Abstract
Auxin acts synergistically with cytokinin to control the shoot stem‐cell niche, while both hormones act antagonistically to maintain the root meristem. In aluminum (Al) stress‐induced root growth inhibition, auxin plays an important role. However, the role of cytokinin in this process is not well understood. In this study, we show that cytokinin enhances root growth inhibition under stress by mediating Al‐induced auxin signaling. Al stress triggers a local cytokinin response in the root‐apex transition zone (TZ) that depends on IPTs, which encode adenosine phosphate isopentenyltransferases and regulate cytokinin biosynthesis. IPTs are up‐regulated specifically in the root‐apex TZ in response to Al stress and promote local cytokinin biosynthesis and inhibition of root growth. The process of root growth inhibition is also controlled by ethylene signaling which acts upstream of auxin. In summary, different from the situation in the root meristem, auxin acts with cytokinin in a synergistic way to mediate aluminum‐induced root growth inhibition in Arabidopsis.
Keywords: aluminum, auxin, cytokinin, root growth
Subject Categories: Plant Biology, Signal Transduction
Introduction
Plants rely on their roots to extract water and nutrients from the soil, making the plasticity of root growth an important adaptive trait 1, 2, 3, 4. Through changing root architecture, plants adapt to various environmental cues. Aluminum (Al) stress is one of the widespread constraints on crop production in acid soil. Its effect is readily observed from its inhibition of root growth 5, 6. The root tip, especially the distal part of the root‐apex transition zone (TZ), is the primary site of Al injury, which was shown by propidium iodide (PI)‐stained dead cells 7, 8, 9. Though Al is the most abundant metal in the Earth's crust, only when soil pH drops below 5.5, the released Al3+ in soil solution acts as a limiting factor for plant growth and crop production in most acid soils 10. Acid soils limit crop production about 30–40% of the world's arable land 11, and Al toxicity is a major constraint for crop production on 67% of the total acid soil area 12. Therefore, investigating the molecular mechanism of Al stress‐regulated root growth inhibition not only improves our understanding about root growth plasticity under various environmental cues, it also has the potential usage for crop design or breeding in acid soils.
Phytohormones are intimately involved in the regulation of root growth in response to a variable environment 7, 13, and their interaction is fundamental for the control of root development and growth 14, 15, 16, 17. One of these hormones is cytokinin (CK), which plays an important role in root development 18, 19, 20, 21. In addition to controlling plant growth and development 22, 23, CK is also involved in plant growth adaptation to various stresses 24. The reduced CK content both in A. thaliana ipt (IPT encodes adenosine phosphate isopentenyltransferase) mutants and in over‐expressors of CKX1 (encoding a CK oxidase/dehydrogenase) enhances tolerance to both salinity and drought stress 25, 26, 27, 28. Furthermore, the loss‐of‐function mutants of CK receptor hybrid histidine protein kinases AHK2, AHK3, and CRE1/AHK4 show a greater tolerance to low temperature, salinity, and drought stress than wild‐type (WT) plants 29, 30. Mutants involving the loss of function of certain type‐A ARABIDOPSIS RESPONSE REGULATORs (ARRs), such as arr5, arr6, and arr7 mutants, are more tolerant than WT to low‐temperature stress 29, while type‐B ARR mutants, such as the arr1 arr12 double mutant, are better able to withstand salinity stress 31. It seems that the repression of CK response, and thus CK signaling, is essential for plants to cope with drought stress, and three histidine phosphotransfer proteins (AHPs) such as AHP2, AHP3, and AHP5 control drought response in both abscisic acid (ABA)‐dependent and ABA‐independent manners 32, 33.
The cross talk between CK and auxin regulating root development has been widely studied over the years 34. Auxin acts antagonistically to CK to control root meristem size 19. The CK‐responsive transcription factors ARR1 and ARR12 act to activate SHY2 in the root‐apex transition zone (TZ), which then negatively regulates PIN genes encoding auxin transport facilitators, thus cross‐linking with auxin signaling to regulate root growth 20, 35. In the root, the CK‐responsive transcription factor ARR1, which controls polar auxin transport via the down‐regulation of the auxin influx carrier LIKE AUXIN RESISTANT 2 (LAX2), mediates CK signaling to attenuate auxin response in the quiescent center (QC) and divisions in QC cells 21. In root stem‐cell specification during zygotic embryogenesis, auxin also antagonizes CK signaling through direct transcriptional activation of CK signaling repressors of ARR7 and ARR15 36. Consistent with the opposite role of cytokinin in root and shoot meristem maintenance 37, the interaction between auxin and CK also differs in the activity of the apical meristems in the root and shoot. Auxin acts synergistically with CK through direct transcriptional repression of ARR7 and ARR15 in the control of shoot apical meristems 38. CK also regulates root growth through ethylene signaling. CK‐inhibited root elongation is partially blocked by the action of ethylene inhibitors or in the ethylene‐resistant mutants ein1‐2 (ethylene insensitive 1) and ein2‐1 (ethylene insensitive 2) 39, 40. CK affects root elongation through ethylene signaling, whereas CK controls the root meristem size and also involves ethylene‐independent modulation of polar auxin transport 41, 42.
Hormone‐regulated root growth is not only affected by responding to endogenous developmental signals, but also by mediating environmental stress cues such as aluminum (Al) stress. Phytohormones are known to be involved in the Al stress‐induced inhibition of root growth 7, 43, 44, 45. In A. thaliana, ethylene regulates the synthesis of auxin in the root‐apex TZ 7 and its transport within the root tip 44. Elevated auxin levels enhance the sensitivity of root growth to Al stress by compromising Al detoxification 45. In bean (Phaseolus vulgaris), cross talk between abscisic acid (ABA), ethylene, and CK has been proposed to regulate primary root growth under both Al and drought stress 46. Here, the role of CK and its interplay with auxin in the control of root growth in Al‐stressed A. thaliana seedlings have been clarified.
Results
CK enhances the Al stress‐induced inhibition of root growth
To identify a possible role for CK in Al‐induced inhibition of root growth, we performed experiments via manipulating CK levels or CK signaling through exogenous 6‐benzylaminopurine (BA) application and loss‐ or gain‐of‐function lines (Fig 1A–F). Low levels of BA (< 1 nM) had no effect on the primary root growth of plants, which was only slightly but significantly inhibited (~7%) at a concentration of 2 nM (Fig 1B). Exposure to 5 μM Al in the absence of BA induced a ~20% decrease in root growth, while in the presence of BA, the extent of the inhibition rose in line with the concentration of BA (Fig 1A and B). Treatment with < 4 μM Al stimulated root growth, but when combined with 2 nM BA, the stimulation was less evident and turned to root growth inhibition in the presence of 4 μM Al (Fig 1C).
Figure 1. CK mediates the Al stress‐induced inhibition of root growth.
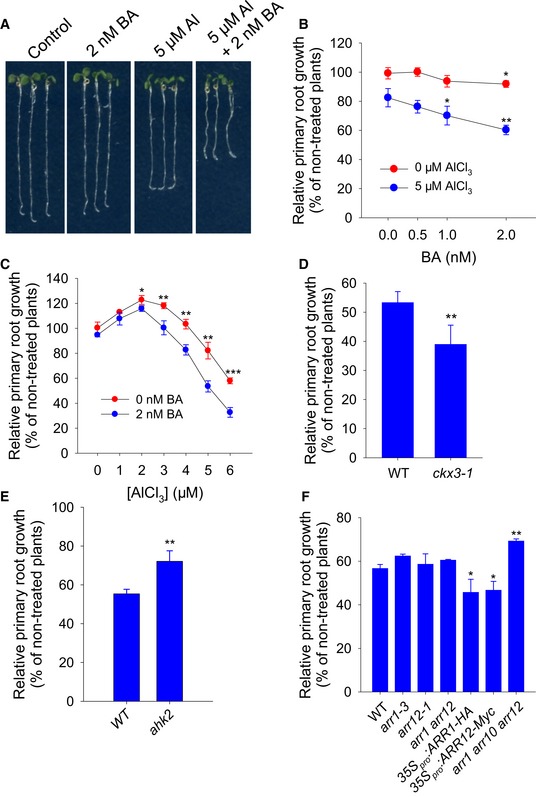
-
A–CRoot growth of WT seedlings subject to Al stress with or without BA. The WT seedlings were exposed to 0 or 5 μM AlCl3 in the presence of 0–2 nM BA (A, B) or 0–6 μM AlCl3 in the presence of 0 or 2 nM BA (C) for 7 days. The data are shown as mean ± SD (n = 3). In (B) and (C), *, **, and ***: means AlCl3 treatment (B) or BA treatment (C) differ significantly at, respectively, P < 0.05, P < 0.01, and P < 0.001 (t‐test). At least 60 seedlings were analyzed from three biological repeats.
-
D–FRoot growth of the WT and the selected CK biosynthetic and signaling mutants after a 7‐day exposure to 0 and 6 μM AlCl3. The data are shown as mean ± SD (n = 3). *, **: means differ significantly within the WT and mutants or over‐expression lines at, respectively, P < 0.05 and P < 0.01 (t‐test). At least 60 seedlings were analyzed from three biological repeats (around 20 seedlings for each repeat).
Next, compared with WT seedling root growth, the ckx3‐1 mutant, which has increased CK levels, was more sensitive to Al stress (Fig 1D). The root growth of the CK receptor single mutants ahk3 and cre1‐12 and the double‐mutant cre1 ahk3 did not show clear phenotypes under Al stress. In contrast, the ahk2 single‐mutant, the cre1 ahk2 double‐mutant, which have defects in CK signaling, and the ipt5 ipt7 double‐mutant seedlings, which have defects in CK biosynthesis, were more resistant to Al stress (Fig 1E and Appendix Fig S1). Similarly, root growth in response to Al stress of type‐B ARR mutants, such as arr1‐3, arr12‐1, and the arr1 arr12 double mutant, was similar to that of WT (Fig 1F). In contrast, the arr1 arr10 arr12 triple mutant was more resistant and the 35S pro :ARR1‐HA and 35S pro :ARR12‐Myc lines were more sensitive to Al stress (Fig 1F). Together, these results suggest that CK enhances the Al‐induced inhibition of root growth.
CK signaling is enhanced in the root‐apex TZ by Al stress
To evaluate whether Al stress influences CK signaling, a robust and sensitive CK synthetic sensor TWO COMPONENT SIGNALING SENSOR new (TCSn):GREEN FLUORESCENT PROTEIN (GFP) (TCSn:GFP) 47 was employed to monitor CK signaling in root tips of Al‐treated seedlings. After a 2‐h exposure to 25 μM Al, an elevated TCSn:GFP signal was detected in the root‐apex TZ epidermis and cortex (Fig 2A and D), the major perception sites of Al toxicity 48. The Al stress‐induced local up‐regulation of CK response in the root TZ was also confirmed by two other CK‐induced genes, ARR3 and ARR4. After a 2‐h exposure to 25 μM Al, a strong elevated GFP signal of ARR3 pro :GFP and ARR4 pro :GFP was detected in the root‐apex TZ epidermis and cortex (Fig 2B, C, E and F). The above observations implied that Al stress induced a local CK response in the root‐apex TZ, thereby compromising root growth. Decreasing the pH of the medium from 5.5 to 4.5 or treating with other ions (such as Cd2+, La3+, or Cu2+) did not affect the expression of TCSn:GFP in the TZ (Appendix Figs S2 and S3), implying that the local induction of CK signaling in the root TZ was specific to Al stress, and not protons or ions in general. Furthermore, the induction of CK response in the root TZ by Al stress is dependent on the concentration of Al3+ in the hydroponic medium since it took around 12 h before we could detect a clear ARR4pro:GFP signal in the root TZ following treatment with 6 μM Al (Appendix Fig S4).
Figure 2. Al stress enhances CK response (as measured by the expression of the CK sensor transgene TCSn:GFP and two CK‐induced genes ARR3 and ARR4) in the root‐apex TZ .
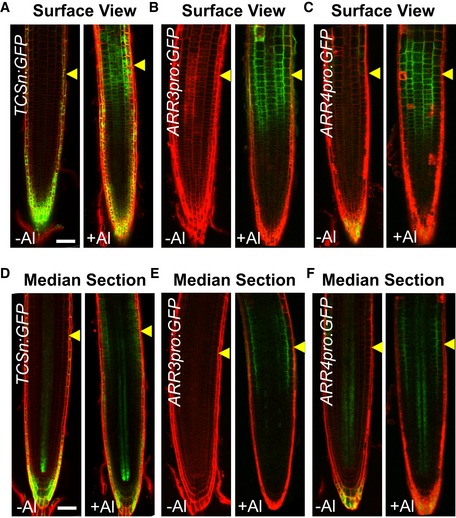
-
A–FThe expression of TCSn:GFP, ARR3pro:GFP, and ARR4pro:GFP in response to AlCl3 stress is detected in the epidermis (A–C) and cortex (D–F) of root‐apex. Six‐day‐old seedlings were exposed or not (control) to 25 μM AlCl3 for 2 h. Cell boundaries appear red following propidium iodide staining. The root‐apex TZ is marked by yellow arrowheads. Scale bar: 100 μm.
To address whether Al stress also regulates type‐B ARR expression, we also examined the expression of ARR1, ARR10, and ARR12 under Al stress. In response to Al stress, a strongly elevated GFP signal was observed in the root‐apex TZ after a 2‐h exposure of ARR1 pro :GFP, ARR10 pro:GFP, and ARR12 pro:GFP transgenic seedlings to Al stress (Appendix Fig S5A–F). This result was also confirmed by qRT–PCR analysis, which showed that ARR1, ARR10, and ARR12 expression was significantly induced in root tips under Al stress (Appendix Fig S5G). These results indicate that Al stress also induces the up‐regulation of CK signaling components besides the synthetic related genes in root TZ.
Al‐induced CK responses in the root‐apex TZ and the resultant root growth inhibition are dependent on auxin signaling
Auxin has been shown to play an important role in Al‐induced root growth inhibition and, as generally accepted, to act antagonistically with CK to control root development 7, 19. To test whether auxin participated in the regulation of the Al stress‐induced CK response in the root‐apex TZ, we first examined the expression of TCSn:GFP in yuc1D (yucca1D) or arf7 arf19 (AUXIN RESPONSE FACTOR 7/19) mutants which display increased auxin levels or reduced auxin signaling, respectively. The results showed that the Al stress‐induced TCSn:GFP signal produced in the root TZ was remarkably enhanced in the yuc1D mutant, while it was highly attenuated in the arf7 arf19 double mutant (Fig 3A and B). Furthermore, the role of auxin in the regulation of the Al stress‐induced CK response in the root‐apex TZ was also examined by exposing the roots to either auxin 1‐naphthalene acetic acid (NAA) or the auxin antagonist α‐(phenylethyl‐2‐one)‐indole‐3‐acetic acid (PEO‐IAA). In the presence of Al stress, the NAA co‐treatment enhanced, while the PEO‐IAA co‐treatment suppressed the up‐regulation of TCSn:GFP (Fig 3C and D, and Appendix Fig S6). However, the Al stress‐induced local auxin response, which was shown by DR5rev:GFP in the root‐apex TZ, was unaffected by the co‐treatment with BA (Appendix Fig S7).
Figure 3. Al stress‐induced up‐regulation of CK response in the root‐apex TZ and inhibition of root growth are regulated by auxin.
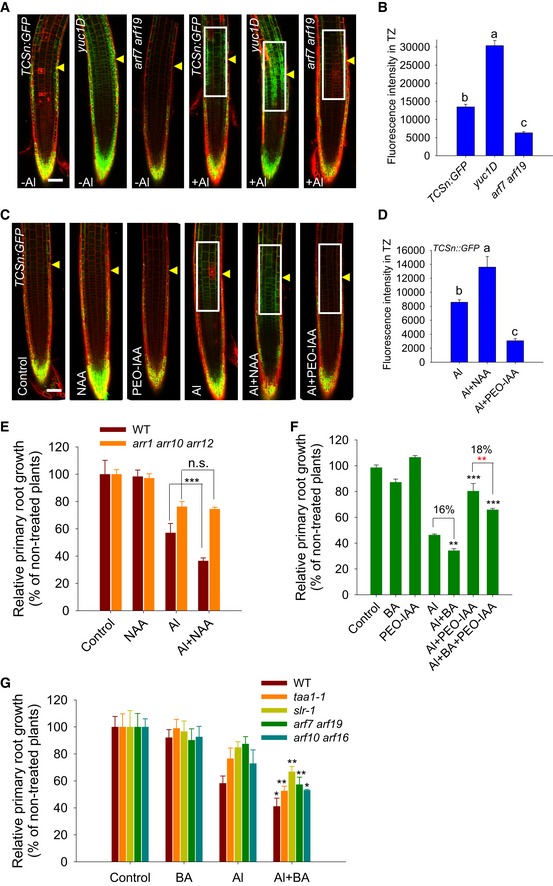
- Six‐day‐old TCSn:GFP, TCSn:GFP/yuc1D, TCSn:GFP/arf7 arf19 seedlings were exposed or not (control) to 25 μM AlCl3 for 2 h. Cell boundaries appear red following propidium iodide staining. The TZ is marked by yellow arrowheads. Scale bar: 100 μm. The detected fluorescence region in (B) is marked by white rectangles.
- Quantification of the Al‐induced fluorescence intensity in the TZ of TCSn:GFP, TCSn:GFP/yuc1D, TCSn:GFP/arf7 arf19 seedlings. The data are shown as mean ± SD (n = 15) with one‐way ANOVA and Tukey's test. Different letters indicate significant differences (P < 0.01).
- The expression of the TCSn:GFP transgene in the epidermis of root‐apex TZ in the presence of Al with NAA or PEO‐IAA co‐treatments. Four‐day‐old transgenic TCSn:GFP seedlings were pre‐treated without or with 100 nM NAA for 2 days; then, the seedlings were continuously treated for 2 h with 0 or 25 μM AlCl3 in the presence of either 100 nM NAA or 15 μM PEO‐IAA. Cell boundaries appear red following propidium iodide staining. The root‐apex TZ is marked by yellow arrowheads. Scale bar: 100 μm. The detected fluorescence region in (D) is marked by white rectangles.
- Quantification of the Al‐induced fluorescence intensity in the TZ of TCSn:GFP seedlings in the presence of NAA or PEO‐IAA co‐treatments. The data are shown as mean ± SD (n = 15) with one‐way ANOVA and Tukey's test. Different letters indicate significant differences (P < 0.01).
- Root growth of WT and the arr1 arr10 arr12 triple mutant in the presence of 6 μM AlCl3 with or without 2.5 nM NAA for 7 days.
- Root growth of WT seedlings after exposure to 6 μM AlCl3 with or without 2 nM BA or 0.5 μM PEO‐IAA for 7 days.
- Root growth of WT seedlings and various mutants in the presence of 6 μM AlCl3 for 7 days with or without 2 nM BA.
The inhibition of root growth induced by Al stress was stronger in NAA‐treated than in non‐treated seedlings, while auxin‐enhanced root growth inhibition in response to Al stress was less obvious in the arr1 arr10 arr12 triple mutant (Fig 3E). In contrast, the alleviated root growth inhibition by the PEO‐IAA co‐treatment in response to Al stress was significantly reduced by the BA co‐treatment (Fig 3F). The BA‐enhanced root growth inhibition in response to Al stress (Fig 1) was still observed in the taa1‐1, slr‐1, arf7 arf19, and arf10 arf16 mutants, which were defective in auxin biosynthesis or auxin signaling 49, 50, 51 (Fig 3G). All these results suggest that CK acts as a downstream signal of auxin in the regulation of the Al stress‐induced inhibition of root growth.
Al‐induced CK responses in the root‐apex TZ and the resultant root growth inhibition are regulated by ethylene
Ethylene has been shown to respond to Al stress and control root growth through the regulation of both TAA1‐ and YUC‐mediated local auxin biosynthesis in root tips 7, 52. Therefore, we also investigated the role of ethylene in the Al‐induced local CK responses in the root‐apex TZ. To this end, we analyzed the local TCSn:GFP induction in response to Al in eto1‐2 (ethylene‐overproducer) single 53 and ein3 eil1 double mutants (ethylene insensitive) 54 (Fig 4A). This revealed that the strong Al stress‐induced TCSn:GFP signal produced in the TZ was remarkably enhanced in the eto1‐2 mutant and attenuated in the ein3 eil1‐1 double‐mutant background (Fig 4A). Subsequently, treatments with the precursor of ethylene biosynthesis 1‐aminocyclopropane‐1‐carboxylic acid (ACC) or the inhibitor of ethylene biosynthesis aminoethoxyvinylglycine (AVG) in Al stress conditions were conducted to confirm the role of ethylene in Al‐induced CK responses in the root‐apex TZ. The results showed that ACC strongly enhanced the TCSn:GFP signal in the root‐apex TZ, while AVG attenuated it in both the epidermis and the cortex (Fig 4A and B, and Appendix Fig S8). Taken together, our results imply that the Al stress‐induced CK response in the root‐apex TZ is dependent on ethylene, with CK acting downstream of ethylene.
Figure 4. Ethylene‐enhanced CK response in the root‐apex TZ and root growth inhibition under Al stress are dependent on auxin signaling.
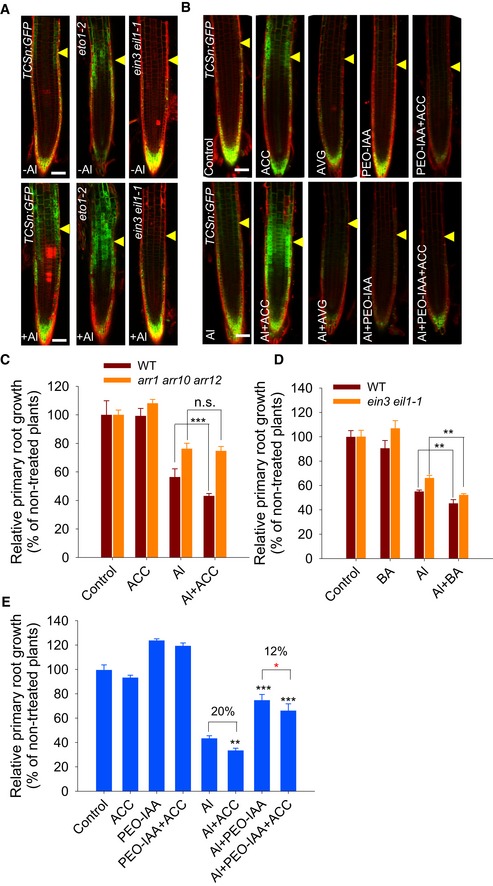
- Six‐day‐old TCSn:GFP, TCSn:GFP/eot1‐2, TCSn:GFP/ein3 eil1‐1 seedlings were exposed or not (control) to 25 μM AlCl3 for 2 h. Cell boundaries appear red following propidium iodide staining. The TZ is marked by yellow arrowheads. Scale bar: 100 μm.
- The expression of the TCSn:GFP transgene in the epidermis of root‐apex TZ in the presence of AlCl3 plus AVG, ACC, PEO‐IAA or ACC and PEO‐IAA co‐treatments. Four‐day‐old transgenic TCSn:GFP seedlings were pre‐treated without or with 1 μM AVG or 1 μM ACC for 2 days; then, the seedlings were continuously treated without or with 1 μM AVG, 1 μM ACC, 15 μM PEO‐IAA, or 1 μM ACC plus 15 μM PEO‐IAA in the absence or presence of 25 μM AlCl3 for 2 h. Control: non‐treated plants. Cell boundaries appear red following propidium iodide staining. The root‐apex TZ is marked by yellow arrowheads. Scale bar: 100 μm.
- Root growth of the WT seedlings and the arr1 arr10 arr12 mutant was affected by a 7‐day exposure to 6 μM AlCl3 with or without 50 nM ACC. ***: means differ significantly with ACC co‐treatment in the presence of AlCl3, P < 0.001 (t‐test). n.s.: not significant.
- Root growth of the WT seedlings and the ein3 eil1‐1 mutant was affected by a 7‐day exposure to 6 μM AlCl3 with or without 2 nM BA. **: means differ significantly with BA co‐treatment in the presence of AlCl3, P < 0.01 (t‐test). n.s.: not significant.
- Root growth of WT seedlings following a 7‐day exposure to 6 μM AlCl3 in the absence or presence of 50 nM ACC or/and 0.5 μM PEO‐IAA. Black **, ***: means differ significantly in the presence of AlCl3, ACC, or/and PEO‐IAA at, respectively, P < 0.01 and P < 0.001 (t‐test); red *: means differ significantly within PEO‐IAA and ACC plus PEO‐IAA in the presence of AlCl3 treatment at P < 0.05.
Consistently, when both WT and the arr1 arr10 arr12 triple‐mutant seedlings were subjected to Al stress in the presence of ACC, the ACC‐enhanced root growth inhibition in response to Al stress was less obvious in the arr1 arr10 arr12 triple mutant (Fig 4C). This suggests that CK acts downstream of ethylene to regulate the Al stress‐induced root growth inhibition. Further supportive evidence was obtained from the co‐treatment of the ein3 eil1‐1 mutant seedlings with BA in response to Al stress. The results showed that exposure to BA still significantly strengthened the Al stress‐induced root growth inhibition in ein3 eil1‐1, as it did that of WT seedlings (Fig 4D).
The ethylene‐enhanced CK response in the root‐apex TZ and inhibition of root growth under Al stress are regulated by auxin
Since Al‐induced CK responses and root growth inhibition are regulated by both auxin and ethylene, we investigated the relationship between these two hormones in this process. The results showed that, in the presence of Al stress, ACC co‐treatment strongly enhanced the TCSn:GFP signal in the root‐apex TZ, while the PEO‐IAA co‐treatment strongly suppressed it (Fig 4B and Appendix Fig S8). The down‐regulation of TCSn:GFP imposed by the PEO‐IAA treatment was not fully reversed by adding ACC (Fig 4B and Appendix Fig S8). The ACC treatment inhibited root growth by ~20%, while the reduction was only ~12% in the presence of PEO‐IAA (Fig 4E). Overall, our results suggested that disrupting auxin signaling attenuated the ACC‐intensified CK response in the root‐apex TZ and Al stress‐induced root growth inhibition.
IPTs are locally induced in the TZ by Al stress and regulate the Al‐induced root growth inhibition
To investigate whether the Al‐induced local CK response in the TZ is the result of local biosynthesis of CK, we first examined the effect of Al stress on the spatial expression of IPTs, which encodes the key enzymes of cytokinin biosynthesis, by using IPT1 pro :GFP, IPT2 pro :GFP, IPT3 pro :IPT3‐GFP, IPT5 pro :GFP, IPT7 pro :GFP, IPT9 pro :GFP transgenic lines. The expression of these transgenes was not detected in the root‐apex TZ; however, a clear GFP signal was observed in the root TZ of these transgenic seedlings after a 2‐h exposure to Al stress (Fig 5A and Appendix Fig S9A).
Figure 5. Al stress up‐regulated the expression of IPTs in the root‐apex TZ that is important for the Al‐induced root growth inhibition.
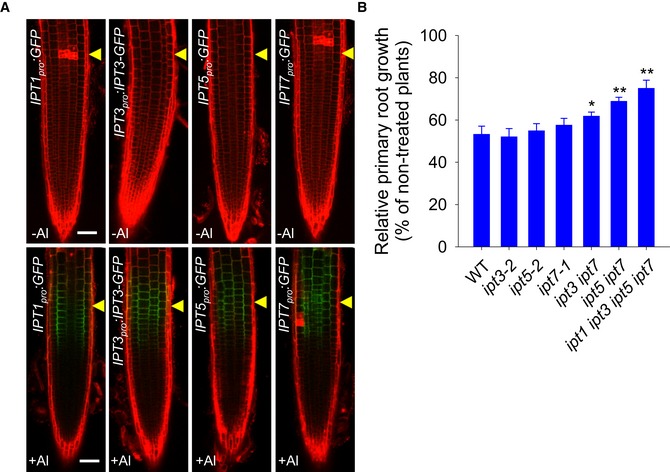
- The expression of the IPT1 pro :GFP, IPT3 pro :IPT3‐GFP, IPT5 pro :GFP, and IPT7 pro :GFP transgenes in the epidermis of root‐apex TZ after a 2‐h exposure to 25 μM AlCl3. Cell boundaries appear red following propidium iodide staining. The root‐apex TZ is marked by yellow arrowheads. Scale bar: 100 μm.
- Relative primary root growth of the WT and IPT mutants as affected by a 7‐day exposure to 6 μM AlCl3. The data are shown as mean ± SD (n = 3). * and **: means differ significantly within the WT and mutants at, respectively, P < 0.05 and P < 0.01 (t‐test). At least 60 seedlings were analyzed from three biological repeats (around 20 seedlings for each repeat).
To further clarify whether CK biosynthesis is involved in the regulation of Al‐induced root growth inhibition, we examined the phenotypes of mutants which have defects in IPT‐regulated CK biosynthesis 26. The single ipt3‐2, ipt5‐2, and ipt7‐1 mutants did not show a significant difference (Fig 5B and Appendix Fig S9B). In contrast, the ipt3 ipt7 and ipt5 ipt7 double mutants and especially the quadruple ipt1 ipt3 ipt5 ipt7 mutant showed a significant reduction in the root growth inhibition compared to the WT control in response to Al stress (Fig 5A). However, only the ipt1 ipt3 ipt5 ipt7 mutant displayed root length phenotype without Al stress (Appendix Fig S9B). These results suggest that Al stress induces a local up‐regulation of IPTs, and thus CK production in the TZ, leading to root growth inhibition.
The local up‐regulation of IPTs induced by Al stress in the root‐apex TZ and root growth inhibition are regulated by auxin
To study the role of auxin in Al‐induced local up‐regulation of IPTs in the root TZ, we examined the local variation of IPT1 pro :GFP, IPT5 pro :GFP, IPT7 pro :GFP expression in response to Al stress in the root TZ of the arf7 arf19 double mutant. This revealed that the Al stress‐induced IPTpro:GFP signals in the root TZ were remarkably attenuated in the arf7 arf19 double mutant (Fig 6A and B). This indicated the critical role of the auxin response factors ARF7 and ARF19 in the local up‐regulation of IPTs induced by Al stress in the root‐apex TZ. Al stress‐induced local up‐regulation of ARF7 pro: GFP in the root‐apex TZ, which was also confirmed by qRT–PCR analysis, further supported its role in this process (Appendix Fig S10).
Figure 6. The Al‐induced local up‐regulation of IPTs in the root‐apex TZ and root growth inhibition are auxin dependent.
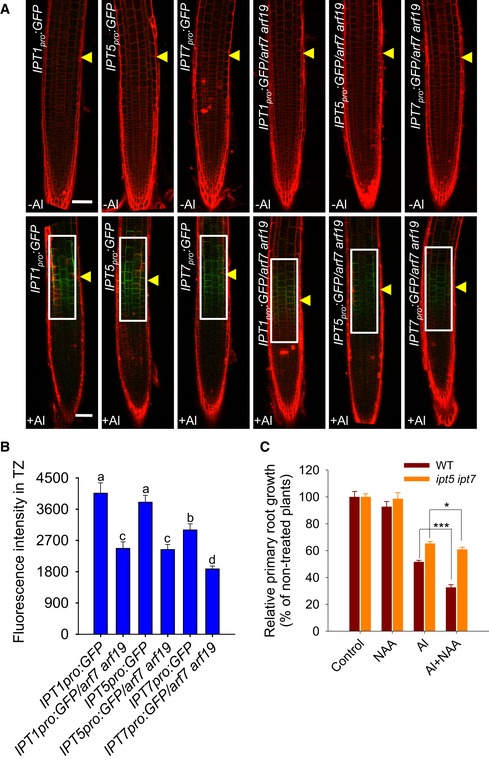
- Six‐day‐old IPT1 pro :GFP, IPT1 pro :GFP /arf7 arf19, IPT5 pro :GFP, IPT5 pro :GFP /arf7 arf19, IPT7 pro :GFP, and IPT7 pro :GFP /arf7 arf19 seedlings were exposed or not (control) to 25 μM AlCl3 for 2 h. Cell boundaries appear red following propidium iodide staining. The TZ is marked by yellow arrowheads. Scale bar: 100 μm. The detected fluorescence region in (B) is marked by white rectangles.
- Quantification of the Al‐induced fluorescence intensity in the TZ of IPT1 pro :GFP, IPT1 pro :GFP /arf7 arf19, IPT5 pro :GFP, IPT5 pro :GFP /arf7 arf19, IPT7 pro :GFP, and IPT7 pro :GFP /arf7 arf19 seedlings. Cell boundaries appear red following propidium iodide staining. The data are shown as mean ± SD (n = 15) with one‐way ANOVA and Tukey's test. Different letters indicate significant differences (P < 0.01).
- Root growth of the WT and the ipt5 ipt7 mutant as affected by a 7‐day exposure to 6 μM AlCl3 with or without 2.5 nM NAA. The data are shown as mean ± SD (n = 3). * and ***: means differ significantly in the presence of AlCl3 and NAA treatment at, respectively, P < 0.05 and P < 0.001 (t‐test). At least 60 seedlings were analyzed from three biological repeats (around 20 seedlings for each repeat).
Next, we examined the Al‐induced local up‐regulation of IPT1 pro :GFP, IPT2 pro :GFP, IPT3 pro :IPT3‐GFP, IPT5 pro :GFP, IPT7 pro :GFP, IPT9 pro :GFP expression in combination with NAA or PEO‐IAA co‐treatment. No clear changes in GFP expression strength and pattern were detected under either NAA or PEO‐IAA treatment. However, the Al‐induced local up‐regulation of IPTpro:GFP signals were strongly enhanced by NAA co‐treatment while they were greatly repressed by PEO‐IAA (Appendix Fig S11). To test the role of IPTs in auxin‐enhanced root inhibition under Al stress, we examined the phenotype of the ipt5 ipt7 double mutant. This revealed that NAA‐enhanced root growth inhibition in response to Al stress was strongly attenuated in the ipt5 ipt7 mutant (Fig 6C). The results suggest that auxin controls the Al‐induced root growth inhibition through the up‐regulation of IPTs and CK responses in the root‐apex TZ.
Ethylene acts upstream of auxin to regulate Al‐induced local up‐regulation of IPTs in the root‐apex TZ and root growth inhibition under Al stress
To study whether the Al‐induced local up‐regulation of IPTs in the root TZ is regulated by ethylene, we examined the local variation of IPT1pro:GFP, IPT5pro:GFP, IPT7pro:GFP transgenic line expression in the root TZ of the ein3 eil1‐1 double mutant under Al stress (Fig 7A). The results showed that strong Al‐induced IPTpro:GFP signals in the root TZ were remarkably attenuated in the ein3 eil1‐1 double mutant (Fig 7A and B). Next, we analyzed Al‐induced local up‐regulation of IPT1pro:GFP, IPT2pro:GFP, IPT3pro:IPT3‐GFP, IPT5pro:GFP, IPT7pro:GFP, IPT9pro:GFP expression in the root TZ in combination with ACC or AVG co‐treatment. We detected no changes in GFP expression strength and pattern under either ACC or AVG treatment (Appendix Fig S12). The Al‐induced local up‐regulation of IPTpro:GFP signals was strongly enhanced by ACC co‐treatment while it was greatly repressed by AVG (Appendix Fig S12). Furthermore, the ACC‐enhanced local induction of IPTpro:GFP signals in the root‐apex TZ in response to Al stress was strongly suppressed by the PEO‐IAA co‐treatment (Appendix Fig S13). In contrast, obvious IPT5pro:GFP and IPT7pro:GFP expression was still observed in the ein3‐1 eil1‐1 background under Al together with auxin treatment (Appendix Fig S14). Taken together, this suggests that the ethylene‐regulated local up‐regulation of IPTs is an auxin‐dependent process. Root growth analysis showed that ACC treatment did not affect or slightly enhanced the root growth inhibition in the ipt5 ipt7 double‐mutant compared to the WT seedlings, in which the root growth inhibition by Al was greatly intensified by ACC (Fig 7C). In summary, the results above suggest that both ethylene and auxin control the Al‐induced root growth inhibition through the up‐regulation of IPTs and CK responses in the root‐apex TZ, with auxin acting downstream of ethylene in this process.
Figure 7. The Al‐induced local up‐regulation of IPTs in the root‐apex TZ and root growth inhibition are regulated by ethylene.
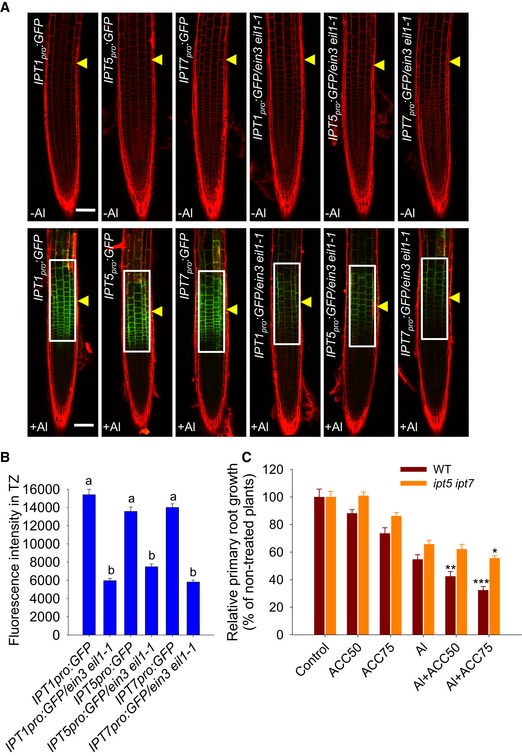
- Six‐day‐old IPT1 pro :GFP, IPT1 pro :GFP/ein3 eil1‐1, IPT5 pro :GFP, IPT5 pro :GFP/ein3 eil1‐1, IPT7 pro :GFP, and IPT7 pro :GFP/ein3 eil1‐1 seedlings were exposed or not (control) to 25 μM AlCl3 for 2 h. Cell boundaries appear red following propidium iodide staining. The TZ is marked by yellow arrowheads. Scale bar: 100 μm. The detected fluorescence region in (B) is marked by white rectangles.
- Quantification of the Al‐induced fluorescence intensity in the TZ of IPT1 pro :GFP, IPT1 pro :GFP/ ein3 eil1‐1, IPT5 pro :GFP, IPT5 pro :GFP /ein3 eil1‐1, IPT7 pro :GFP, and IPT7 pro :GFP /ein3 eil1‐1 seedlings. Cell boundaries appear red following propidium iodide staining. The data are shown as mean ± SD (n = 15) with one‐way ANOVA and Tukey's test. Different letters indicate significant differences (P < 0.01).
- Root growth of the WT and the ipt5 ipt7 mutant was affected by a 7‐day exposure to 6 μM AlCl3 with or without 50 and 75 nM ACC. The data are shown as mean ± SD (n = 3). *, **, and ***: means differ significantly within ACC treatment in the presence of AlCl3 in the WT (dark red) or ipt5 ipt7 mutant (orange) at, respectively, P < 0.05, P < 0.01, and P < 0.001 (t‐test). At least 60 seedlings were analyzed from three biological repeats (around 20 seedlings for each repeat).
ARF7 regulates the expression of IPT5 and IPT7
As mentioned above, the local up‐regulation of IPTs in response to Al stress is remarkably attenuated in the arf7 arf19 double mutant, suggesting that IPTs might be transcriptionally regulated by auxin response factors ARF7 and ARF19. Consistently, the typical auxin response elements (AuxREs), including TGTC, TGTCTC, TGTCGG, and their reverse complement sequence, were found within the promoter sequence of IPT1, IPT5, and IPT7 (Fig 8A). A yeast one‐hybrid analysis showed that ARF7 bound to the IPT5 and IPT7 promoters (Fig 8B), suggesting a direct regulation of IPTs by ARF7. This was also confirmed by a chromatin immunoprecipitation–quantitative PCR (ChIP‐qPCR) assay, which showed a clear association of ARF7 protein with the IPT7 promoter via AuxREs (Fig 8D). Furthermore, we conducted transient dual‐luciferase assays to test whether ARF7 regulates IPT expression. The results showed that over‐expression of ARF7 highly increased the luciferase activity of the IPT5 and IPT7 reporters as compared with the empty vector control, especially in the presence of Al stress (Fig 8C). Taken together, these results indicate that ARF7 proteins directly bind to the promoters of IPT5 and IPT7 and positively regulate the transcription of these genes, thus mediating auxin‐regulated local CK biosynthesis and root growth inhibition in response to Al stress.
Figure 8. ARF7 regulates the expression of IPT5 and IPT7 .
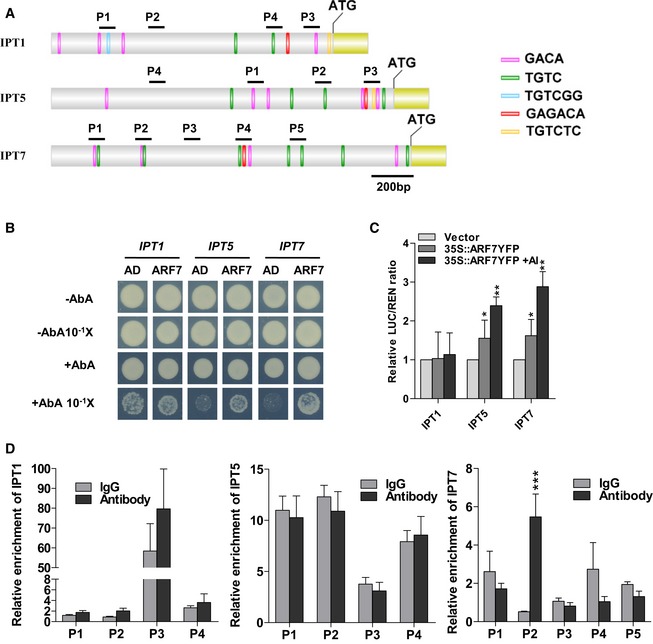
- Promoter structure of the IPT genes (IPT1, IPT5, and IPT7) and fragments used in the ChIP assay. The auxin response elements (AuxREs) TGTC, TGTCGG, and TGTCTC on sense strand of the IPT promoters (IPT1, IPT5, and IPT7) are indicated as green, blue, and yellow bars, respectively. The antisense strand AuxRE elements, GACA and GAGACA, are indicated as pink and red bars. Black lines indicate the promoter regions containing AuxREs sequence or not, which are used in the ChIP assay.
- Physical interactions of ARF7 with IPT5 and IPT7 promoters in Y1H assays. Yeast expression plasmids pGADT7‐ARF7 (ARF7) are introduced into yeast strain Y1H Gold carrying the reporter gene AbAr under the control of the IPT promoters. The transformants were screened for their growth on the yeast synthetic defined media (SD/‐Leu) in the presence of 75 ng/ml aureobasidin A (AbA) for stringent selection. The empty vector pGADT7 (AD) was included as a negative control. Yeast cultures were diluted (1:10 successive dilution series) and spotted onto plates. In the third lane, yeast strains carrying negative control pGADT7 growing in the presence of the toxic AbA are result from the high concentrated yeast cultures without dilution. The binding of ARF7 to IPT1 promoter is a false‐positive since yeast strains carrying the negative control pGADT7 still grow at the same culture dilution.
- ARF7 transactivates the promoters of IPTs in Arabidopsis protoplasts via transient dual‐luciferase assays. The IPT‐LUC reporters are co‐transformed with 35S::ARF7‐YFP plasmid exposed to 10 μM AlCl3 for 12 h or not. The empty vector pBI221 is used as a negative control. * and **: means differ significantly as compared with the respective vector controls at, respectively, P < 0.05 and P < 0.01 (t‐test). Values represent means ± SD (n=3).
- ARF7 associated with the promoters of IPT7 in ChIP‐qPCR assay. Chromatins isolated from 35S::MYC‐ARF7 transgenic line were immunoprecipitated with anti‐MYC antibody followed by qPCR to amplify selected regions. Segments P2, P4, and P3 were used as negative controls of IPT1, IPT5, and IPT7 genes, respectively. Mouse IgG was used as a mock control. Input sample was used to normalize the qPCR results in each ChIP. Error bars indicate standard error mean ± SD (n = 3); asterisks in (D) mark significant differences as compared with the IgG mock control (***P < 0.001, t‐test). The experiments are repeated twice with similar results.
Discussion
Although it has become clear that several phytohormones, such as auxin, ABA, and ethylene, play a part in the regulation of Al stress‐induced root growth inhibition 7, 44, 55, until now, the participation of CK has not been established. CK is a key regulator of a range of plant responses to environmental stress 32, 56, 57. Here, it has been shown that local up‐regulation of CK response in the root‐apex TZ, which is dependent on IPT‐mediated CK biosynthesis, plays an important role in the Al stress‐induced root growth inhibition.
IPTs are responsible for the first step in CK synthesis 23, 58, while CKXs act as an irreversible degrader of CK 59. The root growth of mutants defective in either CK synthesis (ipt3 ipt7; ipt5 ipt7; and ipt1 ipt3 ipt5 ipt7) or signaling (the arr1 arr10 arr12 triple mutant, ahk2‐2, and the cre1 ahk2 double mutant) showed a reduced sensitivity to Al stress (Figs 1E–F and 5B, and Appendix Fig S1). However, the ckx3‐1 mutant was more sensitive (Fig 1D). Using either PEO‐IAA to block auxin signaling or disruption of auxin response factors ARF7 and ARF19 in arf7/19 greatly attenuated the up‐regulation of IPTs and CK response in the root‐apex TZ and alleviated the Al‐induced inhibition of root growth (Figs 3 and 6A and B, and Appendix Fig S11). The externally applied auxin (NAA) treatment strongly enhanced root growth inhibition in response to Al stress; however, this enhancement was less obvious in the ipt5 ipt7 double mutant and arr1 arr10 arr12 triple mutant (Figs 3E and 6C). In addition, BA‐induced inhibition of root growth in Al‐stressed seedlings was not affected by disrupted auxin signaling (Fig 3F and G). The inference is that the Al stress‐induced up‐regulation of IPTs causes enhanced CK response in the root‐apex TZ and inhibition of root growth with CK acting downstream of auxin. In this study, we mainly focused on ARF7 to address the direct role of ARF7‐mediated auxin signaling to control the expression of IPTs. In the ChIP‐qPCR analysis, we confirmed the association of ARF7 with the promoter of IPT7, but not of IPT5 or other IPTs which could be induced by Al stress (Fig 5A and Appendix Fig S9A). In protoplasts, we detected that only IPT5 and IPT7 promoters respond to ARF7 expression. This inconsistency with the attenuated up‐regulation of IPTpro:GFP expressions in the root TZ of the arf7 arf19 double mutant suggests a tissue or cell‐specific manner of ARF7‐regulated IPT expression under Al stress. Alternatively, these differences might reflect a shoot versus root response, as the protoplasts we used are shoot‐derived, or be due to the limitations of the ChIP‐qPCR setup we used. In addition, this might be also a result from redundant functions between ARF7 and ARF19 or other ARFs since ARF proteins often function as heterodimer proteins with other ARFs to mediate auxin signaling 60. In our recent study, we found that ARF10 and ARF16 mediate auxin signaling to control Al‐induced inhibition of root growth 7. Therefore, ARF7 might also have a redundant role with ARF10/ARF16 to regulate IPTs local up‐regulations in root TZ under Al stress. However, ARF10‐ and ARF16‐involved transcriptional activations might be indirect since both ARF10 and ARF16 are classified as transcriptional repressors.
CK‐regulated root growth is mediated via either ethylene signaling or synthesis 39, 61. Ethylene controls plant growth by regulating the transcription of ARR2 62, an example of cross talk between CK and ethylene. Ethylene also influences freezing tolerance in A. thaliana by its interaction with CK signaling 63. The role of ethylene in Al‐induced root growth inhibition has been highlighted recently through the regulation of TAA1‐mediated local auxin biosynthesis in Arabidopsis root 46. In this study, through the manipulation of ethylene levels or ethylene signaling via chemical treatment or mutant analysis, we found that Al stress‐induced up‐regulation of IPTs and CK response in the root‐apex TZ was also regulated by ethylene, which acts upstream of auxin in this process (Figs 4 and 7, and Appendix Figs S8, S12, S13, and S14).
In summary, based on this study and previous reports, we propose a working model of the hormone interactions (Fig 9). The emerging picture is that CK plays a negative regulatory role in Al stress‐regulated root growth. Al stress induces the local up‐regulation of IPTs which is partially dependent on ARF7‐mediated auxin signaling and ethylene signaling which acts upstream of auxin. The up‐regulated local expression of IPTs thus increases CK levels and CK response in the root‐apex TZ and eventually leads to the inhibition of root growth under Al stress. This study provides genetic and cellular evidence to explain the increased cytokinin level under Al stress which was observed in bean seedlings 64. It is also consistent with the changed transcripts that putatively related to auxin, ethylene, and cytokinin under Al treatment 65. However, we could not rule out the possibility that CK might directly or indirectly regulate ethylene biosynthesis through a feedback regulation. Whether CK is involved in Al‐induced ethylene production still needs further studies in the future. Besides plant hormones, cell wall rigidity control and cell cycle checkpoint regulation also play a critical role in Al‐induced root growth inhibition 66, 67. The NAC family transcription factor SOG1 (suppressor of gamma response 1) participates in Al‐inhibited root growth by direct regulation of cell cycle checkpoint genes ATR (Ataxia telangiectasia and RAD3 related) and ALT2 (ALUMINUM TOLERANT 2). Furthermore, SOG1 could induce the expression of DNA damage genes, including BRCA1 and PARP2, through direct interaction with their promoters 67, 68, 69. In addition, organic acids such as citric acid and malate acid, which are transported by the malate transporter ALMT1 (aluminum‐activated malate transporter 1) and the citrate‐transporter MATE (multidrug and toxic compound extrusion), respectively, detoxify Al stress by chelated aluminum ion cations 70, 71. A recent study showed that the auxin‐regulated root growth inhibition under Al stress arises from auxin signaling‐regulated modification of cell wall structure or components 7. Whether auxin and other plant hormones regulate cell wall rigidity and cell cycle checkpoints still requires further investigations.
Figure 9. A network of root growth regulation in response to Al stress.
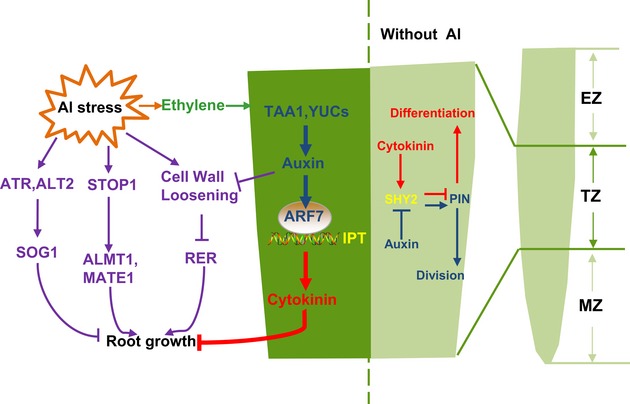
It has been represented that SHY2 can act as a key mediator between auxin and cytokinin in antagonistic manner by maintaining the homeostasis of auxin efflux transporter PINs in the root‐apex TZ and subsequently regulate normal root growth. While under Al stress, the root growth is regulated via three major pathways: (i) via transporters of organic acid (i.e., malate and citrate), such as ALMT1 and MATE1; (ii) via cell wall loosening regulation; (iii) via activating the expression of DNA damage response transcription factor SOG1 through the cell cycle checkpoint genes ATR and ALT2. Here, we propose that the phytohormone regulatory network in response to Al stress is also very important to the Al stress‐induced inhibition of root growth. IPTs‐dependent CK response acts downstream of ARF7‐mediated auxin signaling and synergistically regulates the Al stress‐induced inhibition of root growth. The process is dependent on ethylene signaling which acts upstream of auxin to regulate IPT‐mediated local CK accumulation and CK response. EZ: elongation zone; TZ: transition zone; MZ: meristem zone.
This study also points out a different interaction module in auxin and CK cross talk‐regulated root growth in Arabidopsis under Al stress conditions (Fig 9). Based on previous studies, the current general view is that auxin primarily acts antagonistically with CK to control root development 20, 34, 35, 61. Auxin and CK were identified to act in an antagonistic manner to control root meristem size by promoting cell division and differentiation, respectively 19, 41. To specify the root stem‐cell niche during embryogenesis, auxin represses cytokinin action by activating expression of ARR7 and ARR15, which encode type‐A ARR proteins that repress CK responses 36. However, the interaction between auxin and CK differs in the activity of the apical meristems in the root and shoot. Auxin acts synergistically with CK through direct transcriptional repression of ARR7 and ARR15 in the control of shoot apical meristems 37, 38. In addition, the synergistic interaction between auxin and CK was also recently reported to control root vascular tissue formation 72. Overall, auxin and CK could also act in a synergistic way in root. This raises the intriguing question whether antagonistic or synergistic auxin–CK interactions are tissue or cell‐specific processes in plant development. In addition, it has been reported that nitrate‐regulated plant growth is also mediated through the regulation of cytokinin biosynthesis in Arabidopsis 73. Nutrients such as phosphorus (P) addition could alleviate Al toxicity in plants 74. Furthermore, it seems that environmental stress, such as Al stress, can rewire the auxin–cytokinin cross talk to regulate root growth based on this study. Therefore, it will be interesting to study whether the nutrient status also influences Al‐induced root growth inhibition through the regulation of CK synthesis or signaling.
Materials and Methods
Plant materials and growth conditions
All of the A. thaliana mutants and transgenics employed shared the same (Col‐0) genetic background. The mutants involved were arr1‐3, arr12‐1, arr1 arr12, arr1 arr10 arr12 75, cre1‐12, ahk3, cre1 ahk2, cre1 ahk3 76, ipt3‐2 (KG21969), ipt5‐2 (SALK_133407), ipt7‐1 (SALK_001940), ipt3 ipt5, ipt5 ipt7, ipt3 ipt7, ipt1 ipt3 ipt5 ipt7 26, ckx3‐1 77, ein3 eil1‐1 54, taa1‐1 53, slr‐1 50, 78, arf10 arf16 49, and arf7 arf19 51, and the transgenic lines were IPT3 pro: IPT3‐GFP 26, 35S pro :ARR1‐HA, and 35S pro :ARR12‐Myc 79. The TAA1 product mediates the conversion of tryptophan to indole‐3‐pyruvic acid in the indole‐3‐pyruvic acid pathway 53, 80; SLR encodes IAA14, a member of the Aux/IAA protein family; ARFs are auxin response factors 81. We used previously published transgenics with either the CK sensor TCSn:GFP 47 or the auxin reporter DR5rev:GFP 49. Seedlings were grown on either agar‐solidified medium or hydroponically 2% modified MGRL (pH 5.0) 7 under a 16‐h photoperiod at 22°C.
Treatments and experimental conditions
For all the experiments in this study, we used the low ionic strength of the nutrient solution (2% ionic strength of the MGRL nutrient solution 82). This system has been successfully established by Koyama's laboratory 83 and our laboratory 7. For root growth analysis, the seeds were sown onto polypropylene mesh floating on 2% modified MGRL solution containing Al (total [AlCl3] 0–8 μM, pH 5.0) or Al plus PEO‐IAA, NAA, ACC, 6‐BA, or AVG (pH 5.0) (pH 4.2–5.5) for 7 days. The solution was renewed every 2 days. At day 7, the roots were scanned and primary root length was calculated with ImageJ software.
For short‐term exposure experiments, the seedlings were raised for 6 days in 2% modified MGRL (pH 5.0) solution and then subjected to 25 μM AlCl3, 4 μM CdCl2, 2.5 μM LaCl3, or 1 μM CuCl2 for 2 h in the presence or absence of combinations of NAA, PEO‐IAA, BA, ACC, or AVG. Solutions were replaced every other day. For all the treatments (e.g., −Al/+Al, with chemical or without chemicals), controls were performed at the same time.
Plasmid construction and generation of transgenic lines
The ARR1 pro :GFP, ARR10 pro :GFP (1,467‐bp promoter), and ARR12 pro :GFP (1,654‐bp promoter) were generated using the vector pMDC107 Gateway recombination system (Invitrogen, Carlsbad, CA, USA). For the generation of IPT1 pro :GFP, IPT2 pro :GFP, IPT5 pro :GFP, IPT7 pro :GFP, IPT9 pro :GFP, ARR3 pro :GFP, ARR4 pro :GFP transgenic lines, promoters were amplified from genomic DNA and inserted into pDONR221 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Afterward the ARR3 pro :GFP, ARR4 pro :GFP, and IPT pro :GFP constructs were generated using Gateway technology (www.thermofisher.com/gateway.html) by cloning these promoters into the binary vector pKGWFS7. Primers used for these constructs are listed in Appendix Table S2. Plants were transformed by employing the floral dip method using the Agrobacterium tumefaciens strain GV3101.
Confocal microscopy
Confocal micrographs were captured using a LSM‐700 device (Zeiss, Germany). To visualize the stress‐induced expression of DR5rev::GFP, TCSn:GFP, ARR1 pro :GFP, ARR3 pro :GFP, ARR4 pro :GFP, ARR10 pro :GFP, ARR12 pro :GFP, IPT1 pro :GFP, IPT2 pro :GFP, IPT3 pro :IPT3‐GFP, IPT5 pro :GFP, IPT7 pro :GFP, IPT9 pro :GFP transgenes, seedlings were grown in non‐supplemented nutrient solution for six days and then transferred to 25 μM AlCl3 for 2 h. T3 generations are used in all the experiments. We selected all least three independent lines for each construct to observe the expression pattern with or without Al (25 μM AlCl3 for 2 h) treatment. One representative line was selected for crosses or other chemical treatments. The roots were stained in propidium iodide to distinguish between living and dead cells. Roots were imaged in water supplemented with propidium iodide (PI, 10 μg/ml). PI and green fluorescent protein (GFP) were viewed at excitation wavelengths of 488 nm and 561 nm, respectively. Fluorescence emission was collected at 575 nm for PI and between 500 and 530 nm band pass for GFP. All of the confocal pictures are taken with a single plane, and 10 slices were used for each scanning. The representative images and images used for quantification are all from 10 slice constructions. The quantified fluorescent intensity is an average of selected regions of each seedling by ZEN2011 software, and these images are encoded in 16 bytes.
Yeast one‐hybrid (Y1H) assays
Yeast one‐hybrid (Y1H) assays were carried out by using the Matchmaker Gold Yeast One‐Hybrid Library Screening System (Clontech). To prepare constructs for the yeast one‐hybrid assay, the promoter regions of IPT1, IPT5, and IPT7 were amplified by PCR and cloned into the pAbAi vector. To generate AD‐ARF7, the coding sequence of ARF7 was amplified by PCR with the respective primers and cloned into the pGAD‐T7 vector (Clontech). The yeast one‐hybrid assay was performed according to the Yeast Protocols Handbook (Clontech). Briefly, the bait vector was linearized and introduced into yeast strain Y1H Gold to make a bait‐reporter strain, and then the prey vector was transferred into the aforementioned bait‐reporter yeast strain. Transformants were grown on SD/‐Leu dropout plates containing 75 ng/ml aureobasidin A. Primers used for generating various clones are listed in Appendix Table S2.
Dual‐luciferase transient expression assay in Arabidopsis protoplasts
For dual‐luciferase assays, promoter fragments of IPT, IPT5, and IPT7 were amplified by using specific primers and cloned into the pGreen0800‐LUC vector 84. The ARF7 effector construct was the 35S::ARF7‐YFP. For this construct, the ARF7 coding fragment was amplified by PCR and inserted into pDONR221 (Invitrogen). Then, the fragments were cloned into the Gateway‐compatible vector pBI221 by LR reaction. Firefly luciferase (LUC) reporters (pIPT:LUC) driven by the IPT promoter and 35S promoter driving the full‐length ARF7 cDNA (35S::ARF7‐YFP) was used as an effector, and the empty vector was included as a control. The reporter and effector constructs were co‐transformed into Arabidopsis protoplasts in the presence or absence of Al stress. The LUC and REN activities were measured, and the LUC activity was normalized to REN activity. Protoplasts were isolated from Arabidopsis Col‐0 plants as described 85 and transformed with 35S::ARF7‐YFP effector construct together with IPT1p:LUC, IPT5p:LUC, or IPT7p:LUC reporter constructs by the poly (ethylene glycol)‐mediated method 86. Firefly and Renilla luciferase activities were quantified by using a dual‐luciferase assay kit (Promega) and detected by use of a Synergy 2 multimode microplate (BioTek) as described 85. Primers used for generating various clones are listed in Appendix Table S2.
ChIP‐qPCR
For ChIP‐qPCR assays, promoter fragments of IPT, IPT5, and IPT7 were amplified by using specific primers which are listed in Appendix Table S3. The ARF7 recombinant construct was the 35S::MYC‐ARF7. For this construct, the ARF7 coding fragment was amplified by PCR and inserted into pDONR221 (Invitrogen). Then, the fragments were cloned into the Gateway‐compatible vector pGWB18 by LR reaction. The seedlings of 14‐day‐old 35S::MYC‐ARF7 plants were harvested and cross‐linked with 1% formaldehyde. ChIP was carried out using the EpiQuik Plant ChIP Kit (Epigentek, Brooklyn, NY, USA) with the antibody against MYC (ab9132; Abcam). Input samples and immunoprecipitated samples were analyzed by qPCR. Segments P2, P4, and P3 were used as negative control of IPT1, IPT5, and IPT7 genes, respectively. Mouse IgG was used as a mock control. Input sample was used to normalize the qPCR results in each ChIP analysis.
RNA isolation and quantitative real‐time PCR (qRT–PCR)
The roots were harvested and shock‐frozen in liquid nitrogen. Total RNA was isolated using TriPure isolation reagent (Roche), and first‐strand cDNA was synthesized from 1 μg of total RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche) following the manufacturer's protocol. Quantitative real‐time PCR was performed using the CFX Connect™ Real‐Time System (Bio‐Rad) with FastStart Universal SYBR Green Master (Rox) (Roche). For each qRT assay, we included three technical replicates and did three biological repeats with RNA extracted from at least 100 seedling roots. Values were normalized to the expression levels of UBQ1. The primers used for qRT–PCR are listed in Appendix Table S1.
Statistical analysis
Normality analysis and homogeneity test were performed before statistical analysis, and the unitized data satisfied both normal distribution and homoscedasticity (P > 0.05). Data sets were analyzed using Prism 6 software (GraphPad Software). Comparisons between two groups were made using Student's t‐test. All values were presented as mean ± SD; values of P < 0.05 were considered significant. *, **, *** denote differences significant at, respectively, P < 0.05, < 0.01, and < 0.001. Comparisons between multiple groups were made using one‐way ANOVA and Tukey's test. Different letters indicate significant differences (P < 0.01).
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database and the GenBank/EMBL database under the following accession numbers: CKX3 (AT5G56970, NM_125079), ARR1 (AT3G16857, NM_112561), ARR3 (AT1G59940, NM_104686), ARR4 (AT1G10470, NM_100921), ARR10 (AT4G31920, NM_119343), ARR12 (AT2G25180, NM_128075), CRE1 (AT2G01830, NM_179594), AHK2 (AT5G35750, NM_122966), AHK3 (AT1G27320, NM_102494), TAA1 (AT1G70560, NM_105724), SLR (AT4G14550, NM_117535), ARF7 (AT5G20730, AY008392), ARF10 (AT2G28350, NM_128394), ARF16 (AT4G30080, NM_119154), EIN3 (AT3G20770, NM_112968), EIL1 (AT2G27050, NM_128263), IPT1 (AT1G68460, NM_105517), IPT3 (AT3G63110, NM_116176), IPT5 (AT5G19040, NM_121909), IPT7 (AT3G23630, NM_113267), IPT9 (AT5G20040, NM_122011).
Author contributions
ZD, Z‐BY, and GL conceived the study and designed the experiments; GL, Z‐BY, ZD, JL, BZ, and WM carried out the experiments and analyzed the data. ZD, GL, IDS, Z‐BY, XZ, BM, ZZ, and K‐iH wrote the manuscript. All authors have agreed to the submitted version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Review Process File
Acknowledgements
We thank Prof. Tatsuo Kakimoto, Prof. Jiri Friml, Prof. Hongwei Guo, Prof. Eva Benkova, and Prof. G. Eric Schaller for sharing published materials. We thank Prof. Dolf Weijers for constructive comments on this manuscript. Z.D. is supported by grants from the Ministry of Science and Technology of China (2015CB942901), the National Natural Science Foundation of China (projects 31270327, 31470371, and 31222005), Shandong Provincial Funds for Distinguished Young Scholars (2014JQ201408). Z.‐B.Y. is supported by the National Natural Science Foundation of China (31400227) and the Shandong Provincial Natural Science Foundation project (ZR2014CQ021).
EMBO Reports (2017) 18: 1213–1230
References
- 1. Giehl RF, Gruber BD, von Wiren N (2014) It's time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot 65: 769–778 [DOI] [PubMed] [Google Scholar]
- 2. Gruber BD, Giehl RF, Friedel S, von Wiren N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163: 161–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith S, De Smet I (2012) Root system architecture: insights from Arabidopsis and cereal crops. Philos Trans R Soc Lond B Biol Sci 367: 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian H, De Smet I, Ding Z (2014) Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci 19: 426–431 [DOI] [PubMed] [Google Scholar]
- 5. Horst WJ, Asher CJ, Cakmak I, Szulkiewicz P, Wissemeier AH (1992) Short‐term responses of soybean roots to aluminium. J Plant Physiol 140: 174–178 [Google Scholar]
- 6. Kochian LV, Pineros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66: 571–598 [DOI] [PubMed] [Google Scholar]
- 7. Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z (2014) TAA1‐regulated local auxin biosynthesis in the root‐apex transition zone mediates the aluminum‐induced inhibition of root growth in Arabidopsis . Plant Cell 26: 2889–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44: 437–446 [Google Scholar]
- 9. Sivaguru M, Horst WJ (1998) The distal part of the transition zone is the most aluminum‐sensitive apical root zone of maize. Plant Physiol 116: 153–163 [Google Scholar]
- 10. Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- 11. Haug A, Foy CE (1983) Molecular aspects of aluminum toxicity. Crit Rev Plant Sci 1: 345–373 [Google Scholar]
- 12. Eswaran H, Reich P, Beinroth F (1997) Global distribution of soils with acidity. In Plant‐soil interactions at low pH, Moniz AC, Furlani AMC, Schaffert RE, Fageria NK, Rosolem CA, Cantarella H. (eds), pp 159–164. Campinas, Brazi: Brazilian Society of Soil Science; [Google Scholar]
- 13. Ding Z, De Smet I (2013) Localised ABA signalling mediates root growth plasticity. Trends Plant Sci 18: 533–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett‐Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4: 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munne‐Bosch S, Muller M (2013) Hormonal cross‐talk in plant development and stress responses. Front Plant Sci 4: 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pacifici E, Polverari L, Sabatini S (2015) Plant hormone cross‐talk: the pivot of root growth. J Exp Bot 66: 1113–1121 [DOI] [PubMed] [Google Scholar]
- 17. Vanstraelen M, Benkova E (2012) Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 28: 463–487 [DOI] [PubMed] [Google Scholar]
- 18. Antoniadi I, Plackova L, Simonovik B, Dolezal K, Turnbull C, Ljung K, Novak O (2015) Cell‐type‐specific cytokinin distribution within the Arabidopsis primary root apex. Plant Cell 27: 1955–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dello Ioio R, Linhares FS, Scacchi E, Casamitjana‐Martinez E, Heidstra R, Costantino P, Sabatini S (2007) Cytokinins determine Arabidopsis root‐meristem size by controlling cell differentiation. Curr Biol 17: 678–682 [DOI] [PubMed] [Google Scholar]
- 20. Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- 21. Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ (2013) Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol 23: 1979–1989 [DOI] [PubMed] [Google Scholar]
- 22. Chandler JW, Werr W (2015) Cytokinin‐auxin crosstalk in cell type specification. Trends Plant Sci 20: 291–300 [DOI] [PubMed] [Google Scholar]
- 23. Werner T, Schmulling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12: 527–538 [DOI] [PubMed] [Google Scholar]
- 24. Schafer M, Brutting C, Meza‐Canales ID, Grosskinsky DK, Vankova R, Baldwin IT, Meldau S (2015) The role of cis‐zeatin‐type cytokinins in plant growth regulation and mediating responses to environmental interactions. J Exp Bot 66: 4873–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumoto‐Kitano M, Kusumoto T, Tarkowski P, Kinoshita‐Tsujimura K, Vaclavikova K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105: 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyawaki K, Tarkowski P, Matsumoto‐Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103: 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi‐Shinozaki K, Shinozaki K, Kakimoto T et al (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T (2010) Root‐specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22: 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeon J, Kim NY, Kim S, Kang NY, Novak O, Ku SJ, Cho C, Lee DJ, Lee EJ, Strnad M et al (2010) A subset of cytokinin two‐component signaling system plays a role in cold temperature stress response in Arabidopsis . J Biol Chem 285: 23371–23386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi‐Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis . Proc Natl Acad Sci USA 104: 20623–20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mason MG, Jha D, Salt DE, Tester M, Hill K, Kieber JJ, Schaller GE (2010) Type‐B response regulators ARR1 and ARR12 regulate expression of AtHKT1;1 and accumulation of sodium in Arabidopsis shoots. Plant J 64: 753–763 [DOI] [PubMed] [Google Scholar]
- 32. Nguyen HT, Dawal SZ, Nukman Y, Rifai AP, Aoyama H (2016) An integrated MCDM model for conveyor equipment evaluation and selection in an FMC based on a fuzzy AHP and fuzzy ARAS in the presence of vagueness. PLoS ONE 11: e0153222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishiyama R, Watanabe Y, Leyva‐Gonzalez MA, Ha CV, Fujita Y, Tanaka M, Seki M, Yamaguchi‐Shinozaki K, Shinozaki K, Herrera‐Estrella L et al (2013) Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc Natl Acad Sci USA 110: 4840–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaller GE, Bishopp A, Kieber JJ (2015) The yin‐yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27: 44–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moubayidin L, Perilli S, Dello Ioio R, Di Mambro R, Costantino P, Sabatini S (2010) The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol 20: 1138–1143 [DOI] [PubMed] [Google Scholar]
- 36. Muller B, Sheen J (2008) Cytokinin and auxin interaction in root stem‐cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T (2003) Cytokinin‐deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU (2010) Hormonal control of the shoot stem‐cell niche. Nature 465: 1089–1092 [DOI] [PubMed] [Google Scholar]
- 39. Cary AJ, Liu W, Howell SH (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Street IH, Aman S, Zubo Y, Ramzan A, Wang X, Shakeel SN, Kieber JJ, Schaller GE (2015) Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol 169: 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruzicka K, Simaskova M, Duclercq J, Petrasek J, Zazimalova E, Simon S, Friml J, Van Montagu MC, Benkova E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci USA 106: 4284–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kollmeier M, Felle HH, Horst WJ (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun P, Tian QY, Chen J, Zhang WH (2010) Aluminium‐induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J Exp Bot 61: 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu XF, Lei GJ, Wang ZW, Shi YZ, Braam J, Li GX, Zheng SJ (2013) Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol 162: 1947–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang ZB, Eticha D, Albacete A, Rao IM, Roitsch T, Horst WJ (2012) Physiological and molecular analysis of the interaction between aluminium toxicity and drought stress in common bean (Phaseolus vulgaris). J Exp Bot 63: 3109–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zurcher E, Tavor‐Deslex D, Lituiev D, Enkerli K, Tarr PT, Muller B (2013) A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol 161: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sivaguru M, Liu J, Kochian LV (2013) Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J 76: 297–307 [DOI] [PubMed] [Google Scholar]
- 49. Ding Z, Friml J (2010) Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 107: 12046–12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M (2005) Tissue‐specific expression of stabilized SOLITARY‐ROOT/IAA14 alters lateral root development in Arabidopsis . Plant J 44: 382–395 [DOI] [PubMed] [Google Scholar]
- 51. Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis . Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu G, Gao S, Tian H, Wu W, Robert HS, Ding Z (2016) Local transcriptional control of YUCCA regulates auxin promoted root‐growth inhibition in response to aluminium stress in Arabidopsis . PLoS Genet 12: e1006360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F et al (2011) A small‐molecule screen identifies L‐kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene‐directed auxin biosynthesis and root growth in Arabidopsis . Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene‐response pathway identified in a screen for weak ethylene‐insensitive mutants in Arabidopsis . Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan X, Matsumoto H (2004) Effect of K‐252a and abscisic acid on the efflux of citrate from soybean roots. J Exp Bot 55: 663–671 [DOI] [PubMed] [Google Scholar]
- 56. Ha S, Vankova R, Yamaguchi‐Shinozaki K, Shinozaki K, Tran LS (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17: 172–179 [DOI] [PubMed] [Google Scholar]
- 57. O'Brien JA, Benkova E (2013) Cytokinin cross‐talking during biotic and abiotic stress responses. Front Plant Sci 4: 451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hirose N, Takei K, Kuroha T, Kamada‐Nobusada T, Hayashi H, Sakakibara H (2008) Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot 59: 75–83 [DOI] [PubMed] [Google Scholar]
- 59. Frebort I, Kowalska M, Hluska T, Frebortova J, Galuszka P (2011) Evolution of cytokinin biosynthesis and degradation. J Exp Bot 62: 2431–2452 [DOI] [PubMed] [Google Scholar]
- 60. Piya S, Shrestha SK, Binder B, Stewart CN Jr, Hewezi T (2014) Protein‐protein interaction and gene co‐expression maps of ARFs and Aux/IAAs in Arabidopsis . Front Plant Sci 5: 744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chae HS, Faure F, Kieber JJ (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hass C, Lohrmann J, Albrecht V, Sweere U, Hummel F, Yoo SD, Hwang I, Zhu T, Schafer E, Kudla J et al (2004) The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis . EMBO J 23: 3290–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type‐A ARR genes in Arabidopsis . Plant Cell 24: 2578–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Massot N, Nicander B, Barceló J, Poschenrieder C, Tillberg E (2002) A rapid increase in cytokinin levels and enhanced ethylene evolution precede Al3+‐induced inhibition of root growth in bean seedlings (Phaseolus vulgaris L.). Plant Growth Regul 37: 105–112 [Google Scholar]
- 65. Kumari M, Taylor GJ, Deyholos MK (2008) Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol Genet Genomics 279: 339–357 [DOI] [PubMed] [Google Scholar]
- 66. Kopittke PM, Moore KL, Lombi E, Gianoncelli A, Ferguson BJ, Blamey FP, Menzies NW, Nicholson TM, McKenna BA, Wang P et al (2015) Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol 167: 1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sjogren CA, Bolaris SC, Larsen PB (2015) Aluminum‐dependent terminal differentiation of the Arabidopsis root tip is mediated through an ATR‐, ALT2‐, and SOG1‐regulated transcriptional response. Plant Cell 27: 2501–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rounds MA, Larsen PB (2008) Aluminum‐dependent root‐growth inhibition in Arabidopsis results from AtATR‐regulated cell‐cycle arrest. Curr Biol 18: 1495–1500 [DOI] [PubMed] [Google Scholar]
- 69. Nezames CD, Sjogren CA, Barajas JF, Larsen PB (2012) The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum‐dependent root growth inhibition. Plant Cell 24: 608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA 104: 9900–9905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sawaki Y, Iuchi S, Kobayashi Y, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M et al (2009) STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol 150: 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Rybel B, Adibi M, Breda AS, Wendrich JR, Smit ME, Novak O, Yamaguchi N, Yoshida S, Van Isterdael G, Palovaara J et al (2014) Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis . Science 345: 1255215 [DOI] [PubMed] [Google Scholar]
- 73. Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H (2004) AtIPT3 is a key determinant of nitrate‐dependent cytokinin biosynthesis in Arabidopsis . Plant Cell Physiol 45: 1053–1062 [DOI] [PubMed] [Google Scholar]
- 74. Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141: 674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Argyros RD, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE (2008) Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20: 2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmulling T (2011) The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J 67: 157–168 [DOI] [PubMed] [Google Scholar]
- 77. Bartrina I, Otto E, Strnad M, Werner T, Schmulling T (2011) Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M et al (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14‐mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim HJ, Chiang YH, Kieber JJ, Schaller GE (2013) SCF(KMD) controls cytokinin signaling by regulating the degradation of type‐B response regulators. Proc Natl Acad Sci USA 110: 10028–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stepanova AN, Robertson‐Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1‐mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- 81. Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain‐of‐function mutation in the SOLITARY‐ROOT/IAA14 gene of Arabidopsis . Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- 82. Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S (1992) Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S, Shaff JE, Maron LG, Pineros MA, Kochian LV, Koyama H (2007) Characterization of AtALMT1 expression in aluminum‐inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis . Plant Physiol 145: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sun L, Yang ZT, Song ZT, Wang MJ, Sun L, Lu SJ, Liu JX (2013) The plant‐specific transcription factor gene NAC103 is induced by bZIP60 through a new cis‐regulatory element to modulate the unfolded protein response in Arabidopsis . Plant J 76: 274–286 [DOI] [PubMed] [Google Scholar]
- 86. Howell LA, Gulam R, Mueller A, O'Connell MA, Searcey M (2010) Design and synthesis of threading intercalators to target DNA. Bioorg Med Chem Lett 20: 6956–6959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Review Process File


