Abstract
Breast cancer (BC) is a leading cause of cancer-associated mortality in females worldwide. MicroRNAs (miRNAs or miRs), a type of non-coding RNA, have been reported to be important in the regulation of BC onset and progression. Several studies have implicated the role of miR-183 and miR-494 in different types of cancer. However, the biological functions of these miRNAs in BC remain largely unknown. In the present study, the expression of both miRNAs was assessed in the MDA-MB-231 and MDA-MB-468 BC cell lines. It was hypothesized that miR-183 and miR-494 serve an important role in regulating the expression of key genes associated with the metastatic phenotype of BC cells. To further understand their role, the expression of these miRNAs was restored in selected BC cell lines. Functional assays revealed that overexpression of miR-183 or miR-494 modulated the proliferation and migration of MDA-MB-231 and MDA-MB-468 cells in vitro. Additionally, retinoblastoma 1 (RB1) was identified to be a downstream target of both miRNAs by in silico analysis. Western blotting revealed that upregulation of miR-183 was associated with downregulation of RB1 protein in MDA-MB-231 cells. In conclusion, the present results support the hypothesis that miR-183 and miR-494 serve a pivotal role in BC metastasis, and that miR-183 may act as an oncogene by targeting RB1 protein in MDA-MB-231 cells.
Keywords: microRNAs, biomarkers, breast cancer, RB1 protein, cancer cell line, functional assays, in vitro
Introduction
MicroRNAs (miRNAs or miRs) are small non-coding RNAs (~22 nucleotides) that serve important roles in the regulation of gene expression at the post-transcriptional level. The regulation process can occur by degradation or inhibition of translation of their target messenger RNAs through base pairing at 3′ or 5′ untranslated regions (1,2). miRNAs are involved in important biological processes such as cell differentiation, proliferation and death (3). Furthermore, dysregulation of miRNA expression is associated with multiple diseases, including several types of cancer such as lymphoma, glioblastoma, and colorectal, lung and breast cancer (BC) (4).
The importance of miRNAs in cancer is supported by the fact that ~50% of miRNAs are located at fragile sites or genomic regions associated with diseases (5–7), as well as by their ability to act as tumor suppressors or oncogenes (8–10). Additionally, miRNA expression signatures can be used to clearly categorize different solid tumors (11). In BC, miRNA signatures can help to discriminate between normal and cancer tissues (12). Furthermore, miRNAs are involved in cancer initiation and progression, consequently regulating the processes of invasion and metastasis in several types of cancer (13,14). miR-10b and miR-335 were the first miRNAs to be described as promoters or inhibitors of metastasis, respectively (15). In BC, certain miRNAs have been associated with the invasion-metastasis cascade, including let-7, miR-10b, miR-21, miR-34, miR-200 and miR-335 (16).
In a previous study, our group demonstrated that miR-183 and miR-494 are potential biomarkers of metastasis in BC (17). miR-183 is located on chromosome 7q32 and is dysregulated in several types of cancer, including BC, hepatic, colorectal and lung cancer, as well as leukemia and osteosarcoma (18). A recent study reported that miR-183 is downregulated in retinoblastoma tumor tissues and cell lines compared with its expression in their normal counterparts (19). Furthermore, ectopic expression of miR-183 inhibited cell migration and invasion in vitro (19). Similarly, miR-494 was observed to be downregulated in human gastric carcinoma tissues and cell lines, in which the restoration of miR-494 expression decreased the cell proliferation rate (20). These findings indicate that both miRNAs are implicated in the regulation of biological processes that are important in cancer progression, thus demonstrating the requirement for further studies to evaluate their involvement in other cancer types. Therefore, the present study evaluated the expression and functional role of miR-183 and miR-494 in BC cell lines, uncovering new evidence of the involvement of both miRNAs in BC onset and progression.
Materials and methods
Cell culture
Seven BC cell lines (MCF-7, MCF-7/AZ, T47D, BT-20, Hs578T, MDA-MB-231 and MDA-MB-468) were evaluated in the present study. The cell lines, which were a kind donated by Dr Rui M. Reis (Barretos Cancer Hospital, Barretos, Brazil) in August 2012, were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin-streptomycin solution (Sigma-Aldrich; Merck KGaA). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Authentication of all BC-derived cell lines was performed by short tandem repeat DNA typing according to the International Reference Standard for Authentication of Human Cell Lines, as previously reported (21). All the cell lines had their genotype confirmed.
Total RNA isolation
Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA quantification was performed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and RNA quality was assessed using RNA 6000 Nano chips (Agilent Technologies, Inc., Santa Clara, CA, USA) in a 2100 Bioanalyzer (Agilent Technologies, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNA expression was assessed by RT-qPCR using TaqMan® MicroRNA Assays (Thermo Fisher Scientific, Inc.). Reverse transcription of primers to the sequence of miR-183 (5′-UAUGGCACUGGUAGAAUUCACU-3′) and miR-494, (5′-AGGUUGUCCGUGUUGUCUUCUCU-3′) and the complementary DNA synthesis was performed with total RNA (10 ng) using TaqMan® Small RNA Assays (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
The PCR with a final volume of 10 µl was performed at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. All reactions were performed in triplicate a 7900HT Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The analyses were performed using the R statistical computing environment (https://www.r-project.org) according to the 2−ΔΔCq method (22). The small non-coding nucleolar RNA RNU48 provided in the TaqMan® Control MicroRNA Assay kit (Thermo Fisher Scientific, Inc.) was used as a control for the analysis.
miRNA transfection
Homo sapiens (hsa)-miR-183 and hsa-miR-494 Pre-miR™ miRNA Precursors (Ambion; Thermo Fisher Scientific, Inc.) were transfected into MDA-MB-231 and MDA-MB-468 BC cells using siPORT™ Amine Transfection Agent (Thermo Fisher Scientific, Inc.) at a final concentration of 20 nM, according to the manufacturer's protocol. Cells transfected with the Pre-miR™ miRNA Precursor negative control (Ambion; Thermo Fisher Scientific, Inc.) were used as a control for the transfection. The efficiency of overexpression of miR-183 and miR-494 was evaluated at 24, 48 and 72 h after transfection by RT-qPCR.
Proliferation and migration assays
Proliferation and migration assays were carried out in the xCELLigence RTCA DP Instrument (Roche Applied Science, Rotkreuz, Switzerland), using the E-Plate for measuring proliferation and the CIM-Plate for measuring migration (both from Roche Applied Science). The xCELLigence system transforms automatically the impedance of electron flow caused by cells in a cell index (CI) value according to the formula CI = (impedance at time point n-impedance in the absence of cells) / nominal impedance value (23). The experiments were performed according to the manufacturer's protocol. For the proliferation assay, concentrations of 6×103 and 8×103 MDA-MB-231 and MDA-MB-468 cells, respectively, were used. For the migration assay, 2×104 cells were used for both BC cell lines. The CI value was monitored for 72 h in the proliferation assay and for 24 h in the migration assay.
In silico target prediction of miR-183 and miR-494
Target prediction was performed using the miRWalk2.0, Micro T4, miRMap, PITA and RNAhybrid algorithms in the comparative platform of miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html). The criteria for miRNA target gene selection was identification in the above algorithms and presence in both miRNAs. The targets were then filtered by Breast Neoplasm (code C2910) National Cancer Institute disease term using the cancer gene index of Reactome FI Cytoscape Plugin 4 (http://wiki.reactome.org/index.php/Reactome_FI_Cytoscape_Plugin). Finally, targets were enriched by biological process using BiNGO Cytoscape plugin using a hypergeometric test with false discovery rate correction (P≤0.05) with the GoSlim Gene Ontology dataset (24).
Western blot assay
Cells transfected with each miRNA were lysed directly on ice using lysis buffer (50 mM Tris pH 7.6–8.0, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 mM sodium pyrophosphate and 1% NP-40) supplemented with a protease inhibitor cocktail (1 mM dithiothreitol, 1 µg/ml leupeptin hemisulfate, 1 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride and 1 mM EDTA). The lysates were centrifuged at 10,000 × g for 15 min at 5°C and the supernatant was stored at −80°C. The soluble proteins were quantitated using Bradford Reagent (Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocol. Total protein (20 µg) was separated by SDS-PAGE (10% gel) and transferred onto a Hybond-C-supported nitrocellulose membrane (GE Healthcare Life Sciences, Chalfont, UK) using a Mini-PROTEAN Trans-Blot® Turbo Transfer System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 100 V for 30 min. Membranes were blocked using TBS with 0.1% Tween-20 and 5% powdered milk at room temperature for 1 h, and then incubated at 5°C overnight in a 1:500-dilution of anti-RB1 mouse polyclonal antibody (#sc-102, Santa Cruz Biotechnology, Inc. MA, USA) in blocking buffer. Upon washing with TBS-T (0.1%), the membranes were probed using an anti-mouse anti-rabbit secondary antibody (#7076, Cell Signaling Technology, Inc., Danvers, MA, USA) at dilution 1:5,000 for 2 h at room temperature. Subsequently, specific binding was detected with enhanced chemiluminescence (GE Healthcare Life Sciences). The detection of the chemiluminescent signal was performed in the gel documentation system ImageQuant LAS 4000 Mini (GE Healthcare Life Sciences). Subsequently, the labeled bands were analyzed and quantified using ImageJ software (version 1.49v; NIH-Scion Corporation, Bethesda, MD, USA) and bar graphs represent densitometric analysis of each band that were plotted using the gplots R package (https://cran.r-project.org/web/packages/gplots/index.html) (25,26). Normalization was performed using β-tubulin as the endogenous control (#3873, Cell Signaling Technology, Inc.) at dilution 1:5,000 for 2 h at room temperature.
Statistical analysis
Statistical analysis was performed using the R statistical computing environment (https://www.r-project.org). Data are displayed as mean values ± standard error of ≥3 independent experiments. Differences were evaluated with the Student's t-test. P<0.05 was considered to indicate a significant difference.
Results
Overexpression and functional analysis of mir-183 in BC cell lines
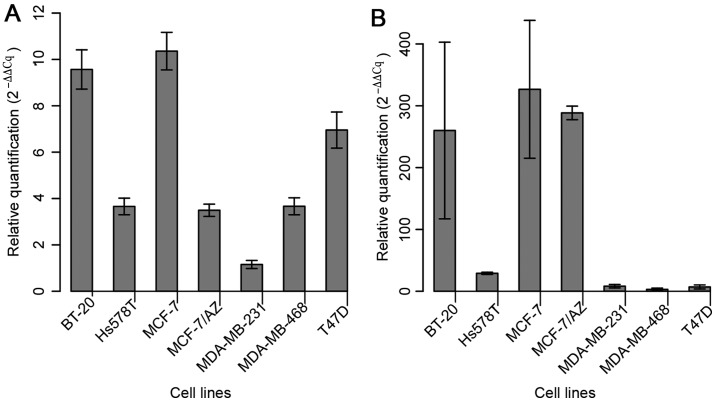
The expression of miR-183 was assessed in the aforementioned seven BC cell lines in order to select those with the lowest levels of miR-183 to further evaluate its role in gain-of-function assays (Fig. 1A). MDA-MB-231 cells exhibited the lowest basal level of miR-183; considering that this cell line represents a model of the basal-like molecular subtype (more aggressive) of BC, it was selected for the functional approach. Among the cell lines that displayed the second lowest level of expression (Hs578T, MCF-7/AZ and MDA-MB-468), MDA-MB-468 was selected due to its low expression level of miR-494 (Fig. 1B).
Figure 1.
The expression of (A) miR-183 and (B) miR-494 was evaluated by reverse transcription-quantitative polymerase chain reaction in a panel of breast cancer cell lines. miR, microRNA.
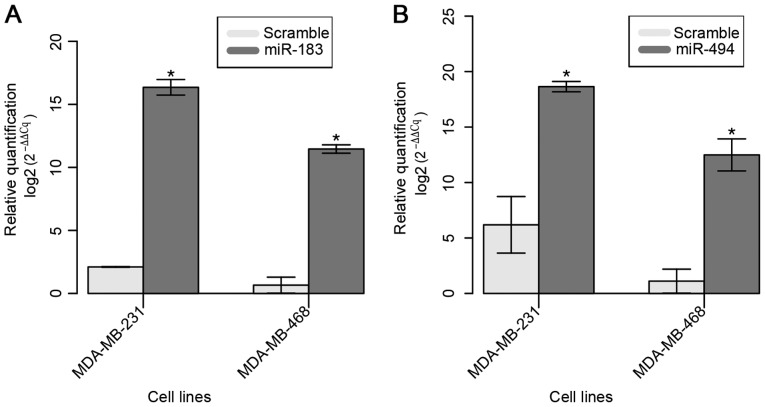
The restoration of miR-183 expression in the selected two BC cell lines was confirmed 24 h after Pre-miR™ miRNA Precursor transfection (Fig. 2A). A mean 15-fold increase in the expression of miR-183 was observed in transfected cell lines compared with that in parental cell lines.
Figure 2.
Expression of miR-183 and miR-494 by reverse transcription-quantitative polymerase chain reaction at 24 h post-transfection. (A) miR-183 and (B) miR-494 were overexpressed in MDA-MB-231 cells compared with their expression levels in control cells. Data are displayed as mean values ± standard error of ≥3 independent experiments. Differences were evaluated with the Student's t-test. *P<0.05. miR, microRNA.
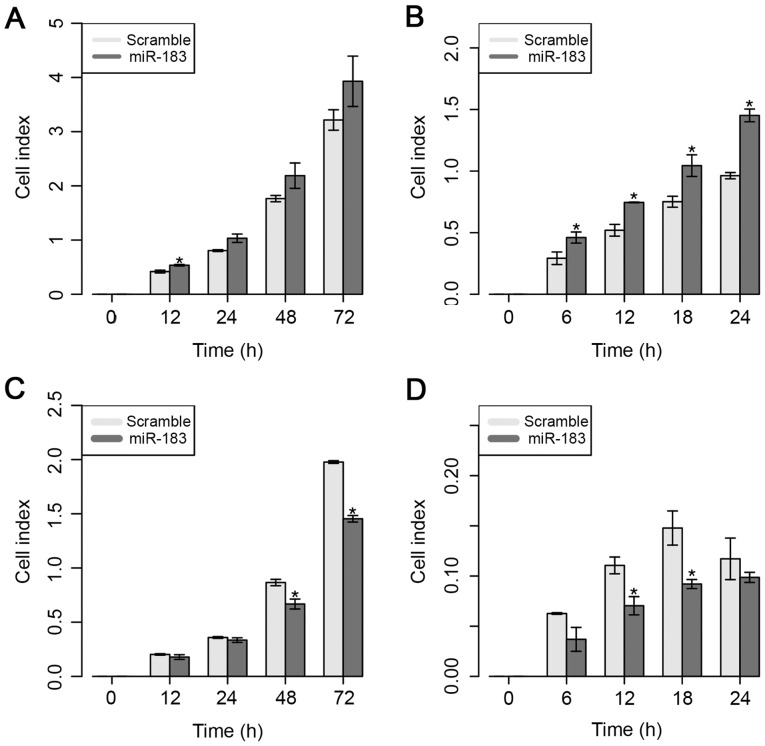
Next, the effect of ectopic expression of miR-183 on the proliferative and migratory capacities of MDA-MB-231 cells was evaluated, as shown in Fig. 3. Overexpression of miR-183 induced a significant increase in proliferative capacity in vitro at 12 h; however, a higher proliferative rate at 24 and 48 h was also observed, even though these findings were not significant (Fig. 3A). Regarding the migratory ability in vitro, the present results demonstrated that the overexpression of miR-183 significantly increased the migratory ability from 6 to 24 h post-transfection (Fig. 3B).
Figure 3.
Functional assays following miR-183 overexpression. (A) Proliferation was evaluated in the MDA-MB-231 cell line. (B) A migration assay was conducted in the MDA-MB-231 cell line. (C) A proliferation assay was performed in the MDA-MB-468 cell line. (D) Migration was assessed in the MDA-MB-468 cell line. Data are displayed as mean values ± standard error of ≥3 independent experiments. Differences were evaluated with the Student's t-test. *P<0.05. miR, microRNA.
By contrast, overexpression of miR-183 significantly reduced cell proliferation at 48 and 72 h in the MDA-MB-468 cell line (Fig. 3C). Similarly, the present results demonstrated that overexpression of miR-183 also significantly inhibited cell migration at 12 and 18 h post-transfection (Fig. 3D). These results suggest that the overexpression of miR-183 significantly increased the proliferative and migratory abilities of both cell lines, which suggests the potential role of this miRNA in BC cellular behavior.
Overexpression and functional analysis of miR-494 in the MDA-MB-231 BC-derived cell line
The aforementioned seven BC cell lines were used to evaluate the basal expression of miR-494. The results indicated that the MDA-MB-231 and MDA-MB-468 cell lines had the lowest basal levels of miR-494; therefore, these lines were selected for the functional evaluation of this miRNA (Fig. 1B).
The restoration of miR-494 expression in both cell lines was confirmed at 24 h after Pre-miR™ miRNA Precursor transfection (Fig. 2B). A mean increase of ~10-fold in miR-183 expression was observed compared with that in the parental cell lines.
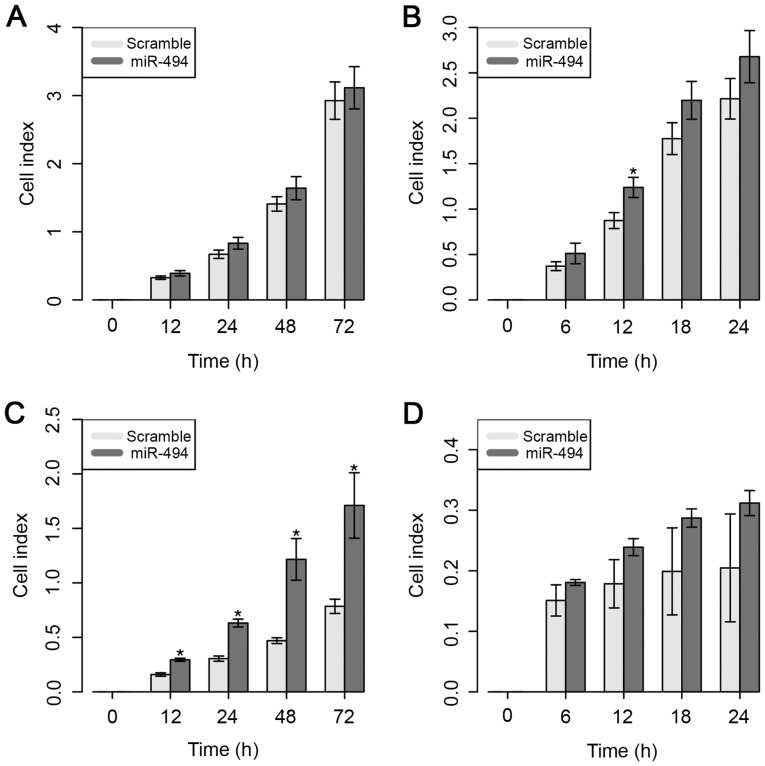
Despite the higher CI value, the ectopic expression of miR-494 did not result in a significant difference in the cell proliferative ability of MDA-MB-231 cells (Fig. 4A). However, overexpression of miR-494 significantly increased the cell migratory capacity of MDA-MB-231 cells, but only at 12 h after in vitro transfection (Fig. 4B).
Figure 4.
Functional assays following miR-494 overexpression. (A) Proliferation and (B) migration assays were conducted in the MDA-MB-231. (C) Proliferation and (D) migration assays were conducted in the MDA-MB-468 cell line. Data are displayed as mean values ± standard error of at ≥3 independent experiments. Differences were evaluated with the Student's t-test. *P<0.05. miR, microRNA.
The results for the MDA-MB-468 cell line demonstrated that cell proliferation was significantly increased 12 h after overexpression of miR-494 (Fig. 4C). However, no significant impact on cell migratory ability was observed in this cell line (Fig. 4D).
These results suggest that the overexpression of miR-494 does not necessarily control the ability of the cells to migrate, but appears to be involved in the significant inhibition of cell proliferative ability in the MDA-MB-231 cell line.
Identification and evaluation of RB1 protein expression by western blotting
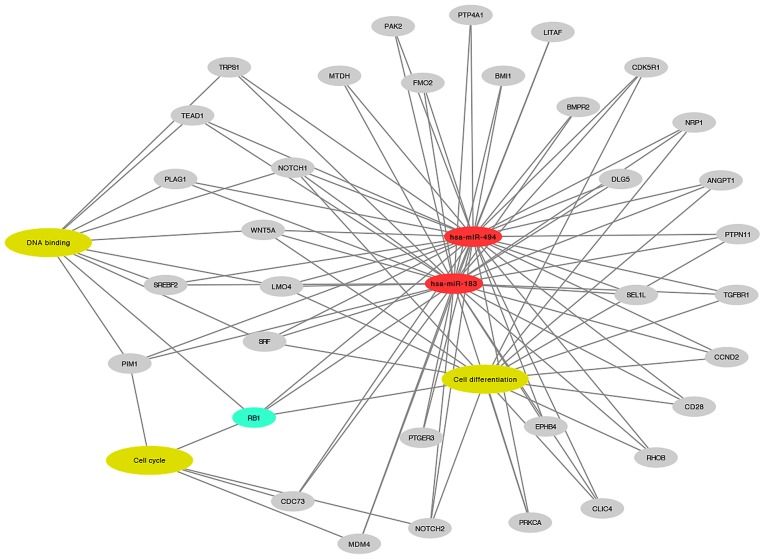
In silico analysis highlighted several targets of miR-183 and miR-494, as shown in Fig. 5. RB1 was selected for western blot validation due to its involvement in enriched biological processes associated with BC, including cell differentiation (P=4.09×10−7), cell cycle (P=3.58×10−3) and DNA binding (P=2.65×10−2). Among these three processes, RB1 was the only target identified to be involved in cell differentiation and cell cycle progression.
Figure 5.
Network interactions of the common targets of miR-183 and miR-494. RB1 was observed to be involved in cancer-associated biological processes. miR, microRNA; RB1, retinoblastoma 1; hsa, Homo sapiens.
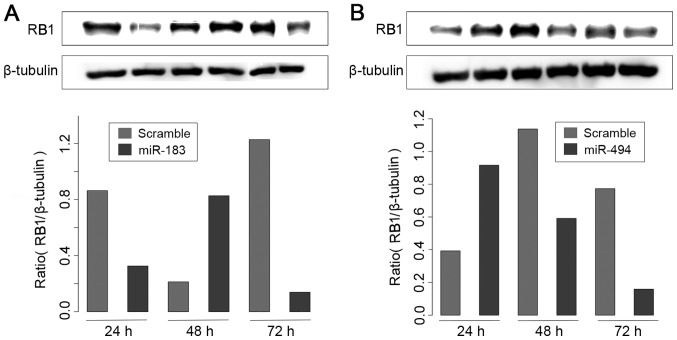
It was observed that transfection of miR-183 resulted in a significant reduction in RB1 protein levels in MDA-MB-231 cells (Fig. 6A). By contrast, miR-494 transfection did not produce an effect on RB1 protein levels (Fig. 6B). These results suggest that miR-183 may act as a potential regulator of RB1 protein, at least in a subset of BC cell lines.
Figure 6.
RB1 protein expression analysis by western blotting following overexpression of (A) miR-183 and (B) miR-494 in the MDA-MB-231 cell line. Two western blot experiments were performed and the bar graphs demonstrate the average of the two density results.
Discussion
Several miRNAs have been associated with BC progression (12). However, the mechanism involved in miRNA-controlled metastasis is not completely understood yet (16). Evidence from a previous study by our group (17) and from studies about the roles of miR-183 and miR-494 in other cancer types (18–20) suggests that these miRNAs are dysregulated in BC.
Considering the expression of these two miRNAs in a panel of BC cell lines, MDA-MB-231 and MDA-MB-468 were selected for functional ectopic overexpression assays due to the low levels of expression of both miRNAs in these two cell lines. According to Subik et al (27), the MDA-MB-231 cell line is characterized as a triple-negative/basal B mammary carcinoma. Haga and Phinney (28) evaluated the expression of miR-494 in MDA-MB-231 cells by qPCR and revealed a low expression level, thus corroborating the findings of the present study. Additionally, Lowery et al (29) also performed in vitro overexpression of miR-183 but in the T47D cell line. According to the present findings, the T47D cell line was not the best model for the study, compared with the other BC cell lines tested. Indeed, the authors observed that overexpression of miR-183 in the T47-D cell line resulted in an increase in migration, but without a significant difference when compared with that exhibited by the negative control (29).
For the functional assays, the xCELLigence System was selected in the present study. Limame et al (30) performed a comparative analysis between the xCELLigence System and conventional functional cellular assays, showing a strong correlation (>90%) between the two methods. The present results demonstrated that the ectopic overexpression of miR-183 affected the behavior of the MDA-MB-231 and MDA-MB-468 cell lines in a contradictory manner. Overexpression of miR-183 resulted in an increase in the proliferative and migratory capacities of the cells over time (proliferation was significantly increased only at 12 h). By contrast, in MDA-MB-468 cells, the overexpression of miR-183 inhibited cell proliferation after 48 and 72 h, and cell migration after 12 and 18 h compared with the control (scramble miRNA). Similarly, Zhao et al showed that the overexpression of miR-183 in an osteosarcoma cell line with a high invasive capacity (F5M2) led to the inhibition of cell migration and invasion, which is partly in agreement with the results obtained in MDA-MB-468 cells (18). Overexpression of miR-494 had no significant effect on cell proliferation in the MDA-MB-231 cell line; however, an increase in cell migratory ability was observed after 12 h of overexpression in vitro. The overexpression of miR-494 increased the cell proliferative capacity of MDA-MB-468 cells after 12 h, but did not affect their migration. Accordingly, the results obtained with overexpression of miR-494 in the MDA-MB-231 cells were similar to those of a recent study, which noticed that the ectopic expression of this miRNA inhibited cell proliferation in a lung cancer cell line (A549) (31).
The differences in proliferative and migratory behavior between the two cell lines evaluated in the present study can be explained on the basis of their molecular subtype, since the MDA-MB-231 cell line is an invasive ductal carcinoma, while the MDA-MB-468 cell line is a metastatic basal-like adenocarcinoma (32). Consequently, these cell lines have different genotypes with specific mutations in genes that control the mechanisms of cell proliferation and apoptosis, including cyclin-dependent kinase (CDK) inhibitor 2A, BRAF, KRAS, tumor protein P53, phosphatase and tensin homolog, neurofibromin 2, RB1 and SMAD family member 4 (33,34). It has been widely reported that various MDA-MB-468 clones have a homozygous deletion of RB1, which can explain such differences in cellular behavior (35) but this finding has no influence on the results of the present study.
Among the genes evaluated in the present study, miR-183 and miR-494 possibly regulated the expression of RB1, a tumor suppressor that negatively regulates the cell cycle. Cell cycle control by RB1 involves other factors, including CDKs-cyclins, which phosphorylate the RB1 protein, thus rendering it inactive. Inactive RB1 subsequently releases E2 factor, a transcription factor that activates the transcription of several genes that promote the cell cycle transition from G1 to S phase or activate cell proliferation (36). In the present study, RB1 protein expression was negatively regulated at 72 h after overexpression of miR-183 and miR-494 in the MDA-MB-231 cell line. Thus, the inhibition of proliferation induced by miR-183 in MDA-MB-231 cells may be modulated via the downregulation of RB1.
In conclusion, the present study provided evidence that miR-183 and miR-494 may be associated with the progression of BC. Furthermore, the present results suggest a new perspective for future research on the potential role of these miRNAs in the regulation of key metastatic biological processes during BC progression.
Acknowledgements
The authors thank Dr Rene A.C. Vieira (Barretos Cancer Hospital, Brazil) for his suggestions. The present study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP, São Paulo, Brazil (grant nos. 2010/16796-0 and 2012/17111-7) and partially supported by FINEP (grant no. MCTI/FINEP/MS/SCTIE/DECIT-01/2013-FPXII-BIOPLAT).
References
- 1.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011;121:141–158. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 2.Da Sacco L, Masotti A. Recent insights and novel bioinformatics tools to understand the role of microRNAs binding to 5′untranslated region. Int J Mol Sci. 2012;14:480–495. doi: 10.3390/ijms14010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer PS. Cancer genomics: Small RNAs with big impacts. Nature. 2005;435:745–746. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 5.Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers; Proc Natl Acad Sci USA; 2004; pp. 2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia; Proc Natl Acad Sci USA; 2002; pp. 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 9.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets; Proc Natl Acad Sci USA; 2006; pp. 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 13.Galasso M, Sandhu SK, Volinia S. MicroRNA expression signatures in solid malignancies. Cancer J. 2012;18:238–243. doi: 10.1097/PPO.0b013e318258b5f4. [DOI] [PubMed] [Google Scholar]
- 14.Sassen S, Miska EA, Caldas C. MicroRNA: Implications for cancer. Virchows Arch. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negrini M, Calin GA. Breast cancer metastasis: A microRNA story. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valastyan S. Roles of microRNAs and other non-coding RNAs in breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2012;17:23–32. doi: 10.1007/s10911-012-9241-9. [DOI] [PubMed] [Google Scholar]
- 17.Marino AL, Evangelista AF, Macedo T, Silveira HC, Kerr LM, Vieira RA, Longatto AF, Silveira MM. Differential expression profile of microRNAs associated with human breast cancer progression; BMC Proc; 2013; p. P9. [DOI] [Google Scholar]
- 18.Zhao H, Guo M, Zhao G, Ma Q, Ma B, Qiu X, Fan Q. miR-183 inhibits the metastasis of osteosarcoma via downregulation of the expression of Ezrin in F5M2 cells. Int J Mol Med. 2012;30:1013–1020. doi: 10.3892/ijmm.2012.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Wang X, Li Z, Liu H, Teng Y. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014;281:1355–1365. doi: 10.1111/febs.12659. [DOI] [PubMed] [Google Scholar]
- 20.He W, Li Y, Chen X, Lu L, Tang B, Wang Z, Pan Y, Cai S, He Y, Ke Z. miR-494 acts as an anti-oncogene in gastric carcinoma by targeting c-myc. J Gastroenterol Hepatol. 2014;29:1427–1434. doi: 10.1111/jgh.12558. [DOI] [PubMed] [Google Scholar]
- 21.Silva-Oliveira RJ, Silva VA, Martinho O, Cruvinel-Carloni A, Melendez ME, Rosa MN, De Paula FE, de Souza Viana L, Carvalho AL, Reis RM. Cytotoxicity of allitinib, an irreversible anti-EGFR agent, in a large panel of human cancer-derived cell lines: KRAS mutation status as a predictive biomarker. Cell Oncol (Dordr) 2016;39:253–263. doi: 10.1007/s13402-016-0270-z. [DOI] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kammermann M, Denelavas A, Imbach A, Grether U, Dehmlow H, Apfel CM, Hertel C. Impedance measurement: A new method to detect ligand-biased receptor signaling. Biochem Biophys Res Commun. 2011;412:419–424. doi: 10.1016/j.bbrc.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 24.Maere S, Heymans K, Kuiper M. BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girish V, Vijayalakshmi A. Affordable image analysis using NIH Image/ImageJ. Indian J Cancer. 2004;41:47. [PubMed] [Google Scholar]
- 27.Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, Tang P. The expression patterns of ER PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Haga CL, Phinney DG. MicroRNAs in the imprinted DLK1-DIO3 region repress the epithelial-to-mesenchymal transition by targeting the TWIST1 protein signaling network. J Biol Chem. 2012;287:42695–42707. doi: 10.1074/jbc.M112.387761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowery AJ, Miller N, Dwyer RM, Kerin MJ. Dysregulated miR-183 inhibits migration in breast cancer cells. BMC Cancer. 2010;10:502. doi: 10.1186/1471-2407-10-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limame R, Wouters A, Pauwels B, Fransen E, Peeters M, Lardon F, De Wever O, Pauwels P. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS One. 2012;7:e46536. doi: 10.1371/journal.pone.0046536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohdaira H, Sekiguchi M, Miyata K, Yoshida K. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012;45:32–38. doi: 10.1111/j.1365-2184.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 34.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuarai S, Machida T, Kobayashi T, Komatani H, Itadani H, Kotani H. Expression ratio of CCND1 to CDKN2A mRNA predicts RB1 status of cultured cancer cell lines and clinical tumor samples. Mol Cancer. 2011;10:31. doi: 10.1186/1476-4598-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinnam M, Goodrich DW. RB1, development, and cancer. Curr Top Dev Biol. 2011;94:129–169. doi: 10.1016/B978-0-12-380916-2.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]