Abstract
Cells respond to environmental stress in multiple ways. In the germ line, heat shock and nutritive stress trigger the assembly of large ribonucleoprotein (RNP) granules via liquid-liquid phase separation (LLPS). The RNP granules are hypothesized to maintain the quality of oocytes during stress. The goal of this study was to investigate the cellular response to glucose in the germ line and determine if it is an osmotic stress response. We found that exposure to 500 mM glucose induces the assembly of RNP granules in the germ line within 1 h. Interestingly, the RNP granules are maintained for up to 3 h; however, they dissociate after longer periods of stress. The RNP granules include processing body and stress granule proteins, suggesting shared functions. Based on several lines of evidence, the germ line response to glucose largely appears to be an osmotic stress response, thus identifying osmotic stress as a trigger of LLPS. Although RNP granules are not maintained beyond 3 h of osmotic stress, the quality of oocytes does not appear to decrease after longer periods of stress, suggesting a secondary adaptation in the germ line. We used an indirect marker of glycerol and observed high levels after 5 and 20 h of glucose exposure. Moreover, in gpdh-1;gpdh-2 germ lines, glycerol levels are reduced concomitant with RNP granules being maintained for an extended period. We speculate that increased glycerol levels may function as a secondary osmoregulatory adaptive response in the germ line, following a primary response of RNP granule assembly.
Keywords: germ line, C. elegans, glucose, osmotic stress, RNP granules
the ability to respond to environmental stresses is an essential feature of all cells. Heat stress, oxidative stress, anoxia, and osmotic stresses are among those that have received significant attention in terms of their effects on gene expression and physiology, in part because of their implications for human disease (5, 8, 26, 27). The use of model systems has proven instrumental in studying stress responses due to the conservation of pathways and genes from yeast to humans (22, 23, 27, 28).
While many studies to date have focused on the effects of stress on life span or on somatic tissues, such as muscle and neurons, fewer have examined effects on the germ line. Since maintenance of the germ line is essential for propagation of the species, improving our understanding of how the germ line responds and adapts to environmental stresses is critical. One type of cellular response to stress that occurs in the germ line is remodeling of ribonucleoprotein complexes, termed RNP granules (6, 15, 29, 32, 34). RNP granules are a shared feature of germ plasm found in a variety of vertebrate and invertebrate species (38). Often referred to as germ granules, these RNP complexes are enriched for maternal mRNAs and RNA-binding proteins and are hypothesized to play a role in germ cell function (37, 38). In response to arrested meiosis, nutritive stress, or heat shock, germ granules increase in size, and their composition increases in complexity (29). RNA-binding proteins that are normally diffuse throughout the cytoplasm appear to aggregate into granules, or bodies, that are up to 20 times larger than typical germ granules or sponge bodies (31, 34). Recent studies have uncovered the process of liquid-liquid phase separation (LLPS) as a main driver promoting assembly of germ line RNP granules, stress granules, processing (P) bodies, and other related membraneless organelles (3, 11, 13). While in vitro studies using purified protein preparations have identified several triggers of LLPS, the extent to which the in vitro triggers of LLPS function during the formation of gametes is not known.
In the model system, Caenorhabditis elegans, the components of stress-induced RNP granules include P-body proteins, stress granule proteins, germ granule proteins, other RNA-binding proteins, and mRNA (30). The hypothesis for the function of the assembly of large RNP granules in the germ line is to maintain oocyte quality by protecting mRNAs from degradation or early translation. Consistent with this hypothesis, the disruption of RNP granule assembly in meiotically arrested oocytes is correlated with reduced quality of oocytes (25, 39). Recent studies report reduced brood sizes in response to long-term exposure to high concentrations of glucose (21). The purpose of this study was to characterize the response to glucose in the C. elegans germ line. We demonstrate here that high concentrations of glucose induce the assembly of RNP granules in the C. elegans germ line. We show via multiple lines of evidence that the response to glucose supplementation is not specific to glucose and appears to be mediated by an osmotic stress response. We find that the RNP granules are not maintained beyond 3 h of osmotic stress and that they dissociate quickly after recovery from the stress. In contrast to a limited, previous study that exposed worms to high concentrations of salt in liquid (15), we demonstrate that both stress granule and P-body proteins are components of osmotic stress-induced granules. Although RNP granules are not maintained beyond 3 h of osmotic stress, the quality of oocytes does not appear to decrease after longer periods of stress, suggesting a secondary adaptation in the germ line. We show that the expression of Pgpdh-1::GFP increases significantly after 5 and 20 h of osmotic stress, in concert with the decline in RNP granules. Thus we propose a model of biphasic adaptation in the germ line. We suggest that the assembly of RNP granules is the initial adaptation that occurs before high levels of glycerol synthesis and that once glycerol levels reach a threshold, as a second phase of the germ line adaptation to osmotic stress, the RNP granules are no longer needed. This study serves as an important foundation on which to investigate the genetic mechanisms regulating the germ line osmoregulatory response.
METHODS
C. elegans strains.
The wild-type strain N2 var. Bristol was grown on nematode growth media (NGM) plates spread with the Escherichia coli bacterial strain OP50 [or ΔPTS (phosphotransferase system) OP50 when noted]. Cultures were maintained at 20°C as described (4). Additional strains included VP332 [gpdh-1(ok1558); gpdh-2(kb33)], JH2338 unc-119(ed3); axIs1489 [pCG61 pie-1prom:LAP::PAB-1], WH346 [CAR-1::GFP(NH2-terminal GFP); unc-119(+)], JH1985 unc-119(ed3); axIs1436 [pCG33 pie-1prom:LAP::CGH-1], AM1 [osr-1(rm1)], GFP::MEX-3 (15), and fog-2; GFP::MEX-3 (15).
Preparation of glucose-supplemented growth media.
Media contained 0.25% tryptone, 0.3% mM NaCl, 1 mM CaCl2, 1 mM MgSO4, 25 mM KPO4 pH 6.0, 5 µg/ml cholesterol, and 15 g/l agar. The osmolarity of the media was increased by addition of glucose or NaCl as indicated. In some experiments, supplemental glycerol (250 mM) was added to the media.
Image analysis.
GFP expression was detected in live worms mounted in 100 mM levamisole on a 2% agarose pad. Antibodies and staining protocols were as described for MEX-3 (10) (antibody from Dr. Jim Priess). Secondary antibody was from Molecular Probes. Images were collected using ×600 magnification on a Nikon A1R confocal microscope or an Olympus BX51 compound microscope equipped with epifluorescence. The number of worms analyzed for each experiment is noted in the figure legends.
Statistical analysis.
Data are presented as means ± SE. Statistical significance was determined with Fisher’s exact probability test with the Bonferroni correction or one-way ANOVA and Tukey’s test. P < 0.05 was considered to indicate statistical significance.
Viability/oocyte quality assay.
To determine the viability of embryos as a proxy for oocyte quality, synchronized L1-stage, N2 wild-type worms were prepared. Hermaphrodites were grown until they were ~1 day post-L4 stage (young adults) and transferred to 500 mM glucose plates for 1 or 20 h before they were individually picked to NGM plates. To avoid scoring embryos that were in the uterus at the time of the stress, the number of embryos in the uterus was counted and each worm laid that number of embryos before being transferred to a second NGM plate. The worm then laid an additional 20 ± 2 embryos (that should come from the oocytes in the germ line at the time of stress) before being removed from the plate. The number of embryos that hatched into larvae was counted after 36–49 h.
RESULTS AND DISCUSSION
Assembly of RNP granules is an acute germ line response induced by glucose.
Given the deleterious effects of long-term exposure to supplemental glucose on brood size, we first asked if long-term exposure to supplemental glucose at concentrations either below or above the threshold defined by Mondoux et al. (21) induces changes in RNP complexes in the germ line. None of the concentrations of glucose, from 56 to 500 mM, induced detectable changes using either of two marker strains, GFP::MEX-3 and GFP::PAB-1. MEX-3 is a KH domain RNA binding protein, and PAB-1 is poly(A) binding protein, both of which localize to RNP granules in meiotically arrested and heat-stressed oocytes (14, 15). After long-term exposure to supplemental glucose, we scored whether large RNP granules assembled, but we did not observe any differences from the no-stress control worms (data not shown). The reduced brood sizes after long-term exposure to glucose observed by Mondoux et al. may be due to impacts on sperm or fertilization and independent of the effects on oocytes, or long-term glucose supplementation may impact oocytes independently of the assembly of RNP granules.
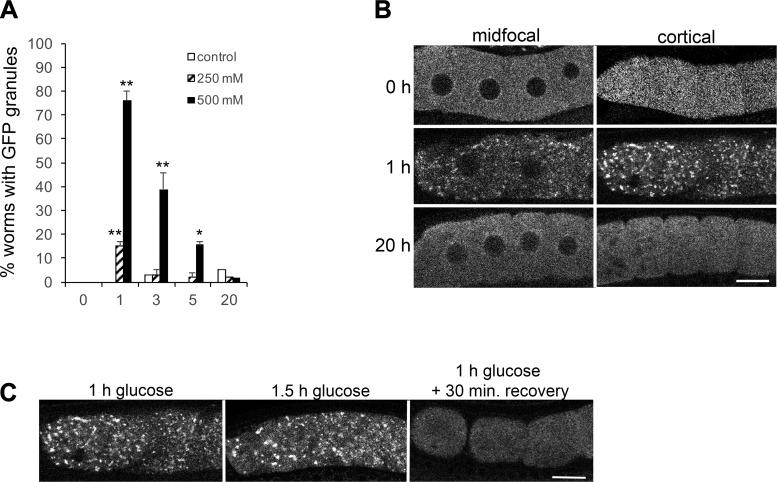
Because the germ line response to heat shock and anoxia occurs within a few hours, we next asked if short-term exposure to glucose induces the assembly of large RNP granules. We found that GFP::MEX-3 granules were detected in 15% of worms exposed to 250 mM glucose for 1 h, and they were observed in 76% of worms exposed to 500 mM glucose for 1 h (Fig. 1A). The granules were enriched in the cortical regions of the oocytes, as can be seen when comparing the midfocal confocal slices to the cortical slices (Fig. 1B); therefore, we show cortical confocal slices of oocytes in all subsequent figures. These data suggest that glucose supplementation induces the assembly of RNP granules in oocytes in a dose-dependent manner. Coupled with the results showing RNP granules are not induced by “metabolically relevant”, low concentrations of glucose, these data showing a maximal response of RNP granules with 500 mM glucose also suggest the response to glucose may primarily be an osmotic stress response. This idea is consistent with previous studies showing MEX-3 granules are induced after osmotic stress is incurred by soaking worms in 500 mM NaCl for 1 h (15). However, the characterization of the osmotic stress response in the prior study was limited, it used a concentration of NaCl that is lethal after a 24-h exposure, and it did not use the standard method of exposing worms to hypertonic agar.
Fig. 1.
Short-term exposure to glucose induces the assembly of large GFP::MEX-3 granules in oocytes. A: 1-h exposure to 250 mM glucose induces the assembly of large GFP::MEX-3 granules in 15% of worms, but they are not maintained after 3, 5, or 20 h. Exposure to 500 mM glucose more robustly induces the assembly of large GFP::MEX-3 granules, but they are not maintained beyond 3 h of exposure. GFP granules were not detected in significant numbers of worms for any of the time-matched negative controls. Values are means ± SE (n = 89–101). **P < 0.001, compared with each time 0 control (for 250 or 500 mM glucose); *P < 0.01, compared with the time 0 control. B: live images of GFP::MEX-3 worms show the change in GFP distribution after 1 h of 500 mM glucose exposure, from being uniform throughout the cytoplasm at time 0, to becoming concentrated in discrete, cortical granules. The GFP granules are not maintained after 20 h of glucose exposure. Scale bar = 10 µm. C: GFP::MEX-3 granules dissociate within 30 min of recovery on nematode growth media plates. GFP::MEX-3 granules are still detected after 1.5 h of glucose exposure; thus the dissociation appears to be an active recovery process (n = 32–50). Scale bar = 10 µm.
In prior experiments with heat stress, we observed an increase in the size and number of RNP granules as the time of heat exposure increased (15). In contrast, as the time of glucose exposure increased in our experiments, we observed a failure of worms to maintain RNP granules. After 3 h of exposure to 500 mM glucose, the percentage of worms with RNP granules had decreased from 76 to 51%. At 5 h, the percent was decreased to 21%, and at 20 h, RNP granules were detected in only 2% of worms (Fig. 1A). The failure to maintain the RNP granules during extended osmotic stress was surprising since RNP granules are maintained during heat stress until the worms die and are maintained for at least 3 days when oocytes are arrested in meiosis (15). The RNP granules induced by heat shock quickly dissociate when the stress is removed (15); therefore, we next asked if the RNP granules induced by glucose were similarly dynamic. After exposing young adults to 500 mM glucose for 1 h, we scored RNP granules in 96% of the worms (Fig. 1C). We then moved half of the worms to normal growth media to recover for 30 min and kept half of the worms on the 500 mM supplemental glucose plates for an additional 30 min. After 30 min of recovery, only 4% of worms had detectable RNP granules; GFP:MEX-3 was fairly uniform throughout the cytoplasm, including in the cortical regions (Fig. 1C). In contrast, after 1.5 h on glucose, RNP granules were detected in 91% of the worms. These results indicate the dissociation of the RNP granules between 60 and 90 min is not simply an adaptation to prolonged exposure to supplemental glucose. We conclude the RNP granules induced by glucose are reversible and dynamic, characteristics shared by the RNP granules induced by other environmental stresses and by meiotic arrest (15).
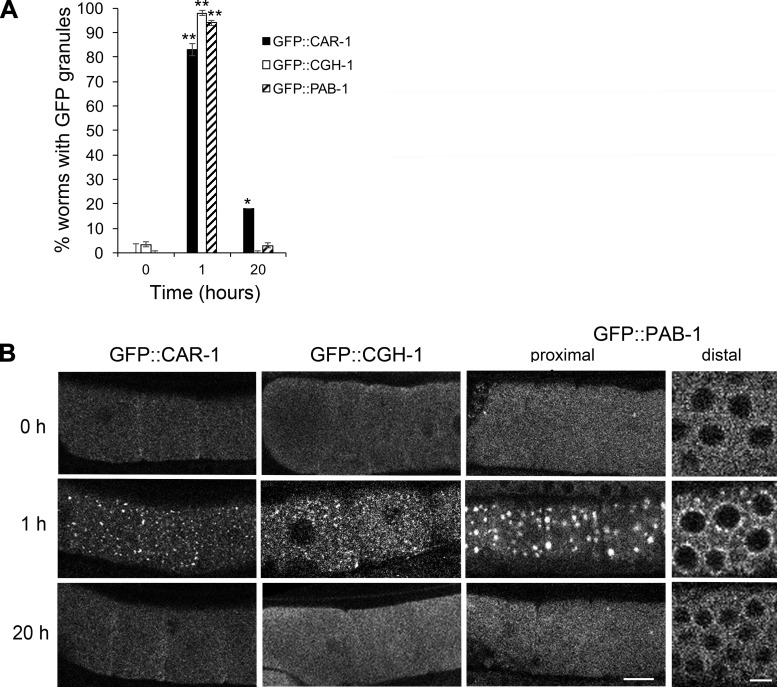
To further characterize the RNP granules induced by supplemental glucose, we interrogated the composition of the granules. Components of P bodies localize to RNP granules induced by heat shock, but these proteins have not been examined after osmotic stress (15). We found that two markers of P bodies, CAR-1 and CGH-1, mirrored the results with GFP::MEX-3. The distribution of each marker was generally uniform throughout the cytoplasm in control, unstressed germ lines (Fig. 2, A and B). After 1 h of exposure to 500 mM glucose, GFP::CAR-1 granules were observed in 83% of worms, and GFP::CGH-1 granules were seen in 98% of worms (Fig. 2, A and B). Since CAR-1 and CGH-1 are decapping proteins, their localization to RNP granules could suggest the large RNP granules are sites of mRNA decapping and decay. However, since P-body proteins appear to also have roles in mRNA storage (2), and many maternal mRNAs need to be maintained in oocytes, we favor the latter interpretation of the granules as storage sites. Interestingly, CAR-1 and CGH-1 granules were detected in only 18% and 3% of worms, respectively, after 20 h of glucose exposure; thus, like MEX-3, the P-body proteins are not maintained in large granules.
Fig. 2.
Glucose supplementation induces P-body and stress granule proteins to assemble into germ line ribonucleoprotein (RNP) granules. A: 1-h exposure to 500 mM glucose induces the assembly of GFP::CAR-1, GFP::CGH-1, and GFP::PAB-1 granules in >75% of worm germ lines. The granules are not maintained after 20 h of glucose exposure. Values are means ± SE (n = 28–59). **P < 0.001, compared with control; *P < 0.05, compared with control. B: live images of GFP::CAR-1, GFP::CGH-1, and GFP::PAB-1 show the assembly of cortical granules in oocytes, and for PAB-1 the assembly of perinuclear granules in the distal germ cells. After 20 h of stress, the distribution of GFP in the germ line is similar to the control, unstressed worms. Scale bars = 7 µm.
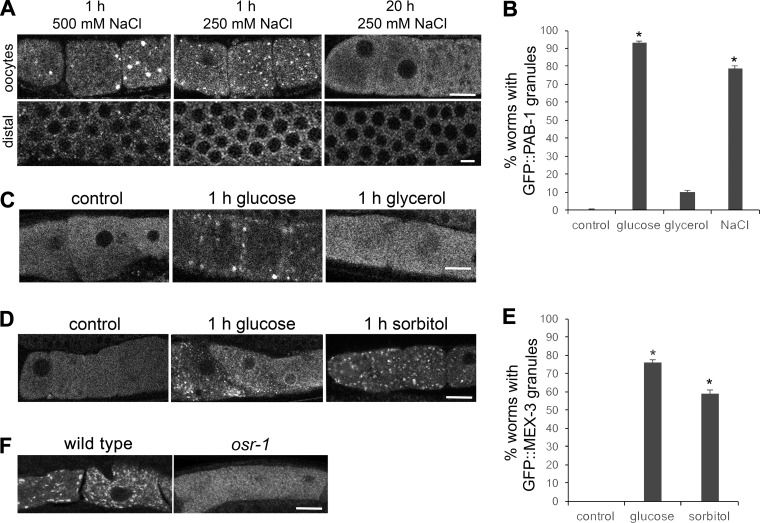
We next assayed the distribution of a marker of stress granules, PAB-1 [poly(A) binding protein]. We observed GFP::PAB-1 granules in 93% of worms after 1 h of exposure to 500 mM glucose, and in 3% of worms after 20 h of stress (Fig. 2, A and B). The PAB-1 marker is expressed strongly in the distal germ line as well as in the proximal germ line, and we detected an induction of perinuclear granules in the distal germ line after 1 h of glucose exposure that was not detected in unstressed worms or after 20 h of stress (Fig. 2B). That this stress granule protein assembles into RNP granules throughout the germ line suggests that the germ line RNP granules may also share functions with stress granules, such as being sites for stalled translation initiation complexes. Interestingly, these results differ from our past results where we did not detect GFP::PAB-1 granules after osmotic stress incurred by soaking worms in 500 mM NaCl (15). The methods differed in several ways between the experiments, including the osmolality of 500 mM glucose in the current experiments is half that of 500 mM NaCl; we exposed worms to hypertonic agar in the current experiments vs. soaking worms in liquid, and responses to glucose may differ compared with salt. We first determined that exposing worms to agar containing 500 mM NaCl for 1 h resulted in GFP::PAB-1 granules in oocytes in 29% of worms, suggesting that the delivery method of NaCl affects the germ line response to osmotic stress, perhaps because efficiency of uptake differs (Fig. 3A). Second, we found that after worms were exposed to agar containing 250 mM NaCl for 1 h, GFP::PAB-1 granules were detected in 79% of oocytes, a similar response as observed with 500 mM glucose (Fig. 3, A and B). With both concentrations of NaCl, we also observed perinuclear granules in the distal germ line (Fig. 3A). The second result suggests that in addition to delivery method, osmolality also affects the germ line response to osmotic stress. Overall, our characterization of the composition of the RNP granules induced by glucose supplementation reveals that these RNP granules have a similar composition as those induced by three previously characterized stresses: meiotic arrest, heat shock, and anoxia. MEX-3 and PAB-1 localize to granules after all four types of stresses (15). CGH-1 localizes to granules in response to three of the stresses, with anoxia undetermined, and CAR-1 localizes to granules after glucose supplementation and meiotic arrest, with heat shock and anoxia undetermined. Although the characterization of anoxia and heat shock is incomplete, no differences in composition are yet known, suggesting that the RNP granules may function similarly in response to a variety of environmental stresses. However, the composition of mammalian stress granules is different in cells subjected to different types of stress (24); therefore, upon future characterization, we may identify stress-specific components.
Fig. 3.
Induction of RNP granules by glucose supplementation reflects an osmotic stress response. A: exposure to 250 mM NaCl for 1 h induces cortical GFP::PAB-1 granules in both oocytes and in the distal germ line, but they are not maintained after 20 h of NaCl stress. B: exposure to 250 mM NaCl for 1 h induces GFP::PAB-1 granules in 79% of germ lines, nearly the level observed after exposure to 500 mM glucose (93%). In contrast, exposure to 250 mM glycerol for 1 h induces GFP::PAB-1 granules in only 11% of germ lines, not a significant increase compared with the control. Values are means ± SE (n = 19–66). *P < 0.001, compared with control. C: exposure to 250 mM glycerol for 1 h does not efficiently induce assembly of GFP::PAB-1 granules compared with exposure to 500 mM glucose. D: exposure to 500 mM sorbitol for 1 h induces the assembly of GFP::MEX-3 granules similar to the exposure to 500 mM glucose. E: sorbitol induces the assembly of GFP::MEX-3 granules in 59% of worms, a level not significantly different from the 76% of worms with granules after exposure to 500 mM glucose for 1 h. Values are means ± SE (n = 32–101). *P < 0.001, compared with control. F: MEX-3 granules are detected in significantly fewer worms in osr-1 mutants (10%) exposed to 1 h of 500 mM glucose, as compared with wild type (60%) (n = 25–29). P < 0.001, compared with wild-type control. Scale bars in A, C, D, and F = 7 µm.
The induction of RNP granules by glucose supplementation reflects an osmotic shock response.
Because the germ line response to glucose supplementation was detected most strongly at relatively high levels of glucose and because 250 mM NaCl, which is equivalent in osmolality as 500 mM glucose, also strongly induced GFP::PAB-1 granules, we probed more in depth to determine if the response to glucose reflects a more general, osmotic shock response. One prediction is that RNP granules would not be maintained after prolonged exposure to 250 mM NaCl, as they are not maintained after prolonged exposure to 500 mM glucose. We found that neither the oocyte nor distal germ line granules were maintained in any worms after 20 h of 250 mM NaCl stress (Fig. 3A). We repeated these experiments using the GFP::MEX-3 strain and saw similar results; oocyte granules were detected in 68% of worms after 1 h of 250 mM NaCl stress and in 3% of worms after 20 h (data not shown). Because dietary glucose and glycerol feeding result in similar phenotypes of decreased life span, we next exposed worms to glycerol (19). While glucose feeding increases both glucose and glycerol levels in worms, glycerol-fed worms have increased levels of glycerol but not glucose, thus suggesting that dietary glucose can be metabolized to glycerol (19). If the germ line response to glucose was a metabolic effect, we might expect glycerol to similarly induce the assembly of RNP granules. However, after exposure of worms to 250 mM glycerol, we detected cortical granules in only 11% of germ lines (Fig. 3, B and C). This difference could be explained by the fact that glucose impacts several metabolic pathways that glycerol may not affect or that exposure to high concentrations of glucose can result in increased glycosylation of proteins, which would not be expected after exposure to glycerol (20). We next exposed GFP::MEX-3 worms to sorbitol, a nonmetabolizable sugar alcohol. After 1 h of exposure to 500 mM sorbitol, 59% of germ lines had detectable GFP::MEX-3 granules; this response was not significantly different from the 76% of germ lines with GFP::MEX-3 granules after 1 h of glucose exposure (Fig. 3, D and E). In combination with reports showing sorbitol treatment can induce the osmotic stress response in yeast and worms, this result is consistent with the idea that a general osmotic stress response mediates the response to 500 mM glucose (7, 16). Taken together, the results with NaCl and sorbitol suggest that the induction of germ line RNP granules in response to glucose likely reflects an osmotic stress response, rather than a glucose-specific metabolic response.
To further test if the RNP granules are induced via an osmotic stress response, we tested osr-1(rm1) worms that are resistant to osmotic stress, as defined by maintenance of normal body volume, motility, and viability upon chronic exposure to high-osmolarity environments (35). We exposed osr-1 worms to 500 mM glucose for 1 h, and after immunostaining for MEX-3 protein, we detected granules in 10% of worms, as compared with wild type in which granules were seen in 60% of worms (Fig. 3F). This result suggests that the osmotic shock response is required for the efficient induction of RNP granules by glucose supplementation. The underlying basis for the osmotic stress resistance of osr-1 is likely the high basal levels of glycerol in these worms (26), raising an interesting possibility of negative regulation of RNP granules by glycerol.
Lastly, we tested whether the ability of the bacteria on the glucose plates to import glucose was needed for the response of the worm germ line to glucose exposure. In certain biological contexts, uptake and/or metabolism of glucose by bacteria modulate stress responses (12). Therefore, we seeded glucose plates with ΔPTS-OP50 mutant bacteria, which are defective in glucose uptake (9). RNP granules were detected in 77% of worms grown on ΔPTS-OP50, compared with 76% of worms grown on OP50, indicating the bacteria do not have a large role in this stress response. In combination, these experiments strongly suggest the germ line response to 500 mM glucose is largely an osmotic stress response, but we cannot rule out a parallel metabolic pathway that contributes to the formation of RNP granules in the germ line.
RNP granule assembly may serve as an initial germ line adaptation to osmotic stress before glycerol levels accumulate.
Our initial hypothesis regarding the function of RNP granules was that they may promote the maintenance of oocyte quality during environmental stress. Given that RNP granules are detected at 1 h of glucose exposure but are not maintained at 20 h of stress, we wondered if oocyte quality diminished after the longer stress or if the RNP granules are needed only during an acute response to osmotic stress. As a proxy for oocyte quality we assayed the hatching of fertilized eggs. To focus on the quality of oocytes at the time of glucose exposure, we avoided measuring the hatching of embryos that were in the uterus at the time of stress and instead assessed embryos arising from oocytes in the gonad arms at the time of stress. In unstressed control worms, 100% of embryos hatched into larvae (n = 128), similar to published reports (1). Oocyte quality did not appear to diminish after 1 h of glucose exposure (99% of embryos hatched, n = 135) or after 20 h of glucose exposure (98% of embryos hatched, n = 138). The high quality of oocytes after 1 h of osmotic stress is consistent with the idea that the induction of RNP granules plays a role in maintaining oocyte quality during stress. However, oocyte quality is not reduced after 20 h of stress, indicating that RNP granules are not required to maintain oocyte quality and suggesting that the germ line may have a secondary adaptation to osmotic stress. In the study by Mondoux et al. (21), brood size was significantly decreased after worms were exposed to 500 mM glucose from the L1 stage until adulthood. Our data suggest that either lifetime exposure to glucose is more deleterious to reproduction than 20 h of exposure during adulthood after the germ line has completely developed, or that the effects on brood size are independent of effects on oocytes. Instead, lifetime glucose exposure may negatively impact sperm or the process of fertilization. In a recent study, exposure to glucose, albeit at a lower concentration than in our assays, resulted in decreased egg-laying without changes in the rate of oogenesis (36).
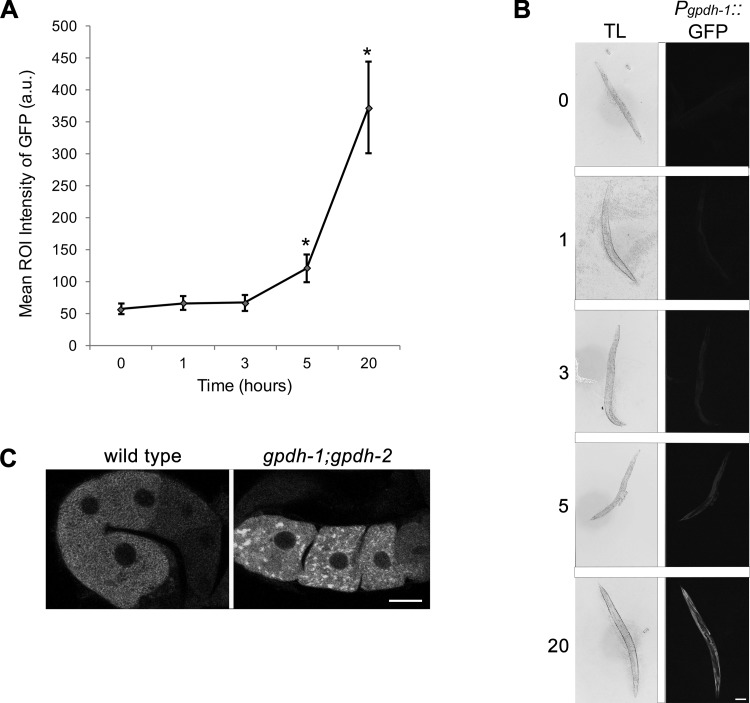
C. elegans is well known to adapt to hypertonic conditions by accumulating the osmolyte glycerol (17). Within 3 h of exposure to hypertonic conditions, glycerol levels increase threefold through upregulation of the glycerol-3-phosphate dehydrogenase genes gpdh-1 and gpdh-2 (18). To ask when glycerol levels increase in our assays, we used the Pgpdh-1::GFP strain and assayed GFP levels after 1, 3, 5, and 20 h of glucose exposure. The mean region of interest intensity in the control worms was 57 arbitrary units (AU), and it was not significantly higher in worms exposed to glucose for 1 or 3 h (Fig. 4, A and B). In contrast, after 5 h of stress, the intensity was 121 AU, and after 20 h, it was 372 AU (Fig. 4, A and B). This time course is similar to that observed in response to 200 mM NaCl, where no significant increase in Pgpdh-1::GFP levels is detected until after 5–6 h of exposure to osmotic stress (18). Our time course also correlates well with that of glycerol accumulation in response to 200 mM NaCl (17); therefore, our data suggest that supplemental glucose induces increased glycerol synthesis starting between 3 and 5 h of stress. To test if high levels of glycerol are required to dissociate RNP granules after 3 h of stress, we used the gpdh-1(ok1558);gpdh-2(kb33) strain, which has deletion alleles of the two catalysts for the rate-limiting step of glycerol biosynthesis (18). When exposed to hypertonic conditions, glycerol accumulation is greatly reduced in gpdh-1;gpdh-2 worms, leading to slow growth and reduced fertility (18). We found that after 5 h of glucose exposure MEX-3 granules were maintained in 73% of gpdh-1;gpdh-2 worms, in contrast to the wild-type control in which MEX-3 granules were maintained in only 15% of worms (Fig. 4C). We also observed MEX-3 granules in 81% of gpdh-1; gpdh-2 worms after 20 h of glucose exposure (data not shown). A trivial explanation for the maintenance of RNP granules would be meiotic arrest in the gpdh-1;gpdh-2 worms. The brood size of gpdh-1;gpdh-2 is reduced from ~225 to ~170, which could be consistent with a slight reduction in the rate of meiosis and ovulation; however, meiosis is clearly not arrested as in feminized worms lacking sperm. Moreover, we do not see any stacking of oocytes or columnar-shaped oocytes as is seen with meiotic arrest. Instead, we favor the interpretation that these results, in combination with the results with osr-1, strongly suggest that RNP granules assemble when osmotic balance is perturbed and either do not form or dissociate when balance is restored. Our data are consistent with published data showing gpdh-1 and gpdh-2 are required for adaptation to hypertonic stress and extend those findings to adaptation in the germ line.
Fig. 4.
Inverse relationship between assembly of RNP granules and glycerol levels. A: levels of GFP in the Pgpdh-1::GFP strain increase significantly after 5 and 20 h of glucose exposure. Values are means ± SE (n = 27–49). *P < 0.001, compared with control. ROI, region of interest; a.u., arbitrary units. B: transmitted light (TL) and GFP fluorescence images of Pgpdh-1::GFP adults after 1, 3, 5, and 20 h of 500 mM glucose exposure. Scale bar = 100 µm. C: MEX-3 granules are detected in only 15% of wild-type worms after 5 h of glucose exposure. In contrast, MEX-3 granules are detected in 73% of gpdh-1;gpdh-2 worms after 5 h of glucose exposure (n = 17–28). Scale bar = 7 µm.
Taken together, our results are consistent with a model in which C. elegans has a biphasic adaptation to osmotic stress in the germ line. Soon after a stress is sensed by the worm, but before glycerol synthesis has increased significantly, the germ line responds by assembling RNP granules in oocytes and in the distal germ line. Once glycerol levels reach a threshold, RNP granules dissociate because they are no longer needed as a germ line adaptation to the osmotic stress. In support of the biphasic model, osmolytes such as glycerol have been shown to promote protein solubility and effectively inhibit RNP granules; for example, trehalose inhibits the aggregation of yeast proteins during heat shock (33). We have described, for the first time, an in-depth characterization of the germ line response to osmotic stress, identified osmotic stress as a trigger of LLPS, and linked the adaptations of RNP granule assembly and glycerol accumulation. These studies provide a critical foundation on which to leverage the identification of pathways regulating osmoregulation in somatic tissues to identify the genetic pathways regulating germ line responses (8). They also raise the intriguing idea that the germ line may be able to sense increased glycerol levels that accumulate in the hypodermis and intestine. Our findings are relevant to the life history of the worm, since its ability to survive desiccation in the wild and subsequently successfully reproduce is imperative. However, osmoregulatory responses are relevant not only for the C. elegans species; thus genetic analysis of this germ line response will likely provide insights into stress responses in other animals and humans.
GRANTS
This work was supported by the National Institute of General Medical Sciences Grant 1R15GM109337-01A1 (to J. A. Schisa). Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.S. conceived and designed research; M.B.D., A.M., and M.P.W. performed experiments; M.B.D., A.M., M.P.W., and J.A.S. analyzed data; M.B.D., A.M., M.P.W., and J.A.S. interpreted results of experiments; M.B.D., A.M., M.P.W., and J.A.S. prepared figures; M.B.D. drafted manuscript; M.B.D., A.M., M.P.W., and J.A.S. edited and revised manuscript; M.B.D., A.M., M.P.W., and J.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Pamela Padilla, Kevin Strange, and James Priess for generously sharing strains and the MEX-3 antibody and Dr. Xantha Karp for helpful discussions and feedback on the manuscript.
REFERENCES
- 1.Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet 4: e1000295, 2008. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Stress-induced reversal of microRNA repression and mRNA P-body localization in human cells. Cold Spring Harb Symp Quant Biol 71: 513–521, 2006. doi: 10.1101/sqb.2006.71.038. [DOI] [PubMed] [Google Scholar]
- 3.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732, 2009. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 4.Brenner S. The genetics of behaviour. Br Med Bull 29: 269–271, 1973. doi: 10.1093/oxfordjournals.bmb.a071019. [DOI] [PubMed] [Google Scholar]
- 5.Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 3: 345–364, 2012. doi: 10.1515/bmc-2012-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burn KM, Shimada Y, Ayers K, Lu F, Hudson AM, Cooley L. Somatic insulin signaling regulates a germline starvation response in Drosophila egg chambers. Dev Biol 398: 206–217, 2015. doi: 10.1016/j.ydbio.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler-Brown D, Choi H, Park S, Ocampo BR, Chen S, Le A, Sutphin GL, Shamieh LS, Smith ED, Kaeberlein M. Sorbitol treatment extends life span and induces the osmotic stress response in Caenorhabditis elegans. Front Genet 6: 316, 2015. doi: 10.3389/fgene.2015.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe KP. Physiological and molecular mechanisms of salt and water homeostasis in the nematode Caenorhabditis elegans. Am J Physiol Regul Integr Comp Physiol 305: R175–R186, 2013. doi: 10.1152/ajpregu.00109.2013. [DOI] [PubMed] [Google Scholar]
- 9.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70: 939–1031, 2006. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper BW, Mello CC, Bowerman B, Hardin J, Priess JR. MEX-3 is a KH domain protein that regulates blastomere identity in early C. elegans embryos. Cell 87: 205–216, 1996. doi: 10.1016/S0092-8674(00)81339-2. [DOI] [PubMed] [Google Scholar]
- 11.Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC, Eckmann CR, Myong S, Brangwynne CP. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci USA 112: 7189–7194, 2015. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia AM, Ladage ML, Dumesnil DR, Zaman K, Shulaev V, Azad RK, Padilla PA. Glucose induces sensitivity to oxygen deprivation and modulates insulin/IGF-1 signaling and lipid biosynthesis in Caenorhabditis elegans. Genetics 200: 167–184, 2015. doi: 10.1534/genetics.115.174631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L, Shorter J. It’s raining liquids: RNA tunes viscoelasticity and dynamics of membraneless organelles. Mol Cell 60: 189–192, 2015. doi: 10.1016/j.molcel.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jud M, Razelun J, Bickel J, Czerwinski M, Schisa JA. Conservation of large foci formation in arrested oocytes of Caenorhabditis nematodes. Dev Genes Evol 217: 221–226, 2007. doi: 10.1007/s00427-006-0130-3. [DOI] [PubMed] [Google Scholar]
- 15.Jud MC, Czerwinski MJ, Wood MP, Young RA, Gallo CM, Bickel JS, Petty EL, Mason JM, Little BA, Padilla PA, Schisa JA. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol 318: 38–51, 2008. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaeberlein M, Andalis AA, Fink GR, Guarente L. High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol Cell Biol 22: 8056–8066, 2002. doi: 10.1128/MCB.22.22.8056-8066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol 286: C785–C791, 2004. doi: 10.1152/ajpcell.00381.2003. [DOI] [PubMed] [Google Scholar]
- 18.Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci USA 103: 12173–12178, 2006. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab 10: 379–391, 2009. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, Liu H, Zhang Z, Ye W, Xu X. The role of N-glycosylation in high glucose-induced upregulation of intercellular adhesion molecule-1 on bovine retinal endothelial cells. Acta Ophthalmol 94: 353–357, 2016. doi: 10.1111/aos.13028. [DOI] [PubMed] [Google Scholar]
- 21.Mondoux MA, Love DC, Ghosh SK, Fukushige T, Bond M, Weerasinghe GR, Hanover JA, Krause MW. O-linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics 188: 369–382, 2011. doi: 10.1534/genetics.111.126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Arriola E, Cárdenas-Rodríguez N, Coballase-Urrutia E, Pedraza-Chaverri J, Carmona-Aparicio L, Ortega-Cuellar D. Caenorhabditis elegans: a useful model for studying metabolic disorders in which oxidative stress is a contributing factor. Oxid Med Cell Longev 2014: 705253, 2014. doi: 10.1155/2014/705253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nostramo R, Herman PK. Deubiquitination and the regulation of stress granule assembly. Curr Genet 62: 503–506, 2016. doi: 10.1007/s00294-016-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol 215: 313–323, 2016. doi: 10.1083/jcb.201609081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson JR, Wood MP, Schisa JA. Assembly of RNP granules in stressed and aging oocytes requires nucleoporins and is coordinated with nuclear membrane blebbing. Dev Biol 353: 173–185, 2011. doi: 10.1016/j.ydbio.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell-Coffman JA. Hypoxia signaling and resistance in C. elegans. Trends Endocrinol Metab 21: 435–440, 2010. doi: 10.1016/j.tem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez M, Snoek LB, De Bono M, Kammenga JE. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet 29: 367–374, 2013. doi: 10.1016/j.tig.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Ryoo HD. Drosophila as a model for unfolded protein response research. BMB Rep 48: 445–453, 2015. doi: 10.5483/BMBRep.2015.48.8.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schisa JA. Effects of stress and aging on ribonucleoprotein assembly and function in the germ line. Wiley Interdiscip Rev RNA 5: 231–246, 2014. doi: 10.1002/wrna.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schisa JA. New insights into the regulation of RNP granule assembly in oocytes. Int Rev Cell Mol Biol 295: 233–289, 2012. doi: 10.1016/B978-0-12-394306-4.00013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schisa JA, Pitt JN, Priess JR. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128: 1287–1298, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Shimada Y, Burn KM, Niwa R, Cooley L. Reversible response of protein localization and microtubule organization to nutrient stress during Drosophila early oogenesis. Dev Biol 355: 250–262, 2011. doi: 10.1016/j.ydbio.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer MA, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1: 639–648, 1998. doi: 10.1016/S1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 34.Snee MJ, Macdonald PM. Dynamic organization and plasticity of sponge bodies. Dev Dyn 238: 918–930, 2009. doi: 10.1002/dvdy.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon A, Bandhakavi S, Jabbar S, Shah R, Beitel GJ, Morimoto RI. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 167: 161–170, 2004. doi: 10.1534/genetics.167.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teshiba E, Miyahara K, Takeya H. Glucose-induced abnormal egg-laying rate in Caenorhabditis elegans. Biosci Biotechnol Biochem 80: 1436–1439, 2016. doi: 10.1080/09168451.2016.1158634. [DOI] [PubMed] [Google Scholar]
- 37.Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. J Androl 31: 53–60, 2010. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voronina E, Seydoux G, Sassone-Corsi P, Nagamori I. RNA granules in germ cells. Cold Spring Harb Perspect Biol 3: a002774, 2011. doi: 10.1101/cshperspect.a002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood MP, Hollis A, Severance AL, Karrick ML, Schisa JA. RNAi screen identifies novel regulators of RNP granules in the Caenorhabditis elegans germ line. G3 (Bethesda) 6: 2643–2654, 2016. doi: 10.1534/g3.116.031559. [DOI] [PMC free article] [PubMed] [Google Scholar]