Abstract
α-Linolenic acid (ALA) supplementation or exercise training can independently prevent hepatic lipid accumulation and reduced insulin signaling; however, this may occur through different mechanisms of action. In the current study, obese Zucker rats displayed decreased phospholipid (PL) content in association with hepatic lipid abundance, and therefore, we examined whether ALA and exercise training would prevent these abnormalities differently to reveal additive effects on the liver. To achieve this aim, obese Zucker rats were fed control diet alone or supplemented with ALA and were sedentary or exercise trained for 4 wk (C-Sed, ALA-Sed, C-Ex, and ALA-Ex). ALA-Sed rats had increased microsomal-triglyceride transfer protein (MTTP), a protein required for lipoprotein assembly/secretion, as well as modestly increased PL content in the absence of improvements in mitochondrial content, lipid accumulation, or insulin sensitivity. In contrast, C-Ex rats had increased mitochondrial content and insulin sensitivity; however, this corresponded with minimal improvements in PL content and hepatic lipid accumulation. Importantly, ALA-Ex rats demonstrated additive improvements in PL content and hepatic steatosis, which corresponded with increased mitochondrial content, MTTP and apolipoprotein B100 content, greater serum triacylglyceride, and insulin sensitivity. Overall, these data demonstrate additive effects of ALA and exercise training on hepatic lipid accumulation, as exercise training preferentially increased mitochondrial content, while ALA promoted an environment conducive for lipid secretion. These data highlight the potential for combination therapy to mitigate liver disease progression.
Keywords: hepatic steatosis, mitochondrial capacity, lipid secretion, phospholipid
obesity is a very strong risk factor for the development of hepatic insulin resistance, a situation that directly impairs whole body glucose homeostasis. Attenuated insulin-mediated signaling within the liver has been partially attributed to an increase in hepatic triacylglyceride (TAG) (14, 47), diacylglyceride (DAG), and ceramide contents (12), as the accumulation of these reactive lipids can activate protein kinase C isoforms and protein phosphatase-2A (PP2A) to antagonize insulin signaling (1, 6, 8, 19, 25, 27, 46, 52–55, 60) and may also activate JNK-mediated kinases (29), which could further compromise hepatic insulin sensitivity (6). Therefore, elucidating strategies that are able to prevent lipid accumulation may help maintain hepatic insulin sensitivity and, in turn, combat the increasing health burden associated with obesity.
While the mechanisms regulating hepatic lipid accumulation remain poorly defined, reduced fatty acid oxidation and an enhanced capacity for lipogenesis have been implicated as key molecular events promoting hepatic lipid storage (14, 23, 42, 59). Interestingly, impaired very-low-density lipoprotein (VLDL) assembly/secretion may also contribute substantially to hepatic lipid retention, as choline deficiency in hepatocytes (61) and ablation of phosphatidylethanolamine N-methyltransferase (20), CTP:phosphocholine cytidylyltransferase (32), or phosphate cytidylyltransferase 2 (38) impair the synthesis of the phospholipids (PLs) phosphatidylcholine and phosphatidylinositol that support VLDL secretion and result in abnormal lipid accumulation. Furthermore, inhibition of microsomal triacylglycerol transfer protein (MTTP) (24) and apolipoprotein B100 (Apo B100) (57), fundamental proteins involved in VLDL assembly and secretion, similarly results in abnormal lipid accumulation. Importantly, while increased hepatic lipid accumulation has been associated with reductions in total PL content (29), the presence of omega-3 (n-3) fatty acids (2, 35), including the essential α-linolenic acid (ALA), can strongly influence hepatic PL content and composition (16, 29, 40). This raises the potential for lifestyle interventions to prevent hepatic lipid accumulation by improving PL profiles that can support lipid metabolism.
In addition to n-3 supplementation, exercise training can independently improve fatty acid contents within the liver and markers of hepatic insulin sensitivity (9, 33, 45, 48). However, the mechanisms underpinning exercise-mediated responses have largely been attributed to the induction of mitochondrial biogenesis/enhanced fatty acid oxidation (16, 42). Therefore, it remains possible that exercise and ALA work through divergent mechanisms that may be additive to reduce abnormal hepatic lipid accumulation and potentially improve insulin sensitivity. However, it is also possible for negative adaptations to occur due to n-3 polyunsaturated fatty acid (PUFA) antioxidant properties that could blunt mitochondrial biogenesis following training (15, 43, 51). Therefore, the current study aimed to determine whether combination therapy involving ALA and exercise training works additively to prevent hepatic lipid accumulation in concert with improved PL composition, and whether this corresponds with increased markers of mitochondrial content, VLDL secretion, and insulin signaling.
MATERIALS AND METHODS
Ethical approval.
All experimental protocols complied with the Canadian Council on Animal Care guidelines and were approved by the Animal Care Committee at the University of Guelph. Five- to 6-wk-old male lean (n = 8) and obese (n = 8) Zucker rats (Charles River, St. Constant, QC, Canada) were housed in a temperature-controlled room with a 12:12 h light-dark cycle and fed chow and water ad libitum. Animals were anesthetized (60 mg/kg sodium pentobarbital) and the liver was snap frozen and stored at −80°C. Following the assessment of basic obese liver characteristics (i.e., lean vs. obese Zucker rats), experiments were repeated in obese Zucker rats only to evaluate possible lifestyle interventions to offset hepatic abnormalities. After a 6-day acclimation period, obese rats were randomly assigned to a control-sedentary (C-Sed), ALA-sedentary (ALA-Sed), control-exercise (C-Ex), or ALA-exercise (ALA-Ex) group (n = 5/group).
Diet and exercise interventions.
Obese rats received either a control diet (AIN93G; Research Diets; 20% protein, 64% carbohydrate, and 16% fat; powdered) or were pair fed an ALA-supplemented diet (AIN93G + 10% flaxseed oil; 20% protein, 54% carbohydrate, 26% fat; powdered) with or without exercise training for 4 wk. Exercise-trained rats (C-Ex and ALA-Ex) were acclimated to motorized treadmills and ran 45 min a day five times per week at the following intensities: 15 m/min, 5% grade (week 1); 15 m/min, 10% grade (week 2); and 15 m/min, 15% grade (weeks 3 and 4).
Insulin-stimulated tissue collection.
To determine Akt phosphorylation as an indicator of insulin action following the dietary intervention, an intravenous bolus of insulin was administered (1.0 U/kg) 15 min before tissue collection.
Liver lipid analyses.
Livers were extracted and immediately weighed and snap frozen. Samples were freeze-dried, powdered under liquid nitrogen, and transferred into glass tubes containing 2 ml of methanol and butylated hydroxytoluene (0.01%), followed by the addition of 4 ml of chloroform and 1.5 ml of water. An internal standard (100 μl) was added containing triheptadecanoate (C17:0 TAG), diheptadecanoate (C17:0 DAG), and diheptadecanoic phosphatidylcholine (C17:0 PC) to correct for assay and extraction losses. Lipids dissolved in chloroform were separated into TAG, DAG, and PL using thin layer chromatography (TLC; Silica Plate 60, 0.25 mm; Merck) in a solvent containing heptane, isopropyl, and acetic acid (60:40:3, vol/vol/vol). Dried silica plates were sprayed using 2′7′-dichlorofluorscein (0.2% solution) in methanol and briefly exposed to ammonia vapors, and bands were visualized under ultraviolet light. Lipid classes (TAG, DAG, and PL) were scraped off plates according to appropriate standards (Sigma, Oakville, ON, Canada) and methylated (14% boron trifloride-methanol), and fatty acid methyl esters were extracted using pentane. Free cholesterol and cholesterol esters were eluted from the gel with chloroform, evaporated under a nitrogen stream, and redissolved in 2-propanol or diethyl ether, respectively. The content of free cholesterol and cholesterol esters was subsequently measured with a commercially available cholesterol diagnostic kit (BioMaxima). Samples were dissolved in hexane and analyzed using a Hewlett-Packard 5890 series II gas chromatograph, an Agilent J&W CP-Sil 88 capillary column (50 m × 0.25 mm inner diameter), and a flame-ionization detector at 220°C for 32 min. Individual fatty acids and standards were identified based on retention times. Total TAG, DAG, and PL were quantified as a sum of identified long chain fatty acids, including myristic (14:0), palmitic (16:0), palmitoleic (16:1n–7), stearic (18:0), oleic (18:1n–9), linoleic (18:2n– 6), linolenic (18:3n–3), arachidic (20:0), arachidonic (20:4n– 6), eicosapentaenoic (20:5n–3), behenic (22:0), docosahexaenoic (22:6n–3), and nervonic (24: 1n–9) acids.
Ceramides and sphingolipids were assessed using HPLC. Lipid extract in chloroform (50 μl) was transferred to a clean tube containing an internal standard 40 pmol of N-palmitoyl-derythro-sphingosine (C17 base). Samples were evaporated under a stream of nitrogen, dissolved in 1.2 ml of 1 M KOH in 90% methanol, and heated at 90°C for 1 h to convert ceramides to sphingosine, without the conversion to other sphingolipids such as sphingomyelin, galactosylceramide, or glucosylceramide, into free sphingoid bases (25). Chloroform and water were added to separate into upper and lower phases, whereby the upper phase was discarded. The lower phase was evaporated under nitrogen, and the free sphingosine was analyzed using HPLC. The calibration curve was prepared with N-palmitoylsphingosine (Avanti Polar Lipids) as a standard. The chloroform extract used for the analysis of ceramide contains small amounts of free sphingoid bases, and therefore, the concentration of ceramide was corrected for the level of free sphingosine measured in the same sample.
Liver glycogens.
Glucose monophosphates were degraded using 0.1 M sodium hydroxide for 10 min at 80°C. Samples were neutralized in a buffer containing 1 M HCl, 0.2 M citric acid, and 0.2 M Na2HPO4·7H2O. Total glycogen content was analyzed using spectrophotometric methods (30).
Citrate synthase activity.
Frozen liver (6–10 mg) was homogenized in Tris buffer (100 mM, pH 8.3) and freeze thawed in liquid nitrogen to lyse the mitochondria. Citrate synthase activity was measured using spectrophotometric methods as previously described (49).
Serum analyses.
Commercially available kits were used to analyze fasting triglyceride (colorimetric assay: Sigma-Aldrich) and cholesterol contents.
Western blotting.
Liver homogenates were diluted (1 μg/μl) and equal amounts of protein (5 μg protein) were loaded for Western blot detection of α-tubulin (Abcam, Cambridge, MA), OXPHOS (Mitoscience, Eugene, OR), total JNK (Cell Signaling), p-JNK (Cell Signaling), total ERK (Cell Signaling), p-ERK(Cell Signaling), catalase (Abcam), SOD2 (Abcam), 4-hydroxynonenal (4HNE; Alpha Diagnostic), oxyblot (Millipore), MTTP (Santa Cruz Biotechnology), diacylglyceride transferase (DGAT; Abcam), glycerol-3-phosphate acyltransferase (GPAT; Abcam), apolipoprotein B100 (Abcam), microsomal triglyceride transfer protein (Santa Cruz Biotechnology), total Akt (Cell Signaling), phospho-Akt ser473 (Cell Signaling), and phospho-Akt thr308 (Cell Signaling). Proteins were separated using SDS-PAGE, gels were cut, and samples for each target were transferred onto the same polyvinylidine difluoride membrane. Membranes were blocked, incubated in appropriate primary and secondary antibodies, and detected using enhanced chemiluminescence (ChemiGenius2 Bioimaging System; SynGene, Cambridge, UK).
Statistics.
All data were analyzed using SigmaPlot software (version 12.0; Systat Software, San Jose, CA). Data from the lean and obese characterization were analyzed using independent samples t-tests. Data from the lifestyle intervention comparisons were analyzed using two-way ANOVA followed by Student-Newman Keuls post hoc analysis. Associations between lipid classes were determined using Pearson r correlational analyses. Statistical significance was determined at P ≤ 0.05.
RESULTS
Obese Zucker rats have lower phosphatidylcholine, phosphatidylethanolamine, and cardiolipin in the liver.
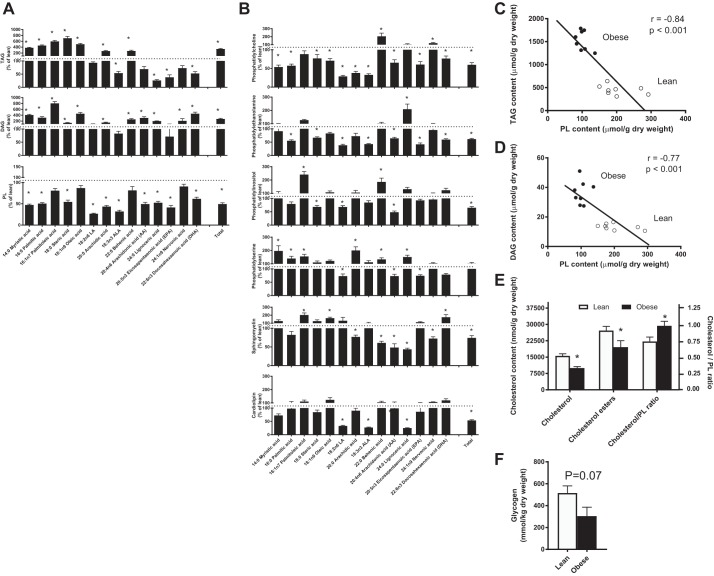
Obese Zucker rats are a well-characterized model of obesity, abnormal hepatic lipid accumulation (3), and insulin resistance. However, the importance of PL profiles in these rodents as a potential contributor to abnormal lipid secretion is unknown. Therefore, we first aimed to characterize hepatic lipid profiles during obesity. Obese rats had elevated total hepatic TAGs (Fig. 1A), DAGs (Fig. 1A), and ceramides (lean: 0.26 ± 0.03 and obese: 0.81 ± 0.13 μmol/g dry wt, P < 0.01). With respect to TAG and DAGs, these findings appeared to be driven by marked increases in saturated fatty acids, in association with reductions in ALA and its desaturase products eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Fig. 1A). In contrast, total PL contents were consistently lower in obese animals, regardless of fatty acid composition (Fig. 1B).
Fig. 1.
Hepatic lipid profiles of obese Zucker rats. Hepatic triacylglycerol (TAG), diacylglycerol (DAG), and phospholipid (PL) contents (A and B), correlations between PL content and TAG (C) and DAG (D) accumulation, and cholesterol/PL ratio (E) in lean and obese Zucker rats. ALA, α-linolenic acid. Data are expressed as means ± SE; n = 8/group. *P < 0.05, significantly different from lean.
Specifically, obese rats had lower PC content, an essential PL for VLDL secretion (61), as well as other important PLs including phosphatidylethanolamine, sphingomyelin, and phosphatidylinositol (Fig. 1B). Together, these PL account for ~80% of membrane composition, and decreased PL content has been shown to directly impair VLDL secretion (61). In support of this, PL content negatively correlated with both hepatic TAG (Fig. 1C) and DAG (Fig. 1D) content. Obese rats also had a higher ratio of cholesterol to PL content (Fig. 1E), which further supports a possible relationship among obesity, PL deficits, lipid accumulation, and an impaired environment for VLDL secretion. Interestingly, cardiolipin, a PL of the inner mitochondrial membrane (7, 36, 37), was also lower in obese rats (Fig. 1B). This was matched by lower markers of mitochondrial content (data not shown) and citrate synthase activity (lean: 30.06 ± 1.97 and obese: 21.90 ± 1.07 μmol·min−1·g−1, P < 0.05), which could contribute to impaired fatty acid oxidation and lipid accumulation. Moreover, liver glycogen content was reduced in obese rodents (Fig. 1F), suggesting impaired insulin suppression of glucose output, and supporting insulin resistance in this model (29). Altogether, these findings suggest decrements in PLs that may contribute to impairments in lipid secretion and fatty acid oxidation, resulting in hepatic TAG, DAG, and ceramide accumulation and possibly insulin resistance.
Establishing the beneficial effects of exercise and ALA within the liver.
We next aimed to identify lifestyle interventions that could possibly prevent the observed PL decrements and lipid accumulation within the liver. We focused on exercise and ALA supplementation, as both interventions have previously been shown to prevent weight gain, promote fatty acid oxidation (5), and alter lipid composition in peripheral tissues (56). In turn, this could contribute to improvements in PLs to support VLDL secretion. In addition, ALA content was lower in almost all lipid fractions examined in obese rats (Fig. 1), and therefore, the observed phenotype may also extend from a deficit of this key essential fatty acid.
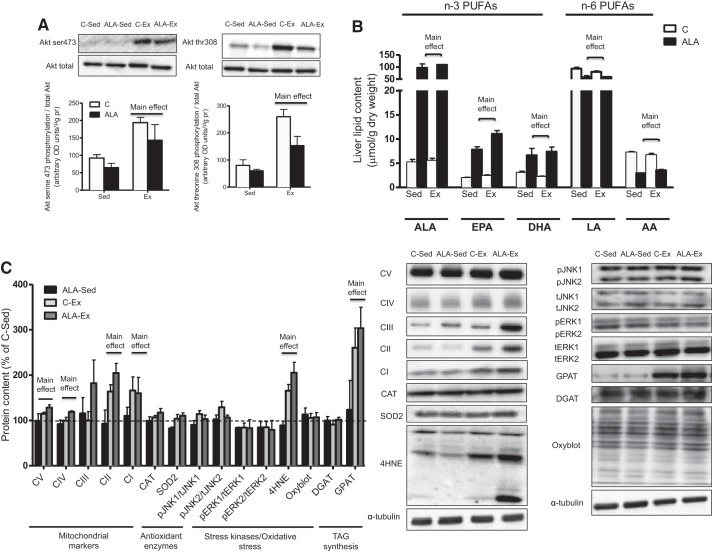
While exercise training decreased body weight, ALA alone had no effect (C-Sed: 422 ± 9.3, ALA-Sed: 401 ± 9.5, C-EX: 379.5 ± 9.1, and ALA-Ex: 381.8 ± 12.5 g, main effect, P < 0.01). In addition, while ALA independently did not alter Akt phosphorylation, exercise training improved insulin-stimulated Akt serine 473 and threonine 308 phosphorylation (Fig. 2A). Given that previous literature has shown that ALA supplementation for a longer period of time improves insulin sensitivity, we next verified that the 4-wk dietary intervention resulted in total PUFA accumulation within the liver. In this regard, the ALA diet (ALA-Sed and ALA-Ex) increased hepatic ALA ~20-fold, while also increasing the ALA desaturation products EPA and DHA ~5-fold (Fig. 2B). These changes appeared to be at the expense of n-6 PUFAs, as LA and AA were reduced in animals fed an ALA fortified diet (Fig. 2B). Therefore, 4 wk of dietary supplementation were sufficient for ALA accumulation within the liver, although these changes did not translate into an improvement in insulin-mediated Akt phosphorylation. We next examined glycogen levels and markers of mitochondrial content, stress kinase activation, and oxidative stress to ascertain if these could provide insight into the varied responses on insulin action following ALA consumption and exercise training. The independent consumption of ALA did not alter glycogen levels or the abundance of any of the proteins examined (Fig. 2C). In contrast, exercise training resulted in the expected increase in markers of mitochondrial content (Fig. 2C), citrate synthase activity (C-Sed: 21.61 ± 0.60, ALA-Sed: 22.18 ± 0.97, C-Ex: 25.46 ± 1.67, and ALA-Ex: 28.68 ± 1.80 μM·min−1·g−1, main effect of exercise, P < 0.05) and liver glycogen levels (C-Sed: 283.56 ± 21.81, ALA-Sed: 284.14 ± 87.12, C-Ex: 403.79 ± 61.43, and ALA-Ex: 418.51 ± 45.10 mmol/kg dry wt, main effect of exercise, P < 0.05). Markers of inflammation and antioxidant capacity were not altered in any group; however, 4HNE was increased in exercise-trained rats (Fig. 2C). Given the expected responses to independent ALA and exercise interventions on hepatic markers of metabolism, we next examined lipid content/composition to determine whether combined ALA-Ex resulted in cumulative effects to support PL contents, composition, and markers of VLDL secretion.
Fig. 2.
Insulin sensitivity, hepatic omega fatty acid composition, and protein markers of stress kinases, lipid peroxidation, oxidative stress, lipid synthesis, and mitochondrial content in control-sedentary (C-Sed), ALA-sedentary (ALA-Sed), control-exercise (C-Ex), or ALA-exercise (ALA-Ex) group obese Zucker rats. AKT phosphorylation (A), hepatic n-3 and n-6 polyunsaturated fatty acids (PUFAs) (B), markers of mitochondrial content, stress kinases, antioxidants, oxidative stress, and TAG synthesis (C) in obese Zucker rats. C, control; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LA, linoleic acid; AA, arachidonic acid; CAT, catalase; SOD, superoxide dismutase; GPAT, glycerol-3-phosphate acyltransferase; DGAT, diacylglyceride transferase; 4HNE, 4-hydroxynonenal; JNK, c-jun NH2-terminal kinases; OD, optical density. Data are expressed as means ± SE; n = 5/group. “Main effect” represents Ex greater than Sed or ALA greater than C.
ALA-Ex resulted in improved hepatic and serum lipid profiles.
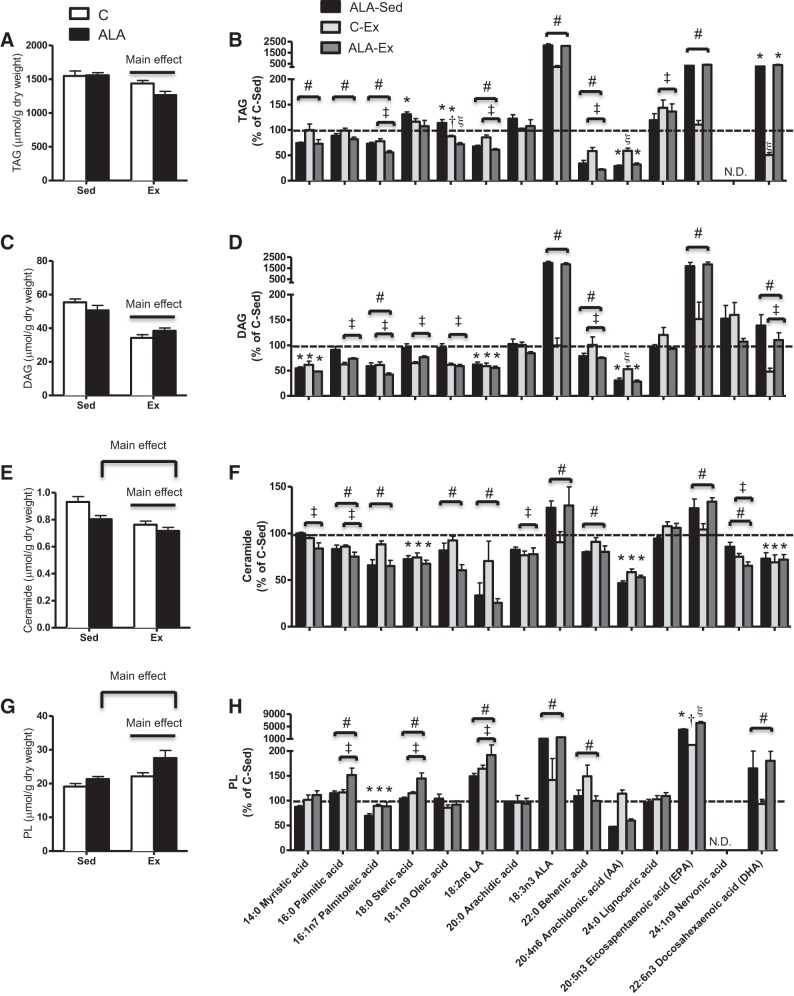
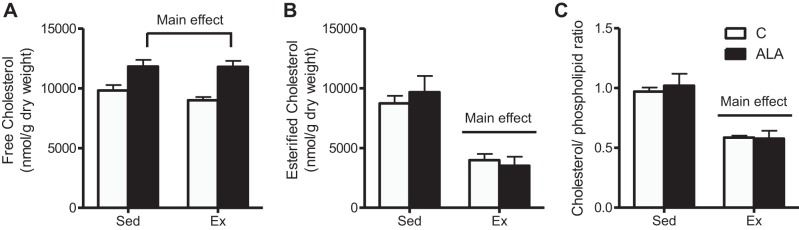
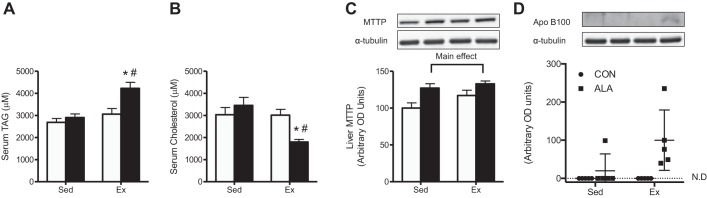
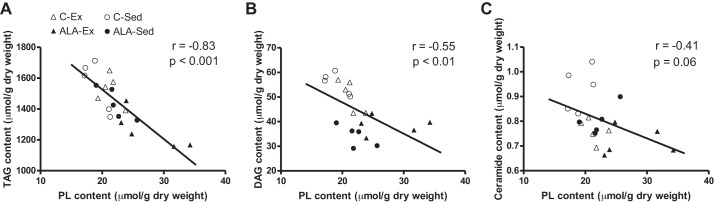
Exercise training reduced the liver weight ~25% (C-Sed: 27.3 ± 1.3, ALA-Sed: 27.5 ± 1.7, C-Ex: 20.3 ± 1.5, and ALA-Ex: 18.7 ± 1.4 g, main effect, P < 0.01) in concert with approximately threefold increase in GPAT protein content (Fig. 2C), a key enzyme involved in TAG (58) and PL (11) synthesis. In addition, exercised rats had lower hepatic TAG (Fig. 3, A and B), DAG (Fig. 3, C and D), and ceramides (Fig. 3, E and F), while ALA-supplemented groups had lowered ceramide contents than rats fed control diet (Fig. 3E). In general, ALA-supplemented groups had lower saturated fatty acids, lower n-6 contents, and higher n-3 contents within TAG, DAG, and ceramides (Fig. 3, B, D, and F). While exercise training increased hepatic PL content, this response was greater following combined exercise and ALA consumption (Fig. 3G). Altogether this appeared to be driven mainly by improved ALA, EPA, and DHA in ALA-supplemented groups, with particular importance of EPA in PL contents following ALA-Ex (Fig. 3H). ALA-supplemented groups also had higher hepatic free cholesterol, while exercised groups (C-Ex and ALA-Ex) had lower esterified cholesterol and cholesterol/PL ratios (Fig. 4, A–C). Altogether, these data suggest that combined ALA-Ex may be advantageous for promoting PL accumulation, which could contribute to improved hepatic lipid secretion (reduced ceramide and strong trend for a reduction in TAG within this group). This supposition is further supported in serum analysis, as TAG was the highest (Fig. 5A) and cholesterol the lowest (Fig. 5B), in the ALA-Ex group. Thus this suggests a greater capacity for VLDL secretion of TAG following the combined ALA-Ex in concert with higher PL content. In support of this, ALA-supplemented groups had higher liver MTTP protein content (Fig. 5C), and apolipoprotein B100 could only be detected in the ALA-supplemented groups (Fig. 5D). Altogether, these data support a greater capacity for lipoprotein assembly and secretion. There were also strong negative relationships between hepatic PL content and TAG (Fig. 6A), DAG (Fig. 6B), and ceramide (Fig. 6C) contents, highlighting the importance of PL content in supporting lipid retention/accumulation within the liver of obese rats.
Fig. 3.
Hepatic neutral, PL, and reactive lipid content from C-Sed, ALA-Sed, C-Ex, and ALA-Ex obese Zucker rats. Total and specific fatty acid content of TAG (A and B), DAG (C and D), ceramides (E and F), and PL (G and H) in obese Zucker rats. Data expressed as means ± SE; n = 5/group. *P < 0.05, significantly different from C-Sed; †P < 0.05, different from ALA-Sed; ξP < 0.05, different from all groups; #main effect for diet; ‡main effect for exercise.
Fig. 4.
Hepatic cholesterol content from C-Sed, ALA-Sed, C-Ex, and ALA-Ex obese Zucker rats. Free cholesterol (A), esterified cholesterol (B), and cholesterol/PL ratio (C) from obese Zucker rats. Data are expressed as means ± SE; n = 5/group. P < 0.05.
Fig. 5.
Serum lipid concentrations and hepatic microsomal triglyceride transfer protein (MTTP) and apolipoprotein B100 protein expression from C-Sed, ALA-Sed, C-Ex, and ALA-Ex obese Zucker rats. Serum TAG (A), serum cholesterol (B), liver MTTP protein (C), and apolipoprotein B100 (D) expression from obese Zucker rats. Data are expressed as means ± SE; n = 5/group. *P < 0.05, significantly different than ALA-Sed; #P < 0.05, significantly different than C-Ex.
Fig. 6.
Pearson r correlational analyses between hepatic PL and lipid accumulation from C-Sed, ALA-Sed, C-Ex, and ALA-Ex obese Zucker rats. PL was negatively associated with TAG (A), DAG (B), and ceramide (C) content from obese Zucker rats. P < 0.05; n = 5/group.
DISCUSSION
Overall, the present data suggest that ALA and exercise alone influence divergent metabolic processes, and combination therapy involving both ALA and exercise training exerted the greatest benefit on hepatic lipid accumulation. Specifically, ALA increased MTTP and PL content in the absence of improvements in hepatic lipid accumulation or insulin sensitivity. In contrast, exercise alone increased mitochondrial content and insulin action with only subtle improvements in PL content and lipid accumulation. While ALA-Ex rats had improved mitochondrial content, MTTP, and apolipoprotein B100 content, they also exhibited an additive response on PL content, which may support mitochondrial function and lipid secretion. Furthermore, our data demonstrate that improvements in hepatic lipid accumulation do not correspond proportionately with decreased markers of stress kinase activation, oxidative stress, or increased insulin sensitivity, divorcing the proposed relationship between these outcomes. Altogether, these data highlight the importance of hepatic mitochondrial capacity, MTTP/apolipoprotein B100 content, and the PL environment in mitigating hepatic lipid accumulation during the progression of obesity.
Effect of ALA supplementation on hepatic steatosis in obese rats.
Chronic ALA and EPA/DHA supplementation has previously been shown to improve tissue lipid profiles, hepatic lipid accumulation, and insulin sensitivity (28, 29). However, the independent administration of ALA in the current study did not improve either of these measures. This is potentially due to shorter ALA supplementation, as previous work showing the beneficial effects of n-3 PUFAs on hepatic lipid steatosis occurred after 8 wk. Regardless, in the present study, ALA supplementation resulted in ALA/EPA/DHA accumulation within the liver and, therefore, reveals that this alone is not sufficient to improve hepatic lipid profiles during obesity development. It is therefore likely that ALA accumulation affects secondary processes, such as gene transcription and ultimately increased protein expression, to influence hepatic lipid accumulation. While it remains possible that ALA increased PPAR-α accumulation to signal mitochondrial biogenesis (13), this clearly did not translate into increased mitochondrial content or citrate synthase activity or reduced TAG levels. However, ALA has also been shown to improve carnitine palmitoyl transferase activity (10) to promote fatty acid oxidation (18), and fish oil supplementation can inhibit the activities of fatty acid synthase, acetyl CoA carboxylase (21), and DGAT (4), which, although speculative, may have contributed to the reduced ceramide accumulation in ALA-supplemented groups. Additionally, ALA supplementation increased MTTP protein content, which corresponded with subtle improvements in total PL content. Together these results suggest a greater capacity to secrete lipids, which may have also contributed to the reduction in ceramide content in obese rodents. Regardless of the potential mechanism of action, ALA did not improve insulin-induced Akt phosphorylation, suggesting the observed reductions in hepatic ceramide content are not sufficient to improve hepatic insulin action. However, it is noteworthy that both ALA and LA supplementation have been shown to improve insulin resistance (29). Therefore, the displacement of total n-6 fatty acids in the current study, including LA, could have masked the effect of ALA-mediated improvements in insulin sensitivity. Overall, while 4 wk of ALA supplementation alone sufficiently increased MTTP, this did not parallel reductions in hepatic TAG accumulation or increased mitochondrial capacity and serum TAG concentrations, suggesting the independent increase in MTTP in the absence of supportive PL contents does not adequately improve hepatic lipid accumulation.
Effects of exercise training on hepatic lipid accumulation and insulin sensitivity in obese rats.
Increased mitochondrial content, and subsequently fatty acid oxidation, have been implicated as major regulators of hepatic lipid accumulation (50). While it has been demonstrated in humans that reductions in mitochondrial content are not required for the induction of insulin resistance (26), there is evidence that increasing mitochondrial content can improve insulin sensitivity (39), possibly by mitigating abnormal lipid storage through increased fat oxidation (31, 42). In addition, the lower PL content in obese rodents could also perpetuate lipid accumulation through impaired cardiolipin-related mitochondrial function (34) and reduced lipid secretion at a cellular level (61). In the present study, while ALA did not alter mitochondrial content, exercise training increased several markers of mitochondrial content/function, which likely promoted fatty acid oxidation to contribute to the observed reduction in hepatic lipids. However, in contrast to ALA supplementation, exercise training did not influence MTTP protein content, and only modestly increased PL content. Combined, these data suggest that exercise training was not sufficient to optimize the capacity for lipid secretion, an assertion supported by the finding that exercise did not alter serum TAG concentrations. Nevertheless, exercise training improved insulin-induced Akt phosphorylation and, therefore, improved hepatic insulin sensitivity independent of hepatic lipids.
Combined ALA and exercise resulted in greatest improvements in PL content and hepatic lipid accumulation.
The present data demonstrate that ALA and exercise interventions display divergent responses within the liver of obese rodents, mainly through elevated MTTP/apolipoprotein B100 protein content and mitochondrial capacity, respectively. With a combined ALA-Ex intervention, animals displayed increases in mitochondrial, MTTP, and apolipoprotein B100 protein contents and the greatest improvements in lipid profiles and PL content. The marked improvement in PL content following ALA-Ex may have supported improved VLDL secretion (i.e., MTTP, apolipoprotein B100), as PL decrements are known to impair this process (61), and ALA-Ex rats displayed elevated circulating serum TAG levels and the largest reduction in liver lipid contents. Moreover, since PL (41) and cholesterol (22) are important constituents of bile, the reduced serum cholesterol following ALA-Ex may suggest greater bile production to reduce hepatic lipids. This is supported by previous work (22), as well as the similar hepatic cholesterol contents among exercised groups. Moreover, as previously mentioned, an increase in the PL cardiolipin could also promote fatty acid oxidation and reduce lipid storage (34). This is especially plausible given the strong inverse relationship between hepatic PL content and various lipid species in obesity and following lifestyle interventions observed in the present study. While it is counterintuitive to consider an elevation in serum TAG to be advantageous during obesity, from the perspective of hepatic lipid contents, ALA-Ex appears to be the most beneficial. This is supported by similar insulin sensitivity between exercised groups, suggesting that circulating lipids following ALA-Ex did not attenuate insulin sensitivity. However, we should acknowledge that both Akt phosphorylation sites following ALA-Ex, although not different with a two-way ANOVA, were lower than C-Ex when analyzed with a t-test, and therefore, the lack of statistical difference could reflect the low sample size and a lack of power. If this possibility is accurate, perhaps this occurred as a result of the increased PUFA content in the PL fraction, as PUFAs are susceptible to oxidative stress (44). In turn, this could have partially blunted improvements in insulin sensitivity following ALA-Ex; however, this is highly speculative and does not account for possible transient changes in oxidative stress during exercise that can activate redox-sensitive processes. Alternatively, it has been suggested that only 10% activation of Akt is required to optimize insulin signaling (17), and therefore, these subtle apparent changes in Akt phosphorylation may have no biological effect on insulin response, particularly since liver glycogen levels were very similar between C-Ex and ALA-Ex groups. Clearly future work determining the ability of insulin to suppress hepatic glucose output is required to directly address this knowledge gap. Overall, while exercise alone promoted subtle improvements in hepatic PL content and reductions in lipid accumulation, ALA-Ex enhanced this response. This is possibly through a combination of increased fatty acid oxidation and lipid secretion, although the exact contribution of either pathway is currently unknown.
Conclusion and perspectives.
Altogether, these findings demonstrate that combined ALA and exercise training yield an additive effect on hepatic lipid accumulation but not insulin signaling in obese rodents. We suggest that this may be due to the combined effects of ALA and exercise on mitochondrial content, MTTP/apolipoprotein B100 expression, and total PL content. In this regard, improvements in all three parameters appear to be most beneficial to support the reductions in hepatic lipid accumulation independent of insulin signaling. Specifically, given that ALA and exercise independently increased MTTP and mitochondrial content, respectively, the additional benefit toward reducing lipid accumulation may be attributed to the increased PL content and apolipoprotein B100 following ALA-Ex. PL levels are important for both mitochondrial (34) and lipid secretion processes (61) and therefore support the strong inverse relationship between PL content and lipid accumulation. Although the current study did not demonstrate additive benefits on insulin sensitivity beyond exercise training alone, perhaps longer exposure to the combined intervention would reveal these improvements, and intensity of exercise should also be considered. Regardless, these data suggest interventions that target lipid PL contents could confer benefits on hepatic lipid metabolism in obese rodents, and this may be linked with improvements in mitochondrial capacity and lipid secretion to support reduced lipid storage and insulin sensitivity.
GRANTS
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) (to G. P. Holloway), and infrastructure was purchased with assistance from the Canadian Foundation for Innovation/Ontario Research Fund. P. M. Miotto is supported by an NSERC graduate scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.M.M., M.H., S.M., A.C., and G.P.H. performed experiments; P.M.M., M.H., A.C., and G.P.H. analyzed data; P.M.M., M.H., R.P., S.M., M.B., A.C., and G.P.H. interpreted results of experiments; P.M.M. and G.P.H. prepared figures; P.M.M. drafted manuscript; P.M.M., M.H., R.P., S.M., M.B., A.C., and G.P.H. edited and revised manuscript; P.M.M., M.H., R.P., S.M., M.B., A.C., and G.P.H. approved final version of manuscript; M.B. and G.P.H. conceived and designed research.
REFERENCES
- 1.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 106: 635–643, 2004. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 3.Argilés JM. The obese Zucker rat: a choice for fat metabolism 1968–1988: twenty years of research on the insights of the Zucker mutation. Prog Lipid Res 28: 53–66, 1989. doi: 10.1016/0163-7827(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 4.Baillie RA, Takada R, Nakamura M, Clarke SD. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids 60: 351–356, 1999. doi: 10.1016/S0952-3278(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 5.Batetta B, Griinari M, Carta G, Murru E, Ligresti A, Cordeddu L, Giordano E, Sanna F, Bisogno T, Uda S, Collu M, Bruheim I, Di Marzo V, Banni S. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J Nutr 139: 1495–1501, 2009. doi: 10.3945/jn.109.104844. [DOI] [PubMed] [Google Scholar]
- 6.Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y, Meikle PJ, Pitson SM, Febbraio MA. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes 61: 3148–3155, 2012. doi: 10.2337/db12-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292: C33–C44, 2007. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 8.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282: 22678–22688, 2007. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 9.Christ CY, Hunt D, Hancock J, Garcia-Macedo R, Mandarino LJ, Ivy JL. Exercise training improves muscle insulin resistance but not insulin receptor signaling in obese Zucker rats. J Appl Physiol (1985) 92: 736–744, 2002. doi: 10.1152/japplphysiol.00784.2001. [DOI] [PubMed] [Google Scholar]
- 10.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 72: 905–911, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Edwards PA, Tabor D, Kast HR, Venkateswaran A. Regulation of gene expression by SREBP and SCAP. Biochim Biophys Acta 1529: 103–113, 2000. doi: 10.1016/S1388-1981(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 12.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51: 679–689, 2010. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab 4: 199–210, 2006. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda N, Azain MJ, Ontko JA. Altered hepatic metabolism of free fatty acids underlying hypersecretion of very low density lipoproteins in the genetically obese Zucker rats. J Biol Chem 257: 14066–14072, 1982. [PubMed] [Google Scholar]
- 15.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gonçalves IO, Maciel E, Passos E, Torrella JR, Rizo D, Viscor G, Rocha-Rodrigues S, Santos-Alves E, Domingues MR, Oliveira PJ, Ascensão A, Magalhães J. Exercise alters liver mitochondria phospholipidomic profile and mitochondrial activity in non-alcoholic steatohepatitis. Int J Biochem Cell Biol 54: 163–173, 2014. doi: 10.1016/j.biocel.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. IRS1-independent defects define major nodes of insulin resistance. Cell Metab 7: 421–433, 2008. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ide T, Murata M, Sugano M. Stimulation of the activities of hepatic fatty acid oxidation enzymes by dietary fat rich in alpha-linolenic acid in rats. J Lipid Res 37: 448–463, 1996. [PubMed] [Google Scholar]
- 19.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs RL, Devlin C, Tabas I, Vance DE. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. J Biol Chem 279: 47402–47410, 2004. doi: 10.1074/jbc.M404027200. [DOI] [PubMed] [Google Scholar]
- 21.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem 277: 8755–8758, 2002. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem 274: 25892–25898, 1999. doi: 10.1074/jbc.274.36.25892. [DOI] [PubMed] [Google Scholar]
- 23.Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci 63: 1393–1409, 2006. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao W, Hui TY, Young SG, Davis RA. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J Lipid Res 44: 978–985, 2003. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund MT, Kristensen M, Hansen M, Tveskov L, Floyd AK, Støckel M, Vainer B, Poulsen SS, Helge JW, Prats C, Dela F. Hepatic mitochondrial oxidative phosphorylation is normal in obese patients with and without type 2 diabetes. J Physiol 594: 4351–4358, 2016. doi: 10.1113/JP272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magkos F, Su X, Bradley D, Fabbrini E, Conte C, Eagon JC, Varela JE, Brunt EM, Patterson BW, Klein S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 142: 1444–1446, 2012. doi: 10.1053/j.gastro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matravadia S, Herbst EA, Jain SS, Mutch DM, Holloway GP. Both linoleic and α-linolenic acid prevent insulin resistance but have divergent impacts on skeletal muscle mitochondrial bioenergetics in obese Zucker rats. Am J Physiol Endocrinol Metab 307: E102–E114, 2014. doi: 10.1152/ajpendo.00032.2014. [DOI] [PubMed] [Google Scholar]
- 29.Matravadia S, Zabielski P, Chabowski A, Mutch DM, Holloway GP. LA and ALA prevent glucose intolerance in obese male rats without reducing reactive lipid content, but cause tissue-specific changes in fatty acid composition. Am J Physiol Regul Integr Comp Physiol 310: R619–R630, 2016. doi: 10.1152/ajpregu.00297.2015. [DOI] [PubMed] [Google Scholar]
- 30.Miotto PM, Holloway GP. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport. Biochem J 473: 2831–2843, 2016. doi: 10.1042/BCJ20160373. [DOI] [PubMed] [Google Scholar]
- 31.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem 278: 21851–21859, 2003. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- 33.Oh S, Tanaka K, Warabi E, Shoda J. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc 45: 2214–2222, 2013. doi: 10.1249/MSS.0b013e31829afc33. [DOI] [PubMed] [Google Scholar]
- 34.Ohtsuka T, Nishijima M, Suzuki K, Akamatsu Y. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J Biol Chem 268: 22914–22919, 1993. [PubMed] [Google Scholar]
- 35.Pachikian BD, Essaghir A, Demoulin JB, Neyrinck AM, Catry E, De Backer FC, Dejeans N, Dewulf EM, Sohet FM, Portois L, Deldicque L, Molendi-Coste O, Leclercq IA, Francaux M, Carpentier YA, Foufelle F, Muccioli GG, Cani PD, Delzenne NM. Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets. PLoS One 6: e23365, 2011. doi: 10.1371/journal.pone.0023365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837: 408–417, 2014. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol 20: 14205–14218, 2014. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlovic Z, Bakovic M. Regulation of phosphatidylethanolamine homeostasis–the critical role of CTP:phosphoethanolamine cytidylyltransferase (Pcyt2). Int J Mol Sci 14: 2529–2550, 2013. doi: 10.3390/ijms14022529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 53: 1714–1721, 2010. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poudyal H, Panchal SK, Ward LC, Brown L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem 24: 1041–1052, 2013. doi: 10.1016/j.jnutbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Rahman K, Hammond TG, Lowe PJ, Barnwell SG, Clark B, Coleman R. Control of biliary phospholipid secretion. Effect of continuous and discontinuous infusion of taurocholate on biliary phospholipid secretion. Biochem J 234: 421–427, 1986. doi: 10.1042/bj2340421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 43.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab Physiol 272: E935–E940, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Saengsirisuwan V, Kinnick TR, Schmit MB, Henriksen EJ. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. J Appl Physiol (1985) 91: 145–153, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 47.Serkova NJ, Jackman M, Brown JL, Liu T, Hirose R, Roberts JP, Maher JJ, Niemann CU. Metabolic profiling of livers and blood from obese Zucker rats. J Hepatol 44: 956–962, 2006. doi: 10.1016/j.jhep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 17: 2162–2168, 2009. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srere PA. Citrate Synthase. New York: Academic, 1969. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- 50.Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Häring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia 51: 648–656, 2008. doi: 10.1007/s00125-008-0938-7. [DOI] [PubMed] [Google Scholar]
- 51.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc 43: 1017–1024, 2011. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 52.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 94: 242–251, 2008. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 55.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59: 2453–2464, 2010. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijaimohan K, Jainu M, Sabitha KE, Subramaniyam S, Anandhan C, Shyamala Devi CS. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci 79: 448–454, 2006. doi: 10.1016/j.lfs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 57.Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJ, Trip MD, Stroes ES. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res 51: 1057–1062, 2010. doi: 10.1194/jlr.M002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wendel AA, Cooper DE, Ilkayeva OR, Muoio DM, Coleman RA. Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J Biol Chem 288: 27299–27306, 2013. doi: 10.1074/jbc.M113.485219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 52: 1081–1089, 2003. doi: 10.2337/diabetes.52.5.1081. [DOI] [PubMed] [Google Scholar]
- 60.Wright LE, Brandon AE, Hoy AJ, Forsberg GB, Lelliott CJ, Reznick J, Löfgren L, Oscarsson J, Strömstedt M, Cooney GJ, Turner N. Amelioration of lipid-induced insulin resistance in rat skeletal muscle by overexpression of Pgc-1β involves reductions in long-chain acyl-CoA levels and oxidative stress. Diabetologia 54: 1417–1426, 2011. doi: 10.1007/s00125-011-2068-x. [DOI] [PubMed] [Google Scholar]
- 61.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 263: 2998–3004, 1988. [PubMed] [Google Scholar]