Abstract
The primed-continuous (PC) phenylalanine (Phe) stable isotope infusion methodology is often used as a proxy for measuring whole body protein breakdown (WbPB) in sepsis. It is unclear if WbPB data obtained by an easy-to-use single IV Phe isotope pulse administration (PULSE) are comparable to those by PC. Compartmental modeling with PULSE could provide us more insight in WbPB in sepsis. Therefore, in the present study, we compared PULSE with PC as proxy for WbPB in an instrumented pig model with Pseudomonas aeruginosa-induced severe sepsis (Healthy: n = 9; Sepsis: n = 13). Seventeen hours after sepsis induction, we compared the Wb rate of appearance (WbRa) of Phe obtained by PC (L-[ring-13C6]Phe) and PULSE (L-[15N]Phe) in arterial plasma using LC-MS/MS and (non)compartmental modeling. PULSE-WbRa was highly correlated with PC-WbRa (r = 0.732, P < 0.0001) and WbPB (r = 0.897, P < 0.0001) independent of the septic state. PULSE-WbRa was 1.6 times higher than PC-WbRa (P < 0.001). Compartmental and noncompartmental PULSE modeling provide comparable WbRa values, although compartmental modeling was more sensitive. WbPB was elevated in sepsis (Healthy: 3,378 ± 103; Sepsis: 4,333 ± 160 nmol·kg BW−1·min−1, P = 0.0002). With PULSE, sepsis was characterized by an increase of the metabolic shunting (Healthy: 3,021 ± 347; Sepsis: 4,233 ± 344 nmol·kg BW−1·min−1, P = 0.026). Membrane transport capacity was the same. Both PC and PULSE methods are able to assess changes in WbRa of plasma Phe reflecting WbPB changes with high sensitivity, independent of the (patho)physiological state. The easy-to-use (non)compartmental PULSE reflects better the real WbPB than PC. With PULSE compartmental analysis, we conclude that the membrane transport capacity for amino acids is not compromised in severe sepsis.
Keywords: protein breakdown, pulse stable isotope method, phenylalanine, sepsis, pig
the primed-constant and continuous infusion methodology (PC) is often used for measuring whole body rate of appearance (WbRa) of amino acids in humans and animals (8, 11, 29). The principle is that a single-pool model (Fig. 1) in which amino acids (e.g., from whole body protein breakdown, WbPB) are appearing is the same pool in which the stable amino acid isotopes are infused. It is assumed that the pool represents mainly the extracellular compartment. In this model, WbRa is calculated by dividing the tracer (stable isotope) infusion rate by the tracer/tracee ratio (TTR) in the extracellular pool at steady state, in which the tracee is the natural occurring amino acid. Arterial blood is mostly used to represent the extracellular pool. The main advantage of measuring WbRa using this method is that when the TTR is low, the effects on tracee metabolism are minimal. Furthermore, when steady state is obtained, only two blood samples are needed (background and at steady state). The main disadvantage is that it might take several hours to reach a tracer steady state, although this can be overcome by correctly priming the tracer pool. The estimation of the pool size for stable tracer studies depends on the size of the plasma and tissue pool of the tracee. In studies in diseased animals or humans, the individual variation of the pool size affects the probability to correctly prime the pool. In sepsis for example, plasma phenylalanine (Phe) concentrations can be increased twofold, while other amino acid concentrations also can be increased many fold up or down (14). Furthermore, it is very difficult to correctly prime the pools of urea, glutamine, taurine, and 3-methylhistidine (16, 27). Moreover, the PC method is highly dependent on the accuracy of the infusion pump. Although the calculation is very simple, WbRa is the only information that can be obtained from the PC method as no further metabolic modeling is possible.
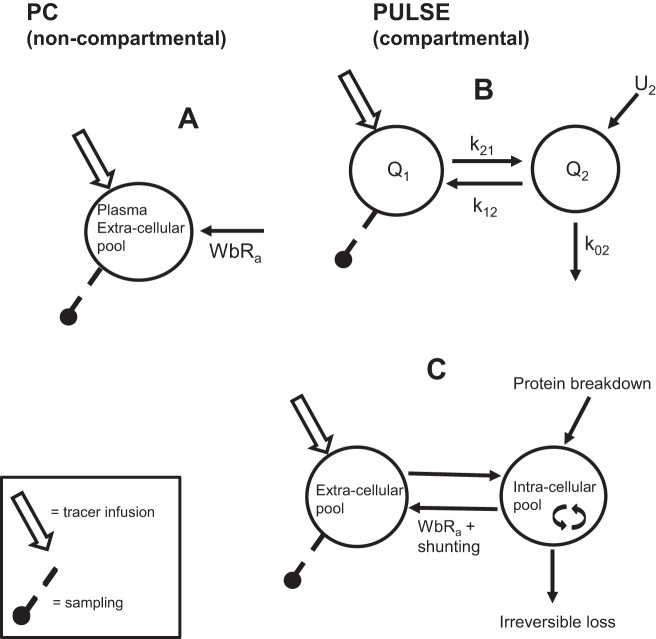
Fig. 1.
Calculation models for whole body protein breakdown. Two calculation models for whole body protein breakdown (WbPB) by determination of whole body rate of appearance (WbRa) of phenylalanine (Phe). A: noncompartmental model using a primed-continuous infusion of L-[ring-13C6]Phe (PC). WbRa is the tracer infusion rate divided by the fraction of tracer found in plasma/extracellular pool (TTR = tracer/tracee ratio) in a tracer steady state. B: compartmental model using bolus infusion, L-[15N]Phe (PULSE) and SAAMII computer modeling. Computer parameters: Q = pool sizes of the compartments; k21 = rate parameter to pool 2, from pool 1; k02 = rate parameter of irreversible loss from pool 2; U2 = rate of appearance of Phe in pool 2. C: physiological assignment of SAAMII computer model. Flux (F12) of Phe from the intracellular pool to the extracellular pool represents the k21 times Q1, and is equal to F21 in physiological steady state; U2 ( = F20) represents WbPB; Flux of Phe from the intracellular pool to hydroxylation and protein synthesis represents the irreversible loss (F02 = k02Q2); WbRa of Phe is the fraction of Phe from protein breakdown that appears in Q1 that is not irreversibly lost. The amount of Phe flux between Q1 and Q2 that is not irreversibly lost is shunt back to Q1 (F21-WbRa).
To overcome these disadvantages, we explored the use of a single pulse of tracer (PULSE) into the extracellular pool (Fig. 1) to measure WbRa (29). In this approach, the shape of the TTR decay curve depends on how many pools are connected to the extracellular pool. For most substrates like amino acids, the intracellular pool is the main secondary pool and the amino acid decay curve therefore reflects two compartments. This TTR decay curve can be used to perform both noncompartmental and compartmental modeling (12, 19, 21). Compartmental modeling enables the calculation of the Ra into the extracellular and the intracellular pools, as well as the fluxes between the pools. The appearance of an essential amino acid (e.g., Phe) into the intracellular pool represents protein breakdown (PB). Advantages of the PULSE method are that only one single tracer dose needs to be administered, no pumps are required, low pharmacy costs, and there is no need to prime. A major disadvantage however is that multiple blood samples are needed to describe the enrichment decay curve accurately. Although the calculations are not as simple as with the PC model, modern modeling/fitting programs are more available and user friendly. For routine Ra measurements, the noncompartmental WbRa calculation in the PULSE method using the fitting of the decay curve with, e.g., the software program GraphPad Prism or add-ins of EXCEL, and additional relative simple calculations of WbRa can be attractive (29). Previously, the computer modeling program SAAM has been used in the compartmental analysis to measure 3-methylhistidine appearance (as indirect marker of myofibrillar muscle PB) (22) or citrulline production in humans (10). Compartmental modeling can be extended to measure the flux between the different metabolic routes (12, 15). PULSE methods with a small amount of tracer are also used to measure tissue-specific fractional protein synthesis (FSR) and breakdown (FBR) (2, 26, 30, 31), but these methods require additional sampling from the tissue compartment.
In the past, comparisons of the tracer PULSE and PC infusion methods were done with theoretically modeling (20), in healthy subjects and animals (19, 22, 31), but not in disease states. In disease conditions with large changes in the tracee pools and in situations in which there is a large effect of the disease on, e.g., whole body protein breakdown, it is unclear if the PULSE method is providing the same clinical information as the more generally used PC method. Specifically in disease states, the additional information coming from the PULSE compartmental modeling can increase the understanding of the clinical situation. Therefore, in the present study, we measured whole body rate of appearance (WbRa) of Phe as proxy for WbPB using the PC protocol in comparison to a single-dose PULSE tracer protocol using Phe stable isotopes during Pseudomonas aeruginosa (PM)-induced severe sepsis in the pig. The severe septic state is used as a disease model in which large changes in tracee pools and substantial effects on WbPB can be expected (23). We hypothesize that both the PC and the PULSE method are able to provide the expected enhanced WbPB information in severe sepsis. We compared the more practical noncompartmental modeling with the PULSE method with the compartmental modeling to obtain this information for a routine setting. Finally, we explored with the compartmental modeling the extra intracellular information (e.g., membrane transport capacity).
MATERIALS AND METHODS
Animals
We used female Yorkshire cross/domestic pigs (20–25 kg BW) in our experimental studies. We housed the pigs in steel pens (2 m × 3 m) in a controlled housing facility (large animal cubicle, room temperature 22–24°C, 12:12- h light-dark cycle, standardized food 1 kg/day (Harlan Teklad Vegetarian Pig/Sow Grower) and provided water ad libitum.
Surgical Procedure
Animals received catheters and a jejunal stoma during a surgical procedure as described in detail previously (6, 18). In brief, during midline laparotomy, we placed catheters for blood sampling into the abdominal aorta and in the caval vein for administering postsurgery medication and experiment-related tracer infusions. We placed a second arterial catheter to monitor mean arterial blood pressure (MAP) and a Swan ganz catheter (5 Fr, no.132F5, Edwards Life Sciences, Irvine, CA) via the right jugular vein to monitor mean pulmonary blood pressure (MPAP). We standardized preoperative and postoperative care (6, 18). During the recovery period (7–10 days) animals were accustomed to a small movable cage (0.9 × 0.5 × 0.3 m) and performed the experiments in this cage in awake animals. This study was approved by the animal experiment ethics committee of University of Arkansas Medical Sciences.
Experimental Design
The experiment started after a recovery period of 7–10 days (Fig. 2). Four hours after the last food intake (half of the daily amount: 0.5 kg), we selected animals for the Sepsis group and the Healthy group in a randomized fashion (Healthy n = 9, Sepsis n = 13). We monitored basal monitoring blood pressures in the preseptic period (t = −2 h to 0 h). At t = 0 h, we induced sepsis by continuous infusion of Pseudomonas aeruginosa (PM, 109 CFU·ml−1·h−1), whereas the Healthy group received an equal volume of 0.9% NaCl solution. We started fluid resuscitation (30 ml·kg body wt−1·h−1) also at t = 0 h and monitored hemodynamics continuously. For 1 h, between 17 and 18 h after the start of PM, we compared the PC tracer protocol with the PULSE tracer protocol and at t = 18 h, we euthanized the pigs with 125 mg/kg pentobarbital sodium and 16 mg/kg phenytoin sodium (Euthanasol) administered via the central vein catheter.
Fig. 2.
Protocol. PC: primed-continuous infusion L-[ring-13C6]phenylalanine (Phe). PULSE: bolus infusion L-[15N]Phe. MPAP, mean pulmonary artery pressure, MAP, mean arterial pressure.
Pseudomonas aeruginosa
For the induction of sepsis, we used a live PM human strain (IRS 12-4-4, Shriners Burns Institute, University of Texas Medical Branch, Galveston, TX). Originally this PM strain was isolated from a burn patient at Brook Army Medical Center in San Antonio, TX. Based on pilot virulence experiment, 109 colony-forming units per hour (CFU/h) in a volume of 1 ml 0.9% NaCl solution was needed to obtain similar cardiovascular responses and hemodynamic variables with characteristics of severe hyperdynamic sepsis. We monitored hemodynamics continuously to ensure that the hyperdynamic state was kept in the expected ranges for severe sepsis (body temp increase of 2–3°C, respiratory rate increased, MPAP increased but <35 mmHg, heart rate increased but <200 beats/min).
Infusion and Sampling Protocol
Stable isotopes.
We used two stable isotopes of Phe: L-[ring-13C6]Phe and L-[15N]Phe (Cambridge Isotopes, Andover, MA) as tracers to study WbRa of Phe with two different tracer infusion protocols. We previously used Phe to determine WbPB (3, 18, 29). We based the prime amount and tracer infusion rates on our previous studies (3). For the PC infusion protocol, we used L-[ring-13C6]Phe. The prime [1.58 µmol/kg body wt (BW)] and infusion (4.32 µmol·kg BW−1·h−1) was given, respectively, in a volume of 2 ml/kg bw and 2 ml·g bw−1·h−1, and started 12 h after the start of PM, 5 h before the PULSE protocol in which we used L-[15N]Phe (26.3 µmol/kg BW in a volume of 0.5 ml/kg bw). We gave all tracers via the central caval vein catheter.
Blood sampling and sample processing.
We took blood samples and directly cooled them on ice. We processed the blood samples within 1 h (3, 4, 6). In brief, for amino acid concentration and enrichment analysis, we centrifuged heparinized blood at 4°C, 5 min, 8,000 g and deproteinized 250 µl plasma with 25 µl trichloroacetic acid solution (TCA, 50% wt/vol) and finally snap frozen in liquid nitrogen and stored at −80°C.
Amino acid concentration and enrichments.
We determined amino acid isotope concentrations and amino acid enrichments (tracer-to-tracee ratios, TTR) on a fully automated LC-ESI-MS system [QTrap 5500 MS (AB Sciex, Foster City, CA] with ExpressHT Ultra LC (Eksigent Div., AB Sciex, Foster City, CA). For concentration and TTR measurements we added 20 µl of TCA deproteinized plasma supernatant to 20 µl L-[U-13C9]Phe internal standard. Within 3 days before the LC-ESI-MS analysis, we derivatized the samples together with external standards at concentrations within the physiological range (calibration curve of concentration) and enriched external standards in the range of expected TTRs (calibration curve for TTRs) with 9-fluorenylmethoxycarbonyl (Fmoc). After neutralization, we injected 160 nl of the solution onto a micro-LC column 0.5 × 100 mm HALO C18, 2.7 μm, 90A pores (ABsciex, Foster City, CA), kept at 35°C. We eluted analytes with a segmentally linear gradient from 35% to 85% acetonitrile in water supplemented with ammonium acetate to 10 µM and 5% isopropanol. We used electrospray triple quadrupole tandem mass spectrometry in multiple reactions monitoring mode for detection. We fragmented the Fmoc amino acid derivatives in the collision cell for detection of either free aminoacyl anions or a fragment larger by 26 atom mass units (coming from the Fmoc derivative) to have the highest sensitivity. We simultaneously did mass analyses for Phe, its tracers and internal standards. We calculated the mass signal areas to enable TTR or tracee concentration calculations.
Calculations
Calculation of Phe tracee concentration and TTR from LCMS-obtained signals.
For concentration calculations, we normalized the tracee signals of the samples and the external standards with their internal l-[13C9]Phe standard. We determined the plasma tracee concentration with the calibration curve external Phe standards. Plasma enrichment (TTR) was determined using the peak area ratio of the tracee and tracer signal and a calibration curve of enriched external standards. For the tracer concentration in the infusions, we used a calibration curve of tracer containing external standards.
Calculation WbRa with PC and PULSE method.
In the present study we compare the calculations of WbRa of Phe into the circulation in a postabsorptive (patho)physiological state, using noncompartmental model in the PC group vs. a compartmental model made up by two compartments in the PULSE group (29) (Fig. 1).
PC.
We derived WbRa from Eq. 1 by using the L-[ring-13C6]Phe isotope. The tracer infusion rate (I) is divided by TTRA. TTRA is the tracer-to-tracee ratio in arterial plasma.
| (1) |
PULSE.
We used noncompartmental analysis in GraphPad Prism (version 6) to perform curve fitting of the exponential decay curve. We found that all data fitted best (R2 and observation) with a two-exponential fit:
| (2) |
The noncompartmental WbRa Eq. 1 is generally applicable to multiple exponential decays. This equation can be written in terms of the parameters of the curve fits as follows:
| (3) |
The two-exponential fit is related to a two-compartmental model in line with what is expected for essential amino acids (29). The two-compartmental analysis of the tracer decay curve follows Michaelis-Menten elimination kinetics (13). The assumption that the rate of change of the amount of tracer in a pool is equal to the rate that tracer enters the pool minus the rate it exits the pool can be stated mathematically, as we described previously (29):
| (4) |
| (5) |
| (6) |
where dq1(t)/dt and dq2(t)/dt are the rates of change of the tracer pool size in pools 1 and 2, respectively, and Q1 the tracee pool size in pool 1. These equations/model (Fig. 1), the measured enrichment data, and the size of the administered tracer pulse are entered into the SAAM II software (see Supplemental Information, available with the online version of this article) that provides the calculated values of k21, k21, k02, and Q1. We converted the k values to whole body rate of appearance (WbRa) or intracellular production as described previously (10, 29). We estimated the transfer rates as fractional rates between pools (k12, k21, k02) and the extracellular pool size (Q1) in all subjects. Assuming that the measurements were done in a physiological steady state, meaning no net loss or production of Phe tracee in Q1 during the experimental period, we calculated the flux (F) from Q2 to Q1 (F12) with the fractional rate of intracellular uptake (k21) from Q1:
| (7) |
The size of the intracellular pool (Q2) can be calculated with the fractional rate of intracellular release (k12):
| (8) |
The irreversible loss from the intracellular pool (F02) is calculated with the fractional rate of irreversible loss (k02):
| (9) |
In a physiological steady state, irreversible loss is equal to appearance of Phe in the intracellular pool. Assuming that the appearance of Phe in the intracellular pool is coming from PB, and PB is occurring intracellularly in the postabsorptive state, we thus determine WbPB:
| (10) |
The fraction of the amount of Phe coming from PB that will appear in the extracellular pool (Q1) is the amount that is not irreversibly lost:
| (11) |
The amount of Phe flux between Q1 and Q2 that is not irreversibly lost is shunt back to Q1:
| (12) |
Statistical Analyses
Results are presented as means ± SE. Graphpad Prism (version 6) was used for statistics. Level of significance was set on P < 0.05. To determine physiological (tracee) or tracer Phe steady state during the experimental period, linear regression was used to determine if the slope of the best fitted line was different from zero. No difference from zero is steady state. To compare data between the Healthy and Sepsis groups, an unpaired t-test was used. To compare physiologically relevant models/parameters, a Pearson correlation test was used. The best-fitted line that describes the relation between both models/parameters was done with linear regression. A shared fitted line was determined when no significant differences were observed between the Healthy and the Sepsis fitted line. Additionally, a Bland-Altman plot was made to determine potential discrepancy between methods (1). For the limits of agreement, 2 times the SD of the average discrepancy was used. Post hoc Wilcoxon Signed Rank test and D’Agostino and Pearson normality tests were used to characterize a discrepancy.
RESULTS
Characteristics/Validation of Tracer Models
Plasma tracer/tracee ratios of both tracer methods.
Figure 3 characterizes the shape of the enrichment (TTR) curve after a PULSE of L-[15N]Phe and the PC infusion enrichment of L-[ring-13C6]Phe in a septic and healthy animal. The exponential decay curves of all individual animals are shown in Figs. 4 and 5. We evaluated the residual errors by visual inspection. We determined goodness of fit with the coefficient of determination (R square): 0.9991 ± 0.0002 (Healthy) and 0.9991 ± 0.002 (Sepsis). We determined, using linear regression, that L-[ring-13C6]Phe tracer steady state (horizontal lines in time during the experimental period) was present. In all animals the TTR was in steady state.
Fig. 3.

Example plasma tracer/tracee ratios. Example of plasma tracer/tracee time courses of arterial phenylalanine (Phe) in a Healthy and a Sepsis animal. PC: steady-state curve, 5 h after the start of a primed-continuous infusion of L-[ring-13C6]Phe tracer; PULSE: a two-exponential decay curve after a bolus infusion tracer with L-[15N]Phe. TTR is tracer/tracee ratio.
Fig. 4.
Individual decay curves of Healthy animals (PULSE). Plasma tracer/tracee (TTR) time courses of arterial Phe in healthy animals. PULSE: a two-exponential decay curve after a bolus infusion tracer with L-[15N]Phe.
Fig. 5.
Individual decay curves of Sepsis animals (PULSE). Plasma tracer/tracee (TTR) time courses of arterial Phe in Sepsis animals. PULSE: A two-exponential decay curve after a bolus infusion tracer with L-[15N]Phe.
Tracee concentrations.
We found that the arterial tracee Phe concentrations during the experimental period (Fig. 6) were stable in the Healthy group but slightly increasing in the Sepsis group (regression slope was different from zero: P = 0.0211). The plasma Phe concentration was increased in the sepsis animals (median over the experimental period: Healthy: 64.6 ± 4.0 µM; Sepsis 113 ± 8.3 µM; P = 0.0002).
Fig. 6.
Phenylalanine (Phe, tracee) concentrations. A: arterial plasma Phe tracee (nontracer) concentrations after administration of L-[15N]Phe pulse. Between 17 and 18 h after start, administration of Pseudomonas aeruginosa. Data are expressed as means ± SD. Healthy n = 9, Sepsis n = 13. Statistics for physiological steady-state: linear regression, significance P < 0.05 if slope was different from zero. Slope in Sepsis group was different from zero (P = 0.0211). B: average tissue Phe concentration from muscle, jejunum, ileum, liver, lung in µmol/kg wet wt. At necropsy, 18 h after start, administration of Pseudomonas aeruginosa. Data are expressed as means ± SE. Healthy n = 9, Sepsis n = 13. Statistics: Sepsis compared with Healthy, unpaired t-test. *Significance, P < 0.05.
Precision of model parameters with PULSE method.
In Table 1 we show the results of the fitting of the decay curves of Phe enrichments performed with Graphpad Prism. For noncompartmental analysis with the PULSE method, the correlation between the fit and the data (R2) should be greater than 0.95 (29). We found that for all fitted curves, the R2 was higher than 0.95. The correlation between the various parameters (dependency) indicates that the use of a two-exponential equation is appropriate.
Table 1.
Precision of decay curves of Phe enrichment
| Healthy |
Sepsis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean Value | Mean SD | Mean CV% | Mean Dependency | Mean Value | Mean SD | Mean CV% | Mean Dependency |
| A1 | 0.371 | 0.025 | 6.7 | 0.932 | 0.319 | 0.084 | 15 | 0.910 |
| A2 | 0.231 | 0.020 | 8.6 | 0.988 | 0.173 | 0.016 | 8.4 | 0.982 |
| B1 | 0.153 | 0.018 | 11 | 0.984 | 0.188 | 0.042 | 18 | 0.981 |
| B2 | 0.021 | 0.002 | 8.5 | 0.956 | 0.020 | 0.002 | 10 | 0.940 |
| R2 | 0.9998 | 0.9991 | ||||||
Mean variations of data of two-exponential fit of individual decay curves of Phe enrichment. Equation: . Fitted with Graphpad Prism. Used fitting constraints: B1 < 0.4, B2 > 0, plateau = 0. R2 is the correlation between the fit and the data. Healthy n = 9; Sepsis n = 13.
The coefficient of variation of each compartmental model parameter should be reasonable and models with coefficients above 100% should be rejected (29). We found for all animals that the parameter estimations performed with SAAM II (Table 2) had all coefficients of variation below 100%.
Table 2.
Precision of compartmental model
| Healthy |
Sepsis |
|||||
|---|---|---|---|---|---|---|
| Parameter | Mean Value | Mean SD | Mean CV% | Mean Value | Mean SD | Mean CV% |
| Q1, µmol | 1121 | 49 | 4.1 | 1655 | 37 | 2.5 |
| k02, min−1 | 0.033 | 0.005 | 14 | 0.028 | 0.003 | 11 |
| k12, min−1 | 0.051 | 0.020 | 26 | 0.044 | 0.006 | 14 |
| k21, min−1 | 0.115 | 0.019 | 14 | 0.105 | 0.008 | 7.0 |
Mean variations of parameters generated from the two-compartmental analysis of individual decay curves of Phe enrichment with computer software SAAM II. Q1 is plasma pool size; k02, k12, k21 are proportionality constants. Healthy n = 9; Sepsis n = 13. Formulas used were the following:
where dq1(t)/dt and dq2(t)/dt are the rates of change of the tracer pool size in pools 1 and 2, respectively.
Whole Body Rate of Appearance Comparisons
We compared healthy and septic animals to determine if both PC and PULSE can be used to observe changes in WbPB (Fig. 7). We found that whole body rate of appearance (WbRa) of Phe was higher in the sepsis group with both methods (PC: P = 0.003; PULSE: P = 0.0001), albeit that WbRa was higher with the PULSE method in both groups (Fig. 7, A and B). Data from the noncompartmental WbRa-PULSE analysis were not different (Healthy: P = 0.788; Sepsis: P = 0.841) from those from the compartmental WbRa-PULSE analysis (Fig. 7B). Although the PC and PULSE WbRa data correlated very well (r = 0.732, P < 0.0001) (Fig. 7C), a systemic difference between the methods was present as reflected by the 1.6 times higher WbRa with the PULSE method, independent of the septic state (Fig. 7D). Although the WbRa compartmental vs. noncompartmental data with the PULSE method correlated highly (r = 0.969, P < 0.0001) (Fig. 7E), the discrepancy did not pass the D’Agostino and Pearson normality test due to higher variability of the data obtained by the noncompartmental PULSE analysis.
Fig. 7.
Effect of sepsis on whole body rate of appearance (WbRa) of phenylalanine (Phe) using two different tracer methods. WbRa as proxy for whole body protein breakdown in sepsis and healthy pigs. Calculated with PC tracer method: primed-continuous infusion of L-[ring-13C6]Phe (A) or with the PULSE tracer method: bolus infusion of L-[15N]Phe (B). Compartmental and noncompartmental data analyses. Data are expressed as means ± SE. Healthy n = 9, Sepsis n = 13. Statistics: sepsis compared with Healthy, unpaired t-test. *Significance, P < 0.05. C: correlation between WbRa-PULSE (compartmental) vs. WbRa-PC. Statistics for correlation: Pearson correlation (r = 0.732), with a likelihood for real correlation (P < 0.0001). Linear regression is used for the prediction of best line (r2 = 0.507, slope 1.61). D: Bland-Altman plot. Ratio vs. the average of the two different tracer methods. The discrepancy (mean ± SD) is 1.628 ± 0.2279. Wilcoxon Signed Rank test was used to determine the discrepancy was different from 1: P < 0.0001. Discrepancy passed D’Agostino and Pearson normality test. E: correlation between WbRa-PULSE (compartmental) vs. WbRa-PULSE (noncompartmental). Statistics for correlation: Pearson correlation (r = 0.969), with a likelihood for real correlation (P < 0.0001). Linear regression is used for the prediction of best line (r2 = 0.9386, slope = 1.005). F: Bland-Altman plot. Difference vs. the average of the two different PULSE data analysis methods. The discrepancy (mean ± SD) is −26.51 ± 108.1. Wilcoxon Signed Rank test was used to determine the discrepancy was different from zero: No significance. Discrepancy did not pass D’Agostino and Pearson normality test.
Additional Metabolic Information with the PULSE Tracer Model
WbRa in comparison with WbPB.
To determine if the WbRa of Phe in the extracellular pool is representing WbPB, we compared it with the WbPB (Fig. 8). Whereas the WbPB was higher (P = 0.002) in the sepsis group, the correlation with WbRa-PULSE was excellent (r = 0.897: P < 0.0001) and was 1.7 times higher than the WbRa-PULSE independent of the septic state.
Fig. 8.
Effect of sepsis on whole body protein breakdown (WbPB). WbPB data obtained with compartmental modeling (PULSE method). A: WbPB. Data are expressed as means ± SE. Healthy n = 9, Sepsis n = 13. Statistics: Sepsis compared with Healthy, unpaired t-test. *Significance, P < 0.0002. B: correlation between WbPB and whole body rate of appearance (WbRa). Statistics: Pearson correlation (r = 0.897), with a likelihood for real correlation (P < 0.0001). Linear regression is used for the prediction of best line (r2 = 0.803, slope = 0.60).
Comparison whole body Phe pools.
Both extracellular and intracellular Phe pool are increased in sepsis (Table 3) and are highly correlated (r = 0.802: P < 0.001), and the intracellular pool is 2.2 times larger than the extracellular pool. A significant relationship is also present between the extracellular Phe pool size and plasma Phe concentration (r = 0.613; P = 0.002).
Table 3.
Whole body metabolic information obtained with PULSE model
| Parameter | Unit | Healthy | Sepsis | P Value |
|---|---|---|---|---|
| Extracellular pool (Q1) | µmol/kg BW | 44 ± 2.2 | 68 ± 5.5 | 0.002 |
| Intracellular pool (Q2) | µmol/kg BW | 105 ± 8.0 | 157 ± 8.2 | 0.0003 |
| Flux (F12 = F21) | µmol·kg BW−1·min−1 | 5.02 ± 0.40 | 6.86 ± 0.42 | 0.006 |
| Shunting (F12-WbRa) | µmol·kg BW−1·min−1 | 3.02 ± 0.35 | 4.23 ± 0.34 | 0.026 |
| Irreversible loss (F02) | µmol·kg BW−1·min−1 | 3.38 ± 0.10 | 4.33 ± 0.16 | 0.0002 |
| Fractional rate of irreversible loss (k02) | %/min | 3.33 ± 0.21 | 2.83 ± 0.15 | 0.065 |
| Fractional rate of intracellular release (k12) | %/min | 5.09 ± 0.69 | 4.43 ± 0.25 | 0.316 |
| Fractional rate of intracellular uptake (k21) | %/min | 11.5 ± 0.71 | 10.6 ± 0.76 | 0.423 |
Mean values of parameters obtained and calculated from the fitting of individual decay curves of Phe enrichment. Values are expressed as means ± SE. Healthy n = 9; Sepsis n = 13. Statistics: unpaired t-test.
Other metabolic parameters.
Besides the increase in Phe pools, we also observed an increase in Phe flux between the extra- and intracellular pools, an increase in the amount of shunting (Table 3), and an increase in irreversible loss. No changes were found in the fractional release, uptake, and irreversible loss of Phe in the intracellular pool.
DISCUSSION
The present study shows that the WbRa obtained by the PULSE method is highly correlated with that of the PC method, albeit the absolute values were higher for the PULSE method but independent of the presence of sepsis. Furthermore, the PULSE method is able to measure protein breakdown using a compartmental model made up of two compartments, and provides additional valuable information, i.e., membrane transport characteristics (fractional release, uptake, irreversible loss). The Phe pools and fluxes between pools are increased in the sepsis animals; however, the transport characteristics between the pools are not changed.
Validation Models
Choice of tracers.
The stable isotope tracers of Phe that can be used for both the PULSE as the PC method are dependent on the choice of analytical method (e.g., LCMS, GCMS or IRMS) and the precision of this method (e.g., higher masses can give more sensitivity, possible use of neutral loss approach). Additionally, in the present study we considered potential chemical interference during the analytical procedures. For instance, if masses of two tracers are too close to each other, contribution of the lower mass (natural abundance) to the higher mass can be expected. Although biological interference (unwanted modifications of tracers in the body) like the potential disappearance of 15N tracer by transamination can occur (24), for the PULSE method it will not interfere with the WbRa (part of irreversible loss). Also, the transamination of phenylalanine in the body and back can be considered very low, because for instance the use of [15N]Phe and [1-13C]Phe gives comparable TTRs (28). This is another reason why phenylalanine is a preferred amino acid in our type of measurements. Any stable isotope tracer of phenylalanine can be used for WbRa, as long as it is possible to measure both high and low enrichments with sufficient precision.
Physiological steady state.
During the 1-h testing period, our animals were all in a physiological steady state and tracer steady state for the PC method. We found that the Phe tracee curves were not significantly affected by the amount of the Phe isotope in the PULSE, although an increasing tracee concentration was found in the septic animals, likely related to the septic condition. Because the increase was minor and the tracer enrichment was in a steady state for the PC method, we did not adjust the PC-WbRa formula with, e.g., the Steele equation correction for non-steady-state measurements (25), which for Phe only has a minor effect on the final results. We suggest that future studies should use small amounts of tracers to reduce the effect on metabolism further.
Fitting.
Because of time limitations and experimental design constraints, we did not take more than six samples to describe the PULSE decay curve in the present study. However, the goodness of fit and CV% of each parameter with Graphpad Prism (for noncompartmental data analysis) as well as SAAM II software (for compartmental data analysis) show high reliable decay curves thanks to very accurate LCMS TTR measurements (CV% < 2%). For the chosen compartmental model (two compartments) (7, 10, 13, 31) and to determine changes in sepsis, this number of samples was sufficient in the present study. In the case of more complex compartmental models (three or more compartments) or less accurate analytical methods, we think that more sample points are necessary.
Identifiability.
The general idea is to use compartments to model various components of the physiological metabolic system. The structure of the model is developed from evaluation of the relationships between the exponential functions of the decay curve and an understanding of the metabolic system to be modeled. Usually the number of exponential terms is directly related to the number of compartments in the model. The minimal number of detectable compartments in the current study is two based on the goodness of fit. The physiological identifiability of the two-compartment PULSE model is not necessary univocal, but this interpretation fits with a generally used two-pool model for amino acid metabolism (29) where Q1 is considered the extracellular pool and Q2 the intracellular pool. If we compare Q1 Phe pool with the plasma Phe pool (plasma Phe concentration times estimated plasma volume per kg: 80 ml/kg blood × plasma fraction) we concluded that the plasma pool is only 9% of the Q1 Phe pool (Healthy: 9.0 ± 2.0%; Sepsis 9.4 ± 2.5%). Assuming that the extracellular concentration is 20% higher in healthy subjects then the arterial plasma concentration (17), we determined that the extravascular Phe pool is ~58% of the Q1 Phe pool (Healthy: 53 ± 4; Sepsis 61 ± 8, weight gain by resuscitation included). Although we assume that Q1 is the extracellular Phe compartment, these calculated pools combined represent only 70% of the Q1 Phe pool. Q2 is the space were Phe is entering in a postabsorptive state from PB and can be used directly for protein synthesis ( = recycling) or hydroxylation that takes place intracellularly. If we compare the Q2 Phe pool with the intracellular pool (calculated with an average Phe concentration in tissue, data in Fig. 8), the intracellular pool represents 61% of the Q2 pool (Healthy: 66 ± 4%; Sepsis 58 ± 3%). At the moment, we do not have a good explanation for these discrepancies (7, 13, 29). It could well be that the above general assumptions (not observations) for the physiological pools are not realistic enough to compare it with the numeric Q1 and Q2 pools in animals that are in resuscitated physiological condition. It could well be that the numeric fast exchanging Q1 Phe pool is partly also intracellular and the slow exchanging Q2 pool is partly also located extracellular. So the numerical Q1 and Q2 pools are likely not strictly physiologically separated. Further research is needed to determine the exact translation of Q1 and Q2 pools to physiological pools.
WbRa PULSE in Relation to WbRa PC
In the present study, we measured WbRa of Phe as an index/proxy for WbPB. We showed that both WbRa-PULSE and PC are related to the total intracellular rate of appearance of Phe from PB in the healthy and disease state. However, the absolute value of WbRa was 1.6 times lower with the PC method.
For both methods, sampling was done in the same arterial plasma pool and TTR was analyzed in the same blood sample on the LC-MS/MS and calibrated with enriched standard curves. Although the exact amount of the tracer administered in the PC model is more error sensitive (e.g., prime is estimated, performance infusion pump, temporary pump errors) than with the PULSE model (single dose with syringe), we think it is unlikely that this can explain the discrepancy in WbRa between the models.
Rakotoambinina et al. (19) also compared the PULSE approach with the PC for the nonessential amino acid taurine and found also 1.8 times higher WbRa with the PULSE approach. One of their explanations was that the area under the curve (AUC) under the decay curve could not well be determined because of the detection level of the used GC-MS. The AUC is related to the final absolute WbRa. In the present study we described the decay curve with 6 time points in a 1-h time window. The reliability of the curves was very high due to accurate LC-MS/MS measurements (CV% < 2%). In practice, the calculation of noncompartmental WbRa with the PULSE method is strongly influenced by the tail of the decay curve because it can influence the area under the curve substantially. The calculation of the pool size Q1 is strongly influenced by the enrichment close to zero. Q1 is used to calculate the compartmental WbRa. Since the noncompartmental and compartmental PULSE approaches give comparable WbRas, we are able to conclude that although the decay curves were fitted with only six sample points, these samples were collected at the right time and provided valid data. Therefore, in the present study a potential inaccuracy of the decay curve fitting cannot explain the WbRa difference between the PULSE and PC method.
The major difference between the PC and PULSE approach is the level of simplicity that is chosen to describe PB by determining WbRa of Phe. The WbRa-PC provides an underestimation of the intracellular appearance (9, 29). This underestimation is related to the fact that the WbRa-PC is greatly limited by the necessary assumption that production appears in the sample compartment (i.e., plasma) in which the tracer is also administered. Physiologically only a few substrates appear directly in plasma, such as glucose and urea. In the postabsorptive state that we studied, the essential amino acid Phe appears intracellularly. The WbRa-PULSE will better represent the dilution both in plasma as well as in tissue. This is related to the fact that the decay curve of the PULSE method has two exponentials that in the compartmental analysis represent most likely the extracellular and intracellular pools. However when we measure changes in PB both WbRa methods will identify these changes, albeit at different turnover rates. We therefore conclude that WbRa-PULSE better reflects the actual absolute PB rate. In case the total amount of PB is of interest in a scientific question, the compartmental PB analysis with the PULSE method will be the only option that is appropriate, because WbRa-PC will not account for the intracellular irreversible loss.
Protein Kinetic Changes in Severe Sepsis Animals Obtained with PULSE Method
WbRa of Phe is related to WbPB, and is considered as important information to compare different physiological conditions. In the present study, both PC as well as PULSE WbRa provide us the same information: WbPB is enhanced in sepsis. The PULSE method provides us more additional information: for instance, how Phe compartments relate to each other (21, 29, 31), and how WbRa relates to WbPB (ratio). A fraction of the difference of those two is the total amount of Phe that is directly reused ( = recycling) for protein synthesis. The other fraction is hydroxylated to tyrosine. An extra tyrosine pulse tracer and the enrichment of the tyrosine metabolite, coming from the chosen Phe-tracer- PULSE, is needed to determine the exact fraction of hydroxylation and subsequently the fraction of Phe recycling and whole body protein synthesis. In this case, it is not preferred to use a [15N]Phe tracer due to potential (but low) interference of transamination (24, 28).
Also with the PULSE method we could determine that the change in WbRa in the current sepsis model is caused by differences in Phe intracellular appearance and not the membrane transport capacity between the pools. We found that membrane transport of Phe in severe sepsis is not compromised, but that only WbPB in sepsis is increased (5, 23) and supports the notion that the PULSE isotope methods provide us with additional physiologically important information about membrane transport.
Conclusion
The increased WbRa found by both the PC method and PULSE method reflects an enhanced WbPB in sepsis. In contrast to the PC method, the easy-to-use PULSE method provides additional information about intra- and extracellular fluxes, cell membrane transport capacity, and WbPB. WbRa-PULSE therefore better reflects the actual absolute PB. Additionally, the noncompartmental PULSE data analysis can be very practical in routine measurements and circumvent the problems of pool priming seen with PC methods but is a little less sensitive than the compartmental PULSE data analysis. In severe sepsis, on the whole body level, membrane transport capacity of amino acids is not compromised and is therefore not a rate-limited factor for protein synthesis.
GRANTS
The project described was supported by National Institute of General Medical Sciences (NIGMS) Grant R01-GM-084447 and by National Center for Research Resources (NCRR) Grant S10-RR-027047.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NCRR.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A.T.H. and N.E.D. performed experiments; G.A.T.H. and N.E.D. analyzed data; G.A.T.H., M.P.E., R.R.W., and N.E.D. interpreted results of experiments; G.A.T.H. prepared figures; G.A.T.H. drafted manuscript; G.A.T.H., M.P.E., R.R.W., and N.E.D. edited and revised manuscript; G.A.T.H., M.P.E., R.R.W., and N.E.D. approved final version of manuscript; N.E.D. conceived and designed research.
ACKNOWLEDGMENTS
We thank J. Spore for skilled technical assistance of sample processing and analyses; Dr. B. Zoer and Dr. R. C. I. Deutz for skilled technical assistance in carrying out the animal experiments; and Dr. J. Thaden for accurate LCMS/MS/MS analyses of amino acids.
Part of the data was presented earlier during an oral presentation at the Experimental Biology 2012 and was published in abstract form (26a).
REFERENCES
- 1.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 8: 135–160, 1999. doi: 10.1191/096228099673819272. [DOI] [PubMed] [Google Scholar]
- 2.Bregendahl K, Yang X, Liu L, Yen JT, Rideout TC, Shen Y, Werchola G, Fan MZ. Fractional protein synthesis rates are similar when measured by intraperitoneal or intravenous flooding doses of L-[ring-2H5]phenylalanine in combination with a rapid regimen of sampling in piglets. J Nutr 138: 1976–1981, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bruins MJ, Deutz NE, Soeters PB. Aspects of organ protein, amino acid and glucose metabolism in a porcine model of hypermetabolic sepsis. Clin Sci (Lond) 104: 127–141, 2003. doi: 10.1042/cs1040127. [DOI] [PubMed] [Google Scholar]
- 4.Bruins MJ, Lamers WH, Meijer AJ, Soeters PB, Deutz NE. In vivo measurement of nitric oxide production in porcine gut, liver and muscle during hyperdynamic endotoxaemia. Br J Pharmacol 137: 1225–1236, 2002. doi: 10.1038/sj.bjp.0704993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruins MJ, Soeters PB, Deutz NE. Endotoxemia affects organ protein metabolism differently during prolonged feeding in pigs. J Nutr 130: 3003–3013, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Carneiro-Filho BA, Bushen OY, Brito GA, Lima AA, Guerrant RL. Glutamine Analogues As Adjunctive Therapy for Infectious Diarrhea. Curr Infect Dis Rep 5: 114–119, 2003. doi: 10.1007/s11908-003-0046-2. [DOI] [PubMed] [Google Scholar]
- 7.Carson ER, Cobelli C, Finkelstein L. The Mathematical Modeling of Metabolic and Endocrine Systems. Ontario, Canada: Wiley, 1983, p. 394. [Google Scholar]
- 8.de Blaauw I, Heeneman S, Deutz NE, von Meyenfeldt MF. Increased whole-body protein and glutamine turnover in advanced cancer is not matched by an increased muscle protein and glutamine turnover. J Surg Res 68: 44–55, 1997. doi: 10.1006/jsre.1997.5007. [DOI] [PubMed] [Google Scholar]
- 9.DiStefano JJ III, Landaw EM. Multiexponential, multicompartmental, and noncompartmental modeling. I. Methodological limitations and physiological interpretations. Am J Physiol Regul Integr Comp Physiol 246: R651–R664, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr 33: 1024–1032, 2014. doi: 10.1016/j.clnu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelen MP, Com G, Wolfe RR, Deutz NE. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. J Cyst Fibros 12: 445–453, 2013. doi: 10.1016/j.jcf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouillet H, Gaudichon C, Mariotti F, Mahé S, Lescoat P, Huneau JF, Tomé D. Compartmental modeling of postprandial dietary nitrogen distribution in humans. Am J Physiol Endocrinol Metab 279: E161–E175, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey K. Compartmental Models and Their Application. London, UK: Academic, 1983, p. 293. [Google Scholar]
- 14.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 89: 142–152, 2009. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 15.Mariotti F, Petzke KJ, Bonnet D, Szezepanski I, Bos C, Huneau JF, Fouillet H. Kinetics of the utilization of dietary arginine for nitric oxide and urea synthesis: insight into the arginine-nitric oxide metabolic system in humans. Am J Clin Nutr 97: 972–979, 2013. doi: 10.3945/ajcn.112.048025. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DE, Downey RS. Measurement of urea kinetics in humans: a validation of stable isotope tracer methods. Am J Physiol Endocrinol Metab 246: E519–E527, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Miller S, Chinkes D, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab 287: E136–E141, 2004. doi: 10.1152/ajpendo.00092.2004. [DOI] [PubMed] [Google Scholar]
- 18.Poeze M, Bruins MJ, Kessels F, Luiking YC, Lamers WH, Deutz NE. Effects of L-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am J Clin Nutr 93: 1237–1247, 2011. doi: 10.3945/ajcn.110.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakotoambinina B, Marks L, Badran AM, Igliki F, Thuillier F, Crenn P, Messing B, Darmaun D. Taurine kinetics assessed using [1,2-13C2]taurine in healthy adult humans. Am J Physiol Endocrinol Metab 287: E255–E262, 2004. doi: 10.1152/ajpendo.00333.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan R. Studying apolipoprotein turnover with stable isotope tracers: correct analysis is by modeling enrichments. J Lipid Res 47: 2738–2753, 2006. doi: 10.1194/jlr.M600302-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rathmacher JA, Nissen SL. Development and application of a compartmental model of 3-methylhistidine metabolism in humans and domestic animals. Adv Exp Med Biol 445: 303–324, 1998. doi: 10.1007/978-1-4899-1959-5_20. [DOI] [PubMed] [Google Scholar]
- 22.Rathmacher JA, Nissen SL, Paxton RE, Anderson DB. Estimation of 3-methylhistidine production in pigs by compartmental analysis. J Anim Sci 74: 46–56, 1996. doi: 10.2527/1996.74146x. [DOI] [PubMed] [Google Scholar]
- 23.Rooyackers O, Kouchek-Zadeh R, Tjäder I, Norberg Å, Klaude M, Wernerman J. Whole body protein turnover in critically ill patients with multiple organ failure. Clin Nutr 34: 95–100, 2015. doi: 10.1016/j.clnu.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Shrawder E, Martinez-Carrion M. Evidence of phenylalanine transaminase activity in the isoenzymes of aspartate transaminase. J Biol Chem 247: 2486–2492, 1972. [PubMed] [Google Scholar]
- 25.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 26.Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol (1985) 107: 34–38, 2009. doi: 10.1152/japplphysiol.91137.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Ten Have GA, Engelen MP, Wolfe RR, Deutz NE. Using the phenylalanine (PHE) stable isotope pulse method to measure intracellular protein breakdown and metabolic shunting in the context of sepsis in the pig. FASEB J 26: 42.1, 2012. [Google Scholar]
- 27.Van Acker BA, Hulsewé KW, Wagenmakers AJ, Deutz NE, Van Kreel BK, Halliday D, Matthews DE, Soeters PB, Von Meyenfeldt MF. Absence of glutamine isotopic steady state: implications for the assessment of whole-body glutamine production rate. Clin Sci (Lond) 95: 339–346, 1998. doi: 10.1042/CS19980053. [DOI] [PubMed] [Google Scholar]
- 28.Wachter U, Tugtekin I, Georgieff M, Radermacher P, Vogt J. Simultaneous determination of plasma enrichments of 1-13C- and 15N-labelled phenylalanine and tyrosine. Isotopes Environ Health Stud 34: 311–316, 1998. doi: 10.1080/10256019808234065. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practise of Kinetic Analysis. New York: Wiley, 2005, p. 274. [Google Scholar]
- 30.Zhang XJ, Chinkes DL, Herndon DN, Wolfe RR. Measurement of protein fractional synthesis and breakdown rates in the skin of rabbits using a subflooding dose method. Metabolism 58: 1239–1247, 2009. doi: 10.1016/j.metabol.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab 283: E753–E764, 2002. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]