Abstract
The present study aimed to quantitate 15 perfluoroalkyl acids (PFAAs) in 125 adult American alligators at 12 sites across the southeastern United States. Of those 15 PFAAs, 9 were detected in 65% to 100% of samples: perfluorooctanoic acid, perfluorononanoic acid, perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid, perfluorotridecanoic acid (PFTriA), perfluorotetradecanoic acid, perfluorohexanesulfonic acid (PFHxS), and perfluorooctane sulfonate (PFOS). Males (across all sites) showed significantly higher concentrations of 4 PFAAs: PFOS (p = 0.01), PFDA (p = 0.0003), PFUnA (p = 0.021), and PFTriA (p = 0.021). Concentrations of PFOS, PFHxS, and PFDA in plasma were significantly different among the sites in each sex. Alligators at both Merritt Island National Wildlife Refuge (FL, USA) and Kiawah Nature Conservancy (SC, USA) exhibited some of the highest PFOS concentrations (medians of 99.5 ng/g and 55.8 ng/g, respectively) in plasma measured to date in a crocodilian species. A number of positive correlations between PFAAs and snout–vent length were observed in both sexes, suggesting that PFAA body burdens increase with increasing size. In addition, several significant correlations among PFAAs in alligator plasma may suggest conserved sources of PFAAs at each site throughout the greater study area. The present study is the first to report PFAAs in American alligators, to reveal potential PFAA hot spots in Florida and South Carolina, and to provide a contaminant of concern when assessing anthropogenic impacts on ecosystem health. Environ Toxicol Chem 2016;9999:1–9. Published 2016 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
Keywords: Perfluorooctane sulfonate (PFOS), Perfluorohexanesulfonic acid (PFHxS), Alligator, Crocodilian, Plasma
INTRODUCTION
Despite being manufactured for more than 50 yr [1], it was not until 2000 that the class of chemicals known as perfluoroalkyl acids (PFAAs) entered the scientific spotlight as a major environmental contaminant of concern [2]. The 2 most commonly known PFAAs, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), were produced by 3M in 1948 and 1947 [1], respectively, the latter of which was subsequently purchased by DuPont in 1951 [3]. A variety of new PFAAs have steadily been introduced to the market since then. Structurally, PFAAs can widely vary; but as a whole, they typically consist of carbon chains of varying length (linear and branched isomers), an acid functional group, and hydrogen atoms substituted with fluorine atoms [4]. The carbon–fluorine bonds are the unique feature of PFAAs and provide chemical and thermal stability [5]. Two well-studied families of PFAAs are carboxylic acids and sulfonic acids [2,6].
The usage of PFAAs has become widespread since the introduction of these chemicals in the 1940s, largely because they exhibit unique surfactant properties that make them attractive components for many consumer-related products, such as nonstick pans, water-repellant surfaces, hair products, plastics, and lubricants [2], as well as firefighting products known as aqueous film-forming foams [7]. Active manufacturing and use of certain PFAAs, such as PFOS and PFOA, have largely ceased as a result of a voluntary phaseout by industry. Current production of fluorinated chemicals includes shorter-chained carboxylic and sulfonic acid substitutes, such as perfluorobutanesulfonic acid (PFBS) and perfluorobutyric acid (PFBA) [8]. In addition, precursor chemicals that have a nonfluorinated structural component attached to a perfluorinated chain may be amenable to microbial or chemical transformation and have the potential to degrade into perfluorinated carboxylic and sulfonic acids over time [9].
The same properties that make PFAAs commercially valuable (e.g., the highly stable carbon–fluorine bonds) also enable them to persist in the environment by resisting chemical, microbial, and photolytic degradation. However, unlike the more lipophilic environmental contaminants, such as organo-chlorine pesticides, polychlorinated biphenyls (PCBs), and brominated flame retardants (PBDEs) that are sequestered in adipose tissue, PFAAs accumulate in the blood and blood-rich organs, such as the liver [10,11]. Conversely, like organochlorine pesticides, PCBs, and PBDEs, PFAAs have also been shown to bioaccumulate and biomagnify in food webs [6]. Increasing PFAA chain length has been shown to correlate with an increasing ability to bioaccumulate [12], and the greatest PFAA concentrations detected in wildlife have been in species occupying high trophic positions [13]. Because PFAAs are bioaccumulative and often observed in higher concentrations in fish-eating marine species [13], humans who consume more fish in their diet may be at higher risk of PFAA exposure than those who consume less fish [14].
Animal studies reveal a wide range of PFAA-related effects that include alterations in liver physiology and serum cholesterol, as well as resulting hepatomegaly, wasting syndromes, neurotoxicity, and immunotoxicity [15–17]. In addition, PFAAs have been mentioned as possible obesogens because of their interaction with peroxisome proliferator–activated receptors[18]. However, although species-specific variations in PFAA excretion rates have been observed [2], the actual mechanism of action of PFAA toxicity is not well understood across species.
Few reports exist on PFAA distribution and body burdens in North American wildlife, and studies of PFAAs in wild reptiles and amphibians have been limited almost exclusively to frogs and sea turtles [19]. Globally, only 3 studies have examined PFAAs in crocodilians [20–22]. Because of their high trophic status, long life span, and high site fidelity, crocodilians are attractive study species for ecotoxicological investigations, particularly those involving exposure and accumulation of persistent environmental contaminants [23–25]. As such, studies examining PFAAs in crocodilians can provide insight into exposure and potential effects in focal species and can identify potential hot spots of PFAA contamination.
In the present study, we examined PFAA concentrations in plasma of wild American alligators (Alligator mississippiensis) from 12 sites in Florida and South Carolina, USA (Figure 1). Because factors such as sex, body size, and location may influence PFAA concentrations in alligators, the relationships between PFAA body burdens and these parameters were also examined.
Figure 1.
Map showing the 12 sites (SC and FL, USA) from which American alligators (Alligator mississippiensis) were sampled in the present study (n = 125) during the years 2012 to 2015. Collection sites are listed in decreasing latitude. WCA = Everglades Water Conservation Area.
MATERIALS AND METHODS
Study area
American alligator plasma samples (n = 125) were collected between 2012 and 2015 as part of multiple ongoing projects examining the biology and ecotoxicology of alligators in Florida and South Carolina [23–25]. In South Carolina, alligator blood samples were collected from the following sites (in order of north to south): Tom Yawkey Wildlife Center (YK; n = 10), Kiawah Island (KA; n = 10), and Bear Island Wildlife Management Area (BI; n = 10) (Figure 1; Supplemental Data, Table S1). In Florida, samples were collected from the following sites (in order from north to south): Lochloosa Lake (LO; n = 10), Lake Woodruff (WO; n = 10), Lake Apopka (AP; n = 10), Merritt Island National Wildlife Refuge (MI; n = 15), St. Johns River (JR; n = 10), Lake Kissimmee (KS; n = 10), Lake Trafford (TR; n = 10), Everglades Water Conservation Area 2A (2A; n = 10), and Everglades Water Conservation Area 3A (3A; n = 10) (Figure 1; Supplemental Data, Table S1).
Sample collection
Immediately following capture of the alligator, a blood sample was collected from the postoccipital sinus of the spinal vein of each animal using a sterile needle and syringe [23–25]. Whole-blood samples were then transferred to 8-mL lithium–heparin Vacutainer blood-collection tubes (BD), stored on ice in the field, and later centrifuged at 2500 rpm at 4 °C for 10 min to obtain plasma, which was stored at −80 °C until analysis. Snout–vent length was measured for each animal as a proxy for size, and sex was determined by cloacal examination of the genitalia [26].
The National Institute of Standards and Technology (NIST) Standard Reference Material® (SRM) 1958 Organic Contaminants in Fortified Human Serum (Freeze-Dried) was used as a control material during PFAA analysis. The freeze-dried human serum SRM 1958 was reconstituted with deionized water according to the instructions on the certificate of analysis [27] and analyzed alongside collected alligator plasma.
Chemicals
Calibration solutions were created by combining 2 solutions produced by the NIST reference materials 8446 Perfluorinated Carboxylic Acids and Perfluorooctane Sulfonamide in Methanol and 8447 Perfluorinated Sulfonic Acids in Methanol. Together, the solution contained 15 PFAAs as follows: PFBA, perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTriA), perfluorotetradecanoic acid (PFTA), PFBS, perfluorohexanesulfonic acid (PFHxS), PFOS, and perfluorooctanesulfonamide (PFOSA).
Internal standards were purchased from Cambridge Isotope Laboratories, RTI International, and Wellington Laboratories to create an internal standard mixture comprised of 11 isotopically labeled PFAAs, as follows: 13C4-PFBA, 13C2-PFHxA, 13C8-PFOA, 13C9-PFNA, 13C9-PFDA, 13C2-PFUnA, 13C2-PFDoA, 18O2-PFBS, 18O2-PFHxS, 13C4-PFOS, and 18O2-PFOSA.
Sample preparation
Samples were extracted using a method described by Reiner et al. [28]. Approximately 1 mL of each alligator plasma sample and SRM 1958 aliquots were thawed and gravimetrically weighed. All samples were then spiked with the internal standard mixture (approximately 600 μL) and gravimetrically weighed. After brief vortex-mixing and 90 min of equilibration, 4 mL of acetonitrile were used to extract the PFAAs from each sample. After sonication and centrifugation, the supernatant was removed from all samples. The collected supernatant was then solvent-exchanged to methanol and further purified using an ENVI-Carb solid-phase extraction cartridge (Supelco). Resulting extracts were evaporated to 1 mL using nitrogen gas prior to being analyzed by liquid chromatography-tandem mass spectrometry.
Samples were analyzed using an Agilent 1100 high-performance liquid chromatographic system coupled to an Applied Biosystems API 4000 triple-quadrupole mass spectrometer with electrospray ionization in negative mode. Samples were examined by liquid chromatography using an Agilent Zorbax Eclipse Plus C18 analytical column (2.1 mm × 150 mm × 5 μm). A ramping liquid chromatography solvent gradient was employed using methanol and deionized water, both containing 20 mmol/L ammonium acetate [28]. Two multiple reaction monitoring transitions for each PFAA were monitored to ensure no interferences with measurements. One was employed for quantitation, and the other was used for confirmation [28].
Quality control
All alligator plasma samples were processed alongside quality control material NIST SRM 1958 to determine the accuracy and precision of the method. The PFAA levels of SRM 1958, processed during our extraction, had to be within the 95% confidence interval as reported on the certificate of analysis. All samples were also processed alongside blanks to assess any background contamination that might be present in the laboratory or a result from the extraction method. Compounds were considered to be above the reporting limit if the mass of an analyte in the sample was greater than the mean plus 3 standard deviations of all blanks.
Statistical methods
All statistical analyses were performed using SPSS statistic 22 (IBM). Statistical tests were performed for the compounds detected in more than 75% of the samples: PFNA, PFDA, PFUnA, PFDoA, PFTriA, PFTA, PFHxS, and PFOS. Unlike previous environmental studies, where PFOA had the second highest concentration measured, PFOA was detected at much lower frequency (detected in only 65% of the samples analyzed). With a full one-third of PFOA measurements falling below the limit of detection, PFOA was excluded from statistical analysis, along with the remaining chemicals (PFHpA, PFHxA, PFPeA, PFBS, and PFBA), which were detected in <2% of the samples. For those PFAAs included in statistical analysis, compounds below the limit of detection were set equal to one-half of the limit of detection prior to running the statistical tests [29].
Sex-based differences of PFAAs in Florida and South Carolina were investigated using univariate analysis of variance with log normally distributed concentration values, and a Friedman’s test was used for the PFAAs with non-normally distributed concentration values. Site was set as the nuisance factor, sex as the treatment, and PFAA concentration as the dependent variable. These tests simulated a randomized block design for the collected data. Other parametric tests employed for data analysis of sex-based differences, on a site-by-site basis, and analysis of site differences for PFAA levels included a t test and one-way analysis of variance when data were normal or log-normal and Friedman rank test, Mann-Whitney U test, and Kruskal-Wallis test when data remained non-normal following log transformation. Pearson correlation and Spearman correlation were used when applicable for correlative measures.
RESULTS AND DISCUSSION
In the present study, we collected a total of 125 plasma samples from alligators at multiple sites in Florida and South Carolina to examine PFAA concentrations in animals from different localities. Of the 15 PFAAs included in the present analysis, all samples contained at least 6 PFAAs. The following 5 PFAAs were detected in every plasma sample analyzed (in order of highest median concentration to lowest median concentration, among all sites): PFOS (median, 11.2 ng/g; range, 1.36–452 ng/g), PFUnA (median, 1.58 ng/g; range, 0.314–18.4 ng/g), PFDA (median; 1.20 ng/g, range; 0.169–15.1 ng/g), PFNA (median, 0.528 ng/g; range, 0.155–1.40 ng/g), and PFHxS (median, 0.288 ng/g; range, 0.057–23.3 ng/g) (Table 1; Supplemental Data, Table S2). Also, PFDoA, PFTriA, PFTA, and PFOA were detected frequently in alligator plasma samples (in more than 96%, 94%, 75%, and 65%, respectively): PFDoA (median, 0.363 ng/g; range, <0.009–7.27 ng/g), PFTriA (median, 0.416 ng/g; range, <0.026–2.60 ng/g), PFTA (median, 0.050 ng/g; range, <0.008–1.38 ng/g), and PFOA (median, 0.064 ng/g; range, <0.008–0.412 ng/g) (Table 1; Supplemental Data, Table S2). The 9 PFAAs commonly measured over the limit of detection resulted in unique fingerprints for each site (Supplemental Data, Figure S1), which are discussed in the section Site differences. The shorter-chain PFAAs (PFHpA, PFHxA, PFPeA, PFBS, and PFBA) were detected infrequently (<2% of the samples) and, therefore, not included in any statistical analysis.
Table 1.
Alligator perfluoroalkyl acid plasma concentrations (ng/g wet mass) at 12 sites from Florida and South Carolina, USA
| Yawkey (n = 10) | Kiawah Island (n = 10) | Bear Island (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Range | Median | n >RLa | Range | Median | n >RLa | Range | Median | n >RLa | |
| PFOA | <0.008b–0.193 | 0.050 | 4 | 0.028–0.298 | 0.126 | 10 | <0.008b–0.193 | <0.100 | 3 |
| PFNA | 0.272–1.32 | 0.620 | 10 | 0.446–1.38 | 1.19 | 10 | 0.155–1.14 | 0.472 | 10 |
| PFDA | 2.27–15.1 | 5.88 | 10 | 3.72–13.6 | 6.26 | 10 | 0.998–3.21 | 1.57 | 10 |
| PFUnA | 1.89–18.4 | 6.25 | 10 | 1.87–7.53 | 3.93 | 10 | 1.05–5.02 | 2.32 | 10 |
| PFDoA | 0.362–3.45 | 1.01 | 10 | 1.32–7.27 | 3.05 | 10 | 0.231–1.88 | 0.559 | 10 |
| PFTriA | <0.070b–1.85 | 0.646 | 8 | 0.420–2.60 | 0.919 | 10 | <0.070b–1.83 | 0.674 | 9 |
| PFTA | <0.082b–0.774 | 0.241 | 7 | 0.198–1.38 | 0.476 | 10 | <0.081b–0.733 | 0.095 | 7 |
| PFHxS | 0.099–0.566 | 0.353 | 10 | 0.313–1.86 | 0.620 | 10 | 0.077–0.824 | 0.304 | 10 |
| PFOS | 4.50–57.0 | 20.2 | 10 | 38.4–98.2 | 55.8 | 10 | 10.0–44.9 | 19.5 | 10 |
|
| |||||||||
| Lochloosa Lake (n = 10) | Lake Woodruff (n = 10) | Lake Apopka (n = 10) | |||||||
|
|
|
|
|||||||
| Range | Median | n >RLa | Range | Median | n >RLa | Range | Median | n >RLa | |
|
| |||||||||
| PFOA | <0.008b–0.132 | 0.071 | 9 | <0.097b–0.184 | 0.062 | 5 | <0.096b–0.152 | 0.126 | 7 |
| PFNA | 0.328–1.19 | 0.676 | 10 | 0.282–1.34 | 0.578 | 10 | 0.251–1.40 | 0.648 | 10 |
| PFDA | 0.238–1.00 | 0.615 | 10 | 0.350–5.06 | 2.01 | 10 | 0.169–2.44 | 1.12 | 10 |
| PFUnA | 0.580–1.56 | 1.03 | 10 | 0.633–3.33 | 1.43 | 10 | 0.614–3.39 | 1.65 | 10 |
| PFDoA | 0.105–0.309 | 0.182 | 10 | <0.166b–0.810 | 0.317 | 9 | <0.157b–0.831 | 0.315 | 9 |
| PFTriA | 0.181–0.580 | 0.309 | 10 | <0.070b–0.854 | 0.259 | 8 | 0.189–1.00 | 0.450 | 10 |
| PFTA | <0.008b–0.060 | 0.018 | 7 | <0.008b–0.146 | 0.029 | 4 | <0.080b–0.194 | 0.049 | 7 |
| PFHxS | 0.069–0.201 | 0.093 | 10 | 0.130–0.623 | 0.445 | 10 | 0.166–0.449 | 0.332 | 10 |
| PFOS | 2.19–6.16 | 4.21 | 10 | 5.89–41.2 | 16.0 | 10 | 1.98–15.8 | 11.4 | 10 |
|
| |||||||||
| Merrit Island (n = 15) | St. Johns River (n = 10) | Lake Kissimmee (n = 10) | |||||||
|
|
|
|
|||||||
| Range | Median | n >RLa | >Range | Median | n >RLa | Range | Median | n >RLa | |
|
| |||||||||
| PFOA | <0.096b–0.412 | 0.155 | 7 | 0.010–0.160 | 0.080 | 10 | <0.008b–0.142 | 0.104 | 9 |
| PFNA | 0.298–1.10 | 0.611 | 15 | 0.250–1.04 | 0.471 | 10 | 0.275–1.18 | 0.642 | 10 |
| PFDA | 0.395–3.50 | 1.02 | 15 | 0.492–1.72 | 1.17 | 10 | 0.417–3.15 | 1.26 | 10 |
| PFUnA | 0.844–5.45 | 1.82 | 15 | 0.655–2.20 | 1.28 | 10 | 0.314–2.47 | 1.03 | 10 |
| PFDoA | <0.543b–1.07 | 0.418 | 14 | 0.156–0.591 | 0.362 | 10 | <0.009b–0.382 | 0.147 | 9 |
| PFTriA | <0.026b–1.42 | 0.654 | 14 | 0.173–0.739 | 0.267 | 10 | 0.122–0.677 | 0.251 | 10 |
| PFTA | <0.080b–0.257 | 0.076 | 6 | <0.008b–0.131 | 0.022 | 8 | <0.009b–0.104 | 0.025 | 9 |
| PFHxS | 0.684–23.3 | 3.83 | 15 | 0.100–0.308 | 0.166 | 10 | 0.338–1.50 | 0.505 | 10 |
| PFOS | 38.6–452 | 99.5 | 15 | 3.41–10.2 | 7.13 | 10 | 6.51–25.1 | 12.2 | 10 |
|
| |||||||||
| Lake Trafford (n = 10) | WCA-2A (n = 10) | WCA-3A (n = 10) | |||||||
|
|
|
|
|||||||
| Range | Median | n >RLa | Range | Median | n >RLa | Range | Median | n >RLa | |
|
| |||||||||
| PFOA | 0.021–0.117 | 0.091 | 10 | <0.008b–0.077 | 0.036 | 2 | <0.008b–0.042 | 0.033 | 6 |
| PFNA | 0.239–0.936 | 0.484 | 10 | 0.189–0.382 | 0.234 | 10 | 0.172–0.388 | 0.301 | 10 |
| PFDA | 0.275–2.05 | 0.885 | 10 | 0.641–2.26 | 0.900 | 10 | 0.406–1.46 | 0.912 | 10 |
| PFUnA | 0.463–2.19 | 0.953 | 10 | 0.958–3.15 | 1.43 | 10 | 0.719–2.48 | 1.45 | 10 |
| PFDoA | 0.073–0.737 | 0.210 | 10 | 0.277–0.949 | 0.392 | 10 | 0.172–0.631 | 0.371 | 10 |
| PFTriA | 0.111–0.528 | 0.304 | 10 | 0.232–0.702 | 0.370 | 10 | 0.162–0.594 | 0.280 | 10 |
| PFTA | <0.008b–0.096 | 0.039 | 9 | 0.031–0.188 | 0.109 | 10 | 0.011–0.148 | 0.042 | 10 |
| PFHxS | 0.071–0.320 | 0.119 | 10 | 0.080–0.172 | 0.112 | 10 | 0.057–0.303 | 0.105 | 10 |
| PFOS | 4.21–14.3 | 7.82 | 10 | 1.36–6.23 | 2.65 | 10 | 1.57–4.71 | 3.81 | 10 |
n >RL indicates the number of samples above the reporting limit (RL).
Values were calculated with one-half the reporting limit substituted for nondetects, as described in Materials and Methods; for values shown as “<,” however, a specified number describe the actual reporting limit.
PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; PFDA = perfluorodecanoic acid; PFUnA = perfluoroundecanoic acid; PFDoA = perfluorododecanoic acid; PFTA = perfluorotetradecanoic acid; PFTriA = perfluorotridecanoic acid; PFHxS = perfluorohexanesulfonic acid; PFOS = perfluorooctane sulfonate; WCA = Water Conservation Area.
Sex differences
As a whole, across all sites, male alligators exhibited significantly higher concentrations of several PFAAs in plasma compared with females as a group: PFOS (p = 0.01), PFDA (p = 0.0003), PFUnA (p = 0.021), and PFTriA (p = 0.021) (Supplemental Data, Figure S2). At some individual sites, however, PFAA concentrations were significantly higher in females (e.g., PFOS at AP, PFUnA at KA).
In a population of captive Chinese alligators (Alligator sinensis), Wang et al. [21] found the highest PFAA concentration in serum to be that of PFUnA rather than PFOS, the PFAA with the highest concentrations in the present study. However, similar to the present study, male Chinese alligators contained significantly higher concentrations of PFOS and PFUnA compared with females. Wang et al. [21] did not find a sex-based difference for PFDA in Chinese alligators. Christie et al. [22] did not find any sex-based differences in their population of Nile crocodiles (Crocodylus niloticus) in South Africa. It is possible that sex-based differences observed for certain PFAAs in alligators are the result of a differential clearance of these contaminants between males and females, as has been observed in rats [30], mice [31], and other mammals [32]. It is also possible that females may offload PFAAs during oviposition, reducing their PFAA body burden compared with males at the same locality. This possibility is supported by studies reporting measurable concentrations of PFAAs in eggs of herring gulls (Larus argentatus) [33] and Nile crocodiles [20], confirming maternal transfer of PFAAs in oviparous species. Sex-specific differences in PFAA concentrations may also be the result of differential habitat use by adult males and females, a phenomenon common among crocodilians [34–37]. In such cases, differences in prey availability and contamination between and among habitats within a site could result in different PFAA exposures in males and females.
Because no sex-specific differences in PFOA, PFNA, PFHxS, PFDoA, and PFTA concentrations were observed as a whole (all sites combined), sex-based differences were examined on a site-by-site basis (Supplemental Data, Table S3). Overall, only a few sites exhibited sex-based differences for these 5 PFAAs (Supplemental Data, Figure S3). At LO, male alligators had significantly higher PFNA (p = 0.016), PFTA (p = 0.032), and PFDoA (p = 0.032) plasma concentrations compared with females; and at MI males had significantly higher PFOA (p = 0.047) plasma concentrations than females. Interestingly, PFHxS was the only PFAA for which females exhibited significantly higher plasma concentrations (YK, p = 0.008; TR, p = 0.008) when compared with males (Supplemental Data, Figure S3). It is important to note that our examination of sex-based differences in PFAA concentrations may have been influenced by small sample sizes; in almost all cases, only 5 males and 5 females were sampled per site.
Site differences
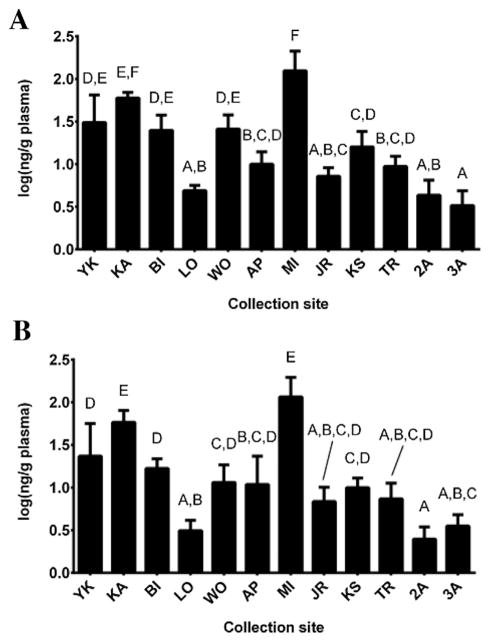
Because sex-based differences in PFAA concentrations were observed among alligator plasma samples, site differences were determined separately for males and females. All of the 9 detected PFAAs displayed at least some minor site differences. The PFAAs that displayed the most notable site differences (the highest number of statistically significant groups between the 12 sites) were PFOS, PFDA, and PFHxS. Of those, PFOS exhibited the greatest statistical difference across sites (Figure 2). This is likely because PFOS is generally the most abundant PFAA in the environment. For male alligators only, concentrations of PFOS in plasma ranged from 1.57 ng/g to 452 ng/g. Concentrations of PFOS were highest at MI (median, 106 ng/g) and KA (median, 56.4 ng/g). Males from MI exhibited significantly higher PFOS concentrations compared with males from all other sites, with the exception of KA. In addition, the individual alligator with the highest overall PFOS concentration measured in the present study (452 ng/g plasma) was from MI. After MI, males from South Carolina (KA, YK, and BI) exhibited higher PFOS concentrations than Florida males, with the exception of WO. Some of the lowest PFOS concentrations observed in males in the present study were measured at sites 2A, 3A, LO, and JR.
Figure 2.
Site comparison of mean (±standard deviation) perfluorooctane sulfonate concentrations (log) in (A) male and (B) female American alligator (Alligator mississippiensis) plasma from multiple sites in Florida and South Carolina. Letters above bars represent statistically significant differences between groups (p <0.05). Samples are listed from left to right in decreasing latitude. 2A/3A = Everglades Water Conservation Areas 2A and 3A; AP = Lake Apopka; BI = Bear Island Wildlife Management Area; JR = St. Johns River; KA = Kiawah Island; KS = Lake Kissimmee; LO = Lochloosa Lake; MI = Merritt Island National Wildlife Refuge; TR = Lake Trafford; WO = Lake Woodruff; YK = Tom Yawkey Wildlife Center.
For female alligators, PFOS concentrations in plasma ranged from 1.36 ng/g to 206 ng/g. Similar to males, females from sites MI (median, 85.5 ng/g) and KA (median, 51.3 ng/g) exhibited significantly higher PFOS concentrations compared with the other sites examined, and the individual female with the highest PFOS concentration was from MI (206 ng/g plasma). After MI and KA, females from the 2 other South Carolina sites (YK and BI) exhibited higher PFOS concentrations than females from Florida, with the exception of WO and AP. Some of the lowest PFOS concentrations observed in females in the present study were measured at sites 2A, 3A, LO, JR, and TR.
The concentrations of PFHxS detected in alligator plasma in the present study exhibited a similar trend to PFOS across sites but on a reduced scale (Supplemental Data, Table S4). For males, PFHxS plasma concentrations ranged from 0.057 ng/g to 23.3 ng/g. Males from MI (median, 3.95 ng/g) had significantly higher PFHxS concentrations than those at any other site examined, and the individual male with the highest PFHxS concentration was from Merritt Island National Wildlife Refuge (23.3 ng/g). Males from Kiawah Island and Lake Kissimmee followed closely but were still statistically grouped with other sites (Lake Apopka, Lake Woodruff, and Bear Island). The lowest PFHxS concentrations in males were typically measured at sites 2A, 3A, Lochloosa Lake, and Lake Trafford. For female alligators, PFHxS concentrations in plasma ranged from 0.069 ng/g to 10.0 ng/g. Similar to males, MI females exhibited significantly higher PFHxS concentrations than those at all other sites. Females from KA and KS had the next highest concentrations but were still statistically grouped with those from other sites (AP, WO, and YK). The lowest PFHxS concentrations in females were typically observed at sites 2A, 3A, and LO.
Across the sampling sites, PFDA had a unique signature, one that varied from the patterns observed for plasma PFOS and PFHxS concentrations (Supplemental Data, Table S4). For male alligators, PFDA concentrations ranged from 0.498 ng/g to 15.1 ng/g. KA males had significantly higher PFDA concentrations overall (median, 6.21 ng/g) compared with all sites, with the exception of YK (median, 6.20 ng/g). Males from YK exhibited the next highest PFDA concentrations, but these were not significantly different from those detected in WO males (median, 2.02 ng/g). Males from many of the remaining sites had similarly low concentrations of PFDA. Overall, LO males (median, 0.792 ng/g) had some of the lowest PFDA concentrations of all the sampling sites. For female alligators, PFDA plasma concentrations ranged from 1.69 ng/g to 14.3 ng/g. The 2 sites with the highest (statistically significant) PFDA concentrations in females were also in South Carolina: KA (median, 6.32 ng/g) and YK (median, 5.55 ng/g). Concentrations of PFDA at BI (median, 1.18 ng/g) and WO (median, 1.84 ng/g) followed closely behind but were not significantly different from the other sites sampled. Like males, LO females (median, 0.501 ng/g) had some of the lowest PFDA concentrations across all sites.
Overall, male and female alligators from both MI and KA exhibited some of the highest PFOS concentrations measured to date in a crocodilian species (median PFOS concentrations in plasma: MI males = 106 ng/g; MI females = 85.5 ng/g; KA males = 56.4 ng/g; KA females = 51.3 ng/g). In comparison, the mean PFOS concentration in serum from captive Chinese alligators was 28.7 ng/mL (28.0 ng/g) [21], whereas the median concentrations in wild Nile crocodiles at several sites in South Africa ranged from 4.31 ng/g to 50.3 ng/g [22]. In a study of other reptiles, loggerhead sea turtles (Caretta caretta) along the coast of South Carolina and Florida exhibited median PFOS plasma concentrations of 2.87 ng/g and 3.80 ng/g, respectively [38]. In another study, hawksbill sea turtles (Eretmochelys imbricata) off of Juno Beach (FL, USA), south of MI, showed higher than expected PFOS levels at 11.9 ng/g [39] when compared with several other species of turtles along the east coast. The authors discuss geographic differences as possible reasons for the higher levels of PFOS in the hawksbill. Comparing the results Keller et al. [39] found for hawksbill with the present results would suggest that there might be a potential source of PFOS off the east coast of Florida. It is possible that, for the present study, the high concentrations of PFOS and PFHxS detected in male and female alligators at MI may be related to the aeronautic facilities located in and around MI, which comprise a large part of Florida’s Kennedy Space Center. The past use of aqueous film-forming foams at Kennedy Space Center may have played a role in PFAAs in the surrounding environment and wildlife. Historically, aqueous film-forming foams have been shown to contain PFAAs, such as PFOS and PFHxS, as well as a number of other proprietary PFAA mixtures [7], and can be resistant to remediation [9]. Perfluoroalkyl acids have been measured in firefighters [40], in wildlife [6], and downstream of their use [41]. Potential sources of PFOS and PFHxS at KA are more speculative. In addition, it should be noted that, with the exclusion of MI, alligators from the South Carolina sites (BI, YK, and KA) had some of the highest PFOS concentrations compared with the Florida sites. In Florida, WO exhibited mid to high concentrations of PFOS, PFHxS, and PFDA compared with other sites sampled. For many years, WO has been used as a reference site for multiple studies on alligator ecotoxicology because of its relatively low concentrations of organochlorine contaminants, such as DDT, its metabolites, and other organochlorine pesticides [42]. The results of the present study indicate that WO would not be a suitable reference site for future studies involving PFAAs. In contrast to WO, sites 2A and 3A, which are located in the Everglades, exhibited some of the lowest concentrations of PFOS and PFHxS measured in Florida. Surprisingly similar levels of PFOS were found in loggerhead sea turtles (3.67 ng/g) from Florida Bay close to the 2A and 3A alligator sampling sites [38]. Interestingly, whereas PFAA concentrations appear to be relatively low in 2A and 3A alligators, the same adult alligators at these sites have been reported to contain some of the highest mercury concentrations in Florida and throughout the range of the species [25,43,44].
Correlations
For all alligators included in the present study, snout–vent length was uniform across sites for males and nearly uniform across sites for females (Supplemental Data, Figure S4). Thus, data from all sites were combined within each sex to investigate relationships between PFAA concentration and alligator snout–vent length. Because MI had a very different pattern of PFAAs compared with the other sites, correlations were run with and without this site included. The significance did not change based on the inclusion or exclusion of this site, and thus it was included in the analysis. Correlations comparing both male snout–vent length and female snout–vent length to PFAAs resulted in a number of significant positive correlations (Table 2). Overall, females exhibited higher correlation coefficients between PFAA concentration and snout–vent length when compared with males. The highest correlation coefficients for females were with PFTriA, which explained 57.0% of the variation, followed closely by PFOS, which explained 55.1% of the variation. In contrast, the highest correlation coefficients for male snout–vent length and PFAA concentration were for PFUnA, which explained 35.5% of the variation, followed closely by PFOS, which explained 33.1% of the variation. This would seem to refute the hypothesis that female alligators may have a lower PFAA burden than male alligators as a result of maternal transfer of PFAAs during oviposition. Collectively, these data suggest that concentrations of some PFAAs in adult American alligators increase with increasing body size in both males and females. Conversely, Wang et al. [21] found that PFAA (specifically PFUnA, PFDA, and PFNA) concentrations decreased with increasing body size (total length). These observed differences between American and Chinese alligators may be the result of many factors, including the combination of including animals from all sites in the present study, interspecific differences in food consumption, growth rate differences, and differences in body size [45], as well as differences in toxicodynamics and toxicokinetics of PFAAs. In addition, differences in diet and numerous environmental variables between wild (present study) and captive [22] alligators may influence growth and body burdens of PFAAs.
Table 2.
Correlation coefficients between plasma perfluoroalkyl acid concentrations and snout–vent length for American alligators (Alligator mississippiensis) sampled in Florida and South Carolina, USAa
| Snout–vent length | PFOA | PFNA | PFDA | PFUnA | PFDoA | PFTA | PFTriA | PFHxS | PFOS |
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 65) | 0.072 | 0.252b,c* | 0.206 | 0.355b** | 0.279b* | 0.209 | 0.273b* | 0.273b* | 0.331b,c** |
| Female (n = 60) | 0.133 | 0.261b,c* | 0.443 b,c** | 0.489b** | 0.469b** | 0.468b,c** | 0.570b** | 0.412b** | 0.551b,c** |
All significant results were positively correlated. Values were calculated using log normal concentrations.
Indicates significant correlation coefficients.
Indicates a correlation coefficient determined using the Pearson correlation. All other correlation coefficients were determined using the Spearman correlation.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; PFDA = perfluorodecanoic acid; PFUnA = perfluoroundecanoic acid; PFDoA = perfluorododecanoic acid; PFTA = perfluorotetradecanoic acid; PFTriA = perfluorotridecanoic acid; PFHxS = perfluorohexanesulfonic acid; PFOS = perfluorooctane sulfonate.
With all sites combined for each sex, significant correlations were observed between different PFAAs measured in plasma, suggesting somewhat similar sources of PFAA contamination across the sampling localities. The varying levels of PFAA contamination from site to site are likely the result of varying distances from these potential PFAA sources. Some correlative relationships between the PFAAs were stronger than others (Table 3). Of all the PFAAs, correlations between PFUnA and PFDoA for male (p <0.01, r = 0.920) and female (p <0.01, r = 0.938) alligators across the sites were the most highly significant relationships observed in the present study.
Table 3.
Correlation coefficients between concentrations of various perfluoroalkyl acids in plasma of American alligators (Alligator mississippiensis) sampled in Florida and South Carolina (nmale = 65, nfemale = 60)a
| PFOA | PFNA | PFDA | PFUnA | PFDoA | PFTriA | PFTA | PFHxS | PFOS | |
|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||
| PFOA | — | 0.615b** | 0.226 | 0.092 | 0.036 | 0.152 | 0.181 | 0.260 | 0.386b** |
| PFNA | — | 0.550b** | 0.322b* | 0.144 | 0.313b* | 0.273b** | 0.339b** | 0.541b** | |
| PFDA | — | 0.840b** | 0.743b** | 0.439b** | 0.654b** | 0.307b* | 0.550b** | ||
| PFUnA | — | 0.920b** | 0.783b** | 0.826b** | 0.445b** | 0.654b** | |||
| PFDoA | — | 0.751b** | 0.846b** | 0.316b* | 0.528b** | ||||
| PFTriA | — | 0.770b** | 0.395b** | 0.489b** | |||||
| PFTA | — | 0.238 | 0.399b** | ||||||
| PFHxS | — | 0.827b** | |||||||
| PFOS | — | ||||||||
| Female | |||||||||
| PFOA | — | 0.648b** | 0.332b* | 0.186 | 0.098 | 0.064 | −0.003 | 0.441b** | 0.440b** |
| PFNA | — | 0.585b** | 0.444b** | 0.365b** | 0.339b** | 0.196 | 0.387b** | 0.538b** | |
| PFDA | — | 0.890b** | 0.827b** | 0.529b** | 0.560b** | 0.337b** | 0.595b** | ||
| PFUnA | — | 0.938b** | 0.684b** | 0.578b** | 0.331b* | 0.691b** | |||
| PFDoA | — | 0.763b** | 0.708b** | 0.226 | 0.635b** | ||||
| PFTriA | — | 0.713b** | 0.190 | 0.598b** | |||||
| PFTA | — | 0.130 | 0.454b** | ||||||
| PFHxS | — | 0.654b** | |||||||
| PFOS | — | ||||||||
Values were calculated using log normal concentrations. All significant correlations resulted in positive correlation coefficients.
Indicates significant correlation coefficients.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
PFOA = perfluorooctanoic acid; PFNA = perfluorononanoic acid; PFDA = perfluorodecanoic acid; PFUnA = perfluoroundecanoic acid; PFDoA = perfluorododecanoic acid; PFTriA = perfluorotridecanoic acid; PFTA = perfluorotetradecanoic acid; PFHxS = perfluorohexanesulfonic acid; PFOS = perfluorooctane sulfonate.
CONCLUSIONS
The present study is the first to quantitate PFAA concentrations in American alligators and one of the few studies to quantitate PFAAs in crocodilians [20–22]. All alligator samples (n = 125) contained the 5 following PFAAs: PFOS (median, 11.2 ng/g; range, 1.36–452 ng/g), PFUnA (median, 1.58 ng/g; range, 0.314–18.4 ng/g), PFDA (median, 1.20 ng/g; range, 0.169–15.1 ng/g), PFNA (median, 0.528 ng/g; range, 0.155–1.40 ng/g), and PFHxS (median, 0.288 ng/g; range, 0.057–23.3 ng/g). The present findings support sex-based differences in PFOS and PFUnA concentrations previously observed in captive Chinese alligators [21], while demonstrating opposite relationships between PFAA concentration and body size for American (wild) and Chinese (captive) alligators. The high number of significant PFAA-to-PFAA correlations suggests common point sources throughout the sampling sites in Florida and South Carolina. The present study also reveals potential hot spots for various PFAAs (e.g., PFOS at KA and MI) that warrant further investigation and provides another contaminant of concern to be combined with organochlorines, metals, and others when assessing overall anthropogenic impacts on ecosystem health.
Acknowledgments
The present project was completed as part of the 2014 Fort Johnson Research Experience for Undergraduates program, operated by the College of Charleston and supported by the US National Science Foundation (DBI-1359079), and as part of the 2015 Summer Undergraduate Research Program, operated by the Medical University of South Carolina and supported by the Marine Biomedicine and Environmental Sciences Director’s Fund. Finally, we thank our collaborators for assistance and support: South Carolina Department of Natural Resources, Tom Yawkey Wildlife Center, Florida Fish and Wildlife Conservation Commission, Kiawah Nature Conservancy, and Integrated Mission Support Service (Titusville, FL, USA). The present work would not have been possible without the passion, guidance, and support of L.J. Guillette, Jr. His mentorship and friendship will be greatly missed.
Footnotes
Supplemental Data—The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3600.
Disclaimer—Certain commercial equipment or instruments are identified to specify adequately the experimental procedures. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the equipment or instruments are the best available for the purpose.
Data availability—Data are available upon request from the author (jessica.reiner@nist.gov). Additional sample information and data are provided in Supplemental Data.
This article includes online-only Supplemental Data.
References
- 1.Renner R. The long and the short of perfluorinated replacements. Environ Sci Technol. 2006;40:12–13. doi: 10.1021/es062612a. [DOI] [PubMed] [Google Scholar]
- 2.Betts KS. Perfluoroalkyl acids—What is the evidence telling us? Environ Health Perspect. 2007;115:A250–A256. doi: 10.1289/ehp.115-a250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paustenbach DJ, Panko JM, Scott PK, Unice KM. A methodology for estimating human exposure to perfluorooctanoic acid (PFOA): A retrospective exposure assessment of a community (1951–2003) J Toxicol Environ Health A. 2006;70:28–57. doi: 10.1080/15287390600748815. [DOI] [PubMed] [Google Scholar]
- 4.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Enviro Assess Manage. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody CA, Field JA. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ Sci Technol. 2000;34:3864–3870. [Google Scholar]
- 6.Houde M, De Silva AO, Muir DCG, Letcher RJ. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environ Sci Technol. 2011;45:7962–7973. doi: 10.1021/es104326w. [DOI] [PubMed] [Google Scholar]
- 7.Place BJ, Field JA. Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ Sci Technol. 2012;46:7120–7127. doi: 10.1021/es301465n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter SK. The shrinking case for fluorochemicals. Chem Eng News. 2015;93:27–29. [Google Scholar]
- 9.Houtz EF, Higgins CP, Field JA, Sedlak DL. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ Sci Technol. 2013;47:8187–8195. doi: 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- 10.Heuvel JPV, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi H, Iwata H, Kim E-Y, Tao L, Kannan K, Amano M, Miyazaki N, Tanabe S, Batoev VB, Petrov EA. Contamination and effects of perfluorochemicals in baikal seal (Pusa sibirica). 1. Residue level, tissue distribution, and temporal trend. Environ Sci Technol. 2008;42:2295–2301. doi: 10.1021/es072054f. [DOI] [PubMed] [Google Scholar]
- 12.Rotander A, Kärrman A, van Bavel B, Polder A, Rigét F, Auđunsson GA, Víkingsson G, Gabrielsen GW, Bloch D, Dam M. Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009. Chemosphere. 2012;86:278–285. doi: 10.1016/j.chemosphere.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 13.Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- 14.Christensen KY, Thompson BA, Werner M, Malecki K, Imm P, Anderson HA. Levels of persistent contaminants in relation to fish consumption among older male anglers in Wisconsin. Int J Hyg Environ Health. 2015;219:184–194. doi: 10.1016/j.ijheh.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol Pathol. 2012;40:300–311. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- 16.Anderson M, Butenhoff J, Chang S-C, Farrar D, Kennedy G, Lau C, Olsen G, Seed J, Wallace K. Perfluoralkyl acids and related chemistries—Toxicokinetics and modes of action. Toxicol Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- 17.Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environ Sci Eur. 2011;23:38–90. [Google Scholar]
- 18.Grün F, Blumberg B. Minireview: The case for obesogens. Mol Endocrinol. 2009;23:1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiner JL, Place BJ. Perfluorinated alkyl acids in wildlife. In: DeWitt JC, editor. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Springer; Cham, Switzerland: 2015. pp. 127–150. [Google Scholar]
- 20.Bouwman H, Booyens P, Govender D, Pienaar D, Polder A. Chlorinated, brominated, and fluorinated organic pollutants in Nile crocodile eggs from the Kruger National Park, South Africa. Ecotoxicol Environ Saf. 2014;104:393–402. doi: 10.1016/j.ecoenv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhang Y, Zhang F, Yeung LW, Taniyasu S, Yamazaki E, Wang R, Lam PK, Yamashita N, Dai J. Age- and gender-related accumulation of perfluoroalkyl substances in captive Chinese alligators (Alligator sinensis) Environ Pollut. 2013;179:61–67. doi: 10.1016/j.envpol.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Christie I, Reiner JL, Bowden JA, Botha H, Cantu TM, Govender D, Guillette MP, Lowers RH, Luus-Powell WJ, Pienaar D. Perfluorinated alkyl acids in the plasma of South African crocodiles (Crocodylus niloticus) Chemosphere. 2016;154:72–78. doi: 10.1016/j.chemosphere.2016.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamlin HJ, Lowers RH, Guillette LJ. Seasonal androgen cycles in adult male American alligators (Alligator mississippiensis) from a barrier island population. Biol Reprod. 2011;85:1108–1113. doi: 10.1095/biolreprod.111.092692. [DOI] [PubMed] [Google Scholar]
- 24.Parrott BB, Bowden JA, Kohno S, Cloy-McCoy JA, Hale MD, Bangma JT, Rainwater TR, Wilkinson PM, Kucklick JR, Guillette LJ. Influence of tissue, age, and environmental quality on DNA methylation in Alligator mississippiensis. Reproduction. 2014;147:503–513. doi: 10.1530/REP-13-0498. [DOI] [PubMed] [Google Scholar]
- 25.Nilsen FM, Parrott BB, Bowden JA, Kassim BL, Somerville SE, Bryan TA, Bryan CE, Lange TR, Delaney JP, Brunell AM. Global DNA methylation loss associated with mercury contamination and aging in the American alligator (Alligator mississippiensis) Sci Total Environ. 2016;545:389–397. doi: 10.1016/j.scitotenv.2015.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allsteadt J, Lang JW. Sexual dimorphism in the genital morphology of young American alligators, Alligator mississippiensis. Herpetologica. 1995;51:314–325. [Google Scholar]
- 27.National Institute of Standards and Tecnhology. Certficiate of analysis: Standard Reference Material ® 1958 Organic Contaminants in Fortified Human Serum (Freeze-Dried) 2015 [cited 2015 July 13]. Available from: https://www-s.nist.gov/m-srmors/certificates/1958.pdf.
- 28.Reiner JL, Phinney KW, Keller JM. Determination of perfluorinated compounds in human plasma and serum standard reference materials using independent analytical methods. Anal Bioanal Chem. 2011;401:2899–2907. doi: 10.1007/s00216-011-5380-x. [DOI] [PubMed] [Google Scholar]
- 29.Keller JM, Kannan K, Taniyasu S, Yamashita N, Day RD, Arendt MD, Segars AL, Kucklick JR. Perfluorinated compounds in the plasma of loggerhead and Kemp’s Ridley sea turtles from the southeastern coast of the United States. Environ Sci Technol. 2005;39:9101–9108. doi: 10.1021/es050690c. [DOI] [PubMed] [Google Scholar]
- 30.Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem Biol Interact. 2001;134:203–216. doi: 10.1016/s0009-2797(01)00155-7. [DOI] [PubMed] [Google Scholar]
- 31.Gannon SA, Johnson T, Nabb DL, Serex TL, Buck RC, Loveless SE. Absorption, distribution, metabolism, and excretion of [1-14C]-perfluorohexanoate ([14C]-PFHx) in rats and mice. Toxicology. 2011;283:55–62. doi: 10.1016/j.tox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. Renal elimination of perfluorocarboxylates (PFCAs) Chem Res Toxicol. 2012;25:35–46. doi: 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- 33.Gebbink WA, Letcher RJ. Linear and branched perfluorooctane sulfonate isomer patterns in herring gull eggs from colonial sites across the Laurentian Great Lakes. Environ Sci Technol. 2010;44:3739–3745. doi: 10.1021/es100474r. [DOI] [PubMed] [Google Scholar]
- 34.Joanen T, McNease L. A telemetric study of nesting female alligators on Rockefeller Refuge, Louisiana. Proceedings, Annual Conference of the Southeast Association of Game and Fish Commissioners; Atlanta, GA, USA. September 27–30, 1970; 1970. pp. 175–193. [Google Scholar]
- 35.Joanen T, McNease L. A telemetric study of adult male alligators on Rockefeller Refuge, Louisiana. Proceedings, Annual Conference of the Southeast Association of Game and Fish Commissioners; Knoxville, TN, USA. October 22–25, 1972; 1972. pp. 252–275. [Google Scholar]
- 36.Hutton J. Movements, home range, dispersal and the separation of size classes in Nile crocodiles. Am Zool. 1989;29:1033–1049. [Google Scholar]
- 37.Tucker A, McCallum H, Limpus C. Habitat use by Crocodylus johnstoni in the Lynd River, Queensland. J Herpetol. 1997;31:114–121. [Google Scholar]
- 38.O’Connell SG, Arendt M, Segars A, Kimmel T, Braun-McNeill J, Avens L, Schroeder B, Ngai L, Kucklick JR, Keller JM. Temporal and spatial trends of perfluorinated compounds in juvenile loggerhead sea turtles (Caretta caretta) along the east coast of the United States. Environ Sci Technol. 2010;44:5202–5209. doi: 10.1021/es9036447. [DOI] [PubMed] [Google Scholar]
- 39.Keller JM, Ngai L, McNeill JB, Wood LD, Stewart KR, O’Connell SG, Kucklick JR. Perfluoroalkyl contaminants in plasma of five sea turtle species: Comparisons in concentration and potential health risks. Environ Toxicol Chem. 2012;31:1223–1230. doi: 10.1002/etc.1818. [DOI] [PubMed] [Google Scholar]
- 40.Laitinen JA, Koponen J, Koikkalainen J, Kiviranta H. Fire-fighters’ exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol Lett. 2014;231:227–232. doi: 10.1016/j.toxlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 41.de Solla SR, De Silva AO, Letcher RJ. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environ Int. 2012;39:19–26. doi: 10.1016/j.envint.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102:680. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heaton-Jones TG, Homer BL, Heaton-Jones D, Sundlof SF. Mercury distribution in American alligators (Alligator mississippiensis) in Florida. J Zoo Wildl Med. 1997;28:62–70. [PubMed] [Google Scholar]
- 44.Chumchal MM, Rainwater TR, Osborn SC, Roberts AP, Abel MT, Cobb GP, Smith PN, Bailey FC. Mercury speciation and biomagnification in the food web of Caddo Lake, Texas and Louisiana, USA, a subtropical freshwater ecosystem. Environ Toxicol Chem. 2011;30:1153–1162. doi: 10.1002/etc.477. [DOI] [PubMed] [Google Scholar]
- 45.Herbert J, Coulson T, Coulson R. Growth rates of Chinese and American alligators. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:909–916. doi: 10.1016/s1095-6433(02)00027-2. [DOI] [PubMed] [Google Scholar]