Abstract
Calcific aortic valve disease (CAVD) is a leading cardiovascular disorder in the elderly. Diseased aortic valves are characterized by sclerosis (fibrosis) and nodular calcification. Sclerosis, an early pathological change, is caused by aortic valve interstitial cell (AVIC) proliferation and overproduction of extracellular matrix (ECM) proteins. However, the mechanism of aortic valve sclerosis remains unclear. Recently, we observed that diseased human aortic valves overexpress growth factor neurotrophin 3 (NT3). In the present study, we tested the hypothesis that NT3 is a profibrogenic factor to human AVICs. AVICs isolated from normal human aortic valves were cultured in M199 growth medium and treated with recombinant human NT3 (0.10 µg/ml). An exposure to NT3 induced AVIC proliferation, upregulated the production of collagen and matrix metalloproteinase (MMP), and augmented collagen deposition. These changes were abolished by inhibition of the Trk receptors. NT3 induced Akt phosphorylation and increased cyclin D1 protein levels in a Trk receptor-dependent fashion. Inhibition of Akt abrogated the effect of NT3 on cyclin D1 production. Furthermore, inhibition of either Akt or cyclin D1 suppressed NT3-induced cellular proliferation and MMP-9 and collagen production, as well as collagen deposition. Thus, NT3 upregulates cellular proliferation, ECM protein production, and collagen deposition in human AVICs. It exerts these effects through the Trk-Akt-cyclin D1 cascade. NT3 is a profibrogenic mediator in human aortic valve, and overproduction of NT3 by aortic valve tissue may contribute to the mechanism of valvular sclerosis.
Keywords: neurotrophin 3, Trk, AVIC, Akt, fibrogenic response
calcific aortic valve disease (CAVD) is an active multifactorial process involving aortic valve leaflet sclerosis (fibrosis) and calcification (10). The initial asymptomatic phases of CAVD primarily involve aortic valve sclerosis. Aortic valve sclerosis is present in ~25% of people over age 65 (38) and is associated with increased risk of cardiovascular events (27). Despite the high prevalence of aortic valve sclerosis, the mechanism underlying its pathogenesis is incompletely understood. Aortic stenosis occurs in the advanced stage of CAVD and is characterized by severe valvular thickening and calcification (17, 23, 26). Impaired leaflet motion and obstructed blood flow eventually cause heart failure or even death (10, 27). However, no pharmacological agent is available for prevention of CAVD progression (33). Aortic valve replacement remains the only therapy for patients with this disease (41).
The aortic valve is composed of aortic valve interstitial cells (AVICs), extracellular matrix (ECM), and overlying endothelial cells. AVICs are the dominant cells in valve leaflets (41). In normal aortic valves, AVICs are considered to be quiescent fibroblast-like cells. Activation of AVICs leads to ECM remodeling, including increased expression of matrix metalloproteinases (MMPs) and collagens (6). While several growth factors, including transforming growth factor-β1 (TGF-β1), have been shown to activate AVICs (13), novel factors responsible for aortic valve sclerosis need to be searched.
Neurotrophins (NTs) are a family of structurally and functionally related growth factors that support the growth and differentiation of developing neurons. NTs include nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). NTs exert their effects on cells by binding to three tropomyosin receptor kinase (Trk) receptors. NGF is known to bind to TrkA. BDNF and NT4 bind to TrkB, and NT3 can bind to TrkA, TrkB, and TrkC. NTs have been found to be important regulators of fibroblast functions, including cell survival, migration, and secretion of cytokines (5, 16, 28). We previously observed that NT3 expression is enhanced in stenotic aortic valves, and NT3 is localized in AVICs and in the extracellular spaces between AVICs (47). Although NT3 is identified as a prosurvival factor and a mitogen for neurons (11, 12, 14, 19, 34), it has also been found to promote human bronchial smooth cell migration and MMP-9 production (5). We found that NT3 is capable of upregulating transforming growth factor-β1 (TGF-β1) production in human AVICs (47). We therefore hypothesize that NT3 promotes the fibrogenic responses in human AVICs.
The purpose of this study was to determine 1) whether NT3 induces human AVIC proliferation, 2) whether NT3 promotes ECM protein production and collagen deposition in human AVICs, and 3) the mechanism by which NT3 exerts its effect on human AVICs.
MATERIALS AND METHODS
Materials.
Polyclonal antibodies against collagen I (cat. no. ab34710), collagen III (cat. no. ab83829), NT3 (cat. no. ab53685), and MMP-2 (cat. no. ab88607) were purchased from Abcam (Cambridge, MA). Recombinant human NT3 was obtained from US Biological (San Antonio, TX). Thymidine analog 5-bromo-2′-deoxyuridine (BrdU) cell proliferation kits and antibodies against phosphorylated Akt (cat. no. 9271), total Akt (cat. no. 9272), MMP-9 (cat. no. 3852), cyclin D1 (cat. no. 2978, clone no. 92G2), and GAPDH (cat. no. 2118, clone no. 14C10) were purchased from Cell Signaling (Beverly, MA). The Trk inhibitor K252a was obtained from Calbiochem (La Jolla, CA). The Akt inhibitor MK-2206 was purchased from Selleckchem (Houston, TX). The cyclin D1 inhibitor arcyriaflavin A and Cell Counting Kit (CCK)-8 were purchased from Enzo Life Sciences International (Plymouth Meeting, PA). Medium 199 was purchased from Lonza (Walkersville, MD). Picro Sirius Red (PSR) and other reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO).
Cell isolation and culture.
Diseased aortic valves were obtained from six patients with CAVD (5 males and 1 female; age: 61.0 ± 4.1 yr) and undergoing aortic valve replacement surgery at the University of Colorado Hospital. Normal aortic valves were collected from explanted hearts of patients (5 males and 1 female; age: 56.9 ± 2.3 yr) with cardiomyopathy and undergoing heart transplantation at the University of Colorado Hospital. The valve leaflets from explanted hearts were thin and had no histological abnormality. All patients gave written informed consent for the use of their aortic valves for this study approved by the University of Colorado Denver Institutional Review Board.
AVICs were isolated and cultured using a previously described method (22). Briefly, valve leaflets were subjected to an initial digestion with a higher concentration of collagenase (2.5 mg/ml) to remove endothelial cells. Then, the remaining tissue was treated with a lower concentration of collagenase (0.8 mg/ml) to free interstitial cells. Cells were collected by centrifugation and cultured in M199 growth medium containing penicillin G, streptomycin, amphotericin B, and 10% fetal bovine serum. AVIC isolates obtained using this modified protocol are lack of endothelial cells as verified by negative von Willebrand factor staining (22). One isolate was obtained from a donor aortic valve and used as an independent sample. Thus six normal AVIC isolates and six diseased AVIC isolates were used for this study. Cells of passage 3 to 6 were used for experiments.
To determine the effect of NT3 on human AVIC proliferation, cells were subcultured on 24-well plates and 96-well plates and treated with different concentrations of NT3 when they reached 50–60% confluence. Cell proliferation was analyzed by BrdU ELISA assay and CCK-8 assay.
To determine the effect of NT3 on Akt activation and cyclin D1 expression, cells were subcultured on 24-well plates and treated when they reached 80–90% confluence. Cells were exposed to NT3 (0.10 µg/ml) for 1–24 h in the presence or absence of Trk inhibitor (K252a, 0.20 µM). The phosphorylation of Akt was analyzed by immunoblotting. To determine the role of Trk and Akt in the expression of cyclin D1 induced by NT3, Trk inhibitor and Akt inhibitor (MK2206; 5 µM) were added to culture medium 1 h before addition of NT3. The levels of cyclin D1 were analyzed by immunoblotting.
To determine the role of Trk receptors, Akt and cyclin D1 in NT3-induced AVIC proliferation, ECM protein production, and collagen deposition, Trk inhibitor (K252a; 0.20 µM), Akt inhibitor (MK-2206; 5.0 µM), or cyclin D1 inhibitor (arcyriaflavin A; 0.25 µM) was added to the cell culture medium 1 h before treatment with NT3. Levels of proliferation were assessed by BrdU ELISA assay and CCK-8 assay. Cellular levels of MMP-9, MMP-2, collagen I, and collagen III were analyzed using immunoblotting. Collagen deposition was analyzed by PSR staining.
Immunoblotting.
Immunoblotting was applied to analyze NT3, cyclin D1, collagen I, collagen III, MMP-9, MMP-2, phosphorylated Akt, total Akt, and GAPDH in cell lysate. Cells were lysed in a sample buffer (100 mmol/l Tris·HCl, pH 6.8, 2% SDS, 0.02% bromophenol blue, and 10% glycerol). Protein samples were separated on gradient (4–20%) mini-gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% skim milk solution for 1 h at room temperature. The blocked membranes were incubated with a primary antibody. After being washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20, the membranes were incubated with a peroxidase-linked secondary antibody specific to the primary antibody. After further washes, membranes were incubated with enhanced chemiluminescence reagents and exposed on X-ray films. Image J (Wayne Rasband, National Institutes of Health, Bethesda, MD) was used to assess the density of bands.
BrdU proliferation assay.
Cell proliferation was analyzed using a BrdU kit according to manufacturer’s protocol. Twenty-four hours before the end of the experiment, BrdU-labeling solution was added to the wells. The amount of BrdU in DNA was subsequently determined by fixation of the cells and incubation with an anti-BrdU-POD antibody followed by colorimetric analysis using Bio-Rad model/680 microplate reader at 450 nm. The results are expressed as the percentage of control value.
CCK-8 assay.
CCK-8 assay was applied to confirm cell proliferation. AVICs were plated in 96-well plates at a density of 50–60% confluence. The experiments were performed in triplicate and started by replacing the medium with fresh medium containing different concentrations of the compounds under investigation. The cells were incubated for 3 days with the compounds. After the incubation was complete, the metabolic activity was determined using the CCK-8 assay according to manufacturer’s protocol. In brief, cells were incubated with CCK-8-labeling reagent at 37°C for 4 h. Formazan dye formation was evaluated by scanning with a multi-well spectrophotometer at 450 nm. The results are expressed as percentage of control value.
PSR staining.
PSR staining specifically identifies collagens and is a useful method for assessment of fibrogenic activity in cultured cells (46). Cells were treated with methanol overnight at −20°C. Following washes with PBS, the cells were incubated in 0.1% PSR for 4 h. Following rinses with 0.1% acetic acid, plates were air dried and examined by microscopy. For quantification of PSR staining, stained cells were treated with 100 µl of 0.1 M sodium hydroxide for 2 h to elute the stain. Optical density was determined using a spectrophotometer (BioTek Instruments) at 540 nm.
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed using StatView software (Abacus Concepts, Calabasas, CA). ANOVA with the post hoc Bonferroni/Dunn test was used to analyze differences between experimental groups, and a t-test was applied to analyze data difference between normal and diseased AVICs. Nonparametric Mann-Whitney U-test was performed to confirm the difference of the two group comparison. For multiple group comparisons, nonparametric Kruskal-Wallis test was performed to confirm the differences. Statistical significance was defined as P ≤ 0.05.
RESULTS
NT3 promotes human AVIC proliferation in a dose-dependent fashion.
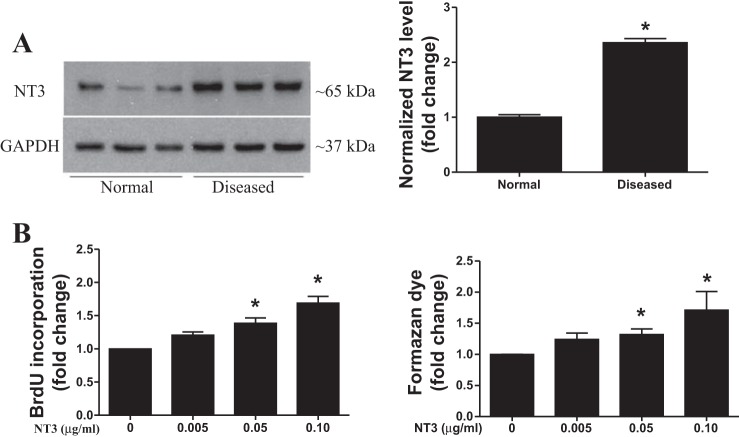
The levels of cell-associated NT3 protein were higher in AVICs from stenotic aortic valves (Fig. 1A). This observation is consistent with our previous report (47). To determine the effect of NT3 on human AVIC proliferation, normal AVICs were treated with recombinant human NT3 (expressed in mammalian cells, endotoxin free, 0.005–0.10 µg/ml) for 3 days. Cell proliferation was analyzed by BrdU ELISA and CCK-8 assay. BrdU incorporation was markedly increased after NT3 stimulation, and NT3 induced BrdU incorporation in a dose-dependent fashion (Fig. 1B). BrdU incorporation increased by 70% in cells treated with 0.10 µg/ml of NT3 compared with the untreated control cells. An increase in the formazan dye formation was confirmed by the CCK-8 assay after treatment with NT3 (Fig. 1B).
Fig. 1.
Neurotrophin 3 (NT3) promotes human aortic valve interstitial cell (AVIC) proliferation. A: representative immunoblot and densitometric data show that AVICs of diseased valves have higher levels of NT3 protein in comparison with normal AVICs. Data are presented as means ± SE of 6 cell isolates from distinct donor valves in each group. Statistical analyses were performed using t-test and confirmed using nonparametric Mann-Whitney U-test. *P < 0.05 vs. normal. B: normal human AVICs were treated with different doses of recombinant human NT3 (0.005–0.10 µg/ml) for 3 days. 5-Bromo-2′-deoxyuridine (BrdU) and Cell Counting Kit (CCK)-8 (formazan dye formation) assays show that NT3 induces AVIC proliferation in a dose-dependent fashion. Data are presented as means ± SE of 6 cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control (without NT3).
NT3 promotes collagen III production and deposition in human AVICs.
The principle ECM components of the native aortic valve are collagen (74% type I, 24% type III, and 2% type V), elastin, and proteoglycans (1). Excessive production of ECM proteins within heart valves results in leaflet thickening and impairs valvular function (43). MMP-2 and MMP-9 are involved in the degradation and reorganization of ECM in heart valves (31, 32, 37). To determine the effect of NT3 on ECM protein production, we treated normal AVICs with NT3 (0.10 µg/ml) for 3 days and applied immunoblotting to analyze the levels of MMP-9, MMP-2, collagen I, and collagen III. Figure 2A shows that NT3 upregulated the levels of MMP-9 and collagen III but had no effect on MMP-2 and collagen I. Thus NT3 selectively upregulates collagen III and MMP-9 levels in human AVICs.
Fig. 2.
NT3 upregulates collagen production in human AVICs. A: normal AVICs were treated with NT3 (0.10 µg/ml) for 3 days. Representative immunoblots of 5 experiments using cell isolates from distinct donor valves show that NT 3 elevates levels of cell-associated matrix metalloproteinase-9 (MMP-9) and collagen III but has no effect on MMP-2 and collagen I levels in the cells. B: normal AVICs were treated with NT3 for 28 days. Picro Sirius Red (PSR) staining was applied to stain collagens. Representative images (scale bar = 150 μm) and spectrophotometric analysis of eluted stain show that cells exposed to NT3 formed a greater amount of collagen deposits. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using t-test and confirmed using nonparametric Mann-Whitney U-test. *P < 0.05 vs. control.
To examine whether NT3 promotes collagen deposition, we applied PSR staining that identifies collagens (46). Cells were treated with NT3 for 28 days. PSR staining and spectrophotometric analysis of eluted PSR stain were applied to assess collagen deposition. The results in Fig. 2B show that collagen deposition was markedly increased following a prolonged exposure to NT3. Thus NT3 induces collagen production and deposition in human AVICs.
NT3 promotes AVIC proliferation and collagen deposition through Trk receptors.
To determine whether Trk receptors play a role in NT3-induced AVIC proliferation, we added a pan Trk inhibitor (K252a; 0.20 µmol/l) to the cell culture medium 1 h before addition of NT3 (0.10 µg/ml). The results of the BrdU assay show that NT3-induced BrdU incorporation was reduced by Trk inhibition, while the vehicle (DMSO) had no effect (Fig. 3). The results of the CCK-8 assay show that formazan dye formation was reduced by Trk inhibition in cells exposed to NT3 (Fig. 3). To confirm the role of Trk receptors and identify the isoform involved, we added Fc chimeras specific to Trk isoforms to AVIC culture 1 h before NT3 treatment. The BrdU and CCK-8 results in Fig. 3 show that inhibition of each of the three Trk isoforms attenuated the effect of NT3 on cell proliferation. Thus all of the three Trk isoforms play a role in mediating NT3-induced AVIC proliferation. Together, the results suggest that Trk receptors mediate NT3-induced AVIC proliferation.
Fig. 3.

The proliferative effect of NT3 on human AVICs is mediated by the Trk receptors. Normal AVICs were treated with NT3 for 3 days in the presence or absence of Trk inhibitor K252a (0.20 µM) or isoform-selective inhibitors (Fc chimeras specific for TrkA, TrkB, and TrkC; 2.0 µg/ml each) for 3 days. Inhibition of Trk markedly reduces NT3-induced BrdU incorporation and formazan dye formation. Each isoform-selective inhibitor attenuates the BrdU incorporation and formazan dye formation induced by NT3. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO.
We applied the Trk inhibitor to evaluate the role of Trk in NT3-induced ECM protein production in human AVICs. K252a (0.20 µmol/l) was added to cell culture medium 1 h before addition of NT3 (0.10 µg/ml). The results in Fig. 4A show that K252a markedly reduced NT3-induced MMP-9 and collagen III production. To determine the role of Trk in NT3-induced collagen deposition, cells were cultured with medium that contained NT3 for 28 days in the presence or absence of the Trk inhibitor. As shown in Fig. 4B, inhibition of Trk decreased collagen deposition in cells treated with NT3. Thus NT3 induces ECM protein production and collagen deposition in human AVICs through the Trk receptor.
Fig. 4.
NT3 upregulates collagen deposition through Trk receptors. A: normal AVICs were treated with NT3 in the presence or absence of the Trk inhibitor K252a (0.20 µM) for 3 days. Representative immunoblots and densitometric data show that inhibition of Trk suppresses NT3-induced expression of collagen III and MMP-9. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO. B: normal AVICs were treated with NT3 in the presence or absence of K252a for 28 days. Representative images of PSR staining (scale bar = 150 μm) and spectrophotometric data show that collagen deposition induced by NT3 is markedly reduced by inhibition of Trk. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO.
Akt and cyclin D1 are involved in the mechanism of Trk-mediated AVIC proliferation.
Akt pathway modulates cellular survival, growth, proliferation, angiogenesis, metabolism, and migration (20). We evaluated the effect of NT3 on Akt activation. The results in Fig. 5A show that NT3 induced rapid phosphorylation of Akt. Furthermore, the Trk inhibitor abolished this effect of NT3.
Fig. 5.
NT3 upregulates cyclin D1 levels through the Trk-Akt pathway. A: normal AVICs were treated with NT3 in the presence or absence of Trk inhibitor for 1 to 24 h. Representative immunoblots of 3 separate experiments show that NT3 induces the phosphorylation of Akt. Inhibition of Trk receptors suppresses NT3-induced Akt phosphorylation. B: normal AVICs were treated with NT3 in the presence or absence of Trk inhibitor (K252a; 0.20 µM). Representative immunoblots and densitometric data show that NT3 upregulates cyclin D1 levels at 24 h and inhibition of Trk suppresses NT3-induced cyclin D1 expression. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO. C: normal AVICs were treated with NT3 in the presence or absence of Akt inhibitor (MK2206; 5.0 µM). Representative immunoblots and densitometric data show that inhibition of Akt reduces NT3-induced cyclin D1 expression. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3.
Cyclin D1 functions as a switch of continuous cell cycle progression and proliferation (36). To determine whether NT3 upregulates cyclin D1 and the role of the Trk-Akt pathway in modulation of cyclin D1 levels, we treated AVICs with Trk inhibitor or Akt inhibitor 1 h before adding NT3. As shown in Fig. 5, B and C, treatment with NT3 increased the levels of cyclin D1 in AVICs at 24 h, and inhibition of either Trk or Akt suppressed the effect of NT3 on cyclin D1 levels. Thus NT3 upregulates the levels of cyclin D1 through the Trk-Akt pathway.
To determine the role of Akt and cyclin D1 in NT3-induced AVIC proliferation, Akt inhibitor or cyclin D1 inhibitor (arcyriaflavin A; 0.25 µM) was added to the cell culture medium 1 h before addition of NT3. The results of BrdU and CCK-8 assays show that inhibition of either Akt or cyclin D1 markedly reduced BrdU incorporation and formazan dye formation following treatment with NT3 (Figs. 6, A and B).
Fig. 6.
Inhibition of Akt or cyclin D1 suppresses NT3-induced AVIC proliferation. A: normal AVICs were treated with NT3 in the presence or absence of Akt inhibitor (MK2206, 5.0 µM) for 3 days. BrdU incorporation and formazan dye formation were reduced by MK2206. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3. B: normal AVICs were treated with NT3 in the presence or absence of cyclin D1 inhibitor (arcyriaflavin A; 0.25 µM) for 3 days. BrdU incorporation and formazan dye formation were reduced by Arcyriaflavin A. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO.
Akt and cyclin D1 mediate NT3-induced collagen deposition in human AVICs.
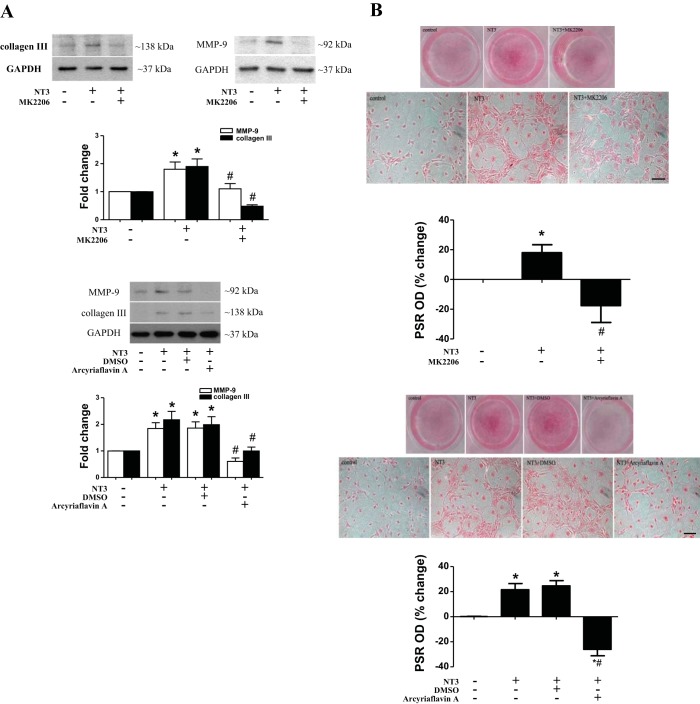
To determine the role of the Akt and cyclin D1 in NT3-induced ECM protein production, Akt inhibitor or cyclin D1 inhibitor was added to the cell culture medium 1 h before stimulation with NT3. The results show that inhibition of either Akt or cyclin D1 reduced MMP-9 and collagen III levels in cells exposed to NT3 (Fig. 7A).
Fig. 7.
Akt and cyclin D1 play a role in mediating NT3-induced collagen deposition. A: normal AVICs were treated with NT3 in the presence or absence of Akt inhibitor (MK2206; 5.0 µM) or cyclin D 1 inhibitor (arcyriaflavin A; 0.25 µM) for 3 days. Representative immunoblots and densitometric data show that inhibition of Akt or cyclin D1 suppresses NT3-induced expression of collagen III and MMP-9. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO. B: normal AVICs were treated with NT3 in the presence or absence of Akt inhibitor or cyclin D1 inhibitor for 28 days. Representative images of PSR staining (scale bar = 150 µm) and spectrophotometric data show that collagen deposition induced by NT3 is markedly reduced by inhibition of Akt or cyclin D1. Data are presented as means ± SE of 5 experiments using different cell isolates from distinct donor valves. Statistical analyses were performed using ANOVA with the post hoc Bonferroni/Dunn test and confirmed using nonparametric Kruskal-Wallis test. *P < 0.05 vs. control; #P < 0.05 vs. NT3 or NT3 + DMSO.
To determine the role of the Akt and cyclin D1 in NT3-induced collagen deposition, cells were treated with NT3 for 28 days in the presence or absence of Akt inhibitor or cyclin D1 inhibitor. As shown in Fig. 7B, NT3-induced collagen deposition was reduced by inhibition of either Akt or cyclin D1. Therefore, Akt and cyclin D1 are involved in the mechanism of NT3-induced ECM protein production and collagen deposition in human AVICs.
DISCUSSION
Aortic stenosis involves valvular sclerosis and calcification. Aortic valve sclerosis, defined as leaflet thickening (fibrosis), occurs in a large number of people age 65 yr and older and is an early sign of the development of aortic stenosis (38). Although moderate aortic valve stenosis does not cause left ventricular outflow obstruction, it is associated with an increased risk of cardiovascular death and myocardial infarction (27). Unfortunately, the cellular and molecular mechanisms of aortic valve stenosis remain incompletely understood. Pharmacological suppression of the development and progression of aortic valve stenosis relies on investigation of the mechanisms.
AVICs are the most prevalent cells in the aortic valve tissue. It is well accepted that AVICs become activated and undergo proliferation in the early stage of aortic valve stenosis (45). AVIC proliferation and overproduction of ECM proteins lead to valvular fibrosis that affects the mechanical properties of aortic valve leaflets (43). Several studies have determined the effect of growth factors, including TGF-β1 and BMP-2, on valvular cell proliferation (24, 39). However, the role of NT3 as a potential growth factor in the aortic valve remains to be evaluated. In this study, we observed that AVICs of stenotic aortic valves express higher levels of NT3 and that NT3 promotes AVIC proliferation in a dose-dependent fashion. In addition, NT3 upregulates collagen III and MMP-9 production, as well as collagen deposition in human AVICs. These results demonstrate for the first time that NT3 is capable of inducing AVIC fibrogenic responses characterized by cellular proliferation, overproduction of ECM proteins, and deposition of collagen. Furthermore, the results demonstrate that a Trk-Akt-cyclin D1 cascade mediates the fibrogenic responses to NT3. These findings suggest that NT3 is a profibrogenic mediator in the aortic valve and overproduction of NT3 in diseased aortic valves may contribute to the mechanism underlying valvular sclerosis.
Accumulation of ECM proteins alters tissue architecture (8). In normal human aortic valves, collagens are present in all three layers but are most abundant in the fibrosa layer (18). Extensive fibrosis with disorganized collagen fibers is observed in diseased valves (3, 9, 21). Interestingly, we observed that NT3 was able to upregulate the levels of collagen III in human AVICs, but had no effect on the level of collagen I. Moreover, we observed greater collagen deposition in AVICs exposed to NT3 for a prolonged period. A previous study reported that in the aortic valves of patients with aortic stenosis, the levels of MMP-1, MMP-2, MMP-3, and MMP-9 are markedly increased (7). We observed that NT3 upregulated MMP-9 but not MMP-2. It appears that multiple mediators are involved in the sclerotic changes in diseased aortic valves and that NT3 is one of the mediators. MMP-9 is known to mediate type III collagen degradation in liver fibrogenesis (42). MMP-9 also has a profibrogenic effect in the lung and kidney (29, 40). The relationship between MMP-9 and collagen III, as well as the role of MMP-9 in collagen deposition, remains unclear from the present study. Further studies are needed to address these issues. Nevertheless, greater production of collagen III induced by NT3 could cause a change in the ratio of this type of collagen to other collagens and may alter valvular ECM components.
As the net rate of cell growth depends on a fine balance between the rates of cell proliferation and cell death, we examined whether reduced cell apoptosis contributes to cell growth. However, no apoptosis is evident in AVIC cultures, and treatment with NT3 does not induce apoptosis (data not shown). It is noteworthy that increased ECM protein levels accompany greater cell density. However, collagen III and MMP-9 levels are normalized by housekeeping protein. In addition, normalized levels of collagen I and MMP-2 are comparable between untreated control cells and cells exposed to NT3. Therefore, NT3 selectively, upregulates collage III and MMP-9. The greater collagen deposition in NT3-treated cells is due to increased production of collagen III.
Trk receptors have been found to play an important role in neurotrophin-induced proliferation and differentiation in human dermal fibroblasts (28). TrkA mediates NGF-induced MMP-2 secretion in human endothelial cells (30) and MMP-9 expression in rat aortic smooth muscle cells (15). Inhibition of TrkB is found to suppress MMP-2 and -9 activities in large cell neuroendocrine carcinoma (25). In the present study, we observed that inhibition of Trk abolishes NT3-induced AVIC proliferation. Inhibition of each of the three Trk isoforms also attenuates the effect of NT3 on AVIC proliferation. More importantly, inhibition of Trk abolishes the production of collagen III and MMP-9, as well as collagen deposition induced by NT3. Thus Trk receptors are critical in mediating the NT3-induced profibrogenic responses in human AVICs.
Akt is believed to be a convergent point of several growth signals. In human lung fibroblasts, the Akt pathway mediates TGF-β1-induced cell proliferation, colony formation, and upregulation of collagen I (48). Knockdown of Akt suppresses collagen I production in human and mouse hepatic stellate cells, leading to attenuation of liver fibrogenesis (44). We observed that NT3 induces rapid phosphorylation of Akt in human AVICs, and inhibition of Trk abolished Akt phosphorylation at each time point examined. Furthermore, NT3-induced AVIC proliferation and collagen deposition are markedly reduced by the selective Akt inhibitor MK2206. Thus Trk links to Akt and the Trk-Akt pathway plays an important role in NT3-induced human AVIC fibrogenic responses. Further studies are needed to identify the Akt isoform that mediates the effect of NT3 in human AVICs.
Cell cycle represents a sequence of events that ultimately contribute to cell proliferation. Cyclin D1 functions as a switch in cell cycle G1/S transition and regulates cell cycle progression and proliferation (36). While its association with tissue fibrosis has been shown by many studies (4, 35), few studies report a role of cyclin D1 in cellular fibrotic responses (2). Our results show that NT3 upregulates the levels of cyclin D1 in human AVICs and inhibition of Trk or Akt reduces the levels of cyclin D1 protein. Furthermore, inhibition of cyclin D1 essentially abolishes NT3-induced fibrogenic responses. These observations indicate that the Trk-Akt pathway is responsible for cyclin D1 upregulation in response to NT3 stimulation and that cyclin D1 plays a mechanistic role in AVIC fibrogenic responses induced by NT3.
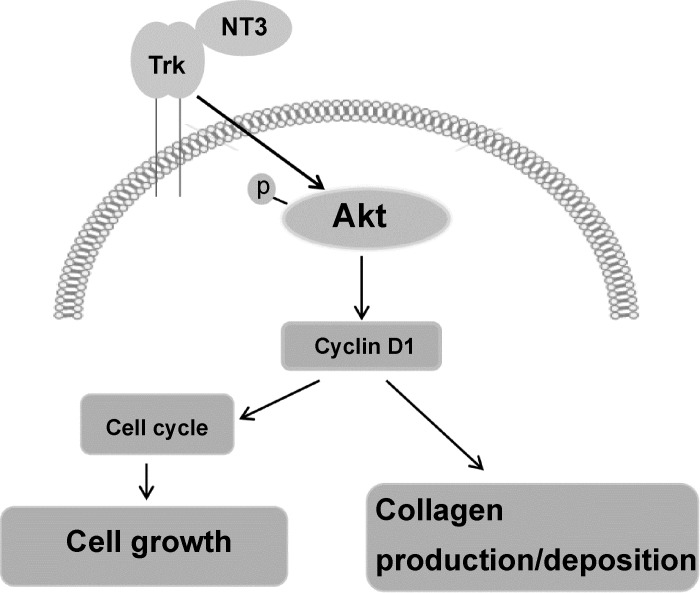
Overall, our study provides evidence that AVICs of diseased aortic valves produce higher levels of NT3 and that a Trk-Akt-cyclin D1 cascade mediates NT3-induced AVIC fibrogenic responses. The findings of the present study suggest that overproduction of NT3 in aortic valve tissue may promote sclerosis (Fig. 8). In view of our previous observation that NT3 upregulates the proosteogenic activity in human AVICs and the current findings, we speculate that NT3 may have a dual role in the pathogenesis of CAVD that involves sclerosis and nodular calcification. However, the role of endogenous NT3 in the pathobiology of CAVD awaits further investigations.
Fig. 8.
Overview of the signaling pathways that mediate the profibrogenic effect of NT3 on human AVICs. NT3 promotes cellular proliferation, extracellular matrix (ECM) protein production and collagen deposition in human AVICs through Trk receptors. A Trk-Akt-cyclin D1 cascade mediates these effects of NT3 and may enhance valvular profibrogenic activity.
Conclusion.
The expression of NT3 is enhanced in aortic valves of patients with CAVD. NT3 stimulates AVIC proliferation and upregulates ECM protein production. NT3 exerts these profibrogenic effects on human AVICs through the Trk-Akt-cyclin D1 cascade.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-106582 and HL-121776. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.M. conceived and designed the research. Q.Y. performed experiments; Q.Y., R.S., L.A., D.A.F., and X.M. analyzed data; Q.Y., R.S., L.A., J.C.C.J., D.A.F., and X.M. interpreted results of experiments; Q.Y., R.S., and X.M. prepared figures; Q.Y., R.S., and X.M. drafted manuscript; R.S., L.A., J.C.C.J., D.A.F., and X.M. edited and revised manuscript; X.M. approved final version of manuscript.
REFERENCES
- 1.Bashey RI, Torii S, Angrist A. Age-related collagen and elastin content of human heart valves. J Gerontol 22: 203–208, 1967. doi: 10.1093/geronj/22.2.203. [DOI] [PubMed] [Google Scholar]
- 2.Chen WC, Lin HH, Tang MJ. Matrix-stiffness-regulated inverse expression of Krüppel-like factor 5 and Krüppel-like factor 4 in the pathogenesis of renal fibrosis. Am J Pathol 185: 2468–2481, 2015. doi: 10.1016/j.ajpath.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Cimini M, Boughner DR, Ronald JA, Aldington L, Rogers KA. Development of aortic valve sclerosis in a rabbit model of atherosclerosis: an immunohistochemical and histological study. J Heart Valve Dis 14: 365–375, 2005. [PubMed] [Google Scholar]
- 4.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the β-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol 308: F358–F365, 2015. doi: 10.1152/ajprenal.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagnell C, Kemi C, Klominek J, Eriksson P, Sköld CM, Eklund A, Grunewald J, Olgart Höglund C. Effects of neurotrophins on human bronchial smooth muscle cell migration and matrix metalloproteinase-9 secretion. Transl Res 150: 303–310, 2007. doi: 10.1016/j.trsl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Durbin AD, Gotlieb AI. Advances towards understanding heart valve response to injury. Cardiovasc Pathol 11: 69–77, 2002. doi: 10.1016/S1054-8807(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 7.Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol 9: 281–286, 2000. doi: 10.1016/S1054-8807(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 8.Eitner F, Floege J. Therapeutic targets for prevention and regression of progressive fibrosing renal diseases. Curr Opin Investig Drugs 6: 255–261, 2005. [PubMed] [Google Scholar]
- 9.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J 26: 1333–1341, 2005. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 10.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111: 3316–3326, 2005. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 11.Henderson TA, Rhoades RW, Bennett-Clarke CA, Osborne PA, Johnson EM, Jacquin MF. NGF augmentation rescues trigeminal ganglion and principalis neurons, but not brainstem or cortical whisker patterns, after infraorbital nerve injury at birth. J Comp Neurol 336: 243–260, 1993. doi: 10.1002/cne.903360207. [DOI] [PubMed] [Google Scholar]
- 12.Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature 344: 339–341, 1990. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 13.Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. 5-HT(2B) antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol 53: 707–714, 2012. doi: 10.1016/j.yjmcc.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalcheim C, Carmeli C, Rosenthal A. Neurotrophin 3 is a mitogen for cultured neural crest cells. Proc Natl Acad Sci USA 89: 1661–1665, 1992. doi: 10.1073/pnas.89.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan KM, Falcone DJ, Kraemer R. Nerve growth factor activation of Erk-1 and Erk-2 induces matrix metalloproteinase-9 expression in vascular smooth muscle cells. J Biol Chem 277: 2353–2359, 2002. doi: 10.1074/jbc.M108989200. [DOI] [PubMed] [Google Scholar]
- 16.Kohyama T, Liu X, Wen FQ, Kobayashi T, Abe S, Ertl R, Rennard SI. Nerve growth factor stimulates fibronectin-induced fibroblast migration. J Lab Clin Med 140: 329–335, 2002. doi: 10.1067/mlc.2002.128347. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 89: 349–379, 2010. doi: 10.1097/MD.0b013e3181fe5648. [DOI] [PubMed] [Google Scholar]
- 18.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis 14: 218–227, 2005. [PubMed] [Google Scholar]
- 19.Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science 247: 1446–1451, 1990. doi: 10.1126/science.2321006. [DOI] [PubMed] [Google Scholar]
- 20.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol 43: 1670–1676, 2004. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC Jr, Fullerton DA. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol 294: C29–C35, 2008. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 23.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res 108: 1392–1412, 2011. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narine K, De Wever O, Van Valckenborgh D, Francois K, Bracke M, DeSmet S, Mareel M, Van Nooten G. Growth factor modulation of fibroblast proliferation, differentiation, and invasion: implications for tissue valve engineering. Tissue Eng 12: 2707–2716, 2006. doi: 10.1089/ten.2006.12.2707. [DOI] [PubMed] [Google Scholar]
- 25.Odate S, Nakamura K, Onishi H, Kojima M, Uchiyama A, Nakano K, Kato M, Tanaka M, Katano M. TrkB/BDNF signaling pathway is a potential therapeutic target for pulmonary large cell neuroendocrine carcinoma. Lung Cancer 79: 205–214, 2013. doi: 10.1016/j.lungcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Otto CM. Calcific aortic valve disease: new concepts. Semin Thorac Cardiovasc Surg 22: 276–284, 2010. doi: 10.1053/j.semtcvs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341: 142–147, 1999. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 28.Palazzo E, Marconi A, Truzzi F, Dallaglio K, Petrachi T, Humbert P, Schnebert S, Perrier E, Dumas M, Pincelli C. Role of neurotrophins on dermal fibroblast survival and differentiation. J Cell Physiol 227: 1017–1025, 2012. doi: 10.1002/jcp.22811. [DOI] [PubMed] [Google Scholar]
- 29.Pardo A, Selman M. Role of matrix metaloproteases in idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair 5, Suppl 1: S9, 2012. doi: 10.1186/1755-1536-5-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem 282: 30485–30496, 2007. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 31.Passi A, Negrini D, Albertini R, Miserocchi G, De Luca G. The sensitivity of versican from rabbit lung to gelatinase A (MMP-2) and B (MMP-9) and its involvement in the development of hydraulic lung edema. FEBS Lett 456: 93–96, 1999. doi: 10.1016/S0014-5793(99)00929-1. [DOI] [PubMed] [Google Scholar]
- 32.Rabkin-Aikawa E, Mayer JE Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol 94: 141–179, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A 90: 1043–1053, 2009. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, Nikolics K, Winslow JW. Primary structure and biological activity of a novel human neurotrophic factor. Neuron 4: 767–773, 1990. doi: 10.1016/0896-6273(90)90203-R. [DOI] [PubMed] [Google Scholar]
- 35.Royce SG, Nold MF, Bui C, Donovan C, Lam M, Lamanna E, Rudloff I, Bourke JE, Nold-Petry CA. Airway Remodeling and Hyperreactivity in a Model of Bronchopulmonary Dysplasia and Their Modulation by IL-1Ra. Am J Respir Cell Mol Biol 55: 858–868, 2016. doi: 10.1165/rcmb.2016-0031OC. [DOI] [PubMed] [Google Scholar]
- 36.Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol 15: 158–163, 2003. doi: 10.1016/S0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 37.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 29: 630–634, 1997. doi: 10.1016/S0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 39.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol 258: 252–263, 2003. doi: 10.1016/S0012-1606(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 40.Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y, Tian X, Wang Y, Wang YM, Cao Q, Wang Y, Lee VW, Wang C, Zheng D, Alexander SI, Thompson E, Harris DC. Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93: 434–449, 2013. doi: 10.1038/labinvest.2013.3. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol 35: 113–118, 2003. doi: 10.1016/S1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 42.Veidal SS, Vassiliadis E, Barascuk N, Zhang C, Segovia-Silvestre T, Klickstein L, Larsen MR, Qvist P, Christiansen C, Vainer B, Karsdal MA. Matrix metalloproteinase-9-mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int 30: 1293–1304, 2010. doi: 10.1111/j.1478-3231.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 43.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 95: 253–260, 2004. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, Lan HY, Sung JJ, Yu J. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget 6: 7325–7338, 2015. doi: 10.18632/oncotarget.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol 50: 561–569, 2011. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB. In vitro models of TGF-beta-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol Renal Physiol 293: F631–F640, 2007. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 47.Yao Q, Song R, Ao L, Zhan Q, Cleveland JC Jr, Yu X, Fullerton DA, Meng X. Over-expression of neurotrophin 3 in human aortic valves affected by calcific disease induces the osteogenic responses via the Trk-Akt pathway. Biochim Biophys Acta 1852: 1940–1949, 2015. doi: 10.1016/j.bbadis.2015.06.021. [DOI] [PubMed] [Google Scholar]