Abstract
By using harmonic radar, we report the complete flight paths of displaced bees. Test bees forage at a feeder or are recruited by a waggle dance indicating the feeder. The flights are recorded after the bees are captured when leaving the hive or the feeder and are released at an unexpected release site. A sequence of behavioral routines become apparent: (i) initial straight flights in which they fly the course that they were on when captured (foraging bees) or that they learned during dance communication (recruited bees); (ii) slow search flights with frequent changes of direction in which they attempt to “get their bearings”; and (iii) straight and rapid flights directed either to the hive or first to the feeding station and then to the hive. These straight homing flights start at locations all around the hive and at distances far out of the visual catchment area around the hive or the feeding station. Two essential criteria of a map-like spatial memory are met by these results: bees can set course at any arbitrary location in their familiar area, and they can choose between at least two goals. This finding suggests a rich, map-like organization of spatial memory in navigating honey bees.
Keywords: dance, communication, localization in navigation, vector orientation, vector map
Navigation of insects is believed to be composed of rather simple, isolated sensory-motor routines that are learned and opportunistically applied to solve quite complicated navigational tasks (refs. 1–4, but see also ref. 5). For example, bees and ants learn the directions and distances of their travels between nest and food sources by path integration (6), use the sun compass to apply these memories, and use landmarks to calibrate measured distances (7, 8). Also, bees can retrieve flight directions from landmarks when the sun compass is not available (9, 10). Importantly, bees are believed to relate all these navigational decisions to the origin of their flight path, the hive (4, 6, 11), and thus may apply a navigational motor routine that brings them back to the hive whenever a landmark is recognized to which they had associated the heading and distance measures of the home directing vector. The prevailing view is that bees do not “compute” the location of a landmark from this vector information but form some simple associations between landmarks and the motor commands that constitute the flight along the actual settings of their dead-reckoning system (bearing and distance to home) when they pass the landmark on their orientation flights.
This view always has been difficult to integrate with published and anecdotal reports that bees are able to find home after displacement within their foraging area. Wolf (12) saw bees flying toward the hive after they had searched around the release site, but he could not exclude that bees headed toward a visual beacon associated with the hive. Gould (13) observed the vanishing bearings of bees that were displaced after they left the hive and flew toward a feeding place. He concluded from his experiments that bees performed a novel shortcut flight to the feeder, and he argued in favor of a “cognitive map” in bee navigation, a capacity that requires that the bee computes its own current localization and that of the goal. Whenever bees were tested that had been trained to a feeder (as in Gould's experiments), bees were seen only to follow the direction of a flight routine that they were in the process of performing when captured (for review, see ref. 4). The first clear indication for a richer form of spatial memory than that expected from learned flight routes was derived from studies in which route training was avoided (11, 14). Under these conditions, bees appeared to refer to a different spatial memory than that developed during intensive route training, namely to the memory they had developed during their orientation flights. Orientation flights are performed by young bees before they start foraging and by experienced foragers after the hive has been displaced (9, 15, 16). Bees explore the landscape around the hive during orientation flights, and thus we can assume that they form an exploratory memory into which those route memories may be integrated, memories that were developed later during repetitive foraging flights. The questions addressed in this article are as follows. What is the structure of this exploratory memory? Do bees localize themselves relative to landmarks, and how do they return home?
To address these questions it is necessary to trace the full flight path of displaced bees, to manipulate the knowledge of bees with respect to flight routes to a feeding place, and to exclude the possibility that bees navigate toward the goal by using a beacon or large structures of the landscape as, for example, the profile of the horizon. We met these requirements by tracing the complete flight paths of displaced bees using a harmonic radar system (17, 18), by testing three groups of bees that differed with respect to their experience with a feeding place, and by performing these experiments in an area with a uniformly flat horizon and devoid of any visual structures marking the hive or the feeder beyond a 60-m radius around them. We found that the test bees followed novel shortcuts and chose between two goals, the hive and the feeder, which suggests a rich and a flexible form of spatial memory.
Materials and Methods
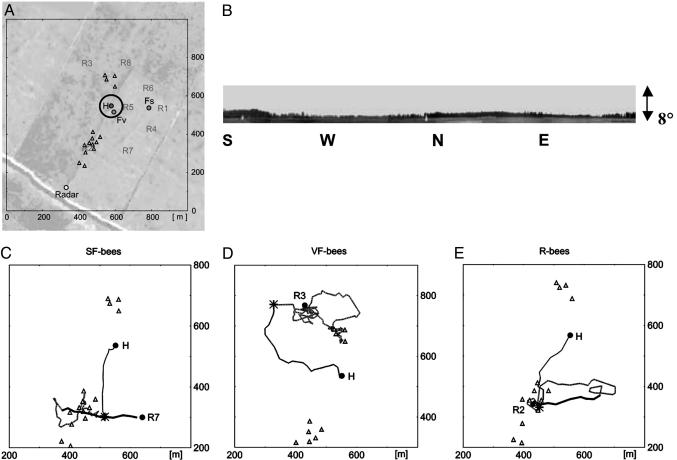
Study Site. The study site was located near Klein Lüben (Brandenburg, Germany, ≈150 km northwest of Berlin), an area that already was used by Lars Chittka and colleagues (19) in experiments on bee navigation. The data were collected during two study periods: July 25 to Aug. 7, 1999, and July 23 to Aug. 4, 2001. A small bee colony (two frames) in an observation hive was set up in a small, low, green tent in flat grassland without any natural landmarks other than the ground structure, which resulted from different mowing times and different soil conditions. Two groups of colored tripod tents (white, yellow, blue, and green; height: 3.8 m, width at the ground: 4 m) were set up as radar-transparent artificial landmarks; one group of 11 tents was between 190 m and 380 m south-southwest of the hive, and the other group of 4 tents was between 110 m and 170 m north-northwest of the hive (Fig. 1A). The profile of the horizon as seen from the hive did not provide any visual cues, because the angular fluctuation of the height above normal varied <1.5° (Fig. 1B). Experienced summer bees were allowed to reorient themselves for 3 days (first study period) or 6 days (second study period) before the experiments started. Very few natural food sources were available. A few patches of yellow composites bloomed in the northeast at distances of >350 m from the hive and showed open petals only for a few hours in the morning. Sparse and widely scattered clover flowered in the whole area in a rather even distribution. Weather conditions were fine throughout the first study period and during most of the second period, with a few cloudy days and 1 half-day with a completely overcast sky. Bees performed their orientation flights under sunny conditions, before the experiments started and after the tents were set up. The location of the hive and the arrangement of the tents were shifted 50 m to the east from the first to the second study period with the intention of testing how an extended landmark (the borderline between two sections of the grassland) influenced navigation performance. To test whether the tents were the only landmarks used by the bee, we shifted all tents to new locations for 2 days during the first study period. The new locations of the tents resembled the old arrangement rotated by 120° around the hive. We also tested the bees under conditions in which all tents were removed.
Fig. 1.
Study site and three representative flight paths. (A) Layout of the experimental site during the first period. Locations: the hive (H), the feeders (Fs, stationary feeder; Fv, variable feeder), the tents (▵), and the radar station (Radar). The circle around the hive has a radius of 60 m (visual angle of 1°). The ground structures resulting from differently mown grass and from soil conditions can be seen in this satellite image. A prominent extended ground structure was the borderline between two fields of grassland mown at different times that stretched from south-southwest to north-northeast and ran through the southern group of tents and the hive location during the first study period. During the second study period, the hive, feeder, tents, and radar station were shifted by 50 m to the east with respect to this borderline. (B) The profile of the horizon as seen from the hive. The maximal elevation above normal is ≈4°, and the angular variance lies within 1.5° and thus cannot be seen by the bees (see the supporting information). (C–E) Three examples of flight paths [SF bees (C), VF bees (D), and R bees (E)] showing the three flight phases: vector flight (bold line in SF and R bees), search flight (dotted line), and homing flight (thin line). The stars mark the homing points at each of the three tracks. (The supporting information provides 39 flight paths from all test groups.)
Recording of Flight Paths. The complete flight paths of bees were recorded by harmonic radar. The harmonic radar system monitors the flight path of an insect carrying the transponder antenna over a distance of up to 900 m and at a temporal resolution of up to 1/3 Hz (18). The data from the radar points were digitized and converted to an x/y coordinate system. The separation between consecutive radar readings depends on the flight speed of the bee and was in the range of 3–18 m under optimal conditions. Because radar points were occasionally lost in the noise, a search program was applied either to indicate incorrect connections between successive radar measurements or to identify lost radar points. Less than 1% of the radar points needed to be corrected by this method. In such rare cases, linear interpolations between safe measurements were applied, and flight paths were corrected by hand. No radar points were lost during the homing flights.
Experimental Groups. Three groups of bees were tested that had the same exploratory memory but differed with respect to route memory: (i) bees trained to a distant (200 m) stationary feeder (SF bees) have extensive route memory; (ii) bees trained to a variable feeder (VF bees) that circled around the hive within a short distance (10 m) lack route memory; and (iii) bees that were recruited (R bees) have “secondhand” route information from observing the recruitment dance (9). SF and VF bees were captured at the feeder after sucking to completion and transported in the dark to the release site. R bees were captured at the hive entrance after attending a dancing bee that indicated a feeder 200 m to the east. Because SF and VF bees were satiated before capture, they were motivated to return to the hive and thus would have applied the heading and distance measures for their return flights to the hive. These feeder-trained bees were loaded with sucrose solution and thus could afford to fly for quite some time before running out of energy. R bees were motivated to search for a new food source but had to fly home quickly because they carried only minimal food supply. A total of 285 radar traces were recorded (first study period: 195; second study period: 90; see also the supporting information, which is published on the PNAS web site). Several bees were released from the same release site two or three times. All data presented in this article refer to the first release.
Visual Orientation. To estimate the distance over which bees are able to visually detect a target and steer toward it, we assumed visual resolution of bees to be close to 1° (20–22). The hive and the experimenter sitting in front of it covered an area <2 × 2 m; therefore, bees should not see any beacon close to the hive over distances >60 m (see the circle around the hive in Fig. 1 A). The groups of tents should be visible to the bees at distances up to 100 m. Also, any structure of the panorama should not have been visible to the bees (Fig. 1B). Two observations support the interpretation that visual resolution is in the range of 1–2°. (i) A bee circled round the radar setup at a constant distance of 70 m. The diameter of the setup was ≈2.5 m, which results in a visual angle of 2°. (ii) Another bee flew up and down the borderline at a height of ≈9 m several times before returning to the hive. The farthest its flights were away from the 2-m-wide borderline (imprints of tractor tires) was 20 m.
To test for any features of the landscape or odor trails that might guide bees toward the hive (or the feeder), we released bees from a colony located 1.8 km away from the study area. If such features would have guided bees without the necessity to learn the landscape around the hive, these bees would have been attracted by the hive or feeder. These bees searched in the study area, but none approached the hive or the feeder, indicating that odor trails, beacons at the hive, or particular structures in the panorama did not provide guiding cues.
Homing Flights. The straightness of flight is an important parameter of goal-directed flights. It was calculated according to an algorithm that determined the transition from search flights to homing flights by calculating both the angular deviation between consecutive measuring points (point-to-point deviation) and the overall deviation for larger stretches of the flight path (long-range deviation). The first criterion is quite obvious: straight flights lead to a smaller angular deviation from point to point than do curved flights. The latter criterion is necessary because large-radius circling flights should also be excluded. To examine the point-to-point deviation, the algorithm started at the point where the flight path crossed a circle at 35 m around the hive (because when very close to the hive, bees tend to perform rather curved flights) and worked backward along the flight path until the angular deviation between three consecutive measurements exceeded 50°. A similar procedure was applied to evaluate the long-range deviation. Whenever the point-to-point deviation exceeded 50°, or the long-range deviation (sum of all normalized angles) exceeded 70°, the bee's radar fix just before this location was declared as the starting point of the straight flight to the hive (homing point).
Statistics. The t test was applied for dependent and independent samples. Circular data were first tested for uniformity by using the Rayleigh test and then analyzed with the Watson F test for two circular means (23).
Supporting Information. Additional procedures and further information and maps pertaining to the radar method and specific flight paths are available in Figs. 5–46 and the associated supporting text, which are published as supporting information on the PNAS web site.
Results
The Structure of Full Flight Paths. Typically, two phases of straight flights, interrupted by one phase of curved flights, can be distinguished (Fig. 1 C–E): an initial straight capture-vector flight (bold line in Fig. 1 C and E) in the compass direction and over the distance of the hive-to-feeder route the bees were pursuing when captured, followed by a curved search flight (dotted line in Fig. 1 C–E) and then by a straight homing flight (thin line in Fig. 1 C–E). These phases also can be distinguished with high concordance on the basis of flight speed, as capture-vector and homing flights were faster than search flights (SF bees: initial flights, 19.1 ± 2.4 km/h; search flights, 12.9 ± 3.5 km/h; homing flights, 19.4 ± 1.8 km/h; t test: vector/initial flights, t = 5.49, df = 212, P < 0.001; homing/search flight, t = 5.70, df = 212, P < 0.01).
Capture-vector flights were apparent only in bees that had the opportunity to establish a route memory, i.e., in SF and R bees but not in VF bees. This finding confirms that capture-vector flights, which are performed first, are based on the heading and distance measures of route memory, either from the bee's own experience or from the information imparted to it by the dance of a returning forager. SF bees always initially performed capture-vector flights. We never saw a SF bee steering directly toward the hive (or the feeding place) from the release site. Thus, if it is available, bees use their route memory during the initial phase. In R bees, we find that information gained by observing a dancing bee is indeed applied in their initial flight phase, thus directly proving von Frisch's (9) finding of information transfer about direction and distance of a food source in the waggle dance. We never saw R bees steering toward the feeder, indicating that only distance and direction from hive to feeder are transmitted in the recruitment dance and not the location of the feeding site.
Search flights were apparent in all three groups of bees. They often consisted of multiple returns to the release site and highly variable, relatively slow, curved flights. The third flight phase was a straight flight to the hive or first to the feeder and then to the hive (see the supporting information and, specifically, Figs. 8–46).
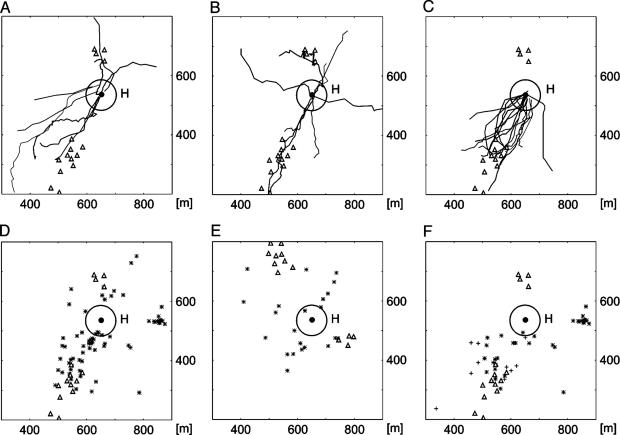
Direct Homing Flights to the Hive. After searching, bees from all groups showed fast and straight homing flights (Fig. 2 A–C). Because these homing flights could be observed in all groups, they must be based on exploratory memory. Importantly, the points where homing flights were initiated (“homing points,” indicated by stars in Fig. 1 C–E) were located well outside the radius around the hive beyond which orientation to a beacon at the hive is possible (60 m, Fig. 2 D–F), i.e., bees initiated homing flights when home was still invisible (see the supporting information). It may be argued that the ground structures around the hive might provide guiding signs from the distance. There was indeed an extended landmark (a borderline between differently mown patches of grassland, see Fig. 1 A) at which the hive was located during the first study period. Of 65 bees crossing this line outside the 60-m radius around the hive, 61 correctly turned toward the hive (17 north of the hive, 34 south of the hive), indicating that they located the hive in the correct direction (Fig. 7). In the second study period, this line did not lead to the hive, and bees did not turn toward the hive when crossing it. Otherwise, homing behavior did not differ during the two study periods, indicating that this grassland borderline was not required as a landmark for homing. This conclusion is supported by the finding that many homing flights in both study periods were initiated in areas far from this extended landmark.
Fig. 2.
Homing flights and homing points of the three test groups. (A–C) Shown are 35 examples of homing flights of SF bees (A), VF bees (B), and R bees (C) tested with the normal arrangement of the tents. The homing flight component of the full trace was detected by using the algorithm described in Materials and Methods. The bees in A and B were released at different sites around the hive, and those in C were released from three release sites in the southeastern to southern part of the study area. The final flight path of each bee is shown with a different line. Flight traces in A and B were recorded in the first study period, and those in C were recorded in the second study period. Because R bees performed very short search flights, and because they were released in the southeastern to southern sector close to the southern groups of tents, it is not surprising that their homing flights are close together. Notice that the borderline was located 50 m to the east as compared with the first study period, and bees did not follow the borderline when homing. (D–F) Localization of homing points for the three experimental groups: SF and VF bees, first study period, normal tent arrangement (D); SF bees, rotated tent arrangement, first study period (E); and R bees, second study period (F). In F, the stars mark the homing flights of R bees under sunny weather conditions, and the crosses indicate those under an overcast sky. The homing points were calculated by using the algorithm described in Materials and Methods. The test with the rotated tents (E) proves that landmarks on the ground are sufficient to allow homing. Notice that most homing points are within the visual range of the tents when the tents are arranged normally, but very few lie close to the tents when they are rotated, indicating that the tents play a role in localization. The results from R bees under an overcast sky show that homing does not require the sun compass.
We were concerned about the possibility that ground structures around the hive might provide guiding posts over larger distances than the estimated 60-m radius around the hive. There were no structures on the ground >2–3 m in diameter close to the hive, and larger patches of grassland with slightly different vegetation were distributed rather evenly in the whole study area. Bees approached the hive from all directions, thus excluding the possibility that they might have seen a particular spatial arrangement of grassland patches. Therefore, we conclude that bees were not guided to the hive by any beacons found close to the hive, in its surroundings, or in the profile of the horizon.
As Fig. 2 D–F also shows, bees had no problems flying back to the hive when the tents were rotated by 120° or removed. They also performed equally well when the sky was overcast. Both observations indicate that the ground structure provided sufficient information for navigation. However, the tents played a role in homing behavior, because the homing points lay well within the visual range of the tents when the tents were arranged normally, but very few homing points lay close to the tents when they were rotated. Thus, when the tents changed their location within the landscape, they ceased to function as landmarks.
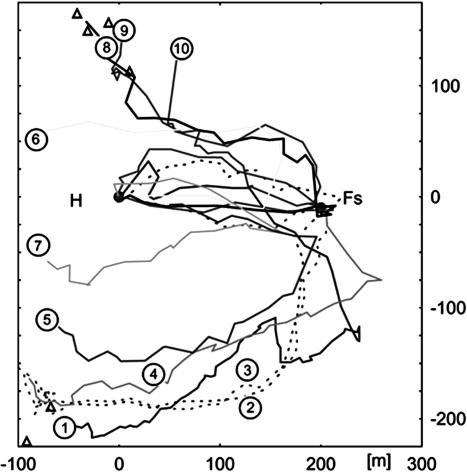
Homing Flights via the Feeder. From a group of 29 SF bees tested under the same conditions with respect to release site and weather, 10 did not perform homing flights directly toward the hive, rather they made a detour via the feeder (Fig. 3). Displaced SF bees, thus, clearly have the option to choose either the hive or the feeder as a goal. It is not clear under which conditions SF bees chose the direct flight or the flight via the feeder, because we did not find a correlation with length or distance flown during searching, the time of transport to the release site (always <15 min), or other parameters (e.g., weather or time of the day).
Fig. 3.
Homing flights via the feeder. Ten SF bees (of 29 bees tested under similar conditions) performed their homing flights via the feeder. Flight paths 2 and 3 come from bees that were tested during the second study period; the other paths come from those tested during the first study period. Bees released at release site 2 are shown by flight paths 1–5, and those released at release site 3 are indicated by flight paths 6–10. The bee from flight path 4 landed at the feeder and flew to the hive after filling its crop. All bees were tested with the normal arrangement of tents under sunny weather conditions. Notice that the coordinates are centered to the hive in an attempt to represent the flight paths of both study periods in one figure.
A closer inspection of Fig. 3 shows that the flight paths toward the feeder may resemble the vector components of their flight from the hive to the feeder (see flight paths 2, 3, 5, 6, 7, and 8). Thus 6 of the 10 bees that flew first to the feeder before returning to the hive may have applied the memory of the direction and distance of their outbound route flights. It is known that bees kept in an enclosure for an hour or longer perform initial straight flights at the release site along the heading from the hive to the feeder (based on our own observations and refs. 24 and 25). Thus bees may switch their motivation to outbound foraging flights when in need of food supply and then refer to the memory of heading and distance of their route flights from hive to feeder. Although we did not find a correlation of flight time or flight distance flown during the search phase in the 10 bees of Fig. 3, we might assume that an essential component in the switch from straight return flights to flights via the feeder to the hive is related to a change in motivation, namely to aim toward the feeder. Close to the feeder they corrected their flights and flew toward the hive. Only one bee landed at the feeder.
Discussion
Tracing the full flight paths of displaced bees clearly demonstrates that novel shortcuts were taken from any place around the hive within the range of orientations flights. Because the three groups of bees tested in our experiments have performed orientations flights but differed with respect to other navigational experience, we conclude that the information they used came from observations during orientation flights. This observation-based memory must have a structure that allows bees to perform novel shortcuts and to choose between two potential goals, the hive and the feeder. As ref. 26 points out, the study of spatial representation in navigating animals requires answers to three major questions: Are apparent shortcuts really novel, can path integration be excluded, and are familiar landmarks recognized from a new angle?
We cannot exclude the possibility that bees already may have returned from a particular location to the hive during their orientation flights, but they have certainly never flown from a particular location to the feeder because the feeder was always approached via direct flights from the hive, and bees always returned from the feeder to the hive in a direct flight. Thus the feeder-directed flights of our test bees were certainly novel, but we can fairly assume that also some proportion of the homing flights to the hive followed novel routes. On-line path integration over short distances can be excluded as a guiding mechanism for homing in our test situation because bees were transported to the release site in the dark. Path integration during orientation flights, however, must have provided the information for relating landmarks to the hive and possibly to each other (see ref. 27 for an explicit model showing how path integration can be used to construct a map). So, do bees use the information on headings and distances to “construct” vectors that localize landmarks, and, if so, do they perform novel shortcuts within a geometric arrangement of loci?
Familiar landmarks are recognizable by bees and associated with the home vector (28). In the case of an extended landmark (in our experiments a border between two textures on the ground), bees are known to follow such a landmark (9), and they do so here, a behavior known also from homing pigeons (29). Besides this extended landmark, which was not essential for homing, such landmarks were local landmarks provided by the ground structure and the tents. They were redundant because removing some of the landmarks (the tents) did not alter navigational performance. Bees reached the landmarks at which they initiated homing flights from many directions, and multiple sequential tests with the same bee started homing flights from different locations. We conclude, therefore, that familiar landmarks are recognized from different angles.
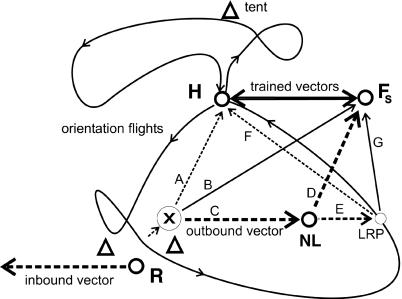
The knowledge established during the path integration process coupled to associative learning of landmarks during multiple observation flights can be used in two ways: localization of places in memory between which the animal navigates along novel routes, a procedure best described as reference to a spatial memory in the form of a map, and some form of integration of two or more memorized headings and distances. Fig. 4 summarizes an attempt to interpret our results as close as possible to the prevailing view of bee navigation. Several operations must be at the disposal of the animal: (i) associations of headings and distance measures toward the hive with a large number of landmarks all around the hive that are recognized from different directions; (ii) shift of motivation (flight to hive or feeder); (iii) reference to the outbound vector components of the route flight from hive to feeder; and (iv) addition and subtraction of the heading and distance components for at least two conditions, those that would lead directly back to the hive and those that lead from the hive to the feeder. It is difficult to imagine that these operations can be done without reference to vectors that relate locations to each other and, thus, make up a map. The question now in bee navigation is not so much whether there is a map-like spatial memory but rather what structure this map has and how it is used. For example, one may ask, do bees report about heading and distance in their dance for a feeding place or do they indicate a location of a feeding place? Obviously, this transmission depends on the receiving animal, the recruit. If it already had experience at this location (which it did not in our experiments), does it retrieve its memory about the properties of the feeding place? In that case, it would evaluate the message of the dance with respect to its knowledge about the indicated place and not only receive information regarding how to steer in a certain direction over a certain distance. In any case, the map-like memory in bees is rich and can be used in a flexible way. Any model of bee navigation thus has to incorporate a strategy based on a map-like representation of the bees' large-scale home range and a freedom to choose between at least two goals. This strategy further suggests that spatial relations between environmental features appear to be coherently represented in a map-like memory in insects, as is the case in other animals and humans (27, 30, 31).
Fig. 4.
A model of the vector map. During observatory flights (curved lines with arrows), bees establish multiple associations between landmarks and the respective return headings and distances to the hive (dotted lines A and F). In addition, SF bees learn the heading and distance between the hive (H) and the feeder (Fs) (double-headed arrow labeled trained vectors). After being released at the release site (R) and applying the information about heading and distance from the feeder to the hive stored in working memory, bees search for a while. When SF bees find themselves at a location ( ) at which a particular heading and distance are retrieved (A), they may either follow this information and return to the hive or switch their motivation and aim for the feeder. In such a situation they retrieve the vector components (heading and distance) of the outbound route flight from the hive-feeder to the feeder (C), which leads them to a new location (NL), or they perform some form of large-scale vector integration that leads them along a novel route to the feeder (B). At the novel location NL they may apply the same vector integration (D) or reach another known place (LRP) from which they go through the same procedure, use the associated flight parameters (F) to fly back to the hive directly or perform vector integration (G). However, our data also are consistent with the possibility that bees establish during orientation flights a relational map-like memory. In that case, they would localize themselves according to local landmarks and views, and they would choose a flight vector to the localizations of the hive or the feeder.
) at which a particular heading and distance are retrieved (A), they may either follow this information and return to the hive or switch their motivation and aim for the feeder. In such a situation they retrieve the vector components (heading and distance) of the outbound route flight from the hive-feeder to the feeder (C), which leads them to a new location (NL), or they perform some form of large-scale vector integration that leads them along a novel route to the feeder (B). At the novel location NL they may apply the same vector integration (D) or reach another known place (LRP) from which they go through the same procedure, use the associated flight parameters (F) to fly back to the hive directly or perform vector integration (G). However, our data also are consistent with the possibility that bees establish during orientation flights a relational map-like memory. In that case, they would localize themselves according to local landmarks and views, and they would choose a flight vector to the localizations of the hive or the feeder.
Supplementary Material
Acknowledgments
We thank Prof. Dr. J. Riley and Dr. D. R. Reynolds for assisting with the field studies and Drs. R. Gallistel, H. Mittelstaedt, B. Gerber, M. Giurfa, and R. Biegler for comments on an earlier version of this manuscript. This study was supported by Deutsche Forschungsgemeinschaft Grants Me 365/25-1 and Me 365/25-2. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC). This work was sponsored by BBSRC Grant 309/S15259.
Abbreviations: SF, stationary feeder; VF, variable feeder; R, recruited.
References
- 1.Wehner, R. (2003) J. Comp. Physiol. A 189, 579-588. [DOI] [PubMed] [Google Scholar]
- 2.Gallistel, C. R. (1998) in Conceptual and Methodological Foundations—Vol. 4 of an Invitation to Cognitive Science, eds. Sternberg, S. & Scarborough, D. (MIT Press, Cambridge, MA), 2nd Ed., pp. 1-51.
- 3.Collett, T. S. & Collett, M. (2002) Nat. Rev. Neurosci. 3, 542-552. [DOI] [PubMed] [Google Scholar]
- 4.Wehner, R. & Menzel, R. (1990) Annu. Rev. Neurosci. 13, 403-414. [DOI] [PubMed] [Google Scholar]
- 5.Mittelstaedt, H. (1983) in Fortschritte der Zoologie (Gustav Fischer, Stuttgart), pp. 197-212.
- 6.Wehner, R. (1992) in Animal Homing, ed. Papi, F. (Chapman & Hall, London), pp. 45-144.
- 7.Wehner, R., Michel, B. & Antonsen, P. (1996) J. Exp. Biol. 199, 129-140. [DOI] [PubMed] [Google Scholar]
- 8.Graham, P. & Collett, T. S. (2002) J. Exp. Biol. 205, 2499-2509. [DOI] [PubMed] [Google Scholar]
- 9.von Frisch, K. (1967) The Dance Language and Orientation of Bees (Harvard Univ. Press, Cambridge, MA).
- 10.Dyer, F. C. & Gould, J. L. (1981) Science 214, 1041-1042. [DOI] [PubMed] [Google Scholar]
- 11.Dyer, F. C. (1998) in Animal Cognition in Nature, eds. Balda, R., Pepperburg, I. & Kamil, A. (Academic, San Diego), pp. 119-154.
- 12.Wolf, E. (1927) Z. Vergl. Physiol. 6, 221-254. [Google Scholar]
- 13.Gould, J. L. (1986) Science 232, 861-863. [DOI] [PubMed] [Google Scholar]
- 14.Menzel, R., Brandt, R., Gumbert, A., Komischke, B. & Kunze, J. (2000) Proc. R. Soc. London B 267, 961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capaldi, E. A. & Dyer, F. C. (1999) J. Exp. Biol. 202, 1655-1666. [DOI] [PubMed] [Google Scholar]
- 16.Capaldi, E. A., Smith, A. D., Osborne, J. L., Fahrbach, S. E., Farris, S. M., Reynolds, D. R., Edwards, A. S., Martin, A., Robinson, G. E., Poppy, G. M., et al. (2000) Nature 403, 537-540. [DOI] [PubMed] [Google Scholar]
- 17.Riley, J. R., Valeur, P., Smith, A. D., Reynolds, D. R., Poppy, G. M. & Löfstedt, C. (1997) J. Insect Behav. 11, 287-296. [Google Scholar]
- 18.Riley, J. R., Smith, A. D., Reynolds, D. R., Edwards, A. S., Osborne, J. L., Williams, I. H., Carreck, N. L. & Poppy, G. M. (1996) Nature 379, 29-30.8538737 [Google Scholar]
- 19.Chittka, L. & Geiger, K. (1995) Anim. Behav. 49, 159-164. [Google Scholar]
- 20.Laughlin, S. B. & Horridge, G. A. (1971) Z. Vergl. Physiol. 74, 329-335. [Google Scholar]
- 21.Wehner, R. (1981) in Handbook of Sensory Physiology VIc, ed. Autrum, H. J. (Springer, Berlin), pp. 287-616.
- 22.Giurfa, M. & Menzel, R. (1997) Curr. Opin. Neurobiol. 7, 505-513. [DOI] [PubMed] [Google Scholar]
- 23.Zar, J. H. (1984) Biostatistical Analysis (Prentice–Hall, Englewood Cliffs, NJ).
- 24.Dyer, F. C., Gill, M. & Sharbowski, J. (2002) Naturwissenschaften 89, 262-264. [DOI] [PubMed] [Google Scholar]
- 25.Wei, C. A., Rafalko, S. L. & Dyer, F. C. (2002) J. Comp. Physiol. A 188, 725-737. [DOI] [PubMed] [Google Scholar]
- 26.Bennett, A. T. D. (1996) J. Exp. Biol. 199, 219-224. [DOI] [PubMed] [Google Scholar]
- 27.Gallistel, C. R. (1990) The Organization of Learning (MIT Press, Cambridge, MA).
- 28.Menzel, R., Geiger, K., Müller, U., Joerges, J. & Chittka, L. (1998) Anim. Behav. 55, 139-152. [DOI] [PubMed] [Google Scholar]
- 29.Lipp, H.-P., Vyssotski, A. L., Wolfer, D. P., Renaudineau, S., Savini, M., Troster, G. & Dell'Omo, G. (2004) Curr. Biol. 14, 1239-1249. [DOI] [PubMed] [Google Scholar]
- 30.Shelton, A. L. & McNamara, T. P. (1997) Psychonom. Bull. Rev. 4, 102-106. [Google Scholar]
- 31.Klatzky, R. L. (1998) in Spatial Cognition: An Interdisciplinary Approach to Representing and Processing Spatial Knowledge, eds. Freksa, C., Habel, C. & Wender, K. F. (Springer, New York), pp. 1-17.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.