Abstract
Fibulin (fbln)-5 is an elastin-binding protein required for assembly and organization of elastic fibers. To examine the potential role of fbln-5 in vascular remodeling and neointima formation, we induced vascular injury by carotid artery ligation in fbln-5–/– mice. Mutant mice displayed an exaggerated vascular remodeling response that was accompanied by severe neointima formation with thickened adventitia. These abnormalities were not observed in elastin+/– mice that exhibited a comparable reduction of vessel extensibility to fbln-5–/– mice. Thus, the severe remodeling response could not be attributed to altered extensibility of the vessel wall alone. Vascular smooth muscle cells cultured from fbln-5–/– mice displayed enhanced proliferative and migratory responses to mitogenic stimulation relative to wild-type cells, and these responses were inhibited by overexpression of fbln-5. These findings demonstrate the importance of the elastic laminae in vascular injury, and reveal an unexpected role of fbln-5 as an inhibitor of vascular smooth muscle cell proliferation and migration.
Keywords: elastic fibers, extracellular matrix, neointima, elastin
Vascular obstructive abnormalities, such as atherosclerosis and restenosis after percutaneous coronary intervention, are triggered by damage to the vessel wall, initiating a series of biological responses, including up-regulation of adhesion molecules, recruitment of inflammatory cells, secretion of cytokines, and activation of smooth muscle cells (SMCs). Activated SMCs secrete growth factors, extracellular matrix proteins, and matrix proteases, thereby altering the microenvironment of the injured vessel wall. Much attention has focused on the signaling pathways responsible for the proliferation and migration of SMCs associated with vascular obstruction, but relatively little is known of the potential contributions of extracellular matrix proteins to these processes (reviewed in ref. 1).
Structural changes of the vessel wall induced by mechanical force or enzymatic digestion also influence the development of vascular obstructive disease (2, 3). The elastic lamina plays a critical role in maintaining the integrity of the vessel wall. Rupture of the external elastic lamina (EEL) is a more potent stimulus for neointima formation than injury involving the internal elastic lamina (IEL) alone after coronary artery stent-induced injury (4). In addition, exposure of the adventitia to the blood lumen by disruption of the EEL during percutaneous angioplasty increases the activation of adventitial myofibroblasts, leading to adventitial fibrosis that eventually constricts the vessel wall (5).
The perception of the elastic lamina (or elastic fibers) as the sole structural component of the vessel wall was recently challenged by the view that elastic fibers actively modulate intercellular signaling. Elastin (eln), a major component of the elastic fibers in the arterial wall, inhibits proliferation of SMCs (6). Eln is secreted as a tropoelastin monomer and subsequently undergoes cross-linking to form an insoluble eln polymer. SMCs cultured from the aortas of eln–/– mice show enhanced proliferation that is inhibited in a dose-dependent manner by recombinant tropoelastin (7). In addition, eln sheath-coated stents were reported to be effective in reducing neointima formation in a porcine coronary injury model of in-stent restenosis (7). Interestingly, overexpression of a variant form of the proteoglycan versican 3 increases expression of tropoelastin mRNA and facilitates elastic fiber assembly within the neointima, thus contributing to a more compact neointima (8). It is conceivable that these proteins may be involved in a negative-feedback loop to inhibit overproliferation of SMCs, either directly or indirectly, by creating a microenvironment nonpermissive for proliferation.
Fibulin (fbln)-5 contains tandem repeats of calcium-binding epidermal growth factor-like domains and localizes on the surface of elastic fibers (9). It binds eln and subsets of integrins and elastic fiber-associated proteins (10–12). Our group and others showed that fbln-5–/– mice exhibited a systemic elastinopathy, including loose skin, emphysematous lungs, and tortuous blood vessels, which is indicative of an essential role for fbln-5 in elastic fiber development in vivo (10, 11). In fbln-5–/– mice, the elastic laminae in the aorta were disorganized, forming multiple aggregates instead of smooth, organized lamellar sheets, thus leading to a loss of integrity of the vessel wall. Recent reports on genetic mutation or variation of the fbln-5 gene in cutis laxa and age-related macular degeneration patients, respectively, underscore the critical role of fbln-5 in assembly and organization of elastic fibers in vivo (13–15).
In addition to its significance in elastic fiber development, the potential involvement of fbln-5 in vascular injury is suggested from the observation that fbln-5 is up-regulated in the late stage of neointimal lesions induced by balloon injury and in the endothelial layer of fibrous atherosclerotic plaques (9). In this study, we investigated the potential role of fbln-5 in vascular injury by comparing the remodeling process of wild-type and fbln-5–/– vessels, using a carotid artery ligation model. We show that fbln-5–/– mice are more susceptible than wild-type mice to severe neointima formation and altered remodeling after carotid artery ligation. Moreover, fbln-5–/– SMCs exhibit increased proliferative and migratory responses to mitogenic stimulation that are suppressed by overexpression of fbln-5. These results suggest that the elastic lamina is critical for maintaining integrity of the vessel wall during vascular injury and reveal an unexpected role of fbln-5 as an inhibitory matrix molecule for SMCs.
Methods
Mice. Fbln-5–/– mice and eln+/– mice were maintained on a C57BL6 × 129/SvEv hybrid background (11, 16). Fbln-5–/– mice and their littermates ranging in age from 6 to 12 months, and closely age-matched eln+/– mice, were used for the studies. All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees.
Adenovirus. A full-length rat fbln-5 cDNA with a FLAG epitope tag was cloned into the pACCMVpLpA (–) loxP-SSP vector (17). A multiplicity of infection of 50 viruses per cell was used in all experiments. Infection efficiency was almost 100% as judged by GFP expression in SMCs after infection, and secretion of fbln-5 into media was confirmed by Western blot analysis.
Carotid Artery Ligation Model. Carotid artery ligation was performed as described (18).
Histology and Immunostaining. Hematoxylin/eosin was used for routine histology, and modified Hart's stain and Masson's trichrome stain were used for elastic fiber and collagen fiber staining, respectively. Immunostaining with anti-SM α-actin (1:400, Sigma) and anti-calponin (1:10,000, Sigma) were performed.
Morphometric Analysis. The left and right carotid arteries were cut 1 mm proximal to the ligature and embedded transversely in paraffin. The first transverse section was designated as level 0 (1,000 μm proximal to the ligature). Sections were obtained at 100, 200, 300, 400, 500, and 900 μm and stained for Hart's. Morphometric analysis was performed at level 400 in each set of ligated and nonligated contralateral carotid arteries (WT, n = 5; fbln-5–/–, n = 6; and eln+/–, n = 5) using nih image software (National Institutes of Health).
BrdUrd Labeling of Carotid Artery. BrdUrd (Sigma) at 100 μg/g of body weight was administered i.p. every day after 48 h of ligation until day 14 when mice were killed to harvest carotid arteries. Serial transverse sections were made at level 0–140 and anti-BrdUrd immunostaining was performed as described (19).
In Situ Hybridization Analysis. 35S-UTP-labeled antisense riboprobes for fbln-5, eln, and SM α-actin were transcribed and hybridized as described (9), and exposed for 28 days.
Electron Microscopy. The left carotid artery from wild-type (n = 4) and fbln-5–/– (n = 5) mice was harvested after 28 days of ligation and prepared for electron microscopic observation as described (20).
Isolation of Vascular SMCs. SMCs were isolated from postnatal day 1 aortas as described in Supporting Methods, which is published as supporting information on the PNAS web site. Three independent preparations were used in this study.
Cell Proliferation Assay. Cells were plated at a density of 1,000 cells per well in 96-well dishes in serum containing media, then serum-starved for 24 h to synchronize the cell cycle, and stimulated with 50 ng/ml human platelet-derived growth factor (PDGF)-BB (Roche Diagnostics) for 24 or 48 h. For overexpression of fbln-5 in fbln-5–/– SMCs, cells were infected with adenovirus expressing rat fbln-5-FLAG for 3 h at 37°C in media containing 10% FBS before plating in 96-well dishes. Cell numbers were quantified using Cell Titer 96 Aqueous One solution (Promega) according to manufacturer's instructions.
Modified Boyden Chamber Migration Assays. Two-well chambers separated by a filter (Chemicon, 8-μm pore size) coated with collagen type I was used in the experiment. A total of 5 × 104 cells were added to the upper chamber and serum-starved for 24 h. PDGF-BB (50 ng/ml) was added to the lower chamber and incubated for 6 h. Cells that migrated on the filter were stained according to the manufacturer's instructions, and cell numbers were counted by microscopy.
In Vitro Scratch Assay. Cells were plated at ≈80% confluency in six-well dishes, allowed to reach confluence, and serum-starved for 24 h. Media was changed to DMEM containing 10% FBS, and a sterile 1-ml pipette tip was used to administer the scratch injury. Cells were allowed to migrate into the cleared zone for 6–12 h and fixed in 3.7% formaldehyde. Still images were captured, and cells were scored based on the distance migrated from the left border of the scratch injury.
Vessel Extensibility Measurement. The isolated left carotid artery was cannulated and mounted on a pressure arteriograph (Living Systems Instrumentation, Burlington, VT) as described (21). Vessels were transilluminated under an inverted microscope connected to a charged-coupled device camera and computerized system, allowing a continuous recording of the vessel diameter.
Statistical Analysis. All data were expressed as the mean ± SEM. Statistical analysis was performed by unpaired Student's t test or ANOVA with a Tukey–Kramer posttest. A Kruskal–Wallis nonparametric test with a Dunn's posttest was used when the SD was significantly different among the group. A P value of <0.05 was considered statistically significant.
Results
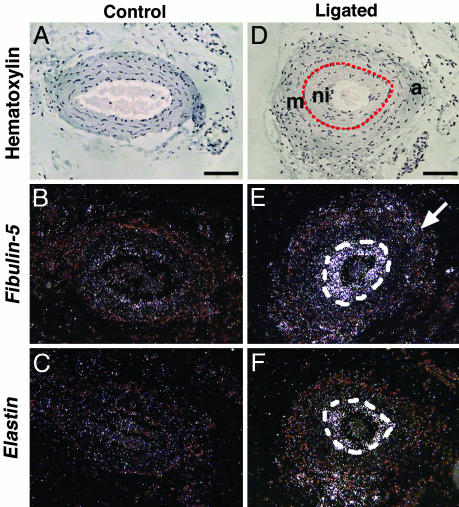
Up-Regulation of fbln-5 and eln in Neointimal Lesions Induced by Carotid Artery Ligation. To begin to explore the potential involvement of fbln-5 in neointima formation, we examined fbln-5 expression in the vessel wall in wild-type mice by in situ hybridization after carotid artery ligation. Neointima was observed on day 28, but not at earlier time points (data not shown) at level 30 (1,030 μm proximal to the ligature), between the endothelial cell layer and IEL (Fig. 1D). The expression of fbln-5, eln, and α-SM actin was barely detectable in the unligated artery (Fig. 1 B and C and data not shown). Fbln-5 and eln expression was increased dramatically in the neointima (Fig. 1 E and F) on postoperative day 28, but not at earlier time points. Mild up-regulation of fbln-5 was also observed in the adventitia (Fig. 1E, arrow). Cells within the neointima also expressed α-SM actin, a marker for early-stage SMCs (data not shown, also see Fig. 6I, which is published as supporting information on the PNAS web site). These findings show that eln and fbln-5 expression is activated in the late stage of neointimal formation.
Fig. 1.
Up-regulation of fbln-5 and eln in the neointimal lesion. In situ hybridization of transverse sections of wild-type carotid arteries before ligation (A–C) and 28 days after ligation (D–F). (A and D) Bright-field images showing hematoxylin counterstaining. The area surrounded by a red line in D and a white line in E and F are the neointima (ni). Sections were hybridized with cRNA probes for fbln-5 (B and E) and eln (C and F). Note the up-regulation of fbln-5 (E) and eln (F) in the neointima. Arrow in E indicates fbln-5 expression in the adventitia. m, media; a, adventitia. (Bars, 80 μm.)
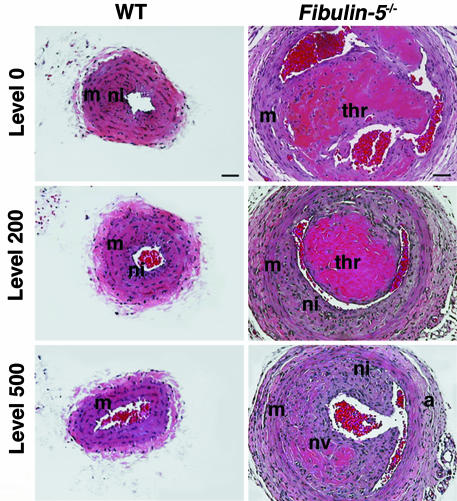
Increased Vessel Diameter and Neointima Formation in fbln-5–/– Mice. The ligated carotid artery undergoes initial outward remodeling, followed by vessel shrinkage and neointima formation, resulting in narrowing of the lumen (22). To examine whether the loss of fbln-5 affected the vascular response to injury, we first compared the remodeling of the vessel 28 days after the ligation in wild-type and fbln-5–/– left carotid arteries. Transverse sections of ligated and contralateral vessels were stained with hematoxylin/eosin (Fig. 2). In the wild-type vessel, the most prominent neointima was observed at level 0 (1,000 μm proximal to the ligature) and significantly decreased as the sections advanced away from the ligature (Fig. 2 Left). The results obtained in the fbln-5+/– mice were identical (data not shown). Fbln-5–/– vessels frequently developed a complex lesion of organized thrombus and SMC proliferation adjacent to the ligature (Fig. 2 Right). Multiple small vessels formed within the organizing thrombus, and a significant neointimal hyperplasia was seen that extended to level 900 (data not shown). The diameter of the fbln-5–/– vessel was significantly larger than that of wild-type vessels, the adventitia surrounding the ligated vessel was hypercellular, and the medial layer was much thinner than that of the wild-type vessel. Comparable vascular lesions developed in younger fbln-5–/– mice (between 2 and 3 months old, data not shown). These data suggested that fbln-5–/– vessels failed to undergo constrictive remodeling at day 28.
Fig. 2.
Vascular remodeling in fbln-5–/– mice after 28 days of ligation. Representative photos of transverse sections at indicated levels stained with hematoxylin/eosin. Wild-type (WT) vessels are shown in Left. Note an increased diameter of the vessel with a thickening of adventitia (a) and thinning of the medial layer (m) in the fbln-5–/– vessel. Thrombus formation (thr) and neovascularization (nv) were observed in the fbln-5–/– mouse. ni, neointima. (Bars, 40 μm.)
Eln and collagen were both present in the neointima of wild-type and fbln-5–/– vessels (Fig. 6 B, G, C, and H). Immunostaining at level 400 revealed that the cells forming the neointimal lesion in the fbln-5–/– vessel were strongly positive for α-SM actin (Fig. 6I, arrow), but only weakly positive for calponin, which is a late-stage differentiation marker for SMCs (Fig. 6J, arrow).
To determine whether there was an increase in cell proliferation at earlier stages of vascular injury in fbln-5–/– mice, we injected BrdUrd i.p. daily, starting 48 h after ligation. At day 14, we killed the animals, and immunostained the transverse sections of the ligated artery with anti-BrdUrd antibody. The number of BrdUrd-positive cells was dramatically increased in the adventitia of the fbln-5–/– vessel (mean ± SEM = 186.6 ± 14.4, n = 5) compared with the wild-type vessel (mean ± SEM = 17.8 ± 3.1, n = 4). A more modest increase in BrdUrd staining was seen in the media. Thus, an exacerbated proliferative response within the adventitia and media may contribute to neointima formation with resulting reduction in the luminal area of the vessel.
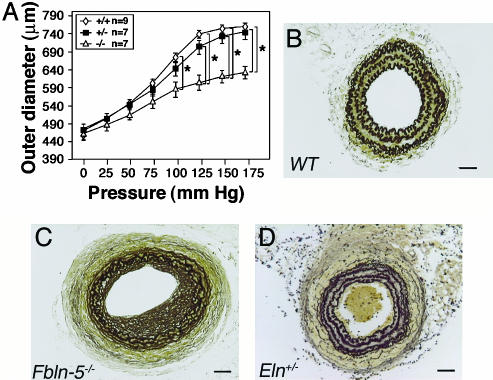
Carotid Arteries of fbln-5–/– and eln+/– Show a Similar Reduction in Extensibility. To determine the potential influence of fbln-5 on vascular mechanical properties, we compared the extensibility of left carotid arteries from wild-type and mutant mice (Fig. 3A). The outer diameter of wild-type and fbln-5+/– arteries extended similarly in response to increasing pressure. In contrast, a significant reduction in the extensibility of fbln-5–/– carotid arteries was found when pressures of >100 mmHg were applied to the vessel (P < 0.05), which was similar to what was previously observed in eln+/– vessels (21). Therefore, we performed carotid artery ligation in eln+/– mice to compare with fbln-5–/– and wild-type mice (Fig. 3 B–D). As shown in Fig. 3D, eln+/– carotid arteries displayed an increased number of lamellar units as previously reported (23). However, unexpectedly, eln+/– mice did not exhibit exacerbated outward remodeling after 28 days of ligation, and the degree of neointima formation was considerably less than was seen in fbln-5–/– mice (Fig. 3C).
Fig. 3.
Reduced vessel extensibility in the fbln-5–/– carotid artery. (A) Pressure-diameter curve. Three measurements were taken for each mouse. At pressures >100 mmHg, there was a significant difference in the outer diameter between wild-type (n = 9) or fbln-5+/– vessels (n = 7) and fbln-5–/– (n = 7) vessels. (B–D) Hart's staining of the transverse sections of ligated arteries. Neointima and vascular remodeling were compared between wild-type (B), fbln-5–/– carotid arteries (C) and eln+/– (D) carotid arteries after 28 days of ligation. Both fbln-5–/– carotid arteries and eln+/– carotid arteries exhibited decreased extensibility (see A and ref. 21); however, there was no outward remodeling or increased neointima formation in the eln+/– vessel. Level shown is 400. (Bars, 40 μm.)
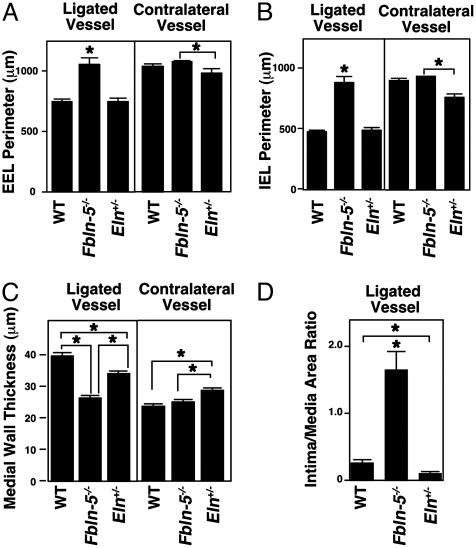
To compare development of vascular remodeling quantitatively between wild-type and mutant mice, we made transverse sections at 1,400 μm proximal to the ligature (at level 400) and performed morphometric analysis. The fbln-5–/–-ligated vessels, but not eln+/– vessels, had an increased perimeter of both the EEL and IEL when compared with wild-type vessels (Fig. 4 A and B). In contrast, no difference was found between fbln-5–/– and wild-type vessels on the contralateral side (Fig. 4 A and B). These results suggested that fbln-5–/– vessels failed to shrink and adjust vessel size after the ligation, which is most likely due to the absence of organized elastic lamina. The thickness of the media was also significantly reduced in the fbln-5–/– vessels, and to a lesser extent in eln+/– vessels (Fig. 4C). This finding suggested that either a change in quality (i.e., disrupted elastic laminae in fbln-5–/– vessels) or quantity (i.e., reduced eln amount in eln+/– vessels) of elastic laminae resulted in failure to maintain the vascular wall thickness in response to cessation of blood flow. We found an increase in wall thickness and a decrease in IEL and EEL perimeter in nonligated contralateral eln+/– vessels when compared with wild-type and fbln-5–/– vessels. This finding may be related to a specific function of eln within the vessel wall, and further examination is required to clarify this phenomenon. As shown in Fig. 4D, a significant increase in intima/media ratio was observed in fbln-5–/– mice but not in eln+/– mice or wild-type mice. Together, these findings suggest a potential regulatory role of fbln-5 in the proliferation and/or migration of vascular cells, in addition to its structural role in the vessel wall.
Fig. 4.
Morphometric analysis of ligated arteries after 28 days of ligation. (A) EEL perimeter of the ligated and contralateral vessels. (B) IEL perimeter of the ligated and contralateral vessels. (C) Thickness of the medial wall of ligated and contralateral vessels. (D) Intima/media area ratio. Wild-type (n = 5), fbln-5–/– vessels (n = 6), and eln+/– (n = 5) vessels were examined at level 400. Three measurements were taken for each parameter from each section. *, P < 0.05.
Disruption of Elastic Fiber Assembly in the Neointima of fbln-5–/– Mice. We wondered whether the accelerated neointima formation in fbln-5–/– mice might be due to insufficient deposition or incorporation of eln into elastic fibers within the neointimal lesion. To investigate this possibility, we examined the neointima of ligated fbln-5–/– vessels and wild-type vessels by electron microscopy. As shown in Fig. 5A, normal solid, amorphous elastic fibers were seen in the neointima of wild-type mice. In contrast, eln deposition and assembly in the neointima of the fbln-5–/– vessel was defective, and resulted in the formation of discontinuous, bead-like structures (Fig. 5B, double arrows). Thus, in the absence of fbln-5, newly formed elastic fibers failed to assemble and mature properly.
Fig. 5.
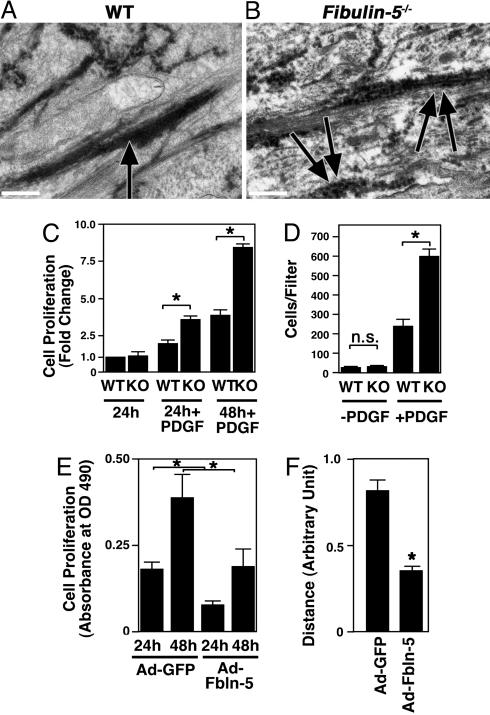
Electron micrographs of neointimal lesions and in vitro analysis of fbln-5–/– SMCs. (A and B) Representative photos of electron micrographic observations of ligated arteries from wild-type (A) and fbln-5–/– vessels (B). Note that elastic fibers are continuous and firm in the wild-type vessel (arrow), whereas elastic fibers are irregular and discontinuous in the mutant (double arrows). (Bars, 0.5 μm.) (C) Cell proliferation assays of wild-type (WT) and fbln-5–/– SMCs. WT cells cultured in the serum free condition for 24 h were assigned a value of 1. A fold increase in the indicated condition was shown to allow comparison between the genotype. Data are from three independent experiments performed in triplicate. *, P < 0.05. (D) Modified Boyden chamber assays. Numbers of WT and fbln-5–/– SMCs that migrated to the filter with or without PDGF-BB (50 ng/ml) were counted. Data are from three independent experiments performed in duplicate. n.s., not significant. (E) Cell proliferation assays of fbln-5–/– SMCs infected with the adenovirus expressing GFP (Ad-GFP) or fbln-5 (Ad-Fbln-5). y axis indicates absorbance at OD490 after 24 or 48 h of culture in PDGF-BB (50 ng/ml). Overexpressions of fbln-5 inhibited proliferation of SMCs. Data are from two independent experiments performed in triplicate. (F) In vitro scratch assays of fbln-5–/– SMCs infected with the adenovirus expressing GFP or fbln-5. The distance from the left border of the scratch injury was measured after 6 h of injury. Six to eight measurements were made for each condition. Data are from two independent experiments.
Suppression of Proliferation and Migration Phenotypes of fbln-5–/– SMCs by Forced Expression of fbln-5. The abnormalities in vessel remodeling in fbln-5–/– mice suggested a role for fbln-5 as a negative regulator of cell proliferation and/or migration. To directly explore the potential influence of fbln-5 in these processes, we isolated primary SMCs from aortas of wild-type pups and fbln-5–/– pups at postnatal day 1. Proliferation of fbln-5–/– SMCs was determined by analyzing the cell number using a tetrazolium compound reduction assay. No difference in proliferation was observed between fbln-5–/– SMCs and wild-type SMCs when cultured under serum-free conditions. However, in the presence of PDGF, fbln-5–/– SMCs showed a 2-fold increase in proliferation compared with wild-type cells (Fig. 5C).
Migration of fbln-5–/– SMCs was examined by using modified Boyden chamber assays (Fig. 5D). No difference in migration was seen between wild-type and fbln-5–/– SMCs when cells were grown in the absence of PDGF. Addition of PDGF resulted in a significantly increased number of fbln-5–/– SMCs that migrated through the filter as compared with wild-type cells.
To examine whether we could suppress the increased proliferation of fbln-5–/– SMCs, fbln-5–/– SMCs were infected with adenovirus expressing rat fbln-5 or GFP, and proliferation was assessed. Fbln-5–/– SMCs expressing fbln-5 showed a significant decrease in cell number after 48 h of incubation with PDGF compared with GFP-infected cells (Fig. 5E). Overexpression of fbln-5 also suppressed serum-induced proliferation of fbln-5–/– SMCs and fbln-5+/– SMCs (data not shown). We did not detect any increase in eln (as judged by Western blots) in fbln-5-overexpressing SMCs compared with GFP-infected cells, suggesting that the suppressive effect of fbln-5 was not due to increased production of eln (data not shown).
Next, fbln-5- and GFP-infected fbln-5–/– cultures were subjected to in vitro scratch assays, and migration toward the wound was assessed after 6 h in the presence of 10% serum. Overexpression of fbln-5 significantly suppressed migration of fbln-5–/– SMCs (Fig. 5F). Similar results were obtained with fbln-5-infected PAC-1 pulmonary vascular SMCs that express a large amount of endogenous fbln-5 (data not shown). It is unlikely that mature elastic fibers were assembled during the relatively short culture period (6–48 h) or under nonconfluent conditions that we used in our cell migration/proliferation assays (24). We propose that the inhibitory effect of fbln-5 is most likely mediated by an elastic fiber-independent mechanism.
Discussion
The results of this study show that fbln-5–/– mice display severe neointima formation and abnormal vascular remodeling after flow cessation-induced vascular injury. Two distinct mechanisms seem to account for the exaggerated vascular remodeling phenotype of fbln-5–/– vessels: (i) loss of structural integrity of the vessel wall and inability to assemble mature elastic fibers within the neointima, and (ii) loss of direct suppressive effects of fbln-5 on SMC proliferation and migration.
Influence of the Elastic Lamina on Vascular Remodeling. Fbln-5–/– vessels failed to undergo constrictive remodeling after 28 days of ligation. The reduction of vessel diameter after ligation-induced injury reflects changes in vessel structure, rather than SMC contraction (18). Our previous finding that fbln-5–/– vessels respond normally to vasoconstrictors supports this notion (11).
Digestion of elastic laminae by matrix metalloprotease (MMP) 2 and MMP 9 is frequently observed during the development of vascular obstructive disease (25), and MMP 2 has been shown to increase intimal hyperplasia by enhancing SMC migration through the media to the intima (26). Thus, the disrupted elastic laminae of the fbln-5–/– vessel wall may facilitate the migration of SMCs. However, reduced extensibility of the vessel wall does not completely account for the exacerbated remodeling phenotype of the fbln-5–/– vessels because eln+/– mice, which showed reduced extensibility of the carotid artery similar to that of fbln-5–/– mice (21), did not develop exaggerated neointima formation. In addition, eln+/– mice show less neointimal lesion formation than wild-type animals in a probe-induced transmural injury model. (R.P.M., unpublished observation). This finding suggests that the remodeling process depends not only on the amount of eln but also on an intact connection between elastic fibers and surrounding cells.
It is interesting to note that fbln-5–/–-ligated vessels frequently developed thrombi. The close relation between thrombus formation and neointima hyperplasia is suggested in vein graft-induced intimal hyperplasia (27), and stent thrombosis has been a serious concern after coronary stenting (28). It has been shown that thrombi induce inflammatory cell accumulation and cytokine release that may perturb the structural integrity within the aneurysm (29). Our recent finding that fbln-5 is a ligand for extracellular superoxide dismutase, thus regulating production of reactive oxygen species within the vascular wall, suggests a potential protective role of fbln-5 in endothelial dysfunction and thrombotic responses (30). In addition, fbln-5 is potentially involved in positive regulation of the fibrinolysis pathway (V. Stepanova, personal communication) that may be attenuated in fbln-5–/– vessels. The possibility that fbln-5–/– vessels cause intraluminar flow that is favorable to thrombus formation also needs to be examined.
Cells within the neointima of fbln-5–/– vessels were positive for α-SM actin, a marker for early SMCs, and weakly positive for calponin, indicating that these cells were highly synthetic. Polymerized eln transmits an antiproliferative signal for SMCs in vitro (7), and an absence of eln enhances proliferation of SMCs in the subendothelial layer of vessels and accounts for early neonatal death in eln–/– mice (16). Although the neointima in fbln-5–/– vessels did contain elastic fibers, a significant difference in the morphology of neointimal elastic fibers suggests that fbln-5 participates in regulation of SMC proliferation by facilitating the assembly of a mature eln-rich matrix within the neointima to suppress overproliferation of SMCs.
We observed an increased thickening and hyperproliferation of the adventitia in fbln-5–/– vessels. The involvement of adventitial fibrosis was proposed as one mechanism for restenosis, and up-regulation of various cytokines was detected in adventitial fibroblasts at early stages of vascular injury (31). In addition, adventitial cells contribute to atherosclerotic lesions by differentiating into SMCs in vivo (32). Up-regulation of fbln-5 in the adventitia at day 28 suggests that fbln-5 may be involved in regulation of the adventitial response to vascular injury. Alternatively, loss of fbln-5 may facilitate recruitment of bone marrow-derived progenitor cells to the injured vessel (33).
Suppression of SMC Proliferation and Migration by fbln-5. The underlying mechanism for an enhanced proliferative response to mitogenic stimuli in fbln-5–/– SMCs appears to be distinct from that of eln–/– SMCs. Eln–/– SMCs show markedly reduced actin stress fibers and contractile proteins, indicating the alteration of SMC phenotype in the absence of eln (7). In contrast, fbln-5 seems to modify the cellular response to mitogenic stimuli.
Several extracellular matrix proteins, including adiponectin, decorin, and vasorin, can antagonize the response of vascular cells to growth factors and cytokines by directly binding to them (34–37). Thus, it is reasonable to speculate that fbln-5 may bind extracellular ligand (s) and prevent them from binding to their receptors. Alternatively, fbln-5 may bind directly to growth factor receptors and block downstream signaling pathways. Further investigation will be required to test these hypotheses.
It is possible that fbln-5 acts as a signaling molecule. Previous studies (38) showed fbln-5 increased cell proliferation of HT1080 fibrosarcoma cells synergistically with TGB-β1, and enhanced fibronectin-induced HT1080 migration and invasion). Fbln-5 has also been shown to bind integrins (αVβ3, αVβ5, and α9β1) in vitro and to mediate cell attachment in an RGD motif-dependent manner in endothelial cells (10, 39). However, it has yet to be established whether fbln-5 can mediate signaling by means of cell-surface integrins. We failed to detect activation of integrin-linked kinase or focal adhesion kinase in fbln-5–/– SMCs after overexpressing fbln-5 (J.A.S., unpublished observation), suggesting that fbln-5 may not act as an integrin-mediated signaling molecule. Integrin β3–/– SMCs show decreased migration in vitro, and loss of αVβ3 expression on vascular cells can inhibit carotid artery ligation-induced neointima formation in vivo (40). The opposite result from our current study suggests that the binding partner of fbln-5 is not limited to the αVβ3-integrin on vascular cells.
The results of the present study underscore the importance of the microenvironment during vascular development and diseases. Therapeutic strategies to alter the microenvironment of the injured vessel so as to suppress proliferation and migration of SMCs warrant further consideration.
Supplementary Material
Acknowledgments
We thank Seiji Yokoyama and Andrew Hall for technical assistance; Jeff Stark and Chris Pomajzl for excellent histology preparations; Alisha Tizenor for assistance with graphics; and Helen Hobbs, Masashi Yanagisawa, Joseph Miano, and Rhonda Bassel-Duby for critical reading of the manuscript. This work was supported in part by the National Institutes of Health (to H.Y. and E.N.O) and the Donald W. Reynolds Center for Clinical Cardiovascular Research (to E.N.O. and R.D.G.).
Abbreviations: EEL, external elastic lamina; fbln, fibulin; eln, elastin; IEL, internal elastic lamina; PDGF, platelet-derived growth factor; SMC, smooth muscle cell.
References
- 1.Owens, G. K., Kumar, M. S. & Wamhoff, B. R. (2004) Physiol. Rev. 84, 767–801. [DOI] [PubMed] [Google Scholar]
- 2.Indolfi, C., Torella, D., Coppola, C., Stabile, E., Esposito, G., Curcio, A., Pisani, A., Cavuto, L., Arcucci, O., Cireddu, M., et al. (2002) Am. J. Physiol. 283, H760–H767. [DOI] [PubMed] [Google Scholar]
- 3.Lijnen, H. R., Van Hoef, B., Vanlinthout, I., Verstreken, M., Rio, M. C. & Collen, D. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2863–2870. [DOI] [PubMed] [Google Scholar]
- 4.Gunn, J., Arnold, N., Chan, K. H., Shepherd, L., Cumberland, D. C. & Crossman, D. C. (2002) Heart 88, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christen, T., Verin, V., Bochaton-Piallat, M., Popowski, Y., Ramaekers, F., Debruyne, P., Camenzind, E., van Eys, G. & Gabbiani, G. (2001) Circulation 103, 882–888. [DOI] [PubMed] [Google Scholar]
- 6.Urban, Z., Riazi, S., Seidl, T. L., Katahira, J., Smoot, L. B., Chitayat, D., Boyd, C. D. & Hinek, A. (2002) Am. J. Hum. Genet. 71, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karnik, S. K., Brooke, B. S., Bayes-Genis, A., Sorensen, L., Wythe, J. D., Schwartz, R. S., Keating, M. T. & Li, D. Y. (2003) Development (Cambridge, U.K.) 130, 411–423. [DOI] [PubMed] [Google Scholar]
- 8.Merrilees, M. J., Lemire, J. M., Fischer, J. W., Kinsella, M. G., Braun, K. R., Clowes, A. W. & Wight, T. N. (2002) Circ. Res. 90, 481–487. [DOI] [PubMed] [Google Scholar]
- 9.Kowal, R. C., Richardson, J. A., Miano, J. M. & Olson, E. N. (1999) Circ. Res. 84, 1166–1176. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura, T., Lozano, P. R., Ikeda, Y., Iwanaga, Y., Hinek, A., Minamisawa, S., Cheng, C. F., Kobuke, K., Dalton, N., Takada, Y., et al. (2002) Nature 415, 171–175. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa, H., Davis, E. C., Starcher, B. C., Ouchi, T., Yanagisawa, M., Richardson, J. A. & Olson, E. N. (2002) Nature 415, 168–171. [DOI] [PubMed] [Google Scholar]
- 12.Liu, X., Zhao, Y., Gao, J., Pawlyk, B., Starcher, B., Spencer, J. A., Yanagisawa, H., Zuo, J. & Li, T. (2004) Nat. Genet. 36, 178–182. [DOI] [PubMed] [Google Scholar]
- 13.Loeys, B., Van Maldergem, L., Mortier, G., Coucke, P., Gerniers, S., Naeyaert, J. M. & De Paepe, A. (2002) Hum. Mol. Genet. 11, 2113–2118. [DOI] [PubMed] [Google Scholar]
- 14.Markova, D., Zou, Y., Ringpfeil, F., Sasaki, T., Kostka, G., Timpl, R., Uitto, J. & Chu, M. L. (2003) Am. J. Hum. Genet. 72, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone, E. M., Braun, T. A., Russell, S. R., Kuehn, M. H., Lotery, A. J., Moore, P. A., Eastman, C. G., Casavant, T. L. & Sheffield, V. C. (2004) N. Engl. J. Med. 351, 346–353. [DOI] [PubMed] [Google Scholar]
- 16.Li, D. Y., Brooke, B., Davis, E. C., Mecham, R. P., Sorensen, L. K., Boak, B. B., Eichwald, E. & Keating, M. T. (1998) Nature 393, 276–280. [DOI] [PubMed] [Google Scholar]
- 17.Aoki, K., Barker, C., Danthinne, X., Imperiale, M. J. & Nabel, G. J. (1999) Mol. Med. 5, 224–231. [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, A. & Lindner, V. (1997) Artherioscler. Thromb. Vasc. Biol. 17, 2238–2244. [DOI] [PubMed] [Google Scholar]
- 19.Clouthier, D. E., Williams, S. C., Yanagisawa, H., Wieduwilt, M., Richardson, J. A. & Yanagisawa, M. (2000) Dev. Biol. 217, 10–24. [DOI] [PubMed] [Google Scholar]
- 20.Davis, E. C. (1993) Lab. Invest. 68, 89–99. [PubMed] [Google Scholar]
- 21.Faury, G., Pezet, M., Knutsen, R. H., Boyle, W. A., Heximer, S. P., McLean, S. E., Minkes, R. K., Blumer, K. J., Kovacs, A., Kelly, D. P., et al. (2003) J. Clin. Invest. 112, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godin, D., Ivan, E., Johnson, C., Magid, R. & Galis, Z. S. (2000) Circulation 102, 2861–2866. [DOI] [PubMed] [Google Scholar]
- 23.Li, D. Y., Faury, G., Taylor, D. G., Davis, E. C., Boyle, W. A., Mecham, R. P., Stenzel, P., Boak, B. & Keating, M. T. (1998) J. Clin. Invest. 102, 1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robb, B. W., Wachi, H., Schaub, T., Mecham, R. P. & Davis, E. C. (1999) Mol. Biol. Cell 10, 3595–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzuya, M. & Iguchi, A. (2003) J. Atheroscler. Thromb. 10, 275–282. [DOI] [PubMed] [Google Scholar]
- 26.Kuzuya, M., Kanda, S., Sasaki, T., Tamaya-Mori, N., Cheng, X. W., Itoh, T., Itohara, S. & Iguchi, A. (2003) Circulation 108, 1375–1381. [DOI] [PubMed] [Google Scholar]
- 27.Torsney, E., Mayr, U., Zou, Y., Thompson, W. D., Hu, Y. & Xu, Q. (2004) Circ. Res. 94, 1466–1473. [DOI] [PubMed] [Google Scholar]
- 28.Kereiakes, D. J., Choo, J. K., Young, J. J. & Broderick, T. M. (2004) Rev. Cardiovasc. Med. 5, 9–15. [PubMed] [Google Scholar]
- 29.Kazi, M., Thyberg, J., Religa, P., Roy, J., Eriksson, P., Hedin, U. & Swedenborg, J. (2003) J. Vasc. Surg. 38, 1283–1292. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, A. D., Itoh, S., Jeney, V., Yanagisawa, H., Fujimoto, M., Ushio-Fukai, M. & Fukai, T. (2004) Circ. Res. 95, 1067–1074. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox, J. N., Okamoto, E. I., Nakahara, K. I. & Vinten-Johansen, J. (2001) Ann. N.Y. Acad. Sci. 947, 68–92. [DOI] [PubMed] [Google Scholar]
- 32.Hu, Y., Zhang, Z., Torsney, E., Afzal, A. R., Davison, F., Metzler, B. & Xu, Q. (2004) J. Clin. Invest. 113, 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schober, A., Knarren, S., Lietz, M., Lin, E. A. & Weber, C. (2003) Circulation 108, 2491–2497. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda, M., Shimomura, I., Sata, M., Arita, Y., Nishida, M., Maeda, N., Kumada, M., Okamoto, Y., Nagaretani, H., Nishizawa, H., et al. (2002) J. Biol. Chem. 277, 37487–37491. [DOI] [PubMed] [Google Scholar]
- 35.Arita, Y., Kihara, S., Ouchi, N., Maeda, K., Kuriyama, H., Okamoto, Y., Kumada, M., Hotta, K., Nishida, M., Takahashi, M., et al. (2002) Circulation 105, 2893–2898. [DOI] [PubMed] [Google Scholar]
- 36.Fischer, J. W., Kinsella, M. G., Clowes, M. M., Lara, S., Clowes, A. W. & Wight, T. N. (2000) Circ. Res. 86, 676–683. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda, Y., Imai, Y., Kumagai, H., Nosaka, T., Morikawa, Y., Hisaoka, T., Manabe, I., Maemura, K., Nakaoka, T., Imamura, T., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 10732–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiemann, W. P., Blobe, G. C., Kalume, D. E., Pandey, A. & Lodish, H. F. (2002) J. Biol. Chem. 277, 27367–27377. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura, T., Ruiz-Lozano, P., Lindner, V., Yabe, D., Taniwaki, M., Furukawa, Y., Kobuke, K., Tashiro, K., Lu, Z., Andon, N. L., et al. (1999) J. Biol. Chem. 274, 22476–22483. [DOI] [PubMed] [Google Scholar]
- 40.Choi, E. T., Khan, M. F., Leidenfrost, J. E., Collins, E. T., Boc, K. P., Villa, B. R., Novack, D. V., Parks, W. C. & Abendschein, D. R. (2004) Circulation 109, 1564–1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.