholar]
- 28. Nguyen CT, Agorio A, Jossier M, Depre S, Thomine S, Filleur S (2015) Characterization of the chloride channel‐like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana . Plant Cell Physiol 57: 764–775 [DOI] [PubMed] [Google Scholar]
- 29. Braun NA, Morgan B, Dick TP, Schwappach B (2010) The yeast CLC protein counteracts vesicular acidification during iron starvation. J Cell Sci 123: 2342–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Courtial B, Barbier‐Brygoo H, Maurel C (2000) Disruption of putative anion channel gene AtCLC‐a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J 21: 259–267 [DOI] [PubMed] [Google Scholar]
- 31. Wege S, De Angeli A, Droillard MJ, Kroniewicz L, Merlot S, Cornu D, Gambale F, Martinoia E, Barbier‐Brygoo H, Thomine S et al (2014) Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci Signal 7: ra65 [DOI] [PubMed] [Google Scholar]
- 32. Kollist H, Nuhkat M, Roelfsema MR (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- 33. Costa A, Gutla PV, Boccaccio A, Scholz‐Starke J, Festa M, Basso B, Zanardi I, Pusch M, Schiavo FL, Gambale F et al (2012) The Arabidopsis central vacuole as an expression system for intracellular transporters: functional characterization of the Cl−/H+ exchanger CLC‐7. J Physiol 590: 3421–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427: 803–807 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


