Abstract
Mutations in the epidermal growth factor receptor (EGFR) are commonly occurring in glioblastoma. Enhanced activation of EGFR can occur through a variety of different mechanisms, both ligand-dependent and ligand-independent. Numerous evidence has suggested that EGFR is overexpressed in most of primary glioblastomas and some of the secondary glioblastomas and is characteristic of more aggressive glioblastoma phenotypes. Additionally, recent studies have revealed that wild-type EGFR, and to a greater extent hyper-activating EGFR mutants induced a substantial upregulation of Fyn expression. Furthermore, it was determined that Fyn expression is upregulated across a panel of patient-derived glioblastoma stem cells (GSCs) relative to normal progenitor controls. Moreover, researchers are continuously involved in elucidation of novel mechanism linking EGFR EGFR-expressing glioblastoma. The present review highlights current aspects of EGFR receptor in glioblastoma and concludes that the concept of EGFR signaling and related receptors and associated factors is evolving, however, it needs detailed evaluation for future clinical applications in cancer patients.
Keywords: epidermal growth factor receptor, glioblastoma, oncology, nervous system
1. Introduction
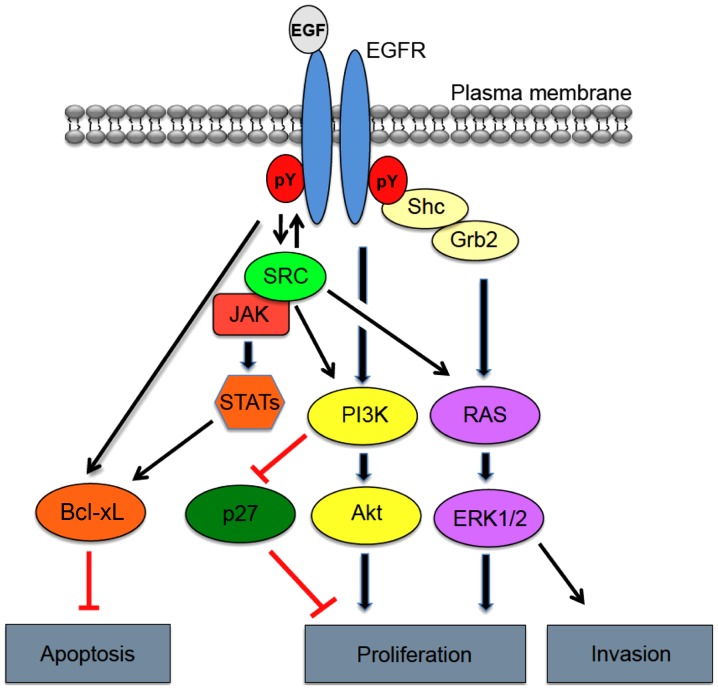
Epidermal growth factor receptor (EGFR), also referred to as HER1/ErbB1, belongs to a larger family of ErbB receptors with tyrosine kinase activity (1,2). Other members of the HER family include ErbB2/HER2, ErbB3/HER3 and ErbB4/HER4. EGFR is frequently overexpressed and/or hyper-activated in human malignancies, including glioblastoma, and therefore EGFR-directed therapeutic strategies are often utilized. Increased activation of EGFR can occur through a variety of different mechanisms, both ligand-dependent and ligand-independent (3–5). Among these mechanisms include: Aberrant enhancement of ligand production; constitutive receptor activation by multiple exon deletion or missense mutations (6); crosstalk with other receptors; increased receptor protein level via gene amplification; and malfunction in receptor degradation. EGFR overexpression and activation are known to significantly impact cancer cell hallmark traits, such as increased cell survival, proliferation and invasion (7) (Fig. 1).
Figure 1.
Epidermal growth factor receptor (EGFR) signaling and related pathways in cancer. Illustration of the gene products involved in transferring signals from the outside of the cell to the nucleus mediated by EGFR, notably through SRC. Grb2, growth factor receptor-bound protein 2; STAT, signal transducer and activator of transcription; PI3K, phosphoinositide 3-kinase.
2. EGFR alterations in glioblastoma
EGFR is overexpressed in ~60% of primary glioblastomas versus only 10% of secondary glioblastomas and is characteristic of more aggressive glioblastoma phenotypes. In addition to overexpression, several alternative mechanisms account for aberrant induction of EGFR activation in glioblastoma, including enhanced autocrine expression of cognate ligands (8). Gene amplification and mutation of EGFR also enhance EGFR activation and occur in upwards of 57% of glioblastomas as determined by the TCGA dataset (9). From a subtype perspective, classical glioblastoma are synonymous with focal amplification of EGFR (~95%), whereas mesenchymal, neural and proneural glioblastomas are associated with reduced rates of EGFR amplification at 29, 67 and 17%, respectively. Mutations of EGFR occur in roughly one-third of all classical tumors and often in mesenchymal, proneural and neural glioblastomas as well (10). Of these mutations, extracellular domain EGFR mutations are most commonly observed in glioblastoma (11).
The most frequently occurring EGFR mutation in glioblastoma, EGFRΔIII, arises from an in-frame deletion of 801 bp in the DNA sequence encoding the extracellular domain, rendering a truncated yet constitutively active form of the receptor (12) EGFRΔIII is a cancer specific mutation, as it not detected in normal tissues, making it an attractive target for therapeutic intervention. Several different studies (12–14) have indicated that EGFRΔIII is expressed in ~50% of glioblastomas that amplify wild-type EGFR (13). Additionally, data mined from the TCGA indicates that EGFRΔIII is most commonly present in the classical tumors (23%), where EGFR amplification is most prevalent.
Despite being constitutively active, EGFRΔIII sustains a low-level signal capable of evading internalization and downregulation, which primarily result from inefficient dimerization (14). In contrast, wild-type EGFR is rapidly degraded following acute stimulation with ligand (15). Though low-level in nature, constitutive signaling downstream of EGFRΔIII leads to increased glioblastoma cell survival in vivo through selective augmentation of various mitogenic factors, namely Akt and repression of apoptosis via enhanced Bcl2 family member expression (16).
EGFRΔIII has also been associated with transformative properties, as INK4A/Arf depleted astrocytes and neural stem cells form high grade tumors in vivo when expressing EGFRΔIII (17). Given this, EGFRΔIII may act as a critical initiating event in tumor development. Not only is EGFRΔIII likely an important factor in gliomagenesis, but the tumorigenic potential of glioma cells in vivo are significantly increased by EGFRΔIII expression when compared to xenografts expressing the wild-type EGFR (18,19). Studies have also shown that EGFRΔIII-expressing glioblastoma cells are approvingly resilient towards both chemotherapy as well as radiation (19–21). Interestingly, recent reports indicate that co-expression of EGFRΔIII and the GSC marker CD133+ defines a population of GSCs harboring the greatest tumor-initiating ability, thus further defining its importance in glioblastoma. Taken together, it is not surprising that EGFRΔIII expression has been strongly associated with a poor survival prognosis for patients whose tumors amplify EGFR (21). In addition to EGFRΔIII, sequence analysis of the EGFR coding region in a cohort of 151 glioblastoma tumors and cell lines identified a number of novel ectodomain missense mutations (22). Approximately 14% of glioblastoma patient samples and 13% of glioblastoma cell lines displayed this form of mutation. Using missense mutants encoding R108K, T263P, A289V, G598V, and L861Q it was determined that these mutations were: i) hyper-phosphorylated receptor in the absence of ligand; ii) accompanied by an increased EGFR gene dosage; and iii) exhibited a stronger transforming phenotype relative to wild-type EGFR as determined by anchorage-independent growth in NIH-3T3 cells. Importantly, of the missense mutations evaluated, EGFR-R108K shares the greatest degree of signaling and behavioral homology to EGFRΔIII, particularly as it relates to therapeutic resistance (23).
3. EGFR therapies in glioblastoma
Overexpression of EGFR has been noted in multiple epithelial tumors, supporting the notion that deregulated EGFR expression and signaling are pivotal events in the origin of human cancers (24). This led to the development of multiple inhibitors of EGFR, including EGFR-targeted monoclonal antibodies (mAB) such as mAB C225 69 and mAB 528 (25). Mechanistically, EGFR-directed mAbs compete with cognate ligands for binding, effectively down-regulating receptor expression and leading to inhibition of cell growth by induction of cell cycle arrest (26). Initially, mAB C225, dubbed cetuximab, demonstrated promising antitumor effects in cell cultures and xenograft models, leading to its implementation as a therapeutic agent (27). Since, cetuximab has been approved for use in metastatic colorectal cancer (CRC) as well as squamous cell carcinoma of the head and neck (HNSCC) (28,29). Cetuximab has additionally been under evaluation in progressive non-small cell lung cancer (NSCLC), where activating mutations of EGFR commonly occur (30). Notably, preclinical studies in glioblastoma cell cultures and mouse models have demonstrated the antitumor and radio-sensitizing effects of cetuximab in this setting (31). Preclinical data also suggest that cetuximab is active against EGFRΔIII, where it binds to and engenders receptor internalization, rendering a reduction in kinase activation (32). Though cetuximab has displayed promising effects in clinical trials involving CRC, HNSCC and NSCLC, phase I/II trials in patients with recurrent glioblastoma have failed to confer any efficacious advantages over standard of care regimens (33). Insufficient intratumoral accumulation of cetuximab was cited in the failed inhibition of EGFR autophosphorylation and degradation in these studies.
Small molecule tyrosine kinase inhibitors (TKIs) that competitively target receptor catalytic activity via the EGFR kinase domain adenosine triphosphate (ATP)-binding pocket, present another approach to targeting EGFR (34). Despite being low in molecular weight and more likely to penetrate the BBB, the specificity of these inhibitors is diminished by the fact that the EGFR ATP-binding pocket shares homology with that of other RTKs, resulting in off-target effects (35). Three TKIs of EGFR (gefitinib, erlotinib and lapatinib) have previously received regulatory approval for use in NSCLC and breast cancer (36). In contrast, several phase II clinical trials evaluating gefitinib, erlotinib or lapatinib in newly diagnosed or recurrent glioblastoma have yielded minimal clinical activity as either a monotherapy or in combination regimens (37–39). The lack of clinical effects were attributed to insufficient inhibition of Akt activation, which correlated most strongly with EGFRΔIII expression and loss of PTEN. Collectively, these findings highlight the need for novel therapeutic targets capable of improving clinical responses in this deadly disease.
4. Dysregulated EGFR signaling networks in glioblastoma
The EGFR family is a complex system involved in growth factor cellular signaling. Phosphorylation of EGFR at the plasma membrane leads to the recruitment of multiple effector proteins via recognition and binding of Src homology 2 (SH2) and phosphotyrosine-binding (PTB) domains to phosphotyrosine motifs on the receptor. Formation of the EGFR signaling complex, in turn, triggers a variety signaling cascades involved in tumor cell proliferation, angiogenesis, motility, differentiation, and survival (Fig. 1) (40). Interestingly, similar substrates are activated downstream of EGFR and EGFRΔIII, but with differing levels of intensity. These pathways include the phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription 3 (STAT3) pathways and Src family kinases (41).
5. PI3K
The class IA PI3Ks form heterodimers that are recruited to trigger RTKs and adaptor proteins through regulatory subunits, including p85a, p55a and p50a, or PIK3R1; p85b or PIKR2; and p55y or PIKR3. p85a associates with EGFR either through ErbB3 heterodimerization or through phosphorylation of EGFR by the SFK c-Src (42). p85a association with EGFR results in a conformational change in p85a, releasing the inhibition of the catalytic subunit p110 of PI3K. PI3K then localizes to the plasma membrane, where it functions to catalyze the formation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) via the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2). The resulting PIP3 is a critical activator of Akt, which consequently phosphorylates, or inhibits, numerous target proteins involved in regulating cellular metabolism, motility and protein synthesis (43). Akt activation additionally results in phosphorylation of Bad, a Bcl family member, which when phosphorylated fails to inhibit the survival protein Bcl-xL, thus precluding apoptotic induction (44). Activation of PI3K can also arise from point mutations, of which ~15% have been catalogued in glioblastoma tumors. These mutations occur most commonly in the adaptor-binding domain (ABD) and less frequently in the C2 helical and kinase domains of the catalytic subunit (PIK3CA) (45). Though mutations in the regulatory subunit (PI3KR1) are uncommon, prior sequencing analysis from the TCGA indicated the presence of 9 such mutations occurring among a cohort of 91 glioblastoma samples. As a result, aberrant PI3K activation and subsequent activation of Akt is observed in upwards of 85% of glioblastoma samples (46). PI3K signaling is negatively regulated by various proteins, most notably PTEN; PTEN, however, is commonly inactivated (~50%) in glioblastoma by either epigenetic silencing or deletion mutation (37). Loss of PTEN, therefore, disrupts the PI3K:PTEN balance resulting in increased Akt activation and uncontrolled cell growth. Given the frequency of PI3K pathway aberrations occurring in glioblastoma, inhibition of its signaling components is an important contribution for a therapeutic avenue. Based on this, the rapamycin analogs, everolimus (Afinitor) and temsirolimus (Torisel), both of which inhibit mammalian target of rapamycin complex 1 (mTORC1) are regulatory-approved for treatment of advanced renal cell carcinoma and have been evaluated in glioblastoma patients. Unfortunately, the clinical application of rapamycin analogs has yielded infrequent and short-lived responses in glioblastoma. Additionally, the PKC/PI3K/AKT inhibitor, enzastaurin, was the first targeted therapy for glioblastoma evaluated in a phase III clinical trial (47).
6. MAPK
Following EGFR activation, the MAPK signaling pathway is triggered by the growth factor receptor-bound protein 2 (Grb2) binding directly to EGFR via Y1068 and Y1086 or indirectly by SHC binding Y1173 and Y1143 (48). Grb2 also houses 2 SH3 domains, allowing for interactions with proline-rich sequences, namely those of son of sevenless (SOS) (49). The Grb2/Shc/EGFR interaction precedes recruitment of SOS to the plasma membrane. SOS is a guanine nucleotide exchange factor, which functions to promote the conversion of Ras-GDP to the active Ras-GTP. Subsequently, Ras activates Raf, a serine-threonine protein kinase, which then phosphorylates and activates MEK1/2, resulting in activation of ERK1/2 (MAPK) (50).
7. Conclusions
The present study concludes that the concept of EGFR signaling and related receptors and associated factors is evolving, however, it needs detailed evaluation for future clinical applications in cancer patients.
References
- 1.Brosseau S, Viala M, Varga A, Planchard D, Besse B, Soria JC. 3rd generation's TKI in lung cancer non-small cell EGFR-mutated having acquired a secondary T790M resistance. Bull Cancer. 2015;102:749–757. doi: 10.1016/j.bulcan.2015.05.001. (In French) [DOI] [PubMed] [Google Scholar]
- 2.Ymer SI, Greenall SA, Cvrljevic A, Cao DX, Donoghue JF, Epa VC, Scott AM, Adams TE, Johns TG. Glioma specific extracellular missense mutations in the first cysteine rich region of epidermal growth factor receptor (EGFR) initiate ligand independent activation. cancers (Basel) 2011;3:2032–2049. doi: 10.3390/cancers3022032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Lorinczi M, Ireton K, Elferink LA. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the cMet-tyrosine kinase. J Biol Chem. 2007;282:16764–16775. doi: 10.1074/jbc.M610835200. [DOI] [PubMed] [Google Scholar]
- 4.Shinomiya N, Gao CF, Xie Q, Gustafson M, Waters DJ, Zhang YW, Woude GF Vande. RNA interference reveals that ligand-independent met activity is required for tumor cell signaling and survival. Cancer Res. 2004;64:7962–7970. doi: 10.1158/0008-5472.CAN-04-1043. [DOI] [PubMed] [Google Scholar]
- 5.Pillay V, Allaf L, Wilding AL, Donoghue JF, Court NW, Greenall SA, Scott AM, Johns TG. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frederick L, Eley G, Wang XY, James CD. Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro Oncol. 2000;2:159–163. doi: 10.1093/neuonc/2.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertotti A, Burbridge MF, Gastaldi S, Galimi F, Torti D, Medico E, Giordano S, Corso S, Rolland-Valognes G, Lockhart BP, et al. Only a subset of Met-activated pathways are required to sustain oncogene addiction. Sci Signal. 2009;2:ra80–ra80. doi: 10.1126/scisignal.2000643. [DOI] [PubMed] [Google Scholar]
- 8.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 9.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Cancer genome atlas research network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100:2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas; Proc Natl Acad Sci USA; 1992; pp. 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 14.Hwang Y, Chumbalkar V, Latha K, Bogler O. Forced dimerization increases the activity of ΔEGFR/EGFRvIII and enhances its oncogenicity. Mol Cancer Res. 2011;9:1199–1208. doi: 10.1158/1541-7786.MCR-11-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt MHH, Furnari FB, Cavenee WK, Bögler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization; Proc Natl Acad Sci USA; 2003; pp. 6505–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagane M, Coufal F, Lin H, Bögler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 17.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/S1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity; Proc Natl Acad Sci USA; 1994; pp. 7727–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavenee WK. Genetics and new approaches to cancer therapy. Carcinogenesis. 2002;23:683–686. doi: 10.1093/carcin/23.5.683. [DOI] [PubMed] [Google Scholar]
- 20.Lammering G, Valerie K, Lin PS, Hewit TH, Schmidt-Ullrich RK. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. 2004;72:267–273. doi: 10.1016/j.radonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Vivanco I, Beroukhim R, Huang JHY, Feng WL, DeBiasi RM, Yoshimoto K, King JC, Nghiemphu P, Yuza Y, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WKA, Gilbert MR, Aldape KA, Wen PY, et al. North American Brain Tumor Consortium: A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-oncol. 2010;12:95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libermann TA, Razon N, Bartal AD, Yarden Y, Schlessinger J, Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44:753–760. [PubMed] [Google Scholar]
- 25.Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: Identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody; Proc Natl Acad Sci USA; 1983; pp. 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waksal HW. Role of an anti-epidermal growth factor receptor in treating cancer. Cancer Metastasis Rev. 1999;18:427–436. doi: 10.1023/A:1006302101468. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 28.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, et al. EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 29.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 30.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, et al. FLEX Study Team: Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 31.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Neurosurgery. Vol. 56. discussion 162; 2005. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo; pp. 155–162. [DOI] [PubMed] [Google Scholar]
- 32.Patel D, Lahiji A, Patel S, Franklin M, Jimenez X, Hicklin DJ, Kang X. Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Anticancer Res. 2007;27(5A):3355–3366. [PubMed] [Google Scholar]
- 33.Stragliotto G, Vega F, Stasiecki P, Gropp P, Poisson M, Delattre JY. Multiple infusions of anti-epidermal growth factor receptor (EGFR) monoclonal antibody (EMD 55,900) in patients with recurrent malignant gliomas. Eur J Cancer. 1996;32A:636–640. doi: 10.1016/0959-8049(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J. Targeting tyrosine kinases in cancer: The second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 35.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, Kubek S, Oldrini B, Chheda MG, Yannuzzi N, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2:458–471. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann M, Krause M, Dikomey E, Dittmann K, Dörr W, Kasten-Pisula U, Rodemann HP. EGFR-targeted anti-cancer drugs in radiotherapy: Preclinical evaluation of mechanisms. Radiother Oncol. 2007;83:238–248. doi: 10.1016/j.radonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- 38.van den Bent MJ, Brandes A, Rampling R, Kouwenhoven M, Kros JM, Carpentier AF, Clement P, Klughammer B, Gorlia T, Lacombe D. Randomized phase II trial of erlotinib (E) versus temozolomide (TMZ) or BCNU in recurrent glioblastoma multiforme (GBM): EORTC 26034. J Clin Oncol. 2005;25:2007. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schaiquevich P, Mason W, Easaw J, Belanger K, Forsyth P, McIntosh L, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: Clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 40.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/S0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 41.Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: Correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 42.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 43.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao TT, Le Francois BG, Goss G, Ding K, Bradbury PA, Dimitroulakos J. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: Potential regulation by targeting rho proteins. Oncogene. 2010;29:4682–4692. doi: 10.1038/onc.2010.219. [DOI] [PubMed] [Google Scholar]
- 45.Gallia GL, Rand V, Siu I-M, Eberhart CG, James CD, Marie SKN, Oba-Shinjo SM, Carlotti CG, Caballero OL, Simpson AJG, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–714. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Wang H, Zhang W, Huang HJ, Liao WSL, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 47.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batzer AG, Rotin D, Ureña JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192–5201. doi: 10.1128/MCB.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 50.Marshall CJ. Cell signalling. Raf gets it together. Nature. 1996;383:127–128. doi: 10.1038/383127a0. [DOI] [PubMed] [Google Scholar]